Abstract
CONTEXT:
As many as 1 in every 110 children in the United States has an autism spectrum disorder (ASD). Secretin is 1 of many medical treatments studied for treating the symptoms of ASDs, but there is currently no consensus regarding which interventions are most effective.
OBJECTIVE:
To systematically review evidence regarding the use of secretin in children with ASDs who are aged 12 years and younger.
METHODS:
We searched the Medline, PsycINFO, and ERIC (Education Resources Information Center) databases from 2000 to May 2010 and reference lists of included articles. Two reviewers independently assessed each study against predetermined inclusion/exclusion criteria. Two reviewers independently extracted data regarding participant and intervention characteristics, assessment techniques, and outcomes and assigned overall quality and strength-of-evidence ratings on the basis of predetermined criteria.
RESULTS:
Evidence from 7 randomized controlled trials supports a lack of effectiveness of secretin for the treatment of ASD symptoms including language and communication impairment, symptom severity, and cognitive and social skill deficits. No studies have resulted in significantly greater improvements in measures of language, cognition, or autistic symptoms when compared with placebo; study authors who reported improvement over time did so equally for both the intervention and placebo groups.
CONCLUSIONS:
Secretin has been studied extensively in multiple randomized controlled trials, and there is clear evidence that it lacks benefit. The studies of secretin included in this review uniformly point to a lack of significant impact of secretin in the treatment of ASD symptoms. Given the high strength of evidence for a lack of effectiveness, secretin as a treatment approach for ASDs warrants no further study.
Keywords: autism spectrum disorders, secretin
Secretin is a gastrointestinal polypeptide used to treat peptic ulcers and in the evaluation of pancreatic function.1,2 Results of animal studies have suggested that secretin affects the central nervous system and may function as a neurotransmitter.3,4 Interest in secretin for the treatment of symptoms of autism spectrum disorders (ASDs) stemmed from a nonblinded, uncontrolled case series of 3 children with ASDs who received synthetic intravenous secretin during a routine endoscopy evaluation for gastrointestinal problems.5 The report noted social, cognitive, and communicative gains after the first infusion and after a second infusion given weeks later.5
As part of an Agency for Healthcare Research and Quality–commissioned comparative effectiveness review, we assessed recent research on the use of secretin to treat ASD symptoms in children between the ages of 2 and 12 years with ASDs.6 Information on other interventions addressed in the full review can be found at www.effectivehealthcare.ahrq.gov.
METHODS
Search Strategy
We searched Medline via the PubMed interface, PsycINFO (psychology and psychiatry literature), and ERIC (Education Resources Information Center) (educational literature) from 2000 to May 2010 using relevant controlled vocabulary terms and key terms related to ASDs (eg, autistic disorder) and therapy-related terms (eg, therapeutics, secretin). We also hand-searched the reference lists of all included articles to identify additional studies and reviewed clinical trials related to therapies for ASDs to identify corresponding articles.
Study Selection
We developed study inclusion and exclusion criteria in consultation with an expert panel of clinicians and educators involved in the care of children with ASDs. We included all study designs and required that studies of secretin include at least 30 participants younger than the age of 12 years with ASDs. We also required that studies be published in the year 2000 or later, after the publication of the Diagnostic and Statistical Manual of Mental Diseases, Fourth Edition (DSM-IV)7 and the widespread implementation of gold-standard ASD-assessment tools including the Autism Diagnostic Observation Schedule,8 and the Autism Diagnostic Interview-Revised.9
Data Extraction
Using standardized forms, 2 investigators independently extracted data regarding study design; descriptions of the study populations, intervention, and comparison groups; and baseline and outcome data, as well as data about harms or adverse effects. We also captured data on the conduct and timing of assessments to inform the assessment of quality. Principal outcomes of interest included effects on core symptoms of ASDs or common comorbid symptoms including sleep, anxiety, hyperactivity, and challenging behavior (eg, irritability/agitation).
Study-Quality Assessment
Two investigators independently assessed each study by using a quality-assessment form developed by the review team with input from experts in the field. We evaluated the following elements with a series of yes/no questions in each domain (eg, were outcomes coded and assessed by people blinded to the intervention status of the participants?):
study design;
diagnostic approach;
participant ascertainment and characterization;
intervention description;
outcomes measurement; and
statistical analysis.
Disagreements between assessors were resolved through discussion to reach consensus. Overall assessment of quality was determined by using a prespecified algorithm that is available in the full report.6
We assessed the strength of evidence of the current research by using methods established in the Evidence-Based Practice Centers' methods guide for effectiveness and comparative effectiveness reviews.10 Assessments are based on consideration of 4 domains: risk of bias; consistency in direction of the effect; directness in measuring intended outcomes; and precision of effect (Table 1). We determined the strength of evidence separately for major intervention-outcome pairs by using a prespecified approach that is described in detail in the full review.6
TABLE 1.
Domains Used to Assess Strength of Evidence
| Risk of bias: Reflects issues in study design and conduct that could result in biased estimates of effect |
| Consistency: Reflects similarity of effect sizes seen across studies; consistency cannot be assessed when only 1 study is available |
| Directness: Reflects the relationship between the intervention and the ultimate health outcome of interest |
| Precision: Reflects the level of certainty around the effect observed |
Data Synthesis
Given considerable heterogeneity in the interventions and outcome measures used in studies that met our inclusion criteria, we did not conduct any meta-analyses. We summarized characteristics of study populations and interventions and used descriptive statistics to report study outcomes.
RESULTS
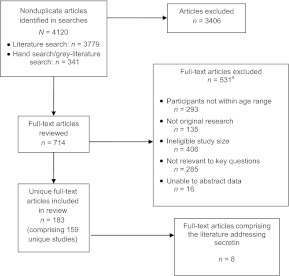
Figure 1 shows the flow of articles retrieved for the review. Among 4120 articles located for the full review, 8 articles11–18 met our inclusion criteria and addressed secretin use in 8 unique populations. Seven studies were randomized controlled trials,11–17 and 1 was a prospective case series.18 We assessed 2 trials as being of good quality,14,15 5 as being of fair quality,11–13,16–17 and the case series as being of poor quality.18
FIGURE 1.
Location of studies that addressed secretin use in children with ASDs.
a The total number of articles in the exclusion categories exceeds the number of articles excluded, because most of the articles fit into multiple exclusion categories.
Of the 8 studies that evaluated the impact of secretin in the treatment of ASDs, 1 study15 was a repeated-dose intervention study. Two studies used synthetic human secretin,11,12 3 used porcine secretin,14,16,17 and 1 used biological secretin;13 1 report did not specify the type of secretin used.18 All of the studies evaluated only short-term outcomes and had follow-up periods that ranged from 3 to 12 weeks. Outcomes assessed included measures of receptive and expressive language, gastrointestinal symptoms, adaptive behavior, cognitive impairments, social skills, and fine motor skills. Participant numbers in the treatment arms of the randomized controlled trials ranged from 21 subjects 12 to 47 subjects.17
No studies revealed significantly greater improvements in measures of language, cognition, or autistic symptoms when compared with placebo. Study authors who reported improvement over time did so equally for both intervention and placebo groups. There also was no benefit demonstrated according to type of secretin (porcine or synthetic).
DISCUSSION
Overall, the studies of secretin included in our review uniformly point to a lack of significant impact of secretin in the treatment of autism symptoms. Similar to our study, a Cochrane review of 13 randomized studies revealed no evidence for the effectiveness of single- or multiple-dose intravenous secretin in children or adults with ASDs.19 Secretin has been studied exhaustively in multiple randomized controlled trials, and there is clear evidence that it lacks benefit for treating children with ASDs.
CONCLUSIONS
Previous studies have demonstrated that secretin is not effective for improving language, cognition, behavior, communication, autism symptom severity, or socialization skills. The strength of evidence for this lack of effectiveness is high. With 7 randomized controlled trials with fair- to good-quality scores and 1 case series that contributes to this evidence base, future studies are unlikely to change the estimate of effect for this treatment. Further studies of secretin in children with ASDs are not warranted.
ACKNOWLEDGMENTS
This project was supported by the Agency for Healthcare Research and Quality (contract number HHSA 290 2007 10065 I). Dr Veenstra-VanderWeele has received research support from the National Institute of Mental Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the Agency for Healthcare Research and Quality.
We acknowledge that all authors of this article meet authorship criteria and have made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; have participated in drafting the article or revising it critically for important intellectual content; and have given final approval of the version to be published.
The authors of this article are responsible for its content. Statements in the report should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services.
FINANCIAL DISCLOSURE: Dr Veenstra-VanderWeele has received research support from Autism Speaks, the American Academy of Child and Adolescent Psychiatry, NARSAD, Seaside Therapeutics, Roche Pharmaceuticals, and Novartis. The other authors have indicated they have no financial relationships relevant to this article to disclose.
COMPANION PAPERS: Companions to this article can be found on pages e1303 and e1312 and online at www.pediatrics.org/cgi/doi/10.1542/peds.2011-0426 and www.pediatrics.org/cgi/doi/10.1542/peds.2011-0427.
- ASD
- autism spectrum disorder
REFERENCES
- 1. Tulassay Z, Bodnar A, Farkas I, Papp J, Gupta R. Somatostatin versus secretin in the treatment of actively bleeding gastric erosions. Digestion. 1992;51(4):211–216 [DOI] [PubMed] [Google Scholar]
- 2. Watanabe Y, Tsumura H, Sasaki H. Effect of continuous intravenous infusion of secretin preparation (secrepan) in patients with hemorrhage from chronic peptic ulcer and acute gastric mucosal lesion (AGML). Gastroenterol Jpn. 1991;26(suppl 3):86–89 [DOI] [PubMed] [Google Scholar]
- 3. Charlton CG, Miller RL, Crawley JN, Handelmann GE, O'Donohue TL. Secretin modulation of behavioral and physiological functions in the rat. Peptides. 1983;4(5):739–742 [DOI] [PubMed] [Google Scholar]
- 4. Fremeau RT, Jr, Jensen RT, Charlton CG, Miller RL, O'Donohue TL, Moody TW. Secretin: specific binding to rat brain membranes. J Neurosci. 1983;3(8):1620–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horvath K, Stefanatos G, Sokolski KN, Wachtel R, Nabors L, Tildon JT. Improved social and language skills after secretin administration in patients with autistic spectrum disorders. J Assoc Acad Minor Phys. 1998;9(1):9–15 [PubMed] [Google Scholar]
- 6. Warren Z, Veenstra-VanderWeele J, Stone W, Bruzek JL, Nahmias AS, Foss-Feig JH, et al. Therapies for Children With Autism Spectrum Disorders. Comparative Effectiveness Review No. 26. (Prepared by the Vanderbilt Evidence-based Practice Center under Contract No. 290-02-HHSA-290-2007-10065-I.) AHRQ Publication No. 11-EHC035-EF. Rockville, MD: Agency for Healthcare Research and Quality; March 2011. Available at: www.effectivehealthcare.ahrq.gov/reports/final.cfm Accessed March 2011 [Google Scholar]
- 7. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- 8. Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223 [PubMed] [Google Scholar]
- 9. Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685 [DOI] [PubMed] [Google Scholar]
- 10. Owens DK, Lohr KN, Atkins D, et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions—Agency for Healthcare Research and Quality and the effective health-care program. J Clin Epidemiol. 2010;63(5):513–523 [DOI] [PubMed] [Google Scholar]
- 11. Levy SE, Souders MC, Wray J, et al. Children with autistic spectrum disorders. I: comparison of placebo and single dose of human synthetic secretin. Arch Dis Child. 2003;88(8):731–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Molloy CA, Manning-Courtney P, Swayne S, et al. Lack of benefit of intravenous synthetic human secretin in the treatment of autism. J Autism Dev Disord. 2002;32(6):545–551 [DOI] [PubMed] [Google Scholar]
- 13. Unis AS, Munson JA, Rogers SJ, et al. A randomized, double-blind, placebo-controlled trial of porcine versus synthetic secretin for reducing symptoms of autism. J Am Acad Child Adolesc Psychiatry. 2002;41(11):1315–1321 [DOI] [PubMed] [Google Scholar]
- 14. Owley T, McMahon W, Cook EH, et al. Multisite, double-blind, placebo-controlled trial of porcine secretin in autism. J Am Acad Child Adolesc Psychiatry. 2001;40(11):1293–1299 [DOI] [PubMed] [Google Scholar]
- 15. Roberts W, Weaver L, Brian J, et al. Repeated doses of porcine secretin in the treatment of autism: a randomized, placebo-controlled trial. Pediatrics. 2001;107(5). Available at: www.pediatrics.org/cgi/content/full/107/5/e71 [DOI] [PubMed] [Google Scholar]
- 16. Coniglio SJ, Lewis JD, Lang C, et al. A randomized, double-blind, placebo-controlled trial of single-dose intravenous secretin as treatment for children with autism. J Pediatr. 2001;138(5):649–655 [DOI] [PubMed] [Google Scholar]
- 17. Dunn-Geier J, Ho HH, Auersperg E, et al. Effect of secretin on children with autism: a randomized controlled trial. Dev Med Child Neurol. 2000;42(12):796–802 [DOI] [PubMed] [Google Scholar]
- 18. Chez MG, Buchanan CP, Bagan BT, et al. Secretin and autism: a two-part clinical investigation. J Autism Dev Disord. 2000;30(2):87–94 [DOI] [PubMed] [Google Scholar]
- 19. Williams KW, Wray JJ, Wheeler DM. Intravenous secretin for autism spectrum disorder. Cochrane Database Syst Rev. 2005;(3):CD003495. [DOI] [PubMed] [Google Scholar]