Abstract
OBJECTIVE:
To assess the efficacy and safety of clonidine hydrochloride extended-release tablets (CLON-XR) combined with stimulants (ie, methylphenidate or amphetamine) for attention-deficit/hyperactivity disorder (ADHD).
PATIENTS AND METHODS:
In this phase 3, double-blind, placebo-controlled trial, children and adolescents with hyperactive- or combined-subtype ADHD who had an inadequate response to their stable stimulant regimen were randomized to receive CLON-XR or placebo in combination with their baseline stimulant medication. Predefined efficacy measures evaluated change from baseline to week 5. Safety was assessed by spontaneously reported adverse events, vital signs, electrocardiogram recordings, and clinical laboratory values. Improvement from baseline for all efficacy measures was evaluated using analysis of covariance.
RESULTS:
Of 198 patients randomized, 102 received CLON-XR plus stimulant and 96 received placebo plus stimulant. At week 5, greater improvement from baseline in ADHD Rating Scale IV (ADHD-RS-IV) total score (95% confidence interval: −7.83 to −1.13; P = .009), ADHD-RS-IV hyperactivity and inattention subscale scores (P = .014 and P = .017, respectively), Conners' Parent Rating Scale scores (P < .062), Clinical Global Impression of Severity (P = .021), Clinical Global Impression of Improvement (P = .006), and Parent Global Assessment (P = .001) was observed in the CLON-XR plus stimulant group versus the placebo plus stimulant group. Adverse events and changes in vital signs in the CLON-XR group were generally mild.
CONCLUSIONS:
The results of this study suggest that CLON-XR in combination with stimulants is useful in reducing ADHD in children and adolescents with partial response to stimulants.
Keywords: α2-adrenergic agonist, attention-deficit/hyperactivity disorder, clonidine–hydrochloride extended-release tablets, psychostimulant
WHAT'S KNOWN ON THIS SUBJECT:
Two small studies have evaluated the safety and efficacy of immediate-release clonidine plus stimulant medication to treat attention-deficit/hyperactivity disorder (ADHD). No previous studies have evaluated the US Food and Drug Administration - approved extended-release formulation of clonidine plus stimulants.
WHAT THIS STUDY ADDS:
In this study information on the safety and efficacy of extended-release clonidine combined with stimulant medication for the treatment of ADHD is provided. The combination of the 2 medications works well to reduce the symptoms of ADHD and is generally well tolerated.
Attention-deficit/hyperactivity disorder (ADHD) is characterized by developmentally inappropriate levels of inattention, impulsivity, and hyperactivity.1 ADHD affects ∼9% of children and adolescents in the United States2 and is often comorbid with other psychiatric disorders.3–5 Stimulant medications (ie, methylphenidate and amphetamine) are often first-line therapies for ADHD,6 but not all patients respond optimally to these medications.7,8 Therefore, a need for additional medication options to treat ADHD in children and adolescents exists.
Clonidine is an α2-adrenergic agonist that has been used to treat ADHD.9–16 A modified oral formulation of clonidine (clonidine hydrochloride extended-release tablets; CLON-XR) (KAPVAY; Shionogi Inc, Florham Park, NJ) was recently approved for treatment of ADHD.17 This modification reduces and delays peak plasma drug concentration compared with the immediate-release oral preperation of clonidie. The efficacy of CLON-XR monotherapy in children and adolescents with ADHD has been demonstrated in Phase 3 clinical trial data.18
An increasing rate of clonidine use in combination with psychostimulants for treating children and adolescents with ADHD has been documented in several studies, with a substantial proportion of stimulant-treated children also receiving clonidine.19,20 Although clonidine is used in combination with stimulants in clinical practice, in few studies have the efficacy and safety of clonidine plus stimulant medication been systematically evaluated for the treatment of neuropsychological disorders. Two small studies (n = 24 and n = 67)10,13 have been conducted in children with ADHD and comorbid aggression or conduct disorder, with mixed results. Given the high rate of treatment with a combination of clonidine plus stimulant in clinical settings,19,20 the efficacy of the extended-release formulation of clonidine as a monotherapy,18 and the need for treatment options for patients with ADHD, the current study was undertaken to assess the efficacy and safety of CLON-XR in combination with stimulants in youth with ADHD.
METHODS
Patient Population
Children and adolescents (aged 6–17 years) with a diagnosis of predominantly hyperactive- or combined-subtype ADHD, on the basis of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision criteria,1 who had a stable regimen of stimulant treatment (ie, methylphenidate or amphetamine) during the previous 4 weeks were included. ADHD was evaluated by using the Kiddie-Sads-Present and Lifetime Version questionnaire 1.0 completed by a trained investigator. Continuous ratings of symptomatology were obtained using the ADHD Rating Scale IV (ADHD-RS-IV) questionnaire.21 To be included in the study, children were required to have inadequate stimulant medication response, defined as a total score ≥ 26 on the ADHD-RS-IV questionnaire after a minimum of 4 weeks on a stable stimulant regimen (ie, ≤10% variation in dose). Additional inclusion criteria were intelligence quotient estimated to be ≥80 by the investigator and a BMI in the ≥5th percentile for the patient's gender and age.
Patients were excluded from participation in the study if they had (1) a current diagnosis or history of a psychiatric disorder that required psychotropic medication or severe comorbid Axis I or Axis II disorder that could interfere with assessment of clonidine efficacy and safety, (2) a history of conduct disorder, (3) a history of syncopal episodes or seizures (except for febrile seizures), (4) current or past drug abuse, (5) a history of clonidine intolerance, or (6) used any investigational drug within 30 days of the study initiation or had a positive drug test (except for ADHD medication). Patients were also excluded if they had a clinically significant illness or abnormality that would increase the safety risk of clonidine or if they had clinically significant electrocardiogram readings. Females of childbearing age who were pregnant or lactating or who refused to use birth control were not allowed to participate. Concomitant use of antihypertensive medications, psychotropic drugs, oral corticosteroids, sedating antihistamines, antidiabetic medications, diet aids, and bronchodilators >3 days per week was not allowed.
The study protocol, amendments, and informed consent form were approved by central and local institutional review boards before study initiation. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice requirements. Written informed consent was obtained from all patients and legal guardians before enrollment.
Study Design
This 8-week, parallel-group, randomized, double-blind, placebo-controlled study was conducted at 22 centers in the United States between March 2008 and February 2009. A screening period of up to 14 days before initiation of dosing was required, during which eligibility was ascertained and baseline efficacy and safety assessments were performed. After baseline assessment, all patients underwent a 1- to 2-week washout period, during which any ADHD medications except methylphenidate- or amphetamine-based products were discontinued. The treatment period consisted of 8 weeks of flexible dose escalation or dose tapering on the basis of patients' response to treatment. Before week 5, physicians were allowed to adjust the dose of CLON-XR by not escalating the dose to the maximal amount for the study (ie, 0.4 mg per day) or by tapering the dose by 0.1 mg per week if safety concerns arose. After week 5, the dose of CLON-XR was tapered by 0.1 mg per week until reaching the lowest possible dose (ie, 0.1 mg per day) on week 8. Investigators were also allowed to adjust the dose of stimulant medication on the basis of the efficacy and safety profile of the patient; however, the category of stimulant could not be altered. Efficacy and safety were assessed during weekly visits.
Patients who completed screening and washout periods were randomly assigned, via block design, to receive total daily doses of CLON-XR of 0.1 to 0.4 mg per day or placebo in addition to the patient's normal regimen of stimulant. Medication (ie, placebo or CLON-XR) was dispensed weekly by the investigator, who was blinded to treatment. At week 1, patients were instructed to take 1 tablet of CLON-XR 0.1 mg or placebo at night in addition to their normal stimulant regimen. Beginning at week 2, the dose of CLON-XR could be increased by increments of 0.1 mg per week to reach the maximal dose. Additional doses of CLON-XR were added to the morning (if > 0.1 mg was administered) or night dosing regimen (eg, patients who received CLON-XR 0.3 mg per day took 1 tablet in the morning and 2 tablets in the evening). Concomitant stimulant medication was prescribed by the patient's regular physician and was obtained from the patient's usual pharmacy. Caregivers recorded the specific amount of CLON-XR and stimulant medication the patient received each week in a medication diary provided by the site personnel. Compliance was assessed by the investigator at each visit via comparison of the number of tablets dispensed for the previous week and the recorded amount of study medication taken.
Study Assessments
The primary efficacy measure was improvement from baseline to week 5 in ADHD-RS-IV total score versus placebo using a last observation carried forward (LOCF) method. The ADHD-RS-IV was completed by the investigator at screening, baseline, and all weekly visits. Additional assessments included improvement in scores from baseline to week 5 compared with placebo on ADHD-RS-IV hyperactivity/impulsivity and inattention subscales, Clinical Global Impression of Severity (CGI-S) and Clinical Global Impression of Improvement (CGI-I) scales, the Conners' Parent Rating Scale, Revised, Long Form (CPRS) hyperactivity and oppositional subscales, and the Parent Global Assessment (PGA) scale using a LOCF method. The ADHD-RS-IV, CGI-S, and CGI-I were completed by the investigator, whereas the CPRS and PGA were evaluated by parents/guardians. Treatment-emergent adverse events (TEAEs), vital signs (eg, blood pressure, heart rate, body temperature), and electrocardiograms were measured throughout the study period.
Statistical Analyses
All predefined primary statistical efficacy analyses were conducted in the intent-to-treat (ITT) population, defined as all patients who were randomly assigned to treatment, took ≥1 dose of study medication, and provided at least 1 efficacy assessment. Sample size calculation using a t test and assuming an effect size of 0.47 (ie, difference of 7 ± 15 [mean ± SD] points on the ADHD-RS-IV total score between groups), at least 90% power, and a 2-sided α level of 0.05 indicated that 100 patients per treatment group were necessary to achieve statistical significance.
Improvement from baseline in ADHD-RS-IV total score to week 5 was evaluated by using analysis of covariance with terms for baseline ADHD-RS-IV score, study site, and treatment group. A LOCF method was used to account for patients who discontinued from the study earlier than week 5. In addition, analysis of covariance with terms for class of concomitant ADHD medication (ie, methylphenidate or amphetamine) was performed on subgroups. P values were derived from 2-sided tests, which were compared with the α level of 0.05 for statistical significance without adjusting for multiple group comparisons. Analyses of secondary end points were similar to those of the primary efficacy variables. Responders were defined posthoc as patients with a ≥30% reduction in ADHD-RS-IV total score from baseline who were considered to be “very much improved” or “much improved” on the investigator-completed CGI-I. Effect size was calculated on the basis of the observed mean differences between the groups and the observed SDs using the unbiased version of Hedges' g. Safety data (ie, TEAEs, vital signs, electrocardiograms) were collected from the defined safety population (ie, all patients who received ≥1 dose of study medication) and were presented as descriptive statistics.
RESULTS
Patient Disposition
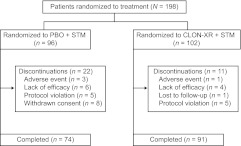
Of 243 patients screened, 198 were randomly assigned to receive CLON-XR plus stimulant (n = 102) or placebo plus stimulant (n = 96) (Fig 1). Reasons for screening failure included not meeting inclusion/exclusion criteria (n = 31), withdrawal of consent (n = 8), being lost to follow-up (n = 4), closure of study enrollment (n = 1), and decision by the study sponsor to exclude the patient (n = 1). One patient in the placebo plus stimulant group was omitted from the ITT population because of a lack of study assessment after baseline. Eight patients (5 in the CLON-XR plus stimulant group and 3 in the placebo plus stimulant group) were enrolled and included in the ITT population despite protocol deviations. Three of these patients had a BMI in the <5th percentile for the patient's age, 1 patient was diagnosed with ADHD of the inattentive subtype, 1 patient had not been on a stable stimulant regimen for 4 weeks before study initiation, and 1 patient had used an investigational topical medication within 30 days before the study. In addition, the class of concomitant psychostimulant used was altered (ie, changed from amphetamine to methylphenidate) in 2 patients (1 per treatment group).
FIGURE 1.
Patient disposition during the treatment phase. PBO indicates placebo; STM, stimulant.
Demographics and the use of concomitant psychostimulants were similar across both groups, and no differences in the use of approved non-ADHD medications (eg, antihistamines) occurred (Tables 1 and 2). Overall compliance was similar at week 4 in the CLON-XR plus stimulant (94%) and the placebo plus stimulant (88%) groups, but a greater percentage of patients in the CLON-XR plus stimulant group (89%) completed the treatment phase of the study compared with the placebo plus stimulant group (77%). Within the CLON-XR plus stimulant group at weeks 4 and 5, 3%, 15%, 68%, and 14% of patients received CLON-XR 0.1 mg per day, CLON-XR 0.2 mg per day, CLON-XR 0.3 mg per day, and CLON-XR 0.4 mg per day, respectively.
TABLE 1.
Patient Demographics and Characteristics (ITT Population)
| Parameter | Patients (N = 197) |
|
|---|---|---|
| Placebo Plus Stimulant (n = 95) | CLON-XR Plus Stimulant (n = 102) | |
| Age, mean (SD), y | 10.5 (2.5) | 10.4 (2.5) |
| Age, n (%), y | ||
| 6–12 | 75 (78.9) | 77 (75.5) |
| >12–17 | 20 (21.1) | 25 (24.5) |
| Gender, n (%) | ||
| Male | 66 (70) | 79 (78) |
| Female | 29 (30) | 23 (22) |
| Race, n (%) | ||
| White | 57 (60.0) | 49 (48.0) |
| Black | 19 (20.0) | 35 (34.3) |
| Hispanic | 11 (11.6) | 11 (10.8) |
| Other | 8 (8.4) | 7 (6.9) |
| Weight, mean (SD), kg | 38.9 (13.6) | 40.2 (18.6) |
| ADHD-RS-IV total score, mean (SD) | 39.0 (7.7) | 38.9 (7.0) |
TABLE 2.
Stable Stimulant Use at Baseline (Safety Population)
| Drug Name | Patients (N = 198) |
|||
|---|---|---|---|---|
| Placebo Plus Stimulant (n = 96) |
CLON-XR Plus Stimulant (n = 102) |
|||
| Patients, n (%) | Mean Dose, mg/d (Range) | Patients, n (%) | Mean Dose, mg/d (Range) | |
| Methylphenidates | ||||
| Any | 59 (62) | NA | 59 (58) | NA |
| Concertaa | 29 (30) | 41.9 (18–72) | 35 (34) | 38.8 (18–72) |
| Focalin XRb | 10 (10) | 18.0 (10–40) | 12 (12) | 16.7 (5–40) |
| Ritalinb | 9 (9) | 17.8 (5–60) | 1 (1) | 30 (NA) |
| Focalinb | 2 (2) | 7.5 (5–10) | 5 (5) | 20 (10–30) |
| Daytranac | 4 (4) | 20.0 (10–30) | 1 (1) | 15 (NA) |
| Metadated | 4 (4) | 27.5 (20–50) | 3 (3) | 23.3 (20–30) |
| Focalin XR and Focalin | 2 (2) | 17.5 (5–15) and 10 (15–20) | 0 (0) | 0 (NA) |
| Ritalin-SRb | 0 (0) | 0 (NA) | 1 (1) | 80 (NA) |
| Amphetamines | ||||
| Any | 37 (38) | NA | 43 (42) | NA |
| Vyvansec | 13 (14) | 51.9 (25–100) | 22 (22) | 48.6 (20–100) |
| Adderall XRc | 13 (14) | 21.5 (10–30) | 17 (17) | 25.9 (5–60) |
| Adderallc | 9 (9) | 23.9 (10–30) | 2 (2) | 45 (30–60) |
| Adderall XR and Adderall | 1 (1) | 30 (NA) and 20 (NA) | 1 (1) | 25 (NA) and 10 (NA) |
| Dexedrinee | 1 (1) | 45 (NA) | 1 (1) | 20 (NA) |
McNeil Pediatrics (Titusville, NJ).
Novartis Pharmaceuticals Corp (East Hanover, NJ).
Shire US Inc (Wayne, PA).
Refers to Metadate CD and Metadate ER, which are both produced by UCB Inc (Smyrna, GA).
GlaxoSmithKline (Research Triangle Park, NC).
Efficacy
Improvement in ADHD-RS-IV total score from baseline was statistically significant between the CLON-XR plus stimulant and the placebo plus stimulant groups beginning at week 2 (ie, first week of potential dose escalation) and was maintained until week 8 (ie, last week of potential dose tapering) (Fig 2). At week 5, patients in the CLON-XR plus stimulant group experienced a significantly greater improvement from baseline in ADHD-RS-IV total score compared with patients in the placebo plus stimulant group (95% confidence interval: −7.83 to −1.13; P = .009) (Table 3). When stimulant class was incorporated into the primary efficacy analysis, no statistically significant difference versus placebo was observed between the CLON-XR plus methylphenidate and CLON-XR plus amphetamine groups (Fig 3). Mean improvement from baseline to week 5 was greater in both the CLON-XR plus methylphenidate (mean change: −14) and the CLON-XR plus amphetamine (mean change: −18) groups versus placebo (mean change: −10 to −14, respectively). Scores from baseline in the inattention and hyperactivity subscales of the ADHD-RS-IV, CPRS, and hyperactivity subscale, CGI-I, CGI-S, and PGA were also significantly improved in the CLON-XR plus stimulant group compared with the placebo plus stimulant group at week 5 (Table 3). A greater percentage of patients in the CLON-XR plus stimulant group were considered responders than patients in the placebo plus stimulant group at week 3 (50% vs 32%; P = .0095), week 4 (52% vs 33%; P = .0065), week 5 (52% vs 32%; P = .0041), week 6 (43% vs 31%; P = .0773), and week 7 (42% vs 25%; P = .0126). Most patients within the safety population did not alter their dosage of concomitant stimulant medication at weeks 4 and 5 in either the CLON-XR plus stimulant (67%) or the placebo plus stimulant group (73%), and no difference between the percentage of patients who increased their dose of stimulant in the CLON-XR plus stimulant (19%) and placebo plus stimulant groups (18%) was observed. However, a greater percentage of patients in the CLON-XR plus stimulant group (15%) decreased their dose of stimulant compared with the placebo plus stimulant group (9%; Fig 4). The overall effect size for CLON-XR plus stimulant versus placebo plus stimulant on the basis of ADHD-RS-IV total score was 0.34.
FIGURE 2.
Improvement in ADHD-RS-IV total score from baseline. Improvement was significantly greater in the CLON-XR plus stimulant group versus the placebo plus stimulant group starting at week 2 and continuing through week 7. PBO indicates placebo; STM, stimulant. aP < .05.
TABLE 3.
Improvement in Efficacy Assessments From Baseline to Week 5
| Efficacy Assessment | Placebo Plus Stimulant (n = 95) | CLON-XR Plus Stimulant (n = 102) | P |
|---|---|---|---|
| ADHD-RS-IV, mean (SD) | |||
| Total change | −11.5 (12.2) | −15.7 (12.3) | .009 |
| Inattention subscale | −5.8 (6.8) | −7.8 (6.8) | .017 |
| Hyperactivity/impulsivity subscale | −5.8 (6.3) | −7.9 (6.7) | .014 |
| CPRS, mean (SD) | |||
| Total change | −27.1 (38.2) | −40.2 (41.4) | .017 |
| Hyperactivity subscale | −3.8 (5.7) | −5.8 (6.5) | .017 |
| Oppositional subscale | −3.6 (6.3) | −5.1 (6.6) | .062 |
| CGI-S, mean (SD) | −1.2 (1.3) | −1.5 (1.2) | .021 |
| CGI-I, mean (SD) | 3.0 (1.2) | 2.5 (1.2) | .006 |
| PGA, mean (SD) | 3.4 (1.4) | 2.7 (1.3) | .001 |
FIGURE 3.
Improvement in ADHD-RS-IV total score from baseline to week 5 in CLON-XR and placebo combined with different classes of stimulants. Improvement in ADHD-RS-IV total score was not statistically significant in patients who received placebo or CLON-XR plus methylphenidate (METH) or amphetamine (AMP). PBO indicates placebo.
FIGURE 4.
Alteration of stimulant medication dosage during the study. A greater percentage of patients in the CLON-XR plus stimulant group (15%) decreased their dose of stimulant compared with patients in the placebo plus stimulant group (10%). PBO indicates placebo; STM, stimulant.
Safety
The percentage of patients who reported ≥1 TEAE thought by the investigator to be related to study medication was 45% and 41% in the CLON-XR plus stimulant and the placebo plus stimulant groups, respectively. Somnolence, headache, fatigue, upper abdominal pain, and nasal congestion were the most commonly reported TEAEs in the CLON-XR plus stimulant group (Table 4). The incidence of TEAEs was numerically higher during weeks 0 to 3 than during weeks 4 to 5 or weeks 6 to 8 for both groups. Within the CLON-XR plus stimulant and the placebo plus stimulant groups, respectively, 50% of patients reported a TEAE during dose titration in both groups, 31% and 23% of patients had a TEAE during weeks 4 and 5, and 26% and 22% of patients reported TEAEs during weeks 6 to 8. Of the 96 patients in the placebo plus stimulant group, 3 (3%) discontinued because of TEAEs (ie, increased heart rate [n = 1], aggression [n = 1], and somnolence [n = 1]), and only 1 of 102 patients (1%) in the CLON-XR plus stimulant group discontinued because of a TEAE (ie, slowed thought processes). Mean systolic and diastolic blood pressure decreased slightly in patients in the CLON-XR plus stimulant group during the study, whereas no change or a 1 mm Hg increase was observed in patients receiving placebo plus stimulant (Table 5). A mean increase in QT interval of 14 milliseconds was observed in the CLON-XR plus stimulant group versus a 1-millisecond increase in the placebo plus stimulant group; slight alterations in this interval were observed in both treatment groups. When QT interval was corrected using Bazett's formula or Fridericia's formula, there were no clinically relevant differences between the placebo plus stimulant and CLON-XR plus stimulant groups in the number of individuals with increased QT interval (Table 5). In addition, no patient had a QT > 450 milliseconds (when QT interval was corrected using Fridericia's formula) or QT > 475 milliseconds (when QT interval was corrected using Bazett's formula).
TABLE 4.
Treatment-Emergent Adverse Events With 5% or Greater Incidence in the CLON-XR + Stimulant (Methylphenidate or Amphetamine) Group (Safety Population)
| Adverse Event | Placebo Plus Stimulant (n = 96), n (%) |
CLON-XR Plus Stimulant (n = 102), n (%) |
||||
|---|---|---|---|---|---|---|
| Any Stimulanta (n = 96) | Methylphenidate (n = 59) | Amphetamine (n = 37) | Any Stimulanta (n = 102) | Methylphenidate (n = 59) | Amphetamine (n = 43) | |
| Somnolenceb | 8 (8) | 6 (10) | 2 (5) | 20 (20) | 13 (22) | 7 (16) |
| Headache | 20 (21) | 12 (20) | 8 (22) | 19 (19) | 10 (17) | 9 (21) |
| Fatiguec | 4 (4) | 2 (3) | 2 (5) | 16 (16) | 7 (12) | 9 (21) |
| Upper abdominal pain | 8 (8) | 6 (10) | 2 (5) | 12 (12) | 4 (7) | 8 (19) |
| Nasal congestion | 6 (6) | 6 (10) | 0 (0) | 9 (9) | 4 (7) | 5 (12) |
| Pharyngolaryngeal pain | 4 (4) | 4 (7) | 0 (0) | 8 (8) | 4 (7) | 4 (9) |
| Cough | 8 (8) | 6 (10) | 2 (5) | 6 (6) | 2 (3) | 4 (9) |
| Irritability | 9 (9) | 6 (10) | 3 (8) | 5 (5) | 1 (2) | 4 (9) |
| Insomnia | 3 (3) | 2 (3) | 1 (3) | 5 (5) | 2 (3) | 2 (5) |
| Increased body temperature | 2 (2) | 1 (2) | 1 (3) | 5 (5) | 1 (2) | 4 (9) |
| Dizziness | 2 (2) | 0 (0) | 2 (5) | 5 (5) | 3 (5) | 2 (5) |
Methylphenidate or amphetamine.
Somnolence includes the terms somnolence and sedation.
Fatigue includes the terms fatigue and lethargy.
TABLE 5.
Cardiac Parameters Throughout the Study (Safety Population)
| Mean HR, bpm (SD) | Blood Pressure, Mean (SD) |
ECG Intervals, Mean (SD) |
|||||
|---|---|---|---|---|---|---|---|
| SBP, mm Hg | DBP, mm Hg | QT, milliseconds | QTcB, milliseconds | QTcF, milliseconds | QRS, milliseconds | ||
| Placebo plus stimulant (n = 96)a | |||||||
| Baseline | 83.8 (12) | 107.9 (12) | 67.8 (9) | 355.8 (21) | 413.2 (16) | 392.8 (12) | 86.1 (6) |
| Week 1 | 85.1 (11) | 108.7 (10) | 67.7 (8) | 353.3 (23) | 414.8 (18) | 392.9 (16) | 85.9 (6) |
| Week 2 | 86.7 (13) | 108.0 (13) | 67.3 (9) | 353.4 (22) | 414.4 (17) | 392.7 (13) | 86.2 (6) |
| Week 3 | 86.0 (13) | 108.8 (10) | 67.7 (7) | 354.1 (24) | 415.6 (17) | 393.4 (14) | 85.9 (3) |
| Week 4 | 86.0 (12) | 108.6 (11) | 68.0 (7) | 356.3 (24) | 414.2 (20) | 393.7 (15) | 86.1 (6) |
| Week 5 | 84.7 (13) | 108.3 (10) | 67.7 (8) | 352.6 (20) | 424.9 (9) | 399.3 (8) | 86.3 (5) |
| Week 6 | 84.3 (11) | 109.0 (10) | 67.6 (7) | 353.7 (23) | 414.3 (21) | 392.7 (16) | 85.7 (6) |
| Week 7 | 86.0 (12) | 108.2 (10) | 66.8 (7) | 355.8 (6) | 421.3 (10) | 398.3 (7) | 84.3 (4) |
| Week 8 | 86.9 (14) | 109.3 (12) | 67.4 (7) | 353.5 (26) | 413.0 (18) | 391.8 (15) | 85.7 (6) |
| Week 9 | 86.9 (11) | 109.5 (10) | 68.1 (7) | 356.5 (23) | 413.8 (21) | 393.5 (17) | 86.9 (6) |
| CLON-XR Plus stimulant (n = 102)a | |||||||
| Baseline | 84.8 (12) | 108.2 (10) | 67.5 (8) | 357.8 (26) | 415.2 (16) | 394.8 (15) | 85.2 (6) |
| Week 1 | 82.5 (12) | 104.7 (11) | 65.7 (8) | 362.0 (25) | 413.8 (18) | 395.5 (16) | 85.6 (6) |
| Week 2 | 79.8 (12) | 104.1 (11) | 63.8 (8) | 367.2 (25) | 409.8 (22) | 394.7 (17) | 85.6 (6) |
| Week 3 | 79.8 (12) | 103.9 (11) | 64.0 (8) | 372.9 (27) | 412.5 (21) | 398.7 (20) | 86.4 (6) |
| Week 4 | 80.2 (14) | 103.5 (11) | 64.4 (8) | 371.1 (29) | 412.2 (22) | 397.6 (18) | 85.9 (6) |
| Week 5 | 80.1 (12) | 104.7 (12) | 66.8 (8) | 380.3 (19) | 408.1 (27) | 398.0 (20) | 88.3 (5) |
| Week 6 | 83.3 (11) | 105.1 (11) | 66.1 (9) | 366.6 (24) | 418.1 (18) | 399.8 (14) | 86.9 (6) |
| Week 7 | 84.0 (13) | 105.0 (11) | 66.3 (8) | 350.5 (21) | 411.6 (18) | 389.8 (12) | 86.4 (7) |
| Week 8 | 86.5 (12) | 110.7 (11) | 69.9 (8) | 352.5 (24) | 419.1 (18) | 395.3 (14) | 84.9 (6) |
| Week 9 | 88.0 (12) | 111.7 (10) | 70.6 (7) | 350.8 (24) | 421.0 (20) | 395.8 (15) | 85.5 (6) |
ECG indicates electrocardiogram; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; QTcB, QT interval corrected using Bazett's formula; QTcF, QT interval corrected using Fridericia's formula.
Sample size n may vary from visit to visit because of missed visits, dropout, or unscheduled visits (eg, 12-lead ECGs were not planned for all patients at weeks 3, 5, and 7).
DISCUSSION
This study is the first in which CLON-XR in combination with stimulants in ADHD youth with inadequate response to stimulants is evaluated. CLON-XR plus stimulant resulted in significantly greater improvement in ADHD-RS-IV total scores at week 5 compared with placebo plus stimulant. Similar efficacy was noted for the hyperactivity and inattention subscales of the ADHD-RS-IV, CGI-I, CGI-S, and PGA scale scores. Treatment with CLON-XR plus stimulant was well tolerated, with 1 patient in the CLON-XR plus stimulant group discontinuing because of TEAEs versus 3 patients in the placebo plus stimulant group. More patients who received CLON-XR plus stimulant experienced TEAEs of somnolence, sedation, and fatigue, and the rates of other TEAEs were comparable across groups. Changes in cardiovascular parameters as a result of treatment with CLON-XR were consistent with known effects of the drug (ie, decreased heart rate and blood pressure) but were not clinically significant.
The efficacy of CLON-XR in combination with stimulants for the treatment of ADHD in children with an inadequate response to stimulants alone is demonstrated by these results. In a previous trial, the efficacy of CLON-XR as monotherapy for treating ADHD in children and adolescents was demonstrated.18 In this trial it also is suggested that CLON-XR used in combination with stimulants for the treatment of ADHD youth is well tolerated. The effect size for the CLON-XR plus stimulant group indicates an incremental benefit for combination treatment in children and adolescents whose symptoms are not ideally controlled with stimulants alone.
There were a number of strengths of this study, including a clinically relevant dosing regimen, a considerable number of female and minority participants, and the inclusion of both children and adolescents. However, several limitations are noted. First, this study excluded patients with significant psychiatric comorbidity. Given the high rates of comorbidity in individuals diagnosed with ADHD,3–5 the sample may therefore not be representative of many patients who are treated for ADHD. Moreover, children with predominantly inattentive-subtype ADHD were excluded from participation. Thus, the safety and efficacy of CLON-XR plus stimulant in this patient population remain unknown. Second, the stimulant medication regimen in this study was allowed to vary on the basis of the response of individual patients to their specific regimen (ie, methylphenidate or amphetamine). Although clinically relevant, this approach made it impossible to determine whether specific combinations of stimulant treatment with CLON-XR were more effective than others. In addition, stimulant medication was not explicitly optimized for each patient during the course of the study. It is unknown what effects CLON-XR would have had if a formal optimization phase had been used. Nevertheless, this approach to dosing accurately reflects actual clinical practice; therefore, the results are generalizable to many children and adolescents with ADHD who respond inadequately to stimulants. Finally, the duration of the study was relatively short. It is important for future work to characterize both the efficacy and safety of long-term combination treatment with CLON-XR and stimulants.
CONCLUSION
The present findings provide evidence for added efficacy of CLON-XR when treating ADHD youth with inadequate response to stimulants. These results indicate that the extended-release formulation of a nonstimulant medication is a useful addition to currently available treatments for patients with ADHD.
ACKNOWLEDGMENTS
This study was supported by Addrenex Pharmaceuticals Inc, a Shionogi company. Medical writing assistance was provided under the direction of Dr Kollins by MedThink SciCom Inc with support from Shionogi Inc.
We thank Drs Nicole Forman and Chao Wang of Shionogi Inc for their assistance with analysis of the clinical trial data and critical review of the manuscript, Dr David Ward for overall safety monitoring during the study, and Ms Kelly Abernathy for supervision of study conduct and data management.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00641329).
FINANCIAL DISCLOSURE: Dr Kollins has received research support, consulting fees, or both from the following: Addrenex Pharmaceuticals Inc, Otsuka Pharmaceutical Co Ltd, Shire, National Institute on Drug Abuse, National Institute of Mental Health, National Institute of Neurological Disorders and Stroke, National Institute of Environmental Health Sciences, and the Environmental Protection Agency; Dr Jain has served as a consultant and advisory board member and has provided research support for Shionogi Pharma Inc and Addrenex Pharmaceuticals Inc; Dr Brams has been a speaker for Shire, Eli Lilly and Company, Ortho-McNeil, and Novartis AG; Dr Findling receives or has received research support, acted as a consultant, and/or served on a speaker's bureau for Abbott Laboratories, Addrenex Pharmaceuticals Inc, AstraZeneca, Biovail Corporation, Bristol-Myers Squibb, Forest Laboratories Inc, GlaxoSmithKline, Johnson and Johnson, KemPharm Inc, H. Lundbeck A/S, Neuropharm Group, Novartis AG, Noven Pharmaceuticals Inc, Organon BioSciences, Otsuka America Pharmaceutical Inc, Pfizer Inc, Rhodes Pharmaceuticals, Sanofi-Aventis US, Schering-Plough, Seaside Therapeutics, Sepracor Inc, Shire, Solvay Pharmaceuticals, Supernus Pharmaceuticals Inc, Validus Pharmaceuticals LLC, and Wyeth; Dr Wigal has received research support, acted as a consultant, or served on a speaker's bureau for Abbott Laboratories, Addrenex Pharmaceuticals Inc, Celgene Corporation, Eli Lilly and Company, Janssen, NextWave Pharmaceuticals Inc, National Institute on Drug Abuse, National Institute of Mental Health, New River Pharmaceuticals Inc, Novartis AG, McNeil Consumer and Specialty Pharmaceuticals, Psychogenics Inc, Quintiles, and Shire; Dr Khayrallah was an employee and shareholder of Addrenex Pharmaceuticals Inc at the time of the study; and Dr Segal has no financial relationships relevant to this article to disclose.
- ADHD
- attention-deficit/hyperactivity disorder
- CLON-XR
- clonidine hydrochloride extended-release tablets
- ADHD-RS-IV
- Attention-Deficit/Hyperactivity Disorder Rating Scale IV
- LOCF
- last observation carried forward
- CGI-S
- Clinical Global Impression of Severity
- CGI-I
- Clinical Global Impression of Improvement
- CPRS
- Conners' Parent Rating Scale
- PGA
- Parent Global Assessment
- TEAE
- treatment-emergent adverse event
- ITT
- intent-to-treat
REFERENCES
- 1. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed Text Revision Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- 2. Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, Kahn RS. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med. 2007;161(9):857–864 [DOI] [PubMed] [Google Scholar]
- 3. Jensen PS, Shervette REI, Xenakis SN, Richters J. Anxiety and depressive disorders in attention deficit disorder with hyperactivity: new findings. Am J Psychiatry. 1993;150(8):1203–1209 [DOI] [PubMed] [Google Scholar]
- 4. Newcorn JH, Halperin JM, Jensen PS, et al. Symptom profiles in children with ADHD: effects of comorbidity and gender. J Am Acad Child Adolesc Psychiatry. 2001;40(2):137–146 [DOI] [PubMed] [Google Scholar]
- 5. Rommelse NN, Altink ME, Fliers EA, et al. Comorbid problems in ADHD: degree of association, shared endophenotypes, and formation of distinct subtypes: implications for a future DSM. J Abnorm Child Psychol. 2009;37(6):793–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Findling RL. Evolution of the treatment of attention-deficit/hyperactivity disorder in children: a review. Clin Ther. 2008;30(5):942–957 [DOI] [PubMed] [Google Scholar]
- 7. Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Leibson CL, Jacobsen SJ. Long-term stimulant medication treatment of attention-deficit/hyperactivity disorder: results from a population-based study. J Dev Behav Pediatr. 2006;27(1):1–10 [DOI] [PubMed] [Google Scholar]
- 8. Pliszka S. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894–921 [DOI] [PubMed] [Google Scholar]
- 9. Tourette's Syndrome Study Group Treatment of ADHD in children with tics: a randomized controlled trial. Neurology. 2002;58(4):527–536 [DOI] [PubMed] [Google Scholar]
- 10. Connor DF, Barkley RA, Davis HT. A pilot study of methylphenidate, clonidine, or the combination in ADHD comorbid with aggressive oppositional defiant or conduct disorder. Clin Pediatr (Phila). 2000;39(1):15–25 [DOI] [PubMed] [Google Scholar]
- 11. Hunt RD, Minderaa RB, Cohen DJ. Clonidine benefits children with attention deficit disorder and hyperactivity: report of a double-blind placebo-crossover therapeutic trial. J Am Acad Child Psychiatry. 1985;24(5):617–629 [DOI] [PubMed] [Google Scholar]
- 12. Agarwal V, Sitholey P, Kumar S, Prasad M. Double-blind, placebo-controlled trial of clonidine in hyperactive children with mental retardation. Ment Retard. 2001;39(4):259–267 [DOI] [PubMed] [Google Scholar]
- 13. Hazell PL, Stuart JE. A randomized controlled trial of clonidine added to psychostimulant medication for hyperactive and aggressive children. J Am Acad Child Adolesc Psychiatry. 2003;42(8):886–894 [DOI] [PubMed] [Google Scholar]
- 14. Nair V, Mahadevan S. Randomised controlled study-efficacy of clonidine versus carbamazepine in children with ADHD. J Trop Pediatr. 2009;55(2):116–121 [DOI] [PubMed] [Google Scholar]
- 15. Palumbo DR, Sallee FR, Pelham WE, Jr, Bukstein OG, Daviss WB, McDermott MP. Clonidine for attention-deficit/hyperactivity disorder: I. Efficacy and tolerability outcomes. J Am Acad Child Adolesc Psychiatry. 2008;47(2):180–188 [DOI] [PubMed] [Google Scholar]
- 16. Schvehla TJ, Mandoki MW, Sumner GS. Clonidine therapy for comorbid attention deficit hyperactivity disorder and conduct disorder: preliminary findings in a children's inpatient unit. South Med J. 1994;87(7):692–695 [DOI] [PubMed] [Google Scholar]
- 17. KAPVAY [package insert]. Florham Park, NJ: Shionogi Inc; 2010 [Google Scholar]
- 18. Jain R, Segal S, Kollins SH, Kayrallah M. Clonidine extended-release tablets for pediatric patients with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50(2):171–179 [DOI] [PubMed] [Google Scholar]
- 19. Efron D, Hiscock H, Sewell JR, et al. Prescribing of psychotropic medications for children by Australian pediatricians and child psychiatrists. Pediatrics. 2003;111(2):372–375 [DOI] [PubMed] [Google Scholar]
- 20. Hazell PL, McDowell MJ, Walton JM. Management of children prescribed psychostimulant medication for attention deficit hyperactivity disorder in the Hunter region of NSW. Med J Aust. 1996;165(9):477–480 [DOI] [PubMed] [Google Scholar]
- 21. DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretation. New York, NY: Guilford Press; 1998 [Google Scholar]