Abstract
OBJECTIVE:
To compare the effectiveness of clindamycin, trimethoprim-sulfamethoxazole, and β-lactams for the treatment of pediatric skin and soft-tissue infections (SSTIs).
METHODS:
A retrospective cohort of children 0 to 17 years of age who were enrolled in Tennessee Medicaid, experienced an incident SSTI between 2004 and 2007, and received treatment with clindamycin (reference), trimethoprim-sulfamethoxazole, or a β-lactam was created. Outcomes included treatment failure and recurrence, defined as an SSTI within 14 days and between 15 and 365 days after the incident SSTI, respectively. Adjusted models stratified according to drainage status were used to estimate the risk of treatment failure and time to recurrence.
RESULTS:
Among the 6407 children who underwent drainage, there were 568 treatment failures (8.9%) and 994 recurrences (22.8%). The adjusted odds ratios for treatment failure were 1.92 (95% confidence interval [CI]: 1.49–2.47) for trimethoprim-sulfamethoxazole and 2.23 (95% CI: 1.71–2.90) for β-lactams. The adjusted hazard ratios for recurrence were 1.26 (95% CI: 1.06–1.49) for trimethoprim-sulfamethoxazole and 1.42 (95% CI: 1.19–1.69) for β-lactams. Among the 41 094 children without a drainage procedure, there were 2435 treatment failures (5.9%) and 5436 recurrences (18.2%). The adjusted odds ratios for treatment failure were 1.67 (95% CI: 1.44–1.95) for trimethoprim-sulfamethoxazole and 1.22 (95% CI: 1.06–1.41) for β-lactams; the adjusted hazard ratios for recurrence were 1.30 (95% CI: 1.18–1.44) for trimethoprim-sulfamethoxazole and 1.08 (95% CI: 0.99–1.18) for β-lactams.
CONCLUSIONS:
Compared with clindamycin, use of trimethoprim-sulfamethoxazole or β-lactams was associated with increased risks of treatment failure and recurrence. Associations were stronger for those with a drainage procedure.
Keywords: antibacterial agents, methicillin-resistant Staphylococcus aureus, abscess, cellulitis, drug therapy
WHAT'S KNOWN ON THIS SUBJECT:
The burden of pediatric skin and soft-tissue infections (SSTIs) is increasing, largely as a result of the widespread community emergence of methicillin-resistant Staphylococcus aureus (MRSA). Optimal antimicrobial management strategies for SSTIs in the era of community-associated MRSA remain unclear.
WHAT THIS STUDY ADDS:
These findings, from a cohort of nearly 50 000 children with incident SSTIs, bring into question the use of trimethoprim-sulfamethoxazole for treatment of purulent SSTIs in community-associated MRSA–prevalent regions in which clindamycin resistance remains low. β-Lactams, however, may still be effective for nonpurulent SSTIs.
The growing burden of community-associated (CA) methicillin-resistant Staphylococcus aureus (MRSA), which is estimated to account for >70% of staphylococcal infections in some regions of the United States, is a major problem in childhood.1–5 A frequent cause of skin and soft-tissue infections (SSTIs),2,4,5 CA-MRSA infections often are more severe and lead to poor clinical outcomes.6,7 CA-MRSA isolates are uniformly resistant to β-lactam antibiotics, previously the most commonly used agents for SSTIs, which makes prompt recognition and initiation of effective empiric therapy for CA-MRSA extremely important. Unfortunately, optimal antimicrobial management strategies for SSTIs in the era of CA-MRSA remain unclear.
Currently, the most commonly recommended, orally administered antibiotics for pediatric SSTIs include clindamycin and trimethoprim-sulfamethoxazole.8–12 However, a paucity of comparative studies of antimicrobial treatment,13,14 as well as studies that suggest that antimicrobial therapy may be unnecessary for drained abscesses,15–17 make treatment decisions difficult. In addition, although studies estimated that nearly one-third of MRSA SSTIs result in ≥1 recurrent event,9,18–20 few studies have attempted to determine the influence of antimicrobial treatment on recurrence risk for pediatric SSTIs. Finally, there are no published, large-scale, randomized trials of antimicrobial treatment for pediatric SSTIs. The objective of this study was to compare the effectiveness of clindamycin, trimethoprim-sulfamethoxazole, and β-lactams with respect to treatment and risk of recurrence after incident SSTIs among a large cohort of children enrolled in the public health care plan in Tennessee in 2004–2007.
METHODS
Data Sources
This study used administrative data files from Tennessee's Medicaid program (TennCare). The TennCare database has been validated extensively and contains information on subject enrollment and claims for hospitalizations, outpatient and emergency department visits, and medications, as described previously.21 International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) diagnostic codes were used to identify potential SSTIs from emergency department and outpatient claims. Claims for drainage procedures were identified on the basis of ICD-9-CM and Current Procedural Terminology (CPT) codes. Data on filled prescriptions were determined from Medicaid pharmacy files.
Study Population
Children 0 to 17 years of age who were enrolled in TennCare between January 1, 2004, and December 31, 2007, were eligible for inclusion if they experienced an incident SSTI, defined on the basis of (1) an outpatient SSTI claim, with or without a drainage procedure (Supplemental Table 4 provides a complete list of eligible ICD-9-CM and CPT codes), (2) no SSTI claims in the previous 365 days (or since birth for those <1 year of age), and (3) a prescription for clindamycin, trimethoprim-sulfamethoxazole, or a β-lactam filled within 2 days after the SSTI claim. All systemic antibiotics identified in the pharmacy data file as either a penicillin or cephalosporin were considered β-lactams and were included. Children were required to have ≥365 days of continuous TennCare enrollment (or enrollment since birth for those <1 year of age) before the incident SSTI and ≥14 days of continuous enrollment after the incident SSTI. Because our primary aim was to compare antibiotic effectiveness, children who received ≥2 different antibiotics within 2 days after an SSTI claim (9960 children who received mupirocin plus another antibiotic and 2418 children who received other combinations) were excluded. To limit potential severity bias, we also excluded children who did not receive an antibiotic after a drainage procedure (N = 2611). Children who were admitted initially to the hospital and those with claims involving burns, foreign-body injuries, or wound or postsurgical infections also were excluded.
Study Outcomes
The primary outcome was an SSTI within 365 days after the incident infection, defined on the basis of (1) an outpatient SSTI claim plus an antibiotic prescription or drainage procedure or (2) hospitalization with SSTI listed as a discharge diagnosis. Subsequent SSTIs were classified as either treatment failures (within 14 days after the incident SSTI) or recurrences (15–365 days after the incident SSTI).
Validation Sample
To assess the validity of our study definition for incident SSTIs, a random sample of cohort children (N = 60) cared for at Vanderbilt University Medical Center was selected for chart review. One record could not be located; of the 59 records reviewed, all except 1 were considered to indicate true incident SSTIs (positive predictive value: 98.3%). Seventy-five percent of cases were classified as abscess/cellulitis, of which 60% were determined to be abscesses. In cases involving drainage, needle drainage (22%) and scalpel incision with subsequent drainage (61%) were the most common approaches.
Statistical Analyses
The primary exposure was antibiotic treatment for the incident SSTI and was classified as clindamycin, trimethoprim-sulfamethoxazole, or a β-lactam. Clindamycin treatment was considered the reference for all analyses. Odds ratios (ORs) for treatment failure were estimated by using logistic regression analyses. Hazard ratios (HRs) for time to recurrence were estimated by using Cox proportional-hazard regression analyses. Covariates in the final models included study year, age, gender, race/ethnicity, and visit diagnosis. We also assessed for effect modification according to gender, age, and race/ethnicity. All models were stratified according to the presence or absence of a drainage procedure. Model diagnostics included Hosmer-Lemeshow goodness-of-fit tests and visualization of logarithm-logarithm survival plots; there was no evidence of misspecification in the final models.
Several sensitivity analyses also were performed. First, to account for children with >1 qualifying incident SSTI in different study years (N = 9074), models using robust SEs and clustering according to patient were created. Second, because a large number of children (N = 9960) were excluded because they received mupirocin in addition to clindamycin, trimethoprim-sulfamethoxazole, or a β-lactam, analyses that included this group of children were performed. Results for these supplemental analyses were unchanged from the results of the overall models and are not presented. Finally, because children who experienced a treatment failure might have received a different antibiotic at the time of the failure event (leading to potential exposure misclassification for the recurrence outcome), analyses that excluded SSTIs that resulted in treatment failures were performed. Stata 10.0 (Stata Corp, College Station, TX) was used for all analyses. This study was approved by the Vanderbilt University institutional review board and the Bureau of TennCare.
RESULTS
Characteristics of Study Population
Of the 47 501 included children with incident SSTIs, 7459 (15.7%) received clindamycin, 10 623 (22.3%) received trimethoprim-sulfamethoxazole, and 29 419 (61.9%) received a β-lactam (Table 1). The duration of antibiotic treatment (mean ± SD) was comparable between the 3 groups (clindamycin, 9.4 ± 2.6 days; trimethoprim-sulfamethoxazole, 9.7 ± 2.6 days; β-lactam, 9.4 ± 2.6 days). During the study period, β-lactam use decreased substantially, accounting for 85.1% and 43.8% of total prescriptions in 2004 and 2007, respectively. There was a modest increase in clindamycin use, from 11.0% in 2004 to 17.7% in 2007, and a dramatic increase in trimethoprim-sulfamethoxazole use, from 3.9% in 2004 to 38.5% in 2007.
TABLE 1.
Baseline Characteristics for Children Who Did or Did Not Receive Drainage, Overall and According to Antibiotic Treatment Group
| Characteristic | N |
n (%) |
||
|---|---|---|---|---|
| Clindamycin | Trimethoprim-Sulfamethoxazole | β-Lactam | ||
| Without drainage procedure | ||||
| Total | 41 094 | 5189 (12.6) | 8417 (20.5) | 27 488 (66.9) |
| Study years | ||||
| 2004 | 10 700 | 913 (8.5) | 340 (3.2) | 9447 (88.3) |
| 2005 | 7828 | 1003 (12.8) | 1015 (13.0) | 5810 (74.2) |
| 2006 | 11 304 | 1635 (14.5) | 2983 (26.4) | 6686 (59.2) |
| 2007 | 11 262 | 1638 (14.5) | 4079 (36.2) | 5545 (49.2) |
| Age | ||||
| 0–2 y | 9267 | 1251 (13.5) | 2139 (23.1) | 5877 (63.4) |
| 3–5 y | 8627 | 943 (10.9) | 1617 (81.7) | 6067 (70.3) |
| 6–10 y | 10 500 | 1187 (11.3) | 2037 (19.4) | 7276 (69.3) |
| 11–17 y | 12 700 | 1808 (14.2) | 2624 (20.7) | 8268 (65.1) |
| Gender | ||||
| Male | 20 187 | 2455 (12.2) | 3930 (19.5) | 13 802 (68.4) |
| Female | 20 907 | 2734 (13.1) | 4487 (21.5) | 13 686 (65.5) |
| Race/ethnicity | ||||
| White | 23 870 | 1309 (5.5) | 5205 (21.8) | 17 356 (72.7) |
| Black | 13 841 | 3463 (25.0) | 2638 (19.1) | 7740 (55.9) |
| Hispanic | 1261 | 136 (10.8) | 188 (14.9) | 937 (74.3) |
| Other | 2122 | 281 (13.2) | 386 (18.2) | 1455 (68.6) |
| With drainage procedure | ||||
| Total | 6407 | 2270 (35.4) | 2206 (34.4) | 1931 (30.1) |
| Study years | ||||
| 2004 | 1222 | 400 (32.7) | 121 (9.9) | 701 (57.4) |
| 2005 | 1222 | 471 (38.5) | 281 (23.0) | 470 (38.5) |
| 2006 | 1823 | 661 (36.3) | 728 (39.9) | 434 (23.8) |
| 2007 | 2140 | 738 (34.5) | 1076 (50.3) | 326 (15.2) |
| Age | ||||
| 0–2 y | 1409 | 563 (40.0) | 526 (37.3) | 320 (22.7) |
| 3–5 y | 1012 | 332 (32.8) | 365 (36.1) | 315 (31.1) |
| 6–10 y | 1410 | 491 (34.8) | 505 (35.8) | 414 (29.4) |
| 11–17 y | 2576 | 884 (34.3) | 810 (31.4) | 882 (34.2) |
| Gender | ||||
| Male | 3180 | 1128 (35.5) | 1086 (34.2) | 966 (30.4) |
| Female | 3227 | 1142 (35.4) | 1120 (34.7) | 965 (29.9) |
| Race/ethnicity | ||||
| White | 2588 | 357 (13.8) | 1236 (47.8) | 995 (38.5) |
| Black | 3428 | 1788 (52.2) | 829 (24.2) | 811 (23.7) |
| Hispanic | 123 | 30 (24.4) | 52 (42.3) | 41 (33.3) |
| Other | 268 | 95 (35.5) | 89 (33.2) | 84 (31.3) |
SSTIs were determined on the basis of a visit with a specific ICD-9-CM code (Supplemental Table 4) and a filled prescription for an antibiotic within 2 days. Treatment failure and recurrence were defined as fulfilling the same criteria (including a new antibiotic) within 14 days and between 15 and 365 days, respectively. Drainage was determined on the basis of CPT and ICD-9-CM procedure codes (Supplemental Table 4) representing incision and drainage.
Ninety-three percent of children who received drainage had a diagnosis of abscess/cellulitis. For children without a drainage procedure, only 61.2% had a diagnosis of abscess/cellulitis; an additional 20.7% had a diagnosis of impetigo. Children who underwent a drainage procedure were more likely to receive either clindamycin (35.4%) or trimethoprim-sulfamethoxazole (34.4%), whereas those who did not undergo a drainage procedure were most likely to receive a β-lactam (66.9%).
Outcomes for Children With Drainage Procedures
Of the 6407 children who underwent drainage procedures, 568 (8.9%) experienced treatment failure, including 4.7% of clindamycin users, 11.2% of trimethoprim-sulfamethoxazole users, and 11.1% of β-lactam users (Table 2). Compared with clindamycin, the odds of treatment failure approximately doubled for both trimethoprim-sulfamethoxazole (adjusted OR: 1.92 [95% confidence interval [CI]: 1.49–2.47]) and β-lactams (adjusted OR: 2.23 [95% CI: 1.71–2.90]).
TABLE 2.
ORs for Treatment Failure, Stratified According to Drainage Status
| Clindamycin | Trimethoprim-Sulfamethoxazole | β-Lactam | |
|---|---|---|---|
| Drainage | |||
| Incident SSTIs, n | 2270 | 2206 | 1931 |
| Treatment failures, n (%) | 107 (4.7) | 246 (11.2) | 215 (11.1) |
| Unadjusted OR (95% CI) | 1.00 (reference) | 2.54 (2.01–3.21) | 2.53 (1.99–3.22) |
| Adjusted OR (95% CI) | 1.00 (reference) | 1.92 (1.49–2.47) | 2.23 (1.71–2.90) |
| No drainage | |||
| Incident SSTIs, n | 5189 | 8417 | 27 488 |
| Treatment failures, n (%) | 253 (4.9) | 739 (8.8) | 1443 (5.3) |
| Unadjusted OR (95% CI) | 1.00 (reference) | 1.88 (1.62–2.18) | 1.08 (0.94–1.24) |
| Adjusted OR (95% CI) | 1.00 (reference) | 1.67 (1.44–1.95) | 1.22 (1.06–1.41) |
SSTIs were determined on the basis of a visit with a specific ICD-9-CM code (Supplemental Table 4) and a filled prescription for an antibiotic within 2 days. Treatment failure was defined as fulfilling the same criteria (including a new antibiotic) within 14 days after the incident event. Drainage was determined on the basis of CPT and ICD-9-CM procedure codes (Supplemental Table 4) representing incision and drainage. ORs were estimated in logistic regression analyses with adjustment for year, age, gender, race/ethnicity, and diagnosis.
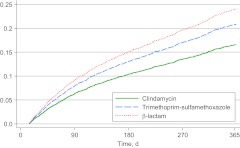
There were 994 recurrences during 4363 person-years of follow-up monitoring (228 events per 1000 person-years) for children who received drainage; the median time to first recurrence was 95 days (interquartile range: 46–195 days) (the numbers of children who experienced ≥1 recurrence by 1, 3, 9, and 12 months of follow-up monitoring are presented in Supplemental Table 5). The rates of recurrence were 173 recurrences per 1000 person-years among clindamycin users, 270 recurrences per 1000 person-years among trimethoprim-sulfamethoxazole users, and 251 recurrences per 1000 person-years among β-lactam users (Table 3). The risk of recurrence was significantly higher among users of trimethoprim-sulfamethoxazole (adjusted HR: 1.26 [95% CI: 1.06–1.49]) and β-lactams (adjusted HR: 1.42 [95% CI: 1.19–1.69]) (Fig 1), compared with clindamycin users. The results of analyses that excluded treatment failures were unchanged from the results with the overall models (Supplemental Table 6). Tests for interaction yielded significant results for gender (Supplemental Tables 7 and 8).
TABLE 3.
HRs for First Recurrence, Stratified According to Drainage Status
| Clindamycin | Trimethoprim-Sulfamethoxazole | β-Lactam | |
|---|---|---|---|
| Drainage | |||
| First recurrences, n (%)a | 280 (12.3) | 359 (16.3) | 355 (18.4) |
| Follow-up period, person-years | 1619 | 1329 | 1415 |
| Events, No. per 1000 person-years | 173.0 | 270.1 | 250.9 |
| Unadjusted HR (95% CI) | 1.00 (reference) | 1.51 (1.29–1.77) | 1.47 (1.26–1.72) |
| Adjusted HR (95% CI) | 1.00 (reference) | 1.26 (1.06–1.49) | 1.42 (1.19–1.69) |
| No drainage | |||
| First recurrences, n (%)a | 610 (11.8) | 1272 (15.1) | 3554 (12.9) |
| Follow-up period, person-years | 3736 | 5136 | 21 015 |
| Events, No. per 1000 person-years | 163.3 | 247.7 | 169.1 |
| Unadjusted HR (95% CI) | 1.00 (reference) | 1.46 (1.32–1.60) | 1.06 (0.97–1.15) |
| Adjusted HR (95% CI) | 1.00 (reference) | 1.30 (1.18–1.44) | 1.08 (0.99–1.18) |
SSTIs were determined on the basis of a visit with a specific ICD-9-CM code (Supplemental Table 4) and a filled prescription for an antibiotic within 2 days. Recurrence was defined as fulfilling the same criteria (including a new antibiotic) between 15 and 365 days after the incident event. Drainage was determined on the basis of CPT and ICD-9-CM procedure codes (Supplemental Table 4) representing incision and drainage. HRs were estimated in Cox proportional-hazard regression analyses with adjustment for year, age, gender, race/ethnicity, and diagnosis.
Percentages reflect the proportion of incident SSTIs in each antibiotic category that resulted in ≥1 recurrence event.
FIGURE 1.
Cumulative hazards for SSTI recurrence for the drainage subgroup. SSTIs were determined on the basis of a visit with a specific ICD-9-CM code (Supplemental Table 4) and a filled prescription for an antibiotic within 2 days. Recurrence was defined as fulfilling the same criteria (including a new antibiotic) between 15 and 365 days after the incident event. Drainage was determined on the basis of CPT and ICD-9-CM procedure codes (Supplemental Table 4) representing incision and drainage. HRs were estimated in Cox proportional-hazard regression analyses with adjustment for year, age, gender, race/ethnicity, and diagnosis.
Outcomes for Children Without Drainage Procedures
Among the 41 094 children without a drainage procedure, 2435 (5.9%) experienced treatment failure, including 4.9% of clindamycin users, 8.8% of trimethoprim-sulfamethoxazole users, and 5.3% of β-lactam users. The adjusted ORs for treatment failure were 1.67 (95% CI: 1.44–1.95) for trimethoprim-sulfamethoxazole and 1.22 (95% CI: 1.06–1.41) for β-lactams, compared with clindamycin.
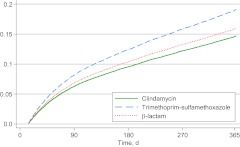
There were 5436 recurrences during 29 887 person-years of follow-up monitoring (182 events per 1000 person-years) among children who did not receive drainage; the median time to first recurrence was 88 days (interquartile range: 41–192 days) (the numbers of children who experienced ≥1 recurrence by 1, 3, 9, and 12 months of follow-up monitoring are presented in Supplemental Table 5). This value included 610 recurrences among clindamycin users (163 recurrences per 1000 person-years), 1272 recurrences among trimethoprim-sulfamethoxazole users (248 recurrences per 1000 person-years), and 3554 recurrences among β-lactam users (169 recurrences per 1000 person-years). The risk of recurrence was significantly higher among trimethoprim-sulfamethoxazole users (adjusted HR: 1.30 [95% CI: 1.18–1.44]) but not among β-lactam users (adjusted HR: 1.08 [95% CI: 0.99–1.18]) (Fig 2). The results of analyses that excluded treatment failures were similar to the results with the overall models (Supplemental Table 6), and tests for interaction yielded nonsignificant results (Supplemental Tables 7 and 8).
FIGURE 2.
Cumulative hazards for SSTI recurrence for the no-drainage subgroup. SSTIs were determined on the basis of a visit with a specific ICD-9-CM code (Supplemental Table 4) and a filled prescription for an antibiotic within 2 days. Recurrence was defined as fulfilling the same criteria (including a new antibiotic) between 15 and 365 days after the incident event. Drainage was determined on the basis of CPT and ICD-9-CM procedure codes (Supplemental Table 4) representing incision and drainage. HRs were estimated in Cox proportional-hazard regression analyses with adjustment for year, age, gender, race/ethnicity, and diagnosis.
DISCUSSION
This is the first large-scale study to investigate the effectiveness of antimicrobial treatment strategies for children with both drained and nondrained SSTIs. In this population-based cohort of nearly 50 000 children from Tennessee, use of trimethoprim-sulfamethoxazole or β-lactams for treatment of incident SSTIs was significantly associated with both treatment failure and recurrence, compared with use of clindamycin.
Among children who received drainage, use of trimethoprim-sulfamethoxazole or a β-lactam doubled the odds of treatment failure, compared with use of clindamycin. In the only other study comparing the effectiveness of clindamycin and trimethoprim-sulfamethoxazole for treatment of MRSA SSTIs among children after drainage, Hyun et al13 demonstrated no difference in the occurrence of treatment failure for >400 hospitalized children discharged with either orally administered clindamycin or trimethoprim-sulfamethoxazole. In contrast to our study, all of the children in that study were hospitalized initially and nearly 90% received parenterally administered clindamycin, which makes it difficult to compare the 2 treatment groups.
In a study examining the epidemiological features of purulent SSTIs in Tennessee, Talbot et al22 reported that, of 182 drained abscesses from a pediatric emergency department that were cultured, 142 (79.7%) were attributable to MRSA. Similarly, data from across the United States demonstrated the predominant organism in pediatric skin abscesses to be MRSA.4,23,24 Therefore, it is likely that the majority of purulent infections in our cohort also were attributable to MRSA. This would explain the increased risk of treatment failure for the β-lactams, which uniformly lack antimicrobial activity against MRSA; however, the mechanism for increased treatment failures in the trimethoprim-sulfamethoxazole group is unknown.
Despite high rates of in vitro susceptibility to trimethoprim-sulfamethoxazole among CA-MRSA isolates,25 failure rates of nearly 20% among adults treated with trimethoprim-sulfamethoxazole for SSTIs in a clinical trial setting16 and as high as 50% among adults with significant comorbidities26,27 have been reported. Moreover, 2 recent randomized trials, although they likely were underpowered to detect small differences, failed to demonstrate superiority of trimethoprim-sulfamethoxazole over placebo for treatment of SSTIs after drainage among both children15 and adults16 (neither study included an active comparator). In the pediatric trial by Duong et al,15 4% of children who received trimethoprim-sulfamethoxazole experienced treatment failure and an additional 13% developed new lesions within 10 days. Trimethoprim-sulfamethoxazole inhibits bacterial growth by blocking key steps in the biosynthesis of thymidine, halting bacterial DNA replication. However, S aureus, through its ability to liberate and to acquire free thymidine from DNA fragments (which are present in high concentrations in abscess fluid), may bypass the antimicrobial effects of trimethoprim-sulfamethoxazole.26 This mechanism might explain the increased risk of treatment failure associated with trimethoprim-sulfamethoxazole.
Although recurrences were common among all children who received drainage, recurrence risk increased by nearly 30% for those who received trimethoprim-sulfamethoxazole and by >40% for those who received β-lactams, compared with clindamycin. Because persistent colonization with S aureus (including CA-MRSA) has been linked to the development of clinical infection,28,29 it is possible that clindamycin is superior at preventing recurrence because it decreases colonization more effectively.30 Another potential mechanism for the increased recurrence risk associated with trimethoprim-sulfamethoxazole may be the development of staphylococcal small-colony variants, slow-growing, intracellular organisms that have been isolated among persons receiving chronic trimethoprim-sulfamethoxazole therapy and patients with recurrent SSTIs.26,31 However, the development of this phenotype among a large population of children with incident SSTIs seems unlikely.
Among children with nondrained SSTIs, the risk of treatment failure remained significantly higher for those treated with either trimethoprim-sulfamethoxazole or β-lactams, although the magnitude of the effect was decreased, compared with children who underwent drainage. Risk of recurrence also was significantly increased among trimethoprim-sulfamethoxazole users but not among users of β-lactams. In contrast to SSTIs that underwent drainage (for which CA-MRSA likely was the predominant organism), SSTIs that did not undergo drainage likely were more heterogeneous. Some children might have had small abscesses that were not amenable to drainage or that drained spontaneously, both likely attributable to CA-MRSA. Nonpurulent SSTIs, however, likely were attributable to a mixture of Streptococcus pyogenes and S aureus. Consistent with this hypothesis, nearly 95% of children who received drainage had an ICD-9-CM–coded diagnosis of abscess/cellulitis, whereas only 60% of those without a drainage procedure had this diagnosis. Trimethoprim-sulfamethoxazole is largely ineffective for streptococcal infections, whereas both clindamycin and β-lactams possess potent activity against S pyogenes. In a recent, nested, case-control study with >2000 children with nondrained, noncultured SSTIs, trimethoprim-sulfamethoxazole doubled the risk of treatment failure, compared with β-lactams, whereas clindamycin conferred no benefit over β-lactams.14 Therefore, the use of β-lactams for nondrained, nonpurulent SSTIs may still be appropriate even in areas with high CA-MRSA prevalence.
Although several prospective treatment trials are ongoing, results likely will not be available for several years; therefore, we selected a retrospective, population-based cohort to investigate the effectiveness of these commonly used antimicrobial agents for pediatric SSTIs. Observational studies that use previously validated administrative data offer several advantages over randomized trials, including larger sample sizes, long-term follow-up monitoring and outcome assessment (in a relatively short period of time), and the ability to assess treatment effectiveness (versus efficacy). The TennCare administrative data files have been used for pharmacoepidemiological research for >30 years and have been validated extensively. In addition, a validation sample confirmed the predictive value of our SSTI definitions.
Despite the study's strengths, several limitations should be discussed, including residual confounding, the lack of microbiologic data, and potential misclassification of antibiotic exposure and outcomes. To control for potential confounding, several patient-level characteristics, as well as study year and diagnosis, were included in the multivariate models. We did find evidence for confounding according to race, because the change in the adjusted models was attributable primarily to this variable. Black individuals were least likely to receive a β-lactam and were most likely to receive clindamycin. The reasons for treatment differences according to race are unclear; however, they might stem from a perceived increased risk for MRSA among black individuals, as well as regional variations in treatment preferences. Although comorbidities were not assessed, there is no indication that children with comorbidities would be more likely to receive one antibiotic over another.
Data on microbiologic causes are important for understanding the mechanisms governing treatment failure and recurrence; even if cultures are obtained, however, results are not immediately available and initial treatment decisions are nearly always empiric. Compared with children who received clindamycin or trimethoprim-sulfamethoxazole, those who received a β-lactam might have had less severe disease, because β-lactams are not recommended for suspected CA-MRSA infections. In contrast, both clindamycin and trimethoprim-sulfamethoxazole are first-line, outpatient, antimicrobial agents when MRSA is a consideration, and both are used frequently in clinical practice. Although we were unable to control for lesion size, location, or character, it is unlikely that these characteristics differed among children who received either clindamycin or trimethoprim-sulfamethoxazole. Confounding according to indication was further limited through restriction of the cohort to outpatient visits for children who received a single antibiotic and stratification according to drainage status. Although it represents a question of great interest, we did not intend to evaluate the effectiveness of drainage alone, because of concerns regarding systematic differences between this group and the group of children who received antimicrobial treatment. However, a posthoc analysis that included children who received drainage alone, compared with clindamycin treatment, revealed an increased risk of treatment failure but not recurrence for this group (Supplemental Table 9). Because of the likely differences mentioned above, however, these results should be interpreted with caution.
We were unable to account for medication noncompliance or antibiotic changes without an additional SSTI visit; however, antibiotic changes without an additional visit should be rare, and prescription filling has been shown to predict medication adherence reliably.32,33 Palatability also is likely a major determinant of drug adherence in children, although clindamycin is notoriously unpalatable, compared with other antibiotics34,35; therefore, it would not be expected that different levels of adherence would explain the decreased risk of treatment failure and recurrence associated with clindamycin. Occasionally, antibiotic changes at subsequent follow-up visits (within 14 days) might have been based on culture results and not a true treatment failure. This would most likely affect children who received a β-lactam, because CA-MRSA causes the vast majority of purulent SSTIs in our region and both clindamycin and trimethoprim-sulfamethoxazole demonstrate good in vitro activity against local CA-MRSA isolates.
It is inevitable that a small proportion of secondary SSTIs that occurred ∼14 days after the incident SSTI were misclassified (treatment failures classified as recurrences and vice versa), although any misclassification would be nondifferential. Among children who did not receive a drainage procedure, it was impossible to determine whether children had purulent infections (ie, abscesses) or nonpurulent cellulitis, because these diagnoses fall under the same ICD-9-CM code. This likely contributed to heterogeneity among these children and may have affected outcome assessment for this group. Similarly, a small number of children who received drainage might have been assigned to the group without drainage if a drainage procedure claim was not filed.
CONCLUSIONS
In the largest pediatric study to date of the comparative effectiveness of antibiotic treatment strategies for incident SSTIs, clindamycin was demonstrated to be superior to both trimethoprim-sulfamethoxazole and β-lactams for the acute treatment and prevention of SSTI recurrence. This effect was most significant for children with purulent SSTIs who underwent drainage. The implications of the current study are twofold. First, our findings call into question the routine use of trimethoprim-sulfamethoxazole for purulent SSTIs in CA-MRSA–prevalent regions where clindamycin resistance remains low. Second, although β-lactams are no longer recommended when MRSA is a consideration, these agents may still be effective for nonpurulent SSTIs such as uncomplicated cellulitis or impetigo. Furthermore, although incision and drainage remains the mainstay of treatment for purulent SSTIs, our data confirm that antimicrobial agents are used frequently after drainage, and they suggest, at least indirectly, a possible additive benefit of appropriate antimicrobial therapy. Additional research, in the form of large, well-designed, randomized, controlled trials, is needed urgently to confirm our findings and to provide additional insight into the mechanisms governing treatment failure and recurrence for pediatric SSTIs in the era of CA-MRSA.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by grant 2T32 HS 013833-07 from the Agency for Healthcare Research and Quality (to Dr Williams).
We thank Kathryn Edwards, MD, and William Schaffner, MD, for critical review of earlier versions of this article.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
- SSTI
- skin and soft-tissue infection
- CA
- community-associated
- MRSA
- methicillin-resistant Staphylococcus aureus
- OR
- odds ratio
- HR
- hazard ratio
- CI
- confidence interval
- ICD-9-CM
- International Classification of Diseases, Ninth Edition, Clinical Modification
- CPT
- Current Procedural Terminology
REFERENCES
- 1. Hersh AL, Chambers HF, Maselli JH, Gonzales R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med. 2008;168(14):1585–1591 [DOI] [PubMed] [Google Scholar]
- 2. Buckingham SC, McDougal LK, Cathey LD, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus at a Memphis, Tennessee Children's Hospital. Pediatr Infect Dis J. 2004;23(7):619–624 [DOI] [PubMed] [Google Scholar]
- 3. National Nosocomial Infections Surveillance System National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32(8):470–485 [DOI] [PubMed] [Google Scholar]
- 4. Kaplan SL, Hulten KG, Gonzalez BE, et al. Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin Infect Dis. 2005;40(12):1785–1791 [DOI] [PubMed] [Google Scholar]
- 5. Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352(14):1436–1444 [DOI] [PubMed] [Google Scholar]
- 6. Gonzalez BE, Martinez-Aguilar G, Hulten KG, et al. Severe staphylococcal sepsis in adolescents in the era of community-acquired methicillin-resistant Staphylococcus aureus. Pediatrics. 2005;115(3):642–648 [DOI] [PubMed] [Google Scholar]
- 7. Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763–1771 [DOI] [PubMed] [Google Scholar]
- 8. Hammond SP, Baden LR. Clinical decisions: management of skin and soft-tissue infection: polling results. N Engl J Med. 2008;359(15):e20. [DOI] [PubMed] [Google Scholar]
- 9. Creech CB, Beekmann SE, Chen Y, Polgreen PM. Variability among pediatric infectious diseases specialists in the treatment and prevention of methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Pediatr Infect Dis J. 2008;27(3):270–272 [DOI] [PubMed] [Google Scholar]
- 10. Daum RS. Clinical practice: skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2007;357(4):380–390 [DOI] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention Outpatient management of MRSA skin and soft tissue infections. Available at: www.cdc.gov/mrsa/treatment/outpatient-management.html Accessed April 22, 2011
- 12. Mascitti KB, Gerber JS, Zaoutis TE, Barton TD, Lautenbach E. Preferred treatment and prevention strategies for recurrent community-associated methicillin-resistant Staphylococcus aureus skin and soft-tissue infections: a survey of adult and pediatric providers. Am J Infect Control. 2010;38(4):324–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hyun DY, Mason EO, Forbes A, Kaplan SL. Trimethoprim-sulfamethoxazole or clindamycin for treatment of community-acquired methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Pediatr Infect Dis J. 2009;28(1):57–59 [DOI] [PubMed] [Google Scholar]
- 14. Elliott DJ, Zaoutis TE, Troxel AB, Loh A, Keren R. Empiric antimicrobial therapy for pediatric skin and soft-tissue infections in the era of methicillin-resistant Staphylococcus aureus. Pediatrics. 2009;123(6). Available at: www.pediatrics.org/cgi/content/full/123/6/e959 [DOI] [PubMed] [Google Scholar]
- 15. Duong M, Markwell S, Peter J, Barenkamp S. Randomized, controlled trial of antibiotics in the management of community-acquired skin abscesses in the pediatric patient. Ann Emerg Med. 2010;55(5):401–407 [DOI] [PubMed] [Google Scholar]
- 16. Schmitz GR, Bruner D, Pitotti R, et al. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection. Ann Emerg Med. 2010;56(3):283–287 [DOI] [PubMed] [Google Scholar]
- 17. Lee MC, Rios AM, Aten MF, et al. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2004;23(2):123–127 [DOI] [PubMed] [Google Scholar]
- 18. Sreeramoju P, Porbandarwalla NS, Arango J, et al. Recurrent skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus requiring operative debridement. Am J Surg. 2011;201(2):216–220 [DOI] [PubMed] [Google Scholar]
- 19. Shastry L, Rahimian J, Lascher S. Community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections in men who have sex with men in New York City. Arch Intern Med. 2007;167(8):854–857 [DOI] [PubMed] [Google Scholar]
- 20. Chen AE, Cantey JB, Carroll KC, Ross T, Speser S, Siberry GK. Discordance between Staphylococcus aureus nasal colonization and skin infections in children. Pediatr Infect Dis J. 2009;28(3):244–246 [DOI] [PubMed] [Google Scholar]
- 21. Ray WA, Griffin MR. Use of Medicaid data for pharmacoepidemiology. Am J Epidemiol. 1989;129(4):837–849 [DOI] [PubMed] [Google Scholar]
- 22. Talbot TR, Nania JJ, Wright PW, Jones I, Aronsky D. Evaluation of the microbiology of soft-tissue abscesses in the era of community-associated strains of methicillin-resistant Staphylococcus aureus: an argument for empirical contact precautions. Infect Control Hosp Epidemiol. 2007;28(6):730–732 [DOI] [PubMed] [Google Scholar]
- 23. Bar-Meir M, Tan TQ. Staphylococcus aureus skin and soft tissue infections: can we anticipate the culture result? Clin Pediatr (Phila). 2010;49(5):432–438 [DOI] [PubMed] [Google Scholar]
- 24. Pickett A, Wilkinson M, Menoch M, Snell J, Yniguez R, Bulloch B. Changing incidence of methicillin-resistant Staphylococcus aureus skin abscesses in a pediatric emergency department. Pediatr Emerg Care. 2009;25(12):831–834 [DOI] [PubMed] [Google Scholar]
- 25. Tillotson GS, Draghi DC, Sahm DF, Tomfohrde KM, Del Fabro T, Critchley IA. Susceptibility of Staphylococcus aureus isolated from skin and wound infections in the United States 2005–07: laboratory-based surveillance study. J Antimicrob Chemother. 2008;62(1):109–115 [DOI] [PubMed] [Google Scholar]
- 26. Proctor RA. Role of folate antagonists in the treatment of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46(4):584–593 [DOI] [PubMed] [Google Scholar]
- 27. Iyer S, Jones DH. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: a retrospective analysis of clinical presentation and treatment of a local outbreak. J Am Acad Dermatol. 2004;50(6):854–858 [DOI] [PubMed] [Google Scholar]
- 28. von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med. 2001;344(1):11–16 [DOI] [PubMed] [Google Scholar]
- 29. Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis. 2004;39(7):971–979 [DOI] [PubMed] [Google Scholar]
- 30. Lipsky BA, Pecoraro RE, Ahroni JH, Peugeot RL. Immediate and long-term efficacy of systemic antibiotics for eradicating nasal colonization with Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 1992;11(1):43–47 [DOI] [PubMed] [Google Scholar]
- 31. von Eiff C, Becker K, Metze D, et al. Intracellular persistence of Staphylococcus aureus small-colony variants within keratinocytes: a cause for antibiotic treatment failure in a patient with Darier's disease. Clin Infect Dis. 2001;32(11):1643–1647 [DOI] [PubMed] [Google Scholar]
- 32. Choo PW, Rand CS, Inui TS, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. 1999;37(9):846–857 [DOI] [PubMed] [Google Scholar]
- 33. Grymonpre R, Cheang M, Fraser M, Metge C, Sitar DS. Validity of a prescription claims database to estimate medication adherence in older persons. Med Care. 2006;44(5):471–477 [DOI] [PubMed] [Google Scholar]
- 34. Steele RW, Russo TM, Thomas MP. Adherence issues related to the selection of antistaphylococcal or antifungal antibiotic suspensions for children. Clin Pediatr (Phila). 2006;45(3):245–250 [DOI] [PubMed] [Google Scholar]
- 35. Steele RW, Thomas MP, Begue RE. Compliance issues related to the selection of antibiotic suspensions for children. Pediatr Infect Dis J. 2001;20(1):1–5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.