Abstract
BACKGROUND:
Prenatal smoke exposure is associated with airway inflammation and asthma in children. It also increases the risk of low birth weight (LBW). LBW is associated with decreased lung function independently of smoking.
OBJECTIVE:
To study the independent and joint effects of prenatal smoking and LBW on childhood asthma.
METHODS:
In 1996, all children aged 7 to 8 years in 3 cities in northern Sweden were invited to an International Study of Asthma and Allergy in Childhood questionnaire survey. This study focused on the follow-up of children aged 11 to 12 years, in which 3389 children (96%) participated. A subset of 2121 children underwent skin-prick testing. Self-reported physician-diagnosed asthma has been clinically validated.
RESULTS:
Mean birth weight was 3360 g in children exposed to prenatal smoking and 3571 g in nonexposed children (P < .001). The association of prenatal smoking with physician-diagnosed asthma was stronger in LBW children (risk ratio: 8.8 [95% confidence interval: 2.1–38]) than in normal birth weight children (risk ratio: 1.3 [95% confidence interval: 1.0–1.8]). LBW alone was not an independent predictor of asthma. These associations were similar in multivariate analysis, and the interaction term LBW × smoking was highly statistically significant.
CONCLUSIONS:
There was a strong interaction of LBW and prenatalsmoking on the risk of physician-diagnosed asthma, which has not been demonstrated previously. This was consistently seen with adjustment for known risk factors, including allergic sensitization. Plausibly, airway inflammation from prenatal smoke exposure induces obstructive symptoms more easily in the underdeveloped airways of LBW children.
Keywords: wheeze, child, prevalence, epidemiology, risk
WHAT'S KNOWN ON THIS SUBJECT:
Smoking during pregnancy is associated with airway inflammation and asthma in the offspring. It also increases the risk of impaired intrauterine growth and low birth weight. Low birth weight, in turn, is associated with decreased lung function independently of smoking.
WHAT THIS STUDY ADDS:
In schoolchildren, the risk of physician-diagnosed asthma from smoking during pregnancy was increased sixfold in low birth weight children compared with children born at normal weight. Likely, smoke-induced airway infammation elicits obstructive symptoms more easily in children with underdeveloped airways.
Asthma, the most common chronic disease of childhood, is caused by interactions between multiple genes and environmental factors.1,2 The development of asthma and atopy is often determined early in life.3 Also, certain prenatal factors, such as impaired intrauterine growth and prenatal tobacco smoke exposure, are well known to affect respiratory health after birth.4,5
Lung development is directly related to fetal growth and length of gestation.6 Low birth weight (LBW), a marker of impaired fetal growth, is associated with lower lung function in infancy as well as in childhood and adulthood.7–9 Respiratory symptoms and asthma are more prevalent in LBW subjects throughout life, and it has been postulated that 15% to 20% of adult asthma is attributable to LBW.5,10–12 Whether this is a direct effect of impaired lung function or mediated via insults such as respiratory tract infections or air pollutants in children with impaired lung function5 has not been determined.
Prenatal maternal smoking, corrected for subsequent smoking, shows an independent dose-response association with current wheeze in infants.13 Lung function at the third day of life, before substantial postnatal exposures have occurred, is impaired in children exposed to prenatal smoke.14 Compared with postnatal smoke exposure, which was associated with diffuse respiratory symptoms, prenatal smoking was more strongly associated with physician-diagnosed asthma.15 After this observation, the idea that exclusive postnatal smoke exposure has any independent effects has been challenged.13
In westernized countries, maternal smoking is the leading cause of LBW.16 Thus, prenatal smoke exposure, LBW, and subsequent reduced lung function and respiratory symptoms are interrelated (Fig 1). Independent effects of prenatal smoking and LBW have been demonstrated.8,17 In 1 study, the risk of respiratory symptoms from LBW was increased by environmental smoke exposure.18 The effect was modest, however, and was not seen for physician-diagnosed asthma and decreased by age.
FIGURE 1.
Associations between prenatal smoking, LBW, and asthma.
To the present authors' knowledge, this relationship has not been further elucidated. We explored the associations between prenatal smoking, LBW, and asthma in a population-based sample of >3000 schoolchildren. The aim was to study the independent and joint effects of prenatal smoking and LBW on asthma in childhood, and we found a strong synergistic effect.
METHODS
Study Population
The Obstructive Lung Disease in Northern Sweden (OLIN) pediatric study has been described in detail previously.19 In 1996, all (N = 3525) schoolchildren in first and second grade (aged 7–8 years) in 3 municipal areas (Kiruna, Luleå, and Piteå) in northern Sweden were invited to participate in a questionnaire study. The children in Kiruna and Luleå (n = 2454) were also invited to undergo skin-prick testing for allergic sensitization. Informed consent was obtained from the children's parents, and the study was approved by the ethics committee of the Umeå University Hospital.
Methods and Definitions
The questionnaire was based on the International Study of Asthma and Allergy in Childhood (ISAAC) questionnaire,20 with added questions about symptoms, physician-diagnoses, medication use, and possible disease determinants.19 Reports of asthma symptoms and physician-diagnosed asthma were clinically validated in 1997.21 The skin-prick tests followed the European Academy of Allergology and Clinical Immunology (EAACI) guidelines.22 The skin-prick test methods and a validation by specific immunoglobulin E have been described in detail.19,23–25
The majority of definitions have been published previously,11,19,21 and only those with special relevance to this article are listed here. These definitions included: (1) physician-diagnosed asthma (“Has the child been diagnosed by a physician as having asthma?”); (2) ever asthma (“Has your child ever had asthma?”); (3) current wheeze (wheeze or whistling in the chest in the last 12 months); (4) LBW (according to the World Health Organization definition, a birth weight <2500 g, as opposed to normal birth weight [NBW]); and (5) prenatal smoking (mother's self-report of smoking in pregnancy [“yes” or “no”]).
Data Analysis
Whereas childhood wheeze is heterogeneous and has a fluctuating time pattern, in preteenagers asthma is more clinically recognizable and has a higher probability of persistence.11 The present article thus focuses on children aged 11 to 12 years and their follow up. Birth weight and prenatal smoking were surveyed at age 7 to 8 years and, hence, analyses of these factors were limited to children participating in both surveys. Prevalence and risk relationships at age 7 to 8 years are shown for comparison. Univariate relationships are displayed as risk ratios (RRs) with 95% confidence intervals (CIs). Comparisons of means used the Student's t test, and univariate analyses used the χ2 test. P < .05 was considered statistically significant. Multivariate relationships by binary logistic regression are displayed as odds ratios (ORs). Four different multivariate models were tested, adjusting for different panels of other known risk factors for asthma in this cohort.11,19
The effect of prenatal smoking was tested in univariate and multivariate analyses with stratification for LBW. In the multivariate analysis the interaction term LBW × smoking was also tested for statistical significance. In another multivariate analysis (Fig 2), 4 exclusive exposure categories were used: no LBW, no prenatal smoking (E00) (reference category); LBW, no prenatal smoking (E10); no LBW, prenatal smoking (E01); and LBW, prenatal smoking (E11). All analyses were performed by using SPSS Statistics 11.5 (SPSS Inc, Chicago, IL).
FIGURE 2.
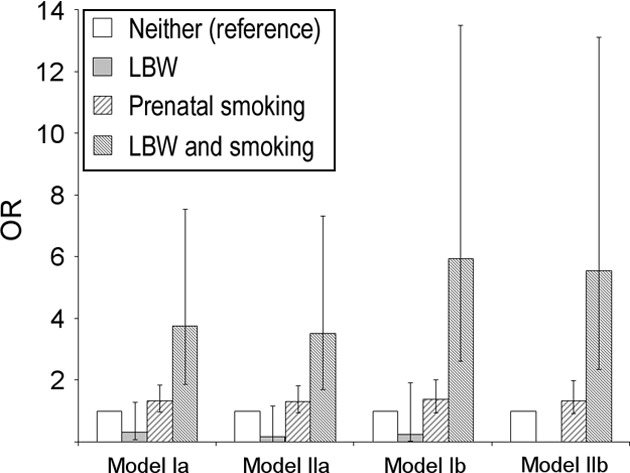
Adjusted ORs for physician-diagnosed asthma at age 11 to 12 years. Four mutually exclusive exposure categories (ie, dummy variables) were analyzed using multivariate regression analysis. Adjustment was made for: parental asthma, respiratory tract infections (model Ia); parental asthma, respiratory tract infections, gender, breastfeeding <3 months, and damp house (model IIa). Models Ib and IIb in addition to their counterparts Ia and IIa also included allergic sensitization.
The attributable proportion due to interaction (AP) was calculated following the method described by Andersson et al and the software provided by the authors at www.epinet.se.26 In the 4 exposure categories noted here, RR (analogous to E) was the relative risk compared with the reference category. RRs were calculated from multivariate regression coefficients:
RESULTS
Basic Characteristics
At age 11 to 12 years, the prevalence of current wheeze and physician-diagnosed asthma was 9.4% and 7.5%, respectively (Table 1). The prevalence of allergic sensitization was 30.1%. Birth weight followed a normal distribution (mean: 3518 g [range: 1064–6620 g]). LBW (ie, <2500 g) was reported by 4.1% whereas 0.4% reported very LBW (<1500 g). Overall, 24.3% of the children had been exposed to prenatal smoking, and 16.6% were exposed both to prenatal smoking and maternal smoking at age 11 to 12 years. Corresponding data at age 7 to 8 years are shown for comparison.
TABLE 1.
Participation and Prevalence of Respiratory Conditions, Allergic Sensitization, Smoking, and LBW
| Age 7–8 y |
Age 11–12 y |
|||
|---|---|---|---|---|
| n | % | n | % | |
| Participation | 3430 | 97.3 | 3389 | 96.5 |
| Current wheeze | 400 | 11.7 | 319 | 9.4 |
| Physician-diagnosed asthma | 197 | 5.7 | 263 | 7.5 |
| Current asthma | 181 | 5.3 | 215 | 6.3 |
| Ever asthma | 218 | 6.4 | 317 | 9.4 |
| Participation in skin test | 2148 | 87.5 | 2155 | 88.4 |
| Allergic sensitization | 443 | 20.6 | 656 | 30.1 |
| Smokinga | ||||
| Prenatal (mother) | 842 | 25.0 | 766 | |
| In first year | ||||
| At least 1 parent | 1224 | 36.7 | 1118 | 36.3 |
| Both parents | 502 | 16.1 | 449 | 15.8 |
| Current | ||||
| At least 1 parent | 1340 | 40.5 | 896 | 29.9 |
| Both parents | 407 | 13.0 | 239 | 7.7 |
| Prenatal and current (mother) | 657 | 19.5 | 541 | 16.6 |
| Prenatal or subsequent (mother) | 1291 | 37.6 | 1284 | 37.9 |
| Birth weighta | ||||
| <2500 g | 139 | 4.1 | 126 | 4.1 |
| <1500 g | 11 | 0.3 | 11 | 0.4 |
In the age 11- to 12-year follow-up, these data were limited to children participating both at ages 7 to 8 and 11 to 12 years.
Univariate Risk Relationships
At age 11 to 12 years, the main outcome, physician-diagnosed asthma, was statistically significantly associated with prenatal smoking (Table 2). This association was greater in LBW children (RR: 8.79 [95% CI: 2.05–37.8]) compared with NBW children (RR: 1.33 [95% CI: 1.01–1.76]). LBW alone was not significantly associated with physician-diagnosed asthma (P = .160). In regression analysis without adjustment, the interaction term LBW × smoking was highly statistically significant (P = .006). This synergistic effect was not seen for current wheeze (P = .350 for the interaction term). At age 7 to 8 years, the synergistic effect of the 2 risk factors on physician-diagnosed asthma was weaker (RR: 2.93 [95% CI: 1.06–8.11]).
TABLE 2.
Prevalence and RR of Physician-Diagnosed Asthma and Current Wheeze at Ages 7 to 8 and 11 to 12 Years According to the Presence of LBW and Prenatal Smoking
| Age 7–8 y |
Age 11–12 y |
|||
|---|---|---|---|---|
| Asthma | Wheeze | Asthma | Wheeze | |
| NBW, % (n) | ||||
| All | 5.5 (171) | 11.0 (345) | 7.7 (221) | 9.5 |
| No smoking | 5.2 (124) | 10.5 (249) | 7.1 (156) | 8.8 (193) |
| Smoking | 6.2 (47) | 12.7 (96) | 9.5 (65) | 11.6 (80) |
| Smoking, RR (95% CI) | 1.19 (0.86–1.65) | 1.21 (0.97–1.51) | 1.33 (1.01–1.76)a | 1.37 (1.04–1.80)a |
| LBW, % (n) | ||||
| All | 10.9 (15) | 23.2 (32) | 11.1 (14) | 7.9 (10) |
| No smoking | 6.1 (5) | 19.5 (16) | 2.4 (2) | 4.9 (4) |
| Smoking | 17.9 (10) | 28.6 (16) | 21.4 (12) | 10.7 (6) |
| Smoking, RR (95% CI) | 2.93 (1.06–8.11)a | 1.46 (0.80–2.68) | 8.79 (2.05–37.8)a | 2.21 (0.65–7.43) |
NBW indicates normal birth weight.
Statistically significant association.
Characteristics of Children Exposed to Smoking in Pregnancy and Born at a LBW
Mothers' prenatal smoking was highly associated with subsequent smoking (Table 3). In children exposed to prenatal smoking, mean birth weight was 3360 g compared with 3571 g in unexposed children (P < .001), and the prevalence of LBW was 6.9% compared with 3.3% in the nonexposed children (P < .001). Breastfeeding <3 months was more prevalent in children born of smoking mothers and among LBW children (P < .001 each). Respiratory tract infections tended to be more prevalent, although not statistically significant (P = .057) in LBW children. The prevalence of other known risk factors for asthma was generally independent of the presence of prenatal smoking and LBW.
TABLE 3.
Characteristics of and Exposures Among 11- to 12-Year-Olds Exposed and Not Exposed, Respectively, to Prenatal Smoking by the Mother
| Prenatal Smoking (N = 766) |
LBW (N = 126) |
|||||
|---|---|---|---|---|---|---|
| Yes | No | Pa | Yes | No | Pa | |
| Prenatal smoking, % | — | — | — | 40.5 | 23.8 | <.001 |
| Smoking first year, % | 90.2 | 18.4 | <.001 | 44.6 | 26.6 | <.001 |
| Smoking, age 7–8 y, % | 79.7 | 15.0 | <.001 | 52.0 | 30.1 | <.001 |
| 0–4 cigarettes per d | 7.1 | 5.0 | — | 8.9 | 5.3 | — |
| 5–14 cigarettes per d | 53.5 | 9.0 | — | 33.3 | 19.4 | — |
| 15–24 cigarettes per d | 18.1 | 1.0 | — | 8.9 | 5.1 | — |
| ≥25 cigarettes per d | 1.1 | 0 | — | 0.8 | 0.2 | — |
| Smoking, age 11–12 y, % | 72.8 | 12.0 | <.001 | 45.1 | 26.2 | <.001 |
| 0–4 cigarettes per d | 8.1 | 4.0 | — | 7.4 | 4.7 | — |
| 5–14 cigarettes per d | 50.9 | 7.0 | — | 33.6 | 17.3 | — |
| 15–24 cigarettes per d | 13.2 | 1.0 | — | 3.3 | 4.0 | — |
| ≥25 cigarettes per d | 0.7 | 0 | — | 0.8 | 0.2 | — |
| Birth weight, mean (SD) | 3360 (553) | 3571 (568) | <.001 | 2126 (351) | 3578 (497) | <.001 |
| LBW, % | 6.9 | 3.3 | <.001 | — | — | — |
| Parental asthma, % | 18.9 | 17.5 | .357 | 22.2 | 17.5 | .173 |
| Allergic sensitization, % | 31.3 | 30.5 | .758 | 28.0 | 30.7 | .620 |
| Respiratory tract infections, % | 59.5 | 57.9 | .442 | 66.7 | 58.1 | .057 |
| Breastfeeding <3 mo, % | 30.9 | 11.6 | <.001 | 35.2 | 15.6 | <.001 |
| Damp house, % | 5.8 | 4.4 | .128 | 4.0 | 4.9 | .649 |
| Male gender, % | 48.8 | 51.5 | 0.202 | 45.2 | 50.9 | 0.211 |
| Birth order, mean (SD) | 1.99 (1.16) | 1.98 (1.06) | 0.765 | 1.87 | 1.99 | 0.237 |
χ2 test for dichotomous outcomes and 1-way analysis of variance for comparisons of means.
Adjusted Risk Relationships
In multivariate analyses, the combination of LBW and prenatal smoking increased the risk of physician-diagnosed asthma at age 11 to 12 years four- to sixfold, which was highly statistically significant (Fig 2). The combined risk was higher in models adjusting for allergic sensitization (models Ib and IIb). Prenatal smoking alone was borderline statistically significant (OR: 1.35 [95% CI: 0.99–1.84]) in model Ia, whereas LBW alone was not independently associated with physician-diagnosed asthma. Accordingly, the interaction term LBW × smoking was highly statistically significant for physician-diagnosed asthma (P = .007, model Ia; P = .010, model Ib; and P = .026, model IIa). This interaction was seen also for the outcome ever-asthma, which provided greater statistical power, thus narrowing the CIs (data not shown). For current wheeze, there were no significant interactions (all P > .1).
Multivariate analyses stratified for LBW confirmed the observed univariate associations and further underlined the presence of an interaction (Table 4). In NBW children, prenatal smoking was a weak but statistically significant risk factor for current wheeze and physician-diagnosed asthma alike (OR: 1.3–1.5). In contrast, in LBW children, prenatal smoking had ORs of >10 for physician-diagnosed asthma. This association was weaker and not statistically significant for current wheeze.
TABLE 4.
Adjusted ORs of Current Wheeze and Physician-Diagnosed Asthma at Age 11 to 12 Years From Prenatal Smoke Exposure, Stratified According to LBW and Analyzed by Using Multiple Logistic Regression
| Prenatal Smoke Exposure |
||||
|---|---|---|---|---|
| Model Iaa | Model IIab | Model Ibc | Model IIbc | |
| Wheeze | ||||
| NBW | 1.34 (1.01–1.77)d | 1.30 (0.97–1.74) | 1.53 (1.08–2.17)d | 1.45 (1.01–2.08)d |
| LBW | 2.44 (0.64–9.23) | 4.41 (0.98–19.8) | 5.24 (0.66–41.8) | —e |
| Asthma | ||||
| NBW | 1.35 (0.99–1.84) | 1.30 (0.94–1.80) | 1.44 (0.98–2.12) | 1.38 (0.92–2.07) |
| LBW | 11.3 (2.40–53.2)d | 27.2 (3.20–231)d | 17.7 (1.75–180)d | —e |
Model Ia included also parental asthma and respiratory tract infections.
Model IIa included also parental asthma, respiratory tract infections, gender, breastfeeding <3 months, and damp house.
Models Ib and IIb, in addition to the covariates in their counterparts Ia and IIa, also included allergic sensitization.
Statistically significant association.
Too few cases to compute meaningful OR.
DISCUSSION
In this large, population-based study of 11- to 12-year-olds, there was a clear interaction between prenatal smoking and LBW on the risk of physician-diagnosed asthma. LBW was not independently associated with physician-diagnosed asthma. Prenatal smoking was an independent but weak risk factor for physician-diagnosed asthma in NBW children, whereas the joint effect of both risk factors increased the risk of physician-diagnosed asthma more than sixfold.
The majority of cases of physician-diagnosed asthma among children exposed to prenatal smoking occurred among NBW children. Conversely, in LBW children almost all cases were seen in those exposed to prenatal smoking. Although this group accounts for only a minority of asthma in the general population, the present study identifies a subgroup at very high risk. Adjusted for potential confounders, the proportion of physician-diagnosed asthma that was attributable to the interaction among children with both risk factors (AP) was estimated at between 82% (model Ia) and 89% (model Ib).
Methodologic Aspects
This study benefits from its size and population-based design, which distinguishes it from previous registry-based studies and studies of selected populations.14,27–31 The study included almost all 11- to 12-year-olds in these 3 municipalities as school attendance is mandatory in Sweden, virtually eliminating selection bias. Through use of an extensive questionnaire, the study cohort was well characterized.11,19,21 The risk analyses were adjusted for numerous potential confounders, including objective data on sensitization, which is an important advantage in countering the co-variance of smoking with other lifestyle factors. Other factors that further minimized bias include the fact that Swedish maternity care is of high standard, weighing of the neonate is a standardized procedure, and birth weight was reported in grams instead of having a specific question regarding LBW.
The main limitations arise from the cross-sectional nature of the study, which, through recall bias, may increase the uncertainty of cause-consequence relationships. Mothers of asthmatic children may underreport smoking, which is a social stigma. Analogous to the “healthy smoker effect,” this would lead to an underestimation of the relationship with asthma and thus not inflate the observed association. Regrettably, questions about conditions related to LBW (eg, gestational length, multiparity, maternal disease) were not included in the questionnaire. This may have lowered the specificity of LBW, and it is possible that certain subgroups of LBW subjects are at further elevated risk of developing asthma.
Because no data were available regarding gestational length or perinatal illnesses, we cannot completely rule out the possibility of some misclassification with bronchopulmonary dysplasia, which is overrepresented in premature children. This should be further explored in future studies. Nevertheless, the interaction of LBW and prenatal smoking was not seen for current wheeze but for physician-diagnosed asthma. A diagnosis of asthma is more specific at age 11 to 12 years compared with age 7 to 8 years, as the heterogeneity of wheezing disorders decreases with age.11 A clinical validation of self-reported physician-diagnosed asthma in our study showed near 100% specificity and >70% sensitivity of the question, when evaluated against a predefined panel of asthma criteria and against Swedish pediatricians' assessments.21 Because this condition reflects a lifetime burden of respiratory symptoms severe, persistent, and specific enough to have rendered the child a physician's diagnosis of asthma, it was chosen as the main outcome.
Interaction of LBW and Prenatal Smoking
The joint effect of LBW and prenatal smoking on physician-diagnosed asthma was clearly higher than would be expected on an additive scale, indicating a multiplicative interaction.26 Although there were only a few cases, the finding was consistently seen in univariate analysis and in different multivariate models. The distribution of potential confounders was largely independent of the presence of LBW and prenatal smoking. Slight differences were seen for breastfeeding and respiratory tract infections, which, when adjusted for, only strengthened the observed interaction effect. Thus, the strong interaction was seemingly not explained by other factors.
The present study is the first, to the authors' knowledge, to demonstrate this strong interaction for physician-diagnosed asthma in schoolchildren. Although numerous studies have addressed the independent effects of LBW and smoking on respiratory outcomes,2–10,12–15,17,27–34 only 1 has previously targeted interactions.18 This study observed a birth cohort up to 7 years of age and found that the risk of respiratory symptoms (wheeze, cough, or lower respiratory tract infection) from LBW was slightly increased by smoke exposure (OR: 1.52 vs 1.21) at 5 years of age.18 The authors concluded that however interesting, the weak interaction was only seen for unspecific symptoms and not asthma diagnoses, and was diminished by increasing age. Our study, in contrast, found a strong interaction for physician-diagnosed asthma that was greater at age 11 to 12 years than at age 7 to 8 years, but found no independent effects of LBW. It cannot be excluded that this disparity between our studies was because of differences in methods and study samples. Nevertheless, another plausible explanation is that by the preteen years, many LBW children have outgrown the unspecific wheeze caused by impaired lung development, whereas clinically relevant asthma may have developed among LBW children exposed to prenatal smoke.
In a recent review, Jaakkola et al5 speculated that an exposure (prenatal smoking) could alter the relationship between fetal growth impairment and asthma. Either prenatal smoking could lead to both LBW and asthma in genetically predisposed people, or the development of asthma could be mediated through an association of prenatal smoking with a subsequent exposure (eg, postnatal smoking). The fact that the interaction was greater at age 11 to 12 years than at age 7 to 8 years may indicate a cumulative effect by age, likely in the form of subsequent smoking among mothers who smoked during pregnancy. However, the study was not designed to separate the effects of smoking at different ages.
Independent Effects of LBW and Prenatal Smoking
There is compelling evidence that LBW affects lung function. In infants (ie, before postnatal exposures had occurred), LBW adjusted for prenatal smoking was associated with impaired lung function.30 Similar effects including decreased gas diffusion capacity have been reported in older children and in adults.7–9 The association of impaired intrauterine growth and LBW with asthma is not clear, however. In a meta-analysis, preterm delivery had ORs between 1.07 and 1.37 for asthma but with no consistent adjustment for prenatal smoking.5 Only very few pediatric studies have found an association between LBW and asthma after adjusting for smoking.12 Others have not been able to demonstrate this association in children7 or in adults.8
In our study, LBW was not an independent risk factor for physician-diagnosed asthma. However, without the interaction term the association of LBW with physician-diagnosed asthma was positive, and this was further strengthened when prenatal smoking was also omitted from the model (data not shown). On the basis of our findings, confounding by the interaction with smoking and smoking per se could explain the differences between previous studies. Future prospective studies of these relationships should further clarify whether LBW is a true independent risk factor for asthma.
In contrast to LBW, prenatal smoking has been linked to asthma symptoms previously. In 1 review, maternal smoking was associated with childhood wheeze but not asthma.32 However, later studies have demonstrated this relationship with postnatal smoking, whereas prenatal smoking was associated with asthma15,27 similar to findings in our own cohort.11 Interestingly, prenatal—as opposed to postnatal—smoke exposure seems to interact with the ADAM33 gene on asthma risk,31 underlining the importance of the timing of smoke exposure. The present study unfortunately was not powered to explore prenatal smoking and LBW in mothers who quit smoking shortly after birth. In addition to these epidemiologic findings, mechanistic studies have further clarified some of the pathways linking prenatal smoking to pediatric asthma. In neonates of smoking mothers, flow-volume ratio at tidal breathing (a predictor of infant wheeze) and lung compliance, adjusted for birth weight, were significantly reduced in newborns.14 Reduced infant airway caliber has been reported in other studies.33 Prenatal smoke exposure affects exhaled nitric oxide,29 increases levels of F2-isoprostanes in infants,34 and also increases T-helper type 2 cell responses in neonates.28
Thus, it seems that although birth weight mainly affects lung function, prenatal and later smoke exposure has immunologic effects associated with the development of asthma. We therefore postulate that airway inflammation from prenatal smoking in an underdeveloped lung owing to intrauterine growth impairment could explain the interaction seen in our study. This could be a direct effect of oxidative stress, or mediated through subsequent infections, allergens, or other exposures. Impaired lung function is a risk factor for respiratory tract infections,6 the history of which tended to be more prevalent (P = .057) in LBW children. Moreover, subsequent smoking may add to the prenatal smoking in LBW children, supported by the fact that the observed interaction was stronger at age 11 to 12 years than at age 7 to 8 years. Maternal smoking exclusively at age 11 to 12 years was not associated with physician-diagnosed asthma (RR: 1.13 [95% CI: 0.70–1.83]), but this relationship is complicated, as mothers who smoke during pregnancy are, in general, heavier smokers.13,28
CONCLUSIONS
This study, to our knowledge, is the first to identify a strong interaction between prenatal smoke exposure and LBW on physician-diagnosed asthma in schoolchildren. The significant augmentation of LBW on the association between prenatal smoking and physician-diagnosed asthma could not be explained by confounding. These new findings help explain the interrelationship between smoking, intrauterine growth impairment, and childhood asthma. Smoke-induced oxidative stress in underdeveloped airways caused by impaired fetal growth is a plausible mechanistic explanation for the development of asthma in these children.
ACKNOWLEDGMENTS
The Swedish Heart-Lung Foundation, the Swedish Foundation for Health Care Science and Allergy Research (Vårdal), US National Institute of Allergy and Infectious Disease (grants AI-20565 and AI-34607), the Swedish Asthma-Allergy Foundation, and Norrbotten′s local health authorities. ALK and Pharmacia-Upjohn helped with providing test material. GlaxoSmithKline provided additional support.
We thank the nurses Kerstin Kemi-Björnström, Lena Gustafsson, and Aina Johnsson for their valuable help with data collection.
Dr Bjerg conceived of and executed the statistical analyses and wrote the manuscript; Dr Hedman collected the data and participated in the interpretation of data; Dr Perzanowski participated in the collection and interpretation of data and aided in the statistical analyses and in drafting the manuscript; Dr Lundbäck initiated and helped coordinated the study and aided in drafting the manuscript; and Dr Rönmark coordinated the study and aided in the statistical analyses and in drafting the manuscript. All authors participated in the manuscript-reviewing process and have approved the final version of the article.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
COMPANION PAPER: A companion to this article can be found on page e913 and online at www.pediatrics.org/cgi/doi/10.1542/peds.2010–2603.
- LBW
- low birth weight
- OLIN
- Obstructive Lung Disease in Northern Sweden Studies
- ISAAC
- International Study of Asthma and Allergies in Childhood
- EAACI
- European Academy of Allergology and Clinical Immunology
- RR
- risk ratio
- CI
- confidence interval
- OR
- odds ratio
- AP
- attributable proportion due to interaction
REFERENCES
- 1. Masoli M, Fabian D, Holt S, Beasley R, Global Initiative for Asthma (GINA) Program The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy. 2004;59(5):469–478 [DOI] [PubMed] [Google Scholar]
- 2. Meyers DA, Postma DS, Stine OC, et al. Genome screen for asthma and bronchial hyperresponsiveness: interactions with passive smoke exposure. J Allergy Clin Immunol. 2005;115(6):1169–1175 [DOI] [PubMed] [Google Scholar]
- 3. Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332(3):133–138 [DOI] [PubMed] [Google Scholar]
- 4. Cook DG, Strachan DP. Health effects of passive smoking. 3. Parental smoking and prevalence of respiratory symptoms and asthma in school age children. Thorax. 1997;52(12):1081–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jaakkola JJ, Ahmed P, Ieromnimon A, et al. Preterm delivery and asthma: a systematic review and meta-analysis. J Allergy Clin Immunol. 2006;118(4):823–830 [DOI] [PubMed] [Google Scholar]
- 6. Stick S. Pediatric origins of adult lung disease. 1. The contribution of airway development to paediatric and adult lung disease. Thorax. 2000;55(7):587–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rona RJ, Gulliford MC, Chinn S. Effects of prematurity and intrauterine growth on respiratory health and lung function in childhood. BMJ. 1993;306(6881):817–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Edwards CA, Osman LM, Godden DJ, Campbell DM, Douglas JG. Relationship between birth weight and adult lung function: controlling for maternal factors. Thorax. 2003;58(12):1061–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hancox RJ, Poulton R, Greene JM, McLachlan CR, Pearce MS, Sears MR. Associations between birth weight, early childhood weight gain and adult lung function. Thorax. 2009;64(3):228–232 [DOI] [PubMed] [Google Scholar]
- 10. Svanes C, Omenaas E, Heuch JM, Irgens LM, Gulsvik A. Birth characteristics and asthma symptoms in young adults: results from a population-based cohort study in Norway. Eur Respir J. 1998;12(6):1366–1370 [DOI] [PubMed] [Google Scholar]
- 11. Bjerg-Bäcklund A, Perzanowski M, Platts-Mills T, Sandström T, Lundbäck B, Rönmark E. Asthma during the primary school ages—prevalence, remission and the impact of allergic sensitization. Allergy. 2006;61(5):549–555 [DOI] [PubMed] [Google Scholar]
- 12. Shaheen SO, Sterne JA, Montgomery SM, Azima H. Birth weight, body mass index and asthma in young adults. Thorax. 1999;54(5):396–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stein RT, Holberg CJ, Sherrill D, et al. Influence of parental smoking on respiratory symptoms during the first decade of life: the Tucson Children's Respiratory Study. Am J Epidemiol. 1999;149(11):1030–1037 [DOI] [PubMed] [Google Scholar]
- 14. Lödrup Carlsen KC, Jaakkola JJ, Nafstad P, Carlsen KH. In utero exposure to cigarette smoking influences lung function at birth. Eur Respir J. 1997;10(8):1774–1779 [DOI] [PubMed] [Google Scholar]
- 15. Gilliland FD, Li YF, Peters JM. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med. 2001;163(2):429–436 [DOI] [PubMed] [Google Scholar]
- 16. Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65(5):663–737 [PMC free article] [PubMed] [Google Scholar]
- 17. Jaakkola JJK, Gissler M. Maternal smoking in pregnancy, fetal development, and childhood asthma. Am J Publ Health. 2004;94(1):136–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caudri D, Wijga A, Gehring U, et al. Respiratory symptoms in the first 7 years of life and birth weight at term: the PIAMA birth cohort. Am J Respir Crit Care Med. 2007;175(10):1078–1085 [DOI] [PubMed] [Google Scholar]
- 19. Rönmark E, Lundbäck B, Jönsson E, Platts-Mills T. Asthma, type-1 allergy and related conditions in 7- and 8-year-old children in northern Sweden: prevalence rates and risk factor pattern. Respir Med. 1998;92(2):316–324 [DOI] [PubMed] [Google Scholar]
- 20. Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8(3):483–491 [DOI] [PubMed] [Google Scholar]
- 21. Rönmark E, Jönsson E, Platts-Mills T, Lundbäck B. Different pattern of risk factors for atopic and nonatopic asthma among children—report from the Obstructive Lung Disease in Northern Sweden study. Allergy. 1999;54(9):926–935 [DOI] [PubMed] [Google Scholar]
- 22. Dreborg S, Backman E, Basomba A, Bousquet J, Malling D. Skin tests used in type i allergy testing. Position paper of European Academy of Allergology and Clinical Immunology. Allergy. 1989;44(Suppl 10):1–59 [Google Scholar]
- 23. Rönmark E, Perzanowski M, Platts-Mills T, Lundbäck B, Obstructive Lung Disease in Northern Sweden Study Group Four-year incidence of allergic sensitization among schoolchildren in a community where allergy to cat and dog dominates sensitization: report from the Obstructive Lung Disease in Northern Sweden Study Group. J Allergy Clin Immunol. 2003;112(4):747–754 [DOI] [PubMed] [Google Scholar]
- 24. Rönmark E, Bjerg A, Perzanowski M, Platts-Mills T, Lundbäck B. Major increase in allergic sensitization in schoolchildren from 1996 to 2006 in northern Sweden. J Allergy Clin Immunol. 2009;124(2):357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perzanowski M, Rönmark E, Platts-Mills TA, Lundbäck B. Effect of cat and dog ownership on sensitization and development of asthma among preteenage children. Am J Respir Crit Care Med. 2002;166(5):696–702 [DOI] [PubMed] [Google Scholar]
- 26. Andersson T, Alfredsson L, Källberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20(7):575–579 [DOI] [PubMed] [Google Scholar]
- 27. Jaakkola JJ, Gissler M. Are girls more susceptible to the effects of prenatal exposure to tobacco smoke on asthma? Epidemiology. 2007;18(5):573–576 [DOI] [PubMed] [Google Scholar]
- 28. Noakes PS, Thomas R, Lane C, et al. Association of maternal smoking with increased infant oxidative stress at 3 months of age. Thorax. 2007;62(8):714–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Frey U, Kuehni C, Roiha H, et al. Maternal atopic disease modifies effects of prenatal risk factors on exhaled nitric oxide in infants. Am J Respir Crit Care Med. 2004;170(3):260–265 [DOI] [PubMed] [Google Scholar]
- 30. Dezateux C, Lum S, Hoo AF, Hawdon J, Costeloe K, Stocks J. Low birth weight for gestation and airway function in infancy: exploring the fetal origins hypothesis. Thorax. 2004;59(1):60–66 [PMC free article] [PubMed] [Google Scholar]
- 31. Reijmerink NE, Kerkhof M, Koppelman GH, et al. Smoke exposure interacts with ADAM33 polymorphisms in the development of lung function and hyperresponsiveness. Allergy. 2009;64(6):898–904 [DOI] [PubMed] [Google Scholar]
- 32. Strachan DP, Cook DG. Health effects of passive smoking. 6. Parental smoking and childhood asthma: longitudinal and case-control studies. Thorax. 1998;53(3):204–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dezateux C, Stocks J, Dundas I, Fletcher ME. Impaired airway function and wheezing in infancy: the influence of maternal smoking and a genetic predisposition to asthma. Am J Respir Crit Care Med. 1999;159(2):403–410 [DOI] [PubMed] [Google Scholar]
- 34. Noakes PS, Holt PG, Prescott SL. Maternal smoking in pregnancy alters neonatal cytokine responses. Allergy. 2003;58(10):1053–1058 [DOI] [PubMed] [Google Scholar]


