Abstract
OBJECTIVE:
To determine the association of delayed acyclovir therapy with death among neonates with herpes simplex virus (HSV) infection.
METHODS:
A multicenter, retrospective, cohort study was conducted between January 1, 2003, and December 31, 2009, with 1086 neonates (age: ≤28 days) with HSV infection from 41 tertiary care children's hospitals. Early acyclovir therapy was defined as initiation of intravenous acyclovir treatment within 1 day after hospital admission, and delayed acyclovir therapy was defined as initiation of treatment >1 and ≤7 days after hospital admission. Multivariate logistic regression models determined the association between delayed acyclovir therapy and death, with the use of propensity scores for each neonate's likelihood of receiving delayed acyclovir treatment to control for differences in illness severity between groups.
RESULTS:
The median age was 10 days. Delayed acyclovir therapy was administered to 262 neonates (24.1%). In most cases (86.2%) of delayed receipt, acyclovir administration occurred on the second or third day of hospitalization. The overall mortality rate was 7.3% (95% confidence interval: 5.8%–9.0%); 9.5% of those who received delayed acyclovir treatment and 6.6% of those who received early acyclovir treatment died. In a multivariate analysis, delayed acyclovir therapy was associated with significantly greater odds of death (adjusted odds ratio: 2.63 [95% confidence interval: 1.36–5.08]) compared with early acyclovir therapy.
CONCLUSIONS:
In this multicenter observational study of neonates with HSV infection, delayed initiation of acyclovir therapy was associated with in-hospital death. Our data support the use of empiric acyclovir therapy for neonates undergoing testing for HSV infection.
Keywords: herpes simplex virus, neonate, acyclovir, therapy
WHAT'S KNOWN ON THIS SUBJECT:
Neonatal herpes simplex virus (HSV) infection, although uncommon, is associated with significant mortality and neurologic morbidity. Acyclovir therapy reduces mortality rates. The association between the timing of acyclovir treatment initiation and mortality is not known.
WHAT THIS STUDY ADDS:
In this multicenter observational study of neonates with HSV infection, delayed initiation of acyclovir therapy was associated with in-hospital death. Our data support the use of empiric acyclovir therapy for neonates undergoing testing for HSV infection.
Herpes simplex virus (HSV) infection, although uncommon among neonates,1,2 causes significant deaths and neurologic morbidity.3,4 Early diagnosis on the basis of clinical examination findings is challenging, because initial symptoms (such as fever or hypothermia) are nonspecific and skin vesicles, which represent the most common overt manifestation of neonatal HSV, are absent for up to 40% of neonates with disseminated or central nervous system disease.4,5
HSV polymerase chain reaction tests, which allow for accurate diagnosis, are widely available.6–8 Therefore, HSV testing is commonly performed for neonates undergoing evaluation with lumbar puncture.1,9 Intravenously administered acyclovir improves outcomes,10 and administration often is initiated at the time of HSV testing. However, most neonates in whom testing is performed do not have HSV.1,2,9 In addition, HSV testing is associated with increased hospital length of stay9 and the accompanying risks of hospitalization, including medication errors and health care-acquired infections. Empiric acyclovir therapy is costly9 and exposes neonates to potential adverse effects such as neutropenia and renal toxicity.10 As a consequence, there is controversy regarding which neonates would benefit from empiric acyclovir treatment.11,12 Should all neonates for whom HSV testing is performed receive empiric acyclovir treatment, or should empiric treatment be reserved for a high-risk subset of patients, such as those with hypotension or disordered coagulation, whose illness severity at presentation has been associated with worse outcomes?4,13
The relationship between delayed initiation of acyclovir therapy and outcomes is not known. Previous studies on this topic used historical comparison groups, were conducted before the advent of HSV polymerase chain reaction testing, and were not designed specifically to study the association of early versus late initiation of acyclovir treatment for infants with diagnosed HSV. The objective of this study was to determine whether delayed acyclovir therapy was associated with death among neonates with HSV.
METHODS
Data Source
Data for this multicenter, retrospective, cohort study were obtained from the Pediatric Health Information System (PHIS), a national database containing resource utilization data from 41 freestanding, tertiary care, children's hospitals. These hospitals are affiliated with the Child Health Corporation of America (Shawnee Mission, KS), a business alliance of children's hospitals. Participating hospitals provide discharge data, including demographic data, diagnoses, and procedures. Billing data detail drugs, radiologic imaging studies, and laboratory tests charged to each patient. Systematic monitoring occurs on an ongoing basis to ensure data quality, as described previously.14,15 The protocol for the performance of this study was reviewed and approved by the Children's Hospital of Philadelphia committees for the protection of human subjects; informed-consent requirements were waived.
Patients
Children aged 28 days or younger with HSV infection were eligible for this study if they were discharged from any of the 41 participating hospitals between January 1, 2003, and December 31, 2009. Patients were considered to have HSV if they received intravenous acyclovir therapy during their hospitalization and were assigned a diagnosis of HSV, as indicated by International Classification of Diseases, Ninth Revision (ICD-9), discharge diagnosis code 054.xx (HSV, with the last 2 digits representing any combination of 1- or 2-digit codes) in any discharge diagnosis field. Patients who received their first dose of intravenous acyclovir treatment >7 days after admission were excluded, because it is likely that such infants were admitted initially with conditions unrelated to HSV. In-born neonates were excluded.
Study Definitions
Subjects with isolated skin, eye, and mouth disease were identified by the presence of ICD-9 code 054.9 (HSV without mention of complications) in the absence of concomitant codes indicating a HSV-associated complication (ie, meningitis or sepsis). Congenital anomalies, grouped according to involved organ system, included cardiac, neurologic, pulmonary, gastrointestinal, and genitourinary anomalies (Appendix). Receipt of mechanical ventilation and medication usage, except for acyclovir therapy, were included only if they occurred on the first day of hospital admission. Medications included blood products, anticonvulsants, vasoactive agent infusions, and acyclovir. Early acyclovir receipt was defined as receipt of intravenously administered acyclovir on the first day of hospitalization (measured by day from midnight to midnight). Delayed acyclovir receipt was defined as receipt of intravenously administered acyclovir >1 and ≤7 days after admission.
Measured Outcomes
The primary outcome measure was death before hospital discharge.
Measured Exposures
The primary exposure of interest was delayed receipt of intravenously administered acyclovir, because the clinical dilemma typically centers on whether acyclovir should be used empirically.
Statistical Analyses
Categorical variables were described by using frequencies and proportions, whereas continuous variables were described by using mean, median, range, and interquartile range (IQR) values. In unadjusted analyses, patient characteristics and clinical outcomes of early and delayed acyclovir receipt were compared by using χ2 tests or Fisher's exact tests for categorical variables and Wilcoxon rank-sum tests for continuous variables.
Propensity scores for delayed receipt of acyclovir were constructed to estimate the probability of delayed acyclovir receipt given an observed set of baseline confounders.16–18 Propensity scores for delayed receipt of acyclovir allow for more-valid estimates of treatment effects, because the models compare patients with similar likelihoods of receiving delayed acyclovir treatment. Variables used to develop the propensity scores included age, gender, race, hospital, month of admission, and year of admission; use of HSV skin testing and an ICD-9 code for HSV without mention of complications, to account for skin disease as a presenting complaint for HSV; and the need for endotracheal intubation, anticonvulsant therapy, vasoactive agent infusions, and blood product transfusions on the first day of hospital admission, to account for severe illness presentation as a reason for receiving acyclovir. The propensity model's calculated c statistic was 0.75.
Multivariate logistic regression analysis was performed to evaluate outcomes associated independently with delayed acyclovir receipt, with adjustment for both propensity scores for delayed receipt of acyclovir and individual covariates.19,20 Because disseminated disease is more common among younger infants, we performed a secondary analysis after stratification according to age, to identify important subgroup effects.
Additional analyses were conducted to address the residual biases inherent in any multicenter observational study; these biases include residual patient-level confounding, hospital-level confounding, and misclassification bias. First, we repeated the analysis while excluding patients with HSV without complications. Second, we examined the association of the timing of acyclovir treatment initiation (by day) with death by using logistic regression to adjust for residual patient-level confounding. If the association of delayed acyclovir therapy and death resulted from a delay in the initiation of appropriate therapy rather than unmeasured differences in illness severity for patients who received delayed versus early acyclovir therapy, then we should detect a time-dependent relationship between the length of delay and mortality rates. Third, we performed conditional logistic regression analysis with adjustment for delayed acyclovir propensity scores and individual covariates and stratification according to hospital, to adjust for hospital-level confounding.
Finally, only tertiary care children's hospitals participated. Some neonates might have had lumbar punctures performed at other hospitals before transfer to these tertiary care facilities. Patients who received delayed acyclovir treatment would be misclassified as receiving early acyclovir treatment if transfer occurred >24 hours after lumbar puncture and acyclovir treatment was not initiated until arrival at the PHIS hospital. To account for this possibility, we performed a subanalysis restricted to neonates with a hospital charge for cerebrospinal fluid (CSF) testing (culture, cell count, or glucose or protein measurement) within 7 days after hospitalization. This analysis would minimize misclassification bias by ensuring that the diagnostic lumbar puncture was performed (and, by inference, the diagnosis of HSV was made) at the PHIS hospital, rather than at the transferring hospital.
All analyses were clustered according to hospital. Two-tailed P values of <.05 were considered statistically significant. Because 262 (24.1%) of 1086 neonates with HSV received delayed acyclovir treatment, we had 80% power (α = .05) to detect an odds ratio (OR) of ≥1.8 for death for patients who received delayed acyclovir treatment, compared with patients who received early acyclovir treatment.
RESULTS
Patient Characteristics
During the study period, 1098 neonates with a discharge diagnosis of HSV received intravenous acyclovir treatment; 12 (1.1%) of those neonates received acyclovir >7 days after hospitalization and thus were excluded. The remaining 1086 neonates were included in this study. There was a median of 25 neonates per hospital (IQR: 18–36 neonates per hospital). Demographic characteristics of patients are presented in Table 1. The median patient age at admission was 10 days (IQR: 6–17 days). One hundred ninety-two patients (17.7%) had an ICD-9 code for HSV without complications, 35 of whom received delayed acyclovir treatment.
TABLE 1.
Characteristics of Patients
| Characteristic |
n (%) |
P | ||
|---|---|---|---|---|
| Overall | Delayed Acyclovir Therapy (N = 262) | Early Acyclovir Therapy (N = 824) | ||
| Age category | .566 | |||
| 0–7 d | 354 (32.6) | 93 (35.5) | 261 (31.7) | |
| 8–14 d | 382 (35.2) | 91 (34.7) | 291 (35.3) | |
| 15–21 d | 226 (20.8) | 53 (20.2) | 173 (21.0) | |
| 22–28 d | 124 (11.4) | 25 (9.5) | 99 (12.0) | |
| Male | 585 (53.9) | 139 (53.0) | 446 (54.1) | .762 |
| Race/ethnicitya | .030 | |||
| Non-Hispanic white | 524 (48.3) | 136 (51.9) | 388 (47.1) | |
| Non-Hispanic black | 216 (19.9) | 47 (17.9) | 169 (20.5) | |
| Hispanic | 142 (13.1) | 43 (16.4) | 99 (12.0) | |
| Other | 128 (11.8) | 19 (7.3) | 109 (13.2) | |
| Missing data | 76 (7.0) | 17 (6.5) | 59 (7.2) | |
| Congenital anomaly | 142 (13.1) | 45 (17.2) | 97 (11.8) | .024 |
| Government payer | 635 (58.5) | 168 (64.1) | 467 (56.7) | .033 |
| HSV without complications | 192 (17.7) | 35 (13.4) | 157 (19.1) | .035 |
| Initial management | ||||
| Skin testing for HSV | 251 (23.1) | 43 (16.4) | 208 (25.2) | .003 |
| Vasoactive agent infusionsb | 61 (5.6) | 11 (4.2) | 50 (6.1) | .252 |
| Anticonvulsant therapyc | 133 (12.3) | 19 (7.3) | 114 (13.8) | .005 |
| Blood product transfusiond | 566(6.1) | 8 (3.0) | 36 (4.4) | .347 |
| Endotracheal intubation | 74 (6.8) | 19 (7.3) | 55 (6.7) | .747 |
Because of rounding, proportions may not total 100.
Race/ethnicity data were reported by parents or legal guardians of the subjects. Other races included Asian, Native American, and other. Race was classified as missing data if race was not reported in the database.
Vasoactive agent infusions included dobutamine, dopamine, epinephrine, and norepinephrine infusions.
Anticonvulsants included diazepam, lorazepam, fosphenytoin, phenytoin, pentobarbital, phenobarbital, and valproate.
Blood product transfusions included administration of packed red blood cells, cryoprecipitate, fresh frozen plasma, or platelets.
Timing of Acyclovir Treatment Initiation
Overall, 262 (24.1%) of 1086 neonates had delayed receipt of acyclovir. In most cases (86.2%) of delayed receipt, acyclovir administration occurred on the second or third day of hospitalization. There was no association between delayed acyclovir administration and the month of admission (χ2 test, P = .505). There was variation across hospitals, however. The median proportion of neonates with delayed acyclovir receipt at any hospital was 23.8%, and values ranged from 1.9% to 76.9% (IQR: 15.4%–31.5%).
Outcome Measures
The overall mortality rate was 7.3% (95% confidence interval [CI]: 5.8%–9.0%); mortality rates were 9.5% (95% CI: 6.3%–13.82%) for those with delayed acyclovir receipt and 6.6% (95% CI: 5.0%–8.5%) for those with early acyclovir receipt (Table 2). With stratification according to age, the mortality rate was highest among neonates aged 7 days or younger, and deaths occurred disproportionately among those with delayed acyclovir receipt (Table 2). Overall, the cumulative mortality rates were 4.4% at 7 days and 6.8% at 28 days after admission. The cumulative mortality rates 7 days after admission were 4.6% for those with delayed acyclovir receipt and 4.4% for those with early acyclovir receipt. The cumulative mortality rates 28 days after admission were 8.8% for those with delayed acyclovir receipt and 6.2% for those with early acyclovir receipt.
TABLE 2.
In-Hospital Deaths Among Neonates With HSV Infection According to Age and Timing of Acyclovir Receipt
|
n/N (%) |
|||
|---|---|---|---|
| Overall | Delayed Acyclovir Therapy | Early Acyclovir Therapy | |
| Overall | 79/1086 (7.3) | 25/262 (9.5) | 54/824 (6.6) |
| Age category | |||
| 0–7 d | 38/354 (10.7) | 15/93 (16.1) | 23/261 (8.8) |
| 8–14 d | 32/382 (8.4) | 9/91 (9.9) | 23/291 (7.9) |
| 15–21 d | 8/226 (3.5) | 1/53 (1.9) | 7/173 (4.1) |
| 22–28 d | 1/124 (0.8) | 0/25 (0) | 1/99 (1.0) |
In unadjusted analyses, receipt of vasoactive agent infusions, anticonvulsant therapy, blood product transfusions, and endotracheal intubation on the first day of hospital admission were associated with death; however, delayed acyclovir receipt was not (Table 3). In the multivariate analysis, delayed acyclovir receipt was associated with greater than twofold higher odds of death (Table 4). In the stratified analysis, delayed acyclovir receipt was associated with higher odds of death among neonates aged 14 days or younger (adjusted OR: 3.2 [95% CI: 1.5–7.0]; P = .003).
TABLE 3.
Univariate Analyses of Risk Factors for Death Among Neonates With HSV Infection
| Characteristic | Unadjusted OR (95% CI) | P |
|---|---|---|
| Age categorya | ||
| 0–7 d | Reference | |
| 8–14 d | 0.76 (0.46–1.25) | .277 |
| 15–21 d | 0.31 (0.14–0.67) | .003 |
| 22–28 d | 0.07 (0.01–0.50) | .008 |
| Male | 0.98 (0.62–1.55) | .917 |
| Race | ||
| Non-Hispanic white | Reference | |
| Non-Hispanic black | 1.06 (0.59–1.92) | .842 |
| Hispanic | 0.64 (0.28–1.47) | .298 |
| Other | 1.41 (0.73–2.72) | .312 |
| Congenital anomaly | 2.10 (1.20–3.67) | .009 |
| Government payer | 2.37 (1.40–4.03) | .001 |
| HSV without complications | 0.58 (0.28–1.18) | .133 |
| Initial management | ||
| Delayed acyclovir therapy | 1.50 (0.92–2.47) | .107 |
| Skin testing for HSV | 0.51 (0.27–0.99) | .048 |
| Vasoactive agent infusions | 27.42 (15.25–49.32) | <.001 |
| Anticonvulsant therapy | 2.89 (1.69–4.94) | <.001 |
| Blood product transfusion | 25.04 (14.16–44.29) | <.001 |
| Endotracheal intubation | 7.70 (4.39–13.49) | <.001 |
Age at hospital admission.
TABLE 4.
Multivariate Analysis of Risk Factors for Death Among Neonates With HSV Infection
| Variable | Adjusted OR (95% CI)a | P |
|---|---|---|
| Delayed acyclovir therapy | 2.62 (1.34–5.09) | .005 |
| Age category | ||
| 0–7 d | Reference | |
| 8–14 d | 0.81 (0.42–1.56) | .525 |
| 15–21 d | 0.37 (0.15–0.95) | .039 |
| 22–28 d | 0.11 (0.02–0.77) | .027 |
The model also adjusted for propensity scores, year, race/ethnicity, vasoactive agent infusions, anticonvulsant therapy, blood product transfusions, endotracheal intubation, HSV testing of skin lesions, and HSV without complications.
Several additional analyses were performed to address residual patient-level confounding, hospital-level confounding, and potential misclassification of exposure. First, the association of delayed acyclovir therapy and death remained significant when patients with HSV without complications were excluded from the analysis (adjusted OR: 2.55 [95% CI: 1.32–4.93]; P = .005). Second, the odds of death increased with each additional day of delay in acyclovir treatment initiation; the mortality rates according to the day of acyclovir treatment initiation were 6.6% for day 1, 8.3% for day 2, 8.8% for day 3, and 16.7% for days 4 to 7 (P for trend = .027). This time-dependent relationship remained significant in an adjusted analysis stratified according to the day of acyclovir treatment initiation (Fig 1). Third, delayed acyclovir treatment remained associated with death when the analysis was stratified according to hospital by using conditional logistic regression (adjusted OR: 2.92 [95% CI: 1.51–5.64]; P = .001). Finally, on-site CSF studies were performed for 437 (40.2%) of 1086 neonates; 20 (4.6%) of those infants died, compared with 59 (9.1%) of 649 infants without a charge for CSF studies within 7 days of hospitalization. In a multivariate analysis restricted to the subset of infants with on-site CSF studies, the strength of the association between delayed acyclovir treatment and death was even stronger (adjusted OR: 9.16 [95% CI: 3.41–24.66]; P < .001).
FIGURE 1.
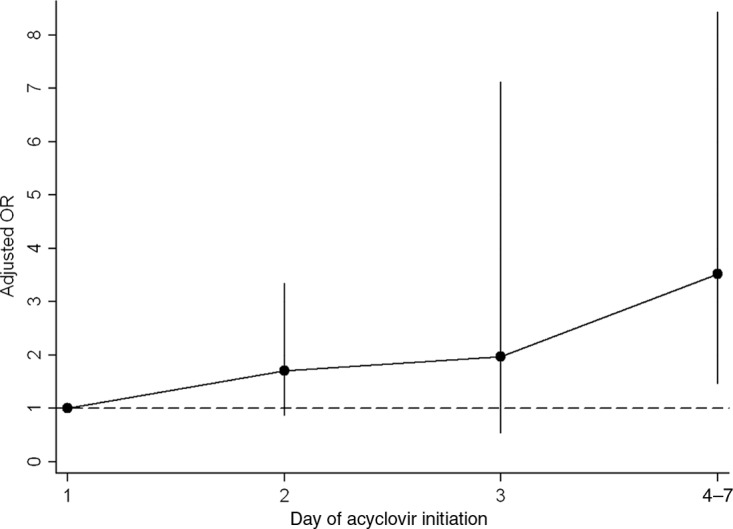
Relationship between the timing of acyclovir treatment initiation and the odds of death. The dashed line represents the reference value (early acyclovir initiation). The circles represent adjusted ORs, and the vertical lines represent 95% CIs. The model was adjusted for propensity scores (for trend in adjusted ORs, P = .003).
DISCUSSION
This multicenter observational study examined the association between delayed administration of acyclovir and death among neonates with HSV. Delayed receipt of acyclovir >1 day after admission was associated with higher in-hospital mortality rates. The association was strongest for neonates aged 14 days or younger, for whom the risk of disseminated infection and consequently the risk of death are greatest. In addition, the response seemed to be time-dependent; each additional day of delay was associated with an even greater mortality rate compared with the previous day.
Neonatal HSV infection is a severe disease with high mortality rates despite intravenous acyclovir therapy.10 An open-label trial compared different acyclovir dosage regimens among 88 neonates with HSV. The 12-month mortality rates for neonates who received “high-dose” acyclovir (60 mg/kg per day), that is, 31% among neonates with disseminated disease and 6% among neonates with central nervous system disease, were threefold lower than those observed with the standard dosage regimen (30 mg/kg per day).10 After publication of that study in 2001, administration of high-dose acyclovir quickly became the standard of care.21 However, the mean duration of symptoms before study enrollment was >5 days.10 This delay from symptom onset to initiation of appropriate therapy presents an additional opportunity to improve clinical outcomes. Earlier initiation of acyclovir therapy for neonates with HSV might lead to even lower mortality rates by preventing progression to more-severe disease. Progression from isolated cutaneous disease to involvement of other sites occurs within 1 week for approximately three-fourths of untreated infants.3,22 Whitley et al5 compared the clinical presentation of neonates with HSV during 2 consecutive time periods, 1973–1981 and 1982–1987. The duration of symptoms before therapy decreased from 6.5 days in the first time period to 4.8 days in the second time period, concomitant with a significant decrease in the number of neonates with disseminated disease and an increase in skin, eye, and mouth disease. The authors postulated that earlier diagnosis in the second period (1982–1987) might have identified more skin, eye, and mouth disease before progression to the disseminated form.5 It is possible that earlier initiation of acyclovir treatment (even 1–2 days sooner) would halt this progression.
Clinicians must weigh the potential risks of empiric therapy for uninfected neonates against the risks of delaying administration of acyclovir to HSV-infected neonates when earlier therapy is associated with lower mortality rates. Because measures of illness severity (eg, vasoactive agent infusions) also were associated with death, the benefits of early acyclovir therapy might be greater earlier in the course of the illness, before neonates require such therapeutic interventions. Some clinicians argue that empiric acyclovir treatment always should accompany HSV testing, especially for infants aged 21 days or younger12; our study supports this approach. It is not clear which neonates should be tested for HSV,23 and there are many factors that influence clinicians' decision to test.1,24 Future studies should attempt to identify the subset of neonates at low risk of HSV, a population that might not require routine HSV testing and empiric acyclovir treatment.
This multicenter observational study has several limitations. Discharge diagnosis coding might be unreliable for specific diseases. However, we included patients who had a discharge diagnosis of HSV and received treatment with acyclovir, which made it likely that the patients truly had HSV infection. Also, because freestanding children's hospitals often do not have birthing units, our results might not be generalizable to premature infants in birthing units, for whom the risk of HSV infection might be higher.25
In addition, there might be unmeasured confounding or residual confounding according to indication for early versus delayed acyclovir therapy, related to clinical presentation. This effect might influence our results in 2 different ways. First, we would expect that patients who received early acyclovir treatment would be sicker than those who received delayed acyclovir treatment. We included variables associated with greater severity of illness (such as receipt of vasoactive agent infusions) in the propensity score and separately as covariates in multivariate analyses. However, we could not account for clinical or laboratory factors, such as hypothermia or disseminated intravascular coagulation, which also might have influenced the timing of acyclovir administration. It is likely that such findings would lead to early administration of acyclovir and thus bias our results toward finding no difference. Because we found an association between delayed acyclovir therapy and mortality rates, it is possible that earlier initiation of acyclovir treatment would be associated with an even greater benefit than found in our study. Second, we cannot exclude the possibility that the early acyclovir treatment group included a disproportionate number of patients with disease limited to cutaneous or mucosal surfaces at presentation. Because outcomes are better with skin, eye, and mouth disease, this would have biased our results toward finding a mortality rate difference when none existed. We minimized the impact of such misclassification by including HSV without complications in our propensity score and as a covariate in our multivariate analyses. We also repeated the analysis with the exclusion of patients with HSV without complications. Furthermore, the odds of death increased with each additional day of delay in the initiation of acyclovir treatment. We would not expect such a progressive increase in the odds of death if the mortality rate differences were attributable solely to differences in the type of disease at presentation.
We also could not verify the dose of acyclovir used. After publication of the study by Kimberlin et al10 in 2001, high-dose acyclovir therapy immediately became the standard of care for neonates with HSV at our institution, as well as at other freestanding children's hospitals. In addition, high-dose acyclovir therapy was recommended by the American Academy of Pediatrics in 2003,21 the first year of our study. Therefore, it is unlikely that deaths in this study were the result of suboptimal acyclovir dosing.
Misclassification of the exposure (ie, delayed acyclovir receipt) might have occurred. Exposure is classified according to day (from midnight to midnight) in the PHIS, rather than 24-hour blocks after admission. Therefore, patients who received acyclovir within 24 hours after hospitalization might have been classified as receiving delayed acyclovir treatment. This misclassification would be most important within the first 24 hours, after which days are more important. This misclassification of patients would be expected to bias our results toward finding no difference; therefore, our results might underestimate the mortality rate difference between patients who received early versus delayed acyclovir treatment. Misclassification of exposure also might have occurred for patients who were transferred from another institution, who would have been classified erroneously as receiving early acyclovir treatment if acyclovir therapy was initiated within 24 hours after transfer to the PHIS-participating hospital but >24 hours after presentation for care. This potential misclassification would have biased our results toward finding no mortality rate difference. When we limited our analysis to patients with on-site CSF studies, the odds of death with delayed acyclovir treatment were increased. Therefore, the mortality rate for neonates who receive delayed acyclovir therapy might be higher than the rate reported in this study. Finally, other important outcomes, such as neurologic morbidity, could not be measured in this study.
CONCLUSIONS
This multicenter observational study found that delayed initiation of acyclovir therapy was associated with in-hospital death among neonates with HSV infection. Our data support the use of empiric acyclovir therapy for neonates undergoing testing for HSV infection.
ACKNOWLEDGMENTS
Dr Shah received support from National Institute of Allergy and Infectious Diseases grant K01 AI73729 and the Robert Wood Johnson Foundation under its Physician Faculty Scholar Program.
APPENDIX.
ICD-9 Discharge Diagnosis Codes Used to Identify Neonates With Congenital Anomalies
| Anomaly Category | ICD-9 Code | Definition |
|---|---|---|
| Neurologic anomalies | 742 | Encephalocele |
| 742.3 | Congenital hydrocephalus | |
| 742.4 | Other anomalies of brain | |
| 742.59 | Other anomalies of spinal cord | |
| 742.8 | Other anomalies of nervous system | |
| 742.9 | Unspecified anomaly of brain, spinal cord, and nervous system | |
| 756.1 | Anomaly of spine, unspecified | |
| Head/neck anomalies | 744.89 | Other anomalies of face and neck |
| 744.9 | Unspecified anomalies of face and neck | |
| 748 | Choanal atresia | |
| 748.1 | Other anomalies of nose | |
| 749 | Cleft palate, unspecified | |
| 749.1 | Cleft lip, unspecified | |
| 749.24 | Bilateral cleft palate with cleft lip, incomplete | |
| 754 | Congenital musculoskeletal deformities of skull, face, and jaw | |
| 756 | Congenital musculoskeletal anomalies of skull and face bones | |
| Cardiac anomalies | 745.1 | Complete transposition of great vessels |
| 745.2 | Tetralogy of Fallot | |
| 745.4 | Ventricular septal defect | |
| 745.5 | Ostium secundum-type atrial septal defect | |
| 745.69 | Other endocardial cushion defect | |
| 746.02 | Stenosis, congenital | |
| 746.3 | Congenital stenosis of aortic valve | |
| 746.6 | Congenital mitral insufficiency | |
| 746.83 | Infundibular pulmonic stenosis | |
| 746.86 | Congenital heart block | |
| 746.87 | Malposition of heart and cardiac apex | |
| 746.89 | Other anomaly of heart | |
| 746.9 | Unspecified anomaly of heart | |
| 747.1 | Coarctation of aorta (preductal) (postductal) | |
| 747.3 | Anomalies of pulmonary artery | |
| 747.69 | Anomalies of other specified sites of peripheral vascular system | |
| 747.9 | Unspecified anomaly of circulatory system | |
| Pulmonary anomalies | 748.3 | Other anomalies of larynx, trachea, and bronchus |
| 748.5 | Agenesis, hypoplasia, and dysplasia of lung | |
| 748.69 | Other anomalies of lung | |
| 756.6 | Anomalies of diaphragm | |
| Gastrointestinal anomalies | 750.5 | Congenital hypertrophic pyloric stenosis |
| 751.1 | Atresia and stenosis of small intestine | |
| 751.2 | Atresia and stenosis of large intestine, rectum, and anal canal | |
| 751.3 | Hirschsprung's disease and other congenital functional disorders of colon | |
| 751.4 | Anomalies of intestinal fixation | |
| 751.5 | Other anomalies of intestine | |
| 751.6 | Unspecified anomaly of gallbladder, bile ducts, and liver | |
| 751.69 | Other anomalies of gallbladder, bile ducts, and liver | |
| 756.79 | Other congenital anomalies of abdominal wall | |
| Genitourinary anomalies | 752.7 | Indeterminate sex and pseudohermaphroditism |
| 752.8 | Other specified anomalies of genital organs | |
| 753.15 | Renal dysplasia | |
| 753.19 | Other specified cystic kidney disease | |
| 753.2 | Unspecified obstructive defect of renal pelvis and ureter | |
| 753.29 | Other obstructive defects of renal pelvis and ureter | |
| 753.3 | Other specified anomalies of kidney | |
| 753.4 | Other specified anomalies of ureter | |
| 753.7 | Anomalies of urachus | |
| 753.8 | Other specified anomalies of bladder and urethra | |
| 753.9 | Unspecified anomaly of urinary system | |
| 758.6 | Gonadal dysgenesis | |
| Musculoskeletal anomalies | 755.59 | Other anomalies of upper limb, including shoulder girdle |
| 756.9 | Other and unspecified anomalies of musculoskeletal system | |
| 757.31 | Congenital ectodermal dysplasia | |
| Genetic anomalies | 758 | Down's syndrome |
| 758.3 | Autosomal deletion syndromes | |
| 758.5 | Other conditions attributable to autosomal anomalies | |
| 759.3 | Situs inversus |
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Robert Wood Johnson Foundation.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
- CI
- confidence interval
- CSF
- cerebrospinal fluid
- HSV
- herpes simplex virus
- ICD-9
- International Classification of Diseases, Ninth Revision
- IQR
- interquartile range
- OR
- odds ratio
- PHIS
- Pediatric Health Information System
REFERENCES
- 1. Cohen DM, Lorch SA, King RL, Hodinka RL, Cohn KA, Shah SS. Factors influencing the decision to test young infants for herpes simplex virus infection. Pediatr Infect Dis J. 2007;26(12):1156–1158 [DOI] [PubMed] [Google Scholar]
- 2. Caviness AC, Demmler GJ, Almendarez Y, Selwyn BJ. The prevalence of neonatal herpes simplex virus infection compared with serious bacterial illness in hospitalized neonates. J Pediatr. 2008;153(2):164–169 [DOI] [PubMed] [Google Scholar]
- 3. Whitley RJ, Nahmias AJ, Soong SJ, Galasso GG, Fleming CL, Alford CA. Vidarabine therapy of neonatal herpes simplex virus infection. Pediatrics. 1980;66(4):495–501 [PubMed] [Google Scholar]
- 4. Kimberlin DW, Lin CY, Jacobs RF, et al. Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics. 2001;108(2):223–229 [DOI] [PubMed] [Google Scholar]
- 5. Whitley RJ, Corey L, Arvin A, et al. Changing presentation of herpes simplex virus infection in neonates. J Infect Dis. 1988;158(1):109–116 [DOI] [PubMed] [Google Scholar]
- 6. Kimura H, Futamura M, Kito H, et al. Detection of viral DNA in neonatal herpes simplex virus infections: frequent and prolonged presence in serum and cerebrospinal fluid. J Infect Dis. 1991;164(2):289–293 [DOI] [PubMed] [Google Scholar]
- 7. Troendle-Atkins J, Demmler GJ, Buffone GJ. Rapid diagnosis of herpes simplex virus encephalitis by using the polymerase chain reaction. J Pediatr. 1993;123(3):376–380 [DOI] [PubMed] [Google Scholar]
- 8. Lakeman FD, Whitley RJ. Diagnosis of herpes simplex encephalitis: application of polymerase chain reaction to cerebrospinal fluid from brain-biopsied patients and correlation with disease. J Infect Dis. 1995;171(4):857–863 [DOI] [PubMed] [Google Scholar]
- 9. Shah SS, Volk J, Mohamad Z, Hodinka RL, Zorc JJ. Herpes simplex virus testing and hospital length of stay in neonates and young infants. J Pediatr. 2010;156(5):738–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kimberlin DW, Lin CY, Jacobs RF, et al. Safety and efficacy of high-dose intravenous acyclovir in the management of neonatal herpes simplex virus infections. Pediatrics. 2001;108(2):230–238 [DOI] [PubMed] [Google Scholar]
- 11. Kimberlin DW. When should you initiate acyclovir therapy in a neonate? J Pediatr. 2008;153(2):155–156 [DOI] [PubMed] [Google Scholar]
- 12. Long SS. In defense of empiric acyclovir therapy in certain neonates. J Pediatr. 2008;153(2):157–158 [DOI] [PubMed] [Google Scholar]
- 13. Whitley R, Arvin A, Prober C, et al. Predictors of morbidity and mortality in neonates with herpes simplex virus infections. N Engl J Med. 1991;324(7):450–454 [DOI] [PubMed] [Google Scholar]
- 14. Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048–2055 [DOI] [PubMed] [Google Scholar]
- 15. Shah SS, Hall M, Srivastava R, Subramony A, Levin JE. Intravenous immunoglobulin in children with streptococcal toxic shock syndrome. Clin Infect Dis. 2009;49(9):1369–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55 [Google Scholar]
- 17. Stürmer T, Joshi M, Glynn RJ, Avorn J, Rothman KJ, Schneeweiss S. A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J Clin Epidemiol. 2006;59(5):437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weitzen S, Lapane KL, Toledano AY, Hume AL, Mor V. Principles for modeling propensity scores in medical research: a systematic literature review. Pharmacoepidemiol Drug Saf. 2004;13(12):841–853 [DOI] [PubMed] [Google Scholar]
- 19. Imbens GW. Nonparametric estimation of average treatment effects under heterogeneity: a review. Rev Econ Stat. 2004;86(1):4–29 [Google Scholar]
- 20. Greenland S. Introduction to regression modeling. In: Rothman KJ, Greenland S. eds. Modern Epidemiology. 3rd ed Philadelphia, PA: Lippincott, Williams & Wilkins; 2008:446–451 [Google Scholar]
- 21. Pickering LK. ed. Red Book: 2003 Report of the Committee on Infectious Diseases. 26th ed Elk Grove Village, IL: American Academy of Pediatrics; 2003:344–353 [Google Scholar]
- 22. Whitley RJ, Nahmias AJ, Visintine AM, Fleming CL, Alford CA. The natural history of herpes simplex virus infection of mother and newborn. Pediatrics. 1980;66(4):489–494 [PubMed] [Google Scholar]
- 23. Caviness AC, Demmler GJ, Selwyn BJ. Clinical and laboratory features of neonatal herpes simplex virus infection: a case-control study. Pediatr Infect Dis J. 2008;27(5):425–430 [DOI] [PubMed] [Google Scholar]
- 24. Davis KL, Shah SS, Frank G, Eppes SC. Why are young infants tested for herpes simplex virus? Pediatr Emerg Care. 2008;24(10):673–678 [DOI] [PubMed] [Google Scholar]
- 25. O'Riordan DP, Golden WC, Aucott SW. Herpes simplex virus infections in preterm infants. Pediatrics. 2006;118(6). Available at: www.pediatrics.org/cgi/content/full/118/6/e1612 [DOI] [PubMed] [Google Scholar]


