Abstract
OBJECTIVE:
To describe the epidemiology and outcomes of children hospitalized with eczema herpeticum and to determine the association with delayed acyclovir on outcomes.
PATIENTS AND METHODS:
This was a multicenter retrospective cohort study conducted between January 1, 2001, and March 31, 2010, of 1331 children aged 2 months to 17 years with eczema herpeticum from 42 tertiary care children's hospitals in the Pediatric Health Information System database. Multivariable linear regression models determined the association between delayed acyclovir therapy and the main outcome measure: hospital length of stay (LOS).
RESULTS:
There were no deaths during the study period. Staphylococcus aureus infection was diagnosed in 30.3% of the patients; 3.9% of the patients had a bloodstream infection. Fifty-one patients (3.8%) required ICU admission. There were 893 patients (67.1%) who received acyclovir on the first day of admission. The median LOS increased with each day delay in acyclovir initiation. In multivariable analysis, delay of acyclovir initiation by 1 day was associated with an 11% increased LOS (95% confidence interval [CI]: 3%–20%; P = .008), and LOS increased by 41% when acyclovir was started on day 3 (95% CI: 19%–67%; P < .001) and by 98% when started on day 4 to 7 (95% CI: 60%–145%; P < .001). Use of topical corticosteroids on day 1 of hospitalization was not associated with LOS.
CONCLUSIONS:
Delay of acyclovir initiation is associated with increased LOS in hospitalized children with eczema herpeticum. Use of topical corticosteroids on admission is not associated with increased LOS. The mortality rate of hospitalized children with eczema herpeticum is low.
Keywords: eczema herpeticum, herpes simplex virus, acyclovir, eczema
WHAT'S KNOWN ON THIS SUBJECT:
Eczema herpeticum is a potentially life-threatening complication of atopic dermatitis. Acyclovir has shown clinical benefit in adult outpatients. The current mortality rate and impact of delayed acyclovir in hospitalized children with eczema herpeticum is unknown.
WHAT THIS STUDY ADDS:
In this multicenter observational study of hospitalized children with eczema herpeticum, no patient died. Delayed initiation of acyclovir therapy was associated with increased length of stay. This study's findings emphasize the importance of early acyclovir therapy for children with eczema herpeticum.
Atopic dermatitis is a common inflammatory skin disease, with a reported prevalence of 15% to 30% in children.1,2 Patients with atopic dermatitis demonstrate an impaired skin barrier that increases susceptibility to bacterial and viral infection of the skin including disseminated herpes simplex virus (HSV) (ie, eczema herpeticum).2,3 Eczema herpeticum can occur with either primary or recurrent HSV infection.4,5 Although eczema herpeticum is rare, a subset of patients with atopic dermatitis seem to have a predisposition to HSV infection because of defects in specific proteins critical for skin barrier function6 and innate immunity.7 Early onset of atopic dermatitis, more extensive skin involvement, eczematous lesions located on the head and neck, and higher immunoglobulin E levels have also been associated with the development of eczema herpeticum.5,8
There is a paucity of epidemiologic and outcome data on eczema herpeticum, and only 1 case series of 14 patients in which hospitalized children are described.9 In addition, whereas studies before the use of acyclovir quoted mortality rates upward of 10% to 50%,10 the current mortality rate of eczema herpeticum has not been reported.
Acyclovir has traditionally been used as treatment for eczema herpeticum.5 The only published study in which therapy is evaluated compared a 5-day course of oral acyclovir with placebo in 60 adults managed in the outpatient setting. Acyclovir was associated with greater clinical improvement as assessed on a 4-point Likert scale and a shorter duration of ulceration compared with placebo.11 There have been no studies in which acyclovir therapy has been evaluated in pediatric patients with eczema herpeticum. In addition, the effect of timing of initiation of acyclovir in hospitalized patients with eczema herpeticum is unknown.
Our objectives, therefore, were to describe the epidemiology of eczema herpeticum in hospitalized patients, including incidence of bacterial infection, requirement for ICU admission, and mortality rate. In addition, we aimed to explore the effect of delayed acyclovir on outcomes in hospitalized pediatric patients with eczema herpeticum.
PATIENTS AND METHODS
Data Source
Data for this retrospective cohort study were obtained from the Pediatric Health Information System, which contains administrative data from 42 freestanding children's hospitals. Data quality and reliability are assured through a joint effort by the Child Health Corporation of America (Shawnee Mission, KS) and Pediatric Health Information System-participating hospitals as described previously.12,13 The protocol for the conduct of this study was reviewed and approved by the Children's Hospital of Philadelphia committees for the protection of human subjects with a waiver of informed consent.
Patients
Children aged 2 months to 17 years with eczema herpeticum were eligible for inclusion if they were discharged from a participating hospital between January 1, 2001, and March 31, 2010. Children younger than 2 months of age were excluded because of the likelihood of perinatally acquired HSV and the rarity of eczema herpeticum at this age. Subjects were included if they had a discharge diagnosis of eczema herpeticum as defined by the presence of International Classification of Diseases, Ninth Revision (ICD-9) discharge diagnosis code 054.0 in any diagnosis field (primary or nonprimary) and received oral or intravenous acyclovir within 7 days of admission. Patients were excluded if they did not receive acyclovir or received acyclovir after 7 days of admission to minimize the inclusion of patients who were misclassified as having eczema herpeticum or who did not have eczema herpeticum on admission. Transfers from another hospital were excluded because of the possibility of misclassification as a result of receipt of acyclovir before transfer.
Validation of Discharge Diagnosis Codes for Eczema Herpeticum
To assess the potential misclassification of the diagnosis of eczema herpeticum when using our subject identification algorithm, we reviewed the medical charts of all patients admitted and discharged from the Children's Hospital of Philadelphia during the study period with a discharge diagnosis of eczema herpeticum (054.0); no patients had a nonprimary diagnosis of eczema herpeticum during the study period. The criteria for the diagnosis of eczema herpeticum were determined a priori as follows: (1) a documented history of atopic dermatitis either previous to or at the time of admission; (2) a clinical description of vesicles or erosions consistent with herpetic infection, such as “punctate erosions” or “grouped erosions,” not limited to the perioral area; and (3) either documentation of a positive test for HSV or diagnosis of eczema herpeticum confirmed by a pediatric dermatologist when HSV testing was not performed. Using these criteria, 79 of 85 patients with ICD-9 code 054.0 had eczema herpeticum. Therefore the positive predictive value of our algorithm for diagnosing eczema herpeticum was 93%. All patients with eczema herpeticum received acyclovir within 7 days of admission, although 5 of 79 did not receive acyclovir on the first day of hospitalization.
Study Definitions
We identified children with bloodstream infections by ICD-9 codes 790.7 and 038.xx (bacteremia and septicemia, respectively). Patients with Staphylococcus aureus infection were identified by ICD-9 codes 041.11 (methicillin-susceptible S aureus), 038.11 (methicillin-susceptible S aureus septicemia), 482.41 (methicillin-susceptible pneumonia caused by S aureus), 041.12 (methicillin-resistant S aureus [MRSA]), 038.12 (MRSA septicemia), 482.42 (methicillin-resistant pneumonia caused by S aureus), and V12.04 (MRSA) in any discharge diagnosis field. Race and ethnicity data of patients were reported by their parents or legal guardians.
Antibiotics (either oral or intravenous) included in this study all have anti-staphylococcal activity, either against methicillin-susceptible S aureus or MRSA (Table 1). Receipt of acyclovir was determined by a billing code for oral acyclovir or valacyclovir or intravenous acyclovir. Systemic corticosteroids included cortisone, dexamethasone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Topical corticosteroids included alclometasone, amcinonide, betamethasone, clobetasol, clocortolone, desonide, desoximetasone, diflorasone, fluocinolone, fluocinonide, flurandrenolide, fluticasone, halcinonide, halobetasol, hydrocortisone, mometasone, prednicarbate, and triamcinolone. Topical calcineurin inhibitors included tacrolimus and pimecrolimus.
TABLE 1.
Characteristics of Patients
| Characteristic | Patients (N = 1331), n (%) |
|---|---|
| Demographics | |
| Age, median (IQR), y | 1 (1–4) |
| Gender | |
| Male | 786 (59.0) |
| Female | 545 (41.0) |
| Race/ethnicitya | |
| Non-Hispanic white | 317 (23.8) |
| Non-Hispanic black | 452 (34.0) |
| Hispanic | 199 (15.0) |
| Other | 261 (19.6) |
| Missing | 102 (7.7) |
| APR-DRG severity classification | |
| Minor | 1289 (96.8) |
| Moderate | 33 (2.5) |
| Major | 5 (0.4) |
| Extreme | 4 (0.3) |
| ICU admission | 51 (3.8) |
| Therapy and laboratory testing | |
| Acyclovir day of initiation | |
| 1 | 893 (67.1) |
| 2 | 340 (25.5) |
| 3 | 61 (4.6) |
| 4–7 | 37 (2.8) |
| Antibiotics (oral or intravenous)b | 1150 (86.4) |
| Topical antibioticsc | 445 (33.4) |
| Systemic corticosteroids | 95 (7.1) |
| Topical corticosteroids | 614 (46.1) |
| Topical calcineurin inhibitors | 65 (4.9) |
| Blood culture | 739 (55.5) |
| Herpes simplex virus testingd | 736 (55.3) |
| Concurrent infections | |
| Bloodstream infection | 52 (3.9) |
| S aureus infection | 403 (30.3) |
Because of rounding, percentages may not total 100. APR-DRG indicates all patient refined-diagnosis related groups.
Race/ethnicity data were reported by parents or legal guardians of the subjects. Other races included Asian, American Indian, and other. Race was classified as missing if it was not reported in the database.
Antibiotics included were clindamycin, sulfamethoxazole and trimethoprim (co-trimoxazole), vancomycin, linezolid, anti-staphylococcal penicillins (nafcillin, cloxacillin, dicloxacillin, oxacillin), β-lactams with β-lactamase inhibitors (amoxicillin trihydrate and potassium clavulanate, ampicillin sodium and sulbactam sodium, piperacillin sodium and tazobactam sodium, ticarcillin disodium and clavulanate potassium), and cephalosporins.
Topical antibiotics included mupirocin, bacitracin, neomycin, and topical antibiotic combinations.
Herpes testing included herpes simplex antibody, herpes simplex virus antigen, herpes culture, and herpes testing unspecified.
Measures of disease severity included admission to the ICU and all patient refined-diagnosis related groups severity classification, labeled as minor, moderate, major, or extreme. All patient refined-diagnosis related groups scores represent illness severity, risk of death, and resource intensity for the entire hospitalization. They are calculated from computer algorithms on the basis of age, gender, diagnoses, procedures, and discharge status.14
Measured Outcomes and Exposures
The primary outcomes of interest in this study were hospital length of stay (LOS) and mortality. The primary exposure of interest was the timing of acyclovir initiation.
Data Analysis
Continuous variables were described using median and interquartile range (IQR) or range values and compared using Wilcoxon rank-sum test. Categorical variables were described using counts and frequencies and compared by using the χ2 test. Multivariable linear regression analysis was performed to assess the independent impact of delayed acyclovir therapy on LOS. Because the LOS data had a skewed distribution, our analyses were performed using logarithmically transformed LOS values as the dependent variable. The resulting β-coefficients were transformed to reflect the percentage difference in LOS between subjects receiving empiric and delayed acyclovir therapy.
Building of the multivariable models began with the inclusion of the variable for initiation of acyclovir by day as per our a priori hypothesis. Variables associated with LOS on univariate analysis (P < .20) were then considered for inclusion as potential confounding factors of the association between delayed acyclovir and LOS.15 These variables were included in the final multivariable model if they remained significant after adjusting for other factors or if their inclusion in the model resulted in a 15% or greater change in the effect size of the primary association of interest (ie, timing of acyclovir initiation and LOS).16 No patient in our validation cohort had eczema herpeticum as a secondary discharge diagnosis code. To assess the effect of potential misclassification introduced by including patients with a secondary discharge diagnosis of eczema herpeticum, we repeated the analysis while restricting the cohort to those with a primary discharge diagnosis of eczema herpeticum.
Statistical significance was determined a priori as a 2-tailed P value of <.05. All analyses were clustered according to hospital.
RESULTS
Patient Characteristics
During the study period, 2127 patients were identified with an ICD-9 discharge diagnosis code of 054.0 in any field. Of these patients, 749 (35.2%) did not receive acyclovir, and 47 (2.2%) patients received acyclovir after day 7 of hospitalization; these patients were excluded. The remaining 1331 patients were included in the study; 1063 had 054.0 as the primary discharge diagnosis. There was a median of 29 patients per hospital (IQR: 16–46). The median patient age was 1 year (IQR: 1–4). The characteristics of the patients are presented in Table 1.
Epidemiology
Eczema herpeticum accounted for 0.006% of hospital admissions, or 1 of every 16 530 admissions. S aureus infection was diagnosed in 30.3% of patients with eczema herpeticum; 9.2% of S aureus infections were attributable to MRSA. Of the patients, 3.9% had a bloodstream infection. Oral or intravenous antibiotics were provided to 1150 patients (86.4%). Fifty-one patients (3.8%) required ICU admission (Table 1). There were no deaths during the study period.
Timing of Acyclovir Initiation
The distribution of acyclovir day of initiation is listed in Table 1. There was no association between day of acyclovir initiation and route of acyclovir administration (P = .85). Patients who received acyclovir on day 1 of hospitalization were more commonly coadministered empiric antibiotics (P < .001), but a larger proportion of patients who received acyclovir after day 1 of admission received antibiotics during the hospitalization (P = .026).
Outcomes
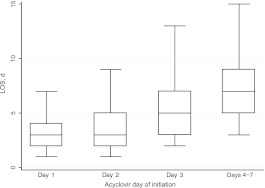
Because no patient died, we could not analyze mortality as an outcome measure. The median hospital LOS was 3 days (IQR: 2–5); 9.2% of patients had a LOS of >7 days. The median LOS increased with each day delay in acyclovir initiation (Fig 1). In unadjusted analysis, acyclovir day of initiation was associated with LOS (Table 2). Antibiotic class was not associated with LOS except for receipt of vancomycin, which was associated with increased LOS (P < .001). There were 294 patients who received topical corticosteroids on day 1 of admission, but empiric topical corticosteroid receipt was not associated with LOS on univariate analysis (P = .96). Use of topical calcineurin inhibitors was not associated with LOS. In multivariable analysis, delay of acyclovir initiation by 1 day was associated with an 11% increased LOS (95% confidence interval [CI]: 3%–20%; P = .008), and LOS increased significantly with each additional day delay (Table 3). Receipt of systemic corticosteroids remained associated with increased LOS on multivariable analysis (18% adjusted increased LOS [95% CI: 2%–36%] P = .03), but receipt of topical corticosteroids at any point during the hospitalization did not.
FIGURE 1.
LOS according to day of acyclovir initiation. Shown is a boxplot with median, IQR, and 1.5× IQR displayed.
TABLE 2.
Univariate Analysis of Factors Associated With Increased LOS in Children With Eczema Herpeticum
| Variable | Unadjusted β Coefficient (95% CI) | P |
|---|---|---|
| Demographics | ||
| Age, y | −0.01 (−0.02 to 0.01) | .35 |
| Gender | ||
| Male | Reference | — |
| Female | 0.01 (−0.06 to 0.08) | .79 |
| Race/ethnicity | ||
| Non-Hispanic white | Reference | — |
| Non-Hispanic black | 0.14 (0.05 to 0.22) | .002 |
| Hispanic | 0.16 (−0.16 to 0.33) | .06 |
| Other | 0.17 (0.04 to 0.31) | .012 |
| APR-DRG severity classification | ||
| Minor | Reference | — |
| Moderate | 0.41 (0.10 to 0.73) | .012 |
| Major | 1.81 (0.28 to 3.34) | .022 |
| Extreme | 2.39 (1.27 to 3.50) | <.001 |
| ICU admission | 0.43 (−0.03 to 0.88) | .065 |
| Therapy and laboratory testing | ||
| Acyclovir day of initiation | ||
| 1 | Reference | — |
| 2 | 0.17 (0.07 to 0.27) | .001 |
| 3 | 0.53 (0.34 to 0.72) | <.001 |
| 4–7 | 0.85 (0.62 to 1.08) | <.001 |
| Antibiotics (oral or intravenous) | 0.58 (0.48 to 0.67) | <.001 |
| Topical antibiotics | 0.30 (0.23 to 0.37) | <.001 |
| Systemic corticosteroids | 0.36 (0.16 to 0.55) | .001 |
| Topical corticosteroids | 0.19 (0.11 to 0.27) | <.001 |
| Topical calcineurin inhibitors | 0.16 (−0.02 to 0.33) | .08 |
| Blood culture | 0.36 (0.27 to 0.45) | <.001 |
| Herpes simplex virus testing | 0.24 (0.10 to 0.37) | .001 |
| Concurrent infections | ||
| Bloodstream infection | 0.98 (0.71 to 1.25) | <.001 |
| S aureus infection | 0.39 (0.33 to 0.45) | <.001 |
APR-DRG indicates all patient refined-diagnosis related groups.
TABLE 3.
Multivariable Analysis of Delayed Acyclovir and LOS in Children With Eczema Herpeticum
| Variable | Acyclovir Day of Initiation |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4–7 | |
| Adjusted β coefficient (95% CI)a,b | Reference | 0.10 (0.03–0.18) | 0.34 (0.18–0.51) | 0.68 (0.47–0.90) |
| Adjusted percent increase in LOS (95% CI)a,b | — | 11 (3–20) | 41 (19–67) | 98 (60–145) |
| P | — | .008 | <.001 | <.001 |
The model was also adjusted for receipt of oral or intravenous antibiotics during the hospitalization, receipt of topical antibiotics, receipt of systemic corticosteroids, bloodstream infection, S aureus infection, testing with blood culture, herpes simplex virus testing, all patient refined-diagnosis related groups severity classification, and race/ethnicity.
Antibiotic class not included in multivariable model because of collinearity with receipt of oral or intravenous antibiotics.
On subanalysis limited to patients with a primary ICD-9 discharge diagnosis code 054.0, the association of delayed acyclovir and increased LOS remained significant (Table 4). In adjusted analysis, receipt of topical corticosteroids at any point during the hospitalization was significant (10% adjusted increased LOS [95% CI: 2%–18%] P = .01), but receipt of topical steroids on the first day of hospitalization was not. Receipt of systemic corticosteroids was not associated with LOS on multivariable analysis.
TABLE 4.
Multivariable Analysis of Delayed Acyclovir and LOS in Children With Primary Discharge Diagnosis Code 054.0
| Variable | Acyclovir Day of Initiation |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4–7 | |
| Adjusted β coefficient (95% CI)a | Reference | 0.11 (0.02–0.20) | 0.35 (0.13–0.56) | 0.56 (0.32–0.80) |
| Adjusted percent increase in LOS (95% CI)a | — | 11 (2–22) | 41 (14–75) | 76 (38–124) |
| P | — | .02 | .002 | <.001 |
The model was also adjusted for receipt of oral or intravenous antibiotics during the hospitalization, receipt of topical antibiotics, receipt of topical corticosteroids, bloodstream infection, S aureus infection, testing with blood culture, herpes simplex virus testing, and race/ethnicity.
DISCUSSION
To our knowledge, this multicenter observational study is the largest study to date in which the characteristics and outcomes of hospitalized children with eczema herpeticum are described and the first in which the association of delayed acyclovir initiation and outcomes in patients with eczema herpeticum is reported. Each additional day delay in acyclovir initiation was associated with greater percent increase in LOS, highlighting the importance of early recognition of eczema herpeticum in children with atopic dermatitis. Despite this increased LOS, outcomes with eczema herpeticum are good, as few children required ICU admission and no children died.
The largest cohorts describing patients with eczema herpeticum before this current study both reported mean ages of 22 years.4,5 The median age of 1 year in our cohort likely reflects the typical age range for atopic dermatitis in the pediatric population, although may suggest more extensive disease in young patients or a higher likelihood of admission given young age. Bacterial colonization, most notably S aureus, in atopic dermatitis and eczema herpeticum is well described,17–19 and septicemia is known to occur.20,21 The prevalence of S aureus infection was 30.3% in our sample, and 3.9% of patients had a bloodstream infection. Because most patients received antibiotics, the prevalence of bacterial superinfection in hospitalized children with eczema herpeticum may be higher than reported here. The low prevalence of MRSA infection is consistent with previous studies that revealed lower rates of MRSA compared with methicillin-susceptible S aureus infection in children with atopic dermatitis.22,23 The low mortality rate in our large sample is important because eczema herpeticum was once considered a potentially life-threatening disease with mortality rates as high as 50%.10
Although acyclovir use has been described in hospitalized children with eczema herpeticum,9,24 the only randomized controlled trial to evaluate the utility of acyclovir enrolled only adult outpatients aged 15 and older.11 Hospitalized children had favorable outcomes in this study with use of acyclovir, but delay in initiation of acyclovir may have resulted in spread of herpetic lesions and prolonged course of disease. Because the median LOS for the sample was 3 days, the results are clinically relevant because a delay in acyclovir initiation until day 3 may increase LOS by an additional 1.2 days or up to 3 days if acyclovir is started on day 4 to 7. This increased LOS may lead to higher costs and increased risk of health care-acquired infections. Empiric antibiotics were more commonly administered to patients who received acyclovir on the first day of hospitalization, which may indicate that patients who received early acyclovir were sicker. A larger proportion of patients who received delayed acyclovir received antibiotics at any point during the hospitalization, possibly because of clinical deterioration or progression of lesions prompting initiation of acyclovir and antibiotics. The increase in LOS does not seem related to receipt of oral versus intravenous acyclovir because there was no difference in route of acyclovir administration between patients with early versus delayed receipt of acyclovir.
There is concern that application of topical corticosteroids to skin affected by eczema herpeticum may facilitate dissemination of HSV and worsened disease,9 although Wollenberg et al5 reported no difference in use of topical corticosteroids preceding admission between patients with eczema herpeticum and control patients with atopic dermatitis. In addition, there was recent use of topical corticosteroids in only 3 of 21 episodes of eczema herpeticum in a prospective cohort study by David et al.19 Receipt of topical corticosteroids on the first day of hospitalization was not associated with LOS in univariate or multivariable analysis in both the entire sample and the subanalysis limited to patients with primary discharge diagnosis code 054.0. Although use of topical corticosteroids at any point during the hospitalization was associated with increased LOS in the subanalysis, it is unclear whether this finding is because of a difference in this subpopulation or to other confounding factors. For instance, topical corticosteroids may be restarted while the patient is receiving acyclovir and clinically improving. These results do provide additional evidence that topical corticosteroid use is not definitively associated with worsened disease in patients with eczema herpeticum, although this issue warrants additional study. Systemic corticosteroids may worsen eczema herpeticum4 and were associated with increased LOS in our primary analysis; this finding also merits additional investigation.
This multicenter observational study has several limitations. Discharge diagnosis coding may be unreliable for specific diseases resulting in misclassification of patients. However, the ICD-9 discharge diagnosis code 054.0 had a positive predictive value of 93% in our validation, making it likely that included patients truly had eczema herpeticum. Although only 55.3% of patients received HSV testing, there is no billing code for HSV polymerase chain reaction, and a larger proportion of the sample likely underwent testing. In addition, we excluded patients who did not receive acyclovir within 7 days of hospitalization to avoid including patients who did not have eczema herpeticum at admission. Patients who did not receive acyclovir were excluded because of likely misclassification. These patients had a median LOS of 1 day, and may have had very mild eczema herpeticum that did not require acyclovir. Our results therefore may not be generalizable to children with mild eczema herpeticum. Finally, our results were similar when limiting the sample to patients with a primary discharge diagnosis code for eczema herpeticum.
In addition, there may be unmeasured confounding or residual confounding by indication for early compared with delayed acyclovir therapy related to clinical presentation. For instance, it is possible that patients who received delayed acyclovir did not have herpetic lesions on day 1 of admission. Of patients with eczema herpeticum in our validation sample, 6.3% did not receive acyclovir on the first day of hospitalization. Although this is lower than the proportion of patients who received delayed acyclovir in our study sample, the progressive increase in LOS with each additional delay in acyclovir initiation would not be expected if the delay was solely because of patients who did not have lesions at admission. It is also probable that sicker patients with more extensive lesions would receive empiric acyclovir. Patients who received acyclovir on day 1 of hospitalization were more commonly administered empiric antibiotics, supporting the notion that these patients were sicker. It is likely that these findings would lead to early administration of acyclovir and thus bias our results toward finding no difference. Because we did find an association between delayed acyclovir therapy and increased LOS, it is possible that earlier initiation of acyclovir would be associated with an even greater benefit than found in our study.
We do not know the duration of symptoms of patients included in this study. Although duration of disease may affect outcomes, longer duration of disease would likely have resulted in earlier initiation of acyclovir because the clinical findings of eczema herpeticum would have been more prominent. Therefore the benefits of acyclovir may be even greater in patients early in the disease course.
CONCLUSIONS
Patients clinically suspected of having eczema herpeticum should receive empiric therapy with acyclovir because there is a statistically significant time-dependent increase in LOS with every day of delay in initiating acyclovir therapy. Topical corticosteroid use on the first day of hospitalization was not associated with an increase in LOS, and additional investigation into the safety of topical steroid use in the context of eczema herpeticum seems warranted. The mortality rate of hospitalized children with eczema herpeticum is low.
ACKNOWLEDGMENTS
This study was funded in part by the Nicholas Crognale Chair for Emergency Medicine (Children's Hospital of Philadelphia), National Institute of Allergy and Infectious Diseases grant K01 AI73729, and the Robert Wood Johnson Foundation under its Physician Faculty Scholar Program (Dr Shah).
Dr Aronson had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; Drs Aronson, Yan, and Shah were responsible for study concept and design; Drs Aronson, Mohamad, and Shah were responsible for data acquisition; Drs Aronson, Yan, and Shah drafted the manuscript; Drs Aronson and Shah were responsible for statistical analysis and study supervision; and all authors were responsible for critical revision of the manuscript for important intellectual content, analysis and interpretation of the data, and final approval of the version to be published.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Robert Wood Johnson Foundation.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
- HSV
- herpes simplex virus
- ICD-9
- International Classification of Diseases, Ninth Revision
- MRSA
- methicillin-resistant Staphylococcus aureus
- LOS
- length of stay
- IQR
- interquartile range
- CI
- confidence interval
REFERENCES
- 1. Laughter D, Istvan JA, Tofte SJ, Hanifin JM. The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43(4):649–655 [DOI] [PubMed] [Google Scholar]
- 2. Bieber T. Atopic dermatitis. N Engl J Med. 2008;358(14):1483–1494 [DOI] [PubMed] [Google Scholar]
- 3. Wollenberg A, Räwer HC, Schauber J. Innate immunity in atopic dermatitis. Clin Rev Allergy Immunol. 2011;41(3):272–810 [DOI] [PubMed] [Google Scholar]
- 4. Bork K, Brauninger W. Increasing incidence of eczema herpeticum: analysis of seventy-five cases. J Am Acad Dermatol. 1988;19(6):1024–1029 [DOI] [PubMed] [Google Scholar]
- 5. Wollenberg A, Zoch C, Wetzel S, Plewig G, Przybilla B. Predisposing factors and clinical features of eczema herpeticum: a retrospective analysis of 100 cases. J Am Acad Dermatol. 2003;49(2):198–205 [DOI] [PubMed] [Google Scholar]
- 6. Gao PS, Rafaels NM, Hand T, et al. Filaggrin mutations that confer risk of atopic dermatitis confer greater risk for eczema herpeticum. J Allergy Clin Immunol. 2009;124(3):507–513, 513.e1–513.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Howell MD, Wollenberg A, Gallo RL, et al. Cathelicidin deficiency predisposes to eczema herpeticum. J Allergy Clin Immunol. 2006;117(4):836–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peng WM, Jenneck C, Bussmann C, et al. Risk factors of atopic dermatitis patients for eczema herpeticum. J Invest Dermatol. 2007;127(5):1261–1263 [DOI] [PubMed] [Google Scholar]
- 9. Novelli VM, Atherton DJ, Marshall WC. Eczema herpeticum: clinical and laboratory features. Clin Pediatr (Phila). 1988;27(5):231–233 [DOI] [PubMed] [Google Scholar]
- 10. Wheeler CE, Jr, Abele DC. Eczema herpeticum, primary and recurrent. Arch Dermatol. 1966;93(2):162–173 [PubMed] [Google Scholar]
- 11. Niimura M, Nishikawa T. Treatment of eczema herpeticum with oral acyclovir. Am J Med. 1988;85(2A):49–52 [PubMed] [Google Scholar]
- 12. Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048–2055 [DOI] [PubMed] [Google Scholar]
- 13. Shah SS, Hall M, Srivastava R, Subramony A, Levin JE. Intravenous immunoglobulin in children with streptococcal toxic shock syndrome. Clin Infect Dis. 2009;49(9):1369–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hughes J. Development of the 3M all patient refined diagnosis related groups (APR-DRGs). Available at: www.ahrq.gov/qual/mortality/hughes.htm Accessed February 26, 2011
- 15. Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129(1):125–137 [DOI] [PubMed] [Google Scholar]
- 16. Concato J, Feinstein AR, Holford TR. The risk of determining risk with multivariable models. Ann Intern Med. 1993;118(3):201–210 [DOI] [PubMed] [Google Scholar]
- 17. Aly R, Maibach HI, Shinefield HR. Microbial flora of atopic dermatitis. Arch Dermatol. 1977;113(6):780–782 [PubMed] [Google Scholar]
- 18. Brook I, Frazier EH, Yeager JK. Microbiology of infected eczema herpeticum. J Am Acad Dermatol. 1998;38(4):627–629 [DOI] [PubMed] [Google Scholar]
- 19. David TJ, Longson M. Herpes simplex infections in atopic eczema. Arch Dis Child. 1985;60(4):338–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ingrand D, Briquet I, Babinet JM, Reinert P, Huraux JM. Eczema herpeticum of the child: an unusual manifestation of herpes simplex virus infection. Clin Pediatr (Phila). 1985;24(11):660–663 [DOI] [PubMed] [Google Scholar]
- 21. Taieb A, Fontan I, Maleville J. Acyclovir therapy for eczema herpeticum in infants. Arch Dermatol. 1985;121(11):1380–1381 [PubMed] [Google Scholar]
- 22. Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics. 2009;123(5). Available at: www.pediatrics.org/cgi/content/full/123/5/e808 [DOI] [PubMed] [Google Scholar]
- 23. Matiz C, Tom WL, Eichenfield LF, Pong A, Friedlander SF. Children with atopic dermatitis appear less likely to be infected with community acquired methicillin-resistant Staphylococcus aureus: the San Diego experience. Pediatr Dermatol. 2011;28(1):6–11 [DOI] [PubMed] [Google Scholar]
- 24. Lai YC, Shyur SD, Fu JL. Eczema herpeticum in children with atopic dermatitis. Acta Paediatr Taiwan. 1999;40(5):325–329 [PubMed] [Google Scholar]