Abstract
OBJECTIVES:
Healthy infants are thought to acquire biliary atresia (BA) in the first weeks of life. Because those diagnosed earlier have better outcomes, we were interested in determining the earliest time BA could be detected. We started by examining the immediate postnatal period, hypothesizing that newborns would not yet have acquired disease and still have normal direct/conjugated bilirubin (DB/CB) levels.
PATIENTS AND METHODS:
Newborn DB/CB levels were obtained retrospectively from birth hospitals. Subjects with BA were born between 2007 and 2010 and cared for at Texas Children's Hospital. Those with BA splenic malformation syndrome or born prematurely were excluded. Control subjects were term newborns who later never developed neonatal liver disease.
RESULTS:
Of the 61 subjects with BA, 56% had newborn DB/CB levels measured. All DB/CB levels exceeded laboratory norms and rose over time. At 24 to 48 hours of life, subjects with BA had mean DB levels significantly higher than those of controls (1.4 ± 0.43 vs. 0.19 ± 0.075 mg/dL, P < .0001), even while their mean total bilirubin (TB) levels remained below phototherapy limits. Finally, despite the elevated DB/CB levels, the majority of patients (79%) had normal DB:TB ratios ≤0.2.
CONCLUSIONS:
Patients with BA have elevated DB/CB levels shortly after birth. To detect affected infants earlier and improve outcomes, the results suggest two possibilities: (1) screen all newborns for elevated DB/CB levels, rather than just those who appear jaundiced; and then (2) follow all newborns with elevated DB/CB levels, rather than just those with DB:TB ratios >0.2.
Keywords: biliary atresia, direct bilirubin, conjugated bilirubin, total bilirubin, newborn bilirubin screening
WHAT'S KNOWN ON THIS SUBJECT:
Infants with biliary atresia (BA) have better outcomes if detected and treated early, typically before 8 weeks of age. Making an early diagnosis is difficult, however, because newborns appear healthy and start developing disease at an unknown time.
WHAT THIS STUDY ADDS:
Patients with BA have elevated direct/conjugated bilirubin (DB/CB) levels at birth. BA could be detected earlier if: (1) all newborns have DB/CB levels measured, including those not jaundiced; and (2) all elevated DB/CB levels are followed, independent of total bilirubin measurements.
Biliary atresia (BA), a disease of unknown etiology, causes significant morbidity in pediatric populations. BA is thought to be acquired by otherwise healthy infants who develop bile duct obstruction weeks after birth and progress to end-stage liver disease over the next 6 to 9 months. If affected infants are identified with BA within the first months, they undergo the Kasai hepatoportoenterostomy (HPE) in an attempt to restore bile flow and delay cirrhosis. If BA is diagnosed later, infants have advanced liver damage, rarely benefit from the Kasai operation, and proceed with liver transplantation (LT) evaluation because no other therapies exist. Unfortunately, because of unsuccessful Kasai operations and/or delayed diagnoses, ∼ half of the patients with BA ultimately require LT, making BA the leading indication for pediatric LT worldwide.1–3
Results of recent studies suggest that LT can be delayed and even avoided if the Kasai hepatoportoenterostomy is performed early, before significant liver damage develops (ie, before 8 weeks).4 However, making such an early diagnosis is difficult. One delay arises because there are no validated early markers to screen for BA The earliest clinical marker–jaundice, is accompanied by a number of pathologic changes in the liver, including bile duct proliferation and fibrosis. Another delay arises because BA is relatively rare. BA occurs in 1 in 8000 to 1 in 12 000 infants and a result, infants with BA are often mistaken initially for infants with more common forms of neonatal jaundice such as breastfeeding jaundice or prolonged newborn “physiologic” jaundice.1
To help clinicians make earlier diagnoses, we aimed to determine the earliest time at which BA can be detected. We started by examining the newborn period. Assuming that BA is acquired, we hypothesized that infants with BA would be unaffected as newborns and have normal direct bilirubin/conjugated bilirubin (DB/CB) levels shortly after birth.
PATIENTS AND METHODS
Patient Selection
This study was approved by the institutional review board at Baylor College of Medicine. Eligible subjects with BA were born between January 1, 2007, and December 31, 2010, and cared for at Texas Children's Hospital, a tertiary care center in Houston, Texas. BA diagnoses were made by liver biopsy and/or intraoperative cholangiogram. Eligible control subjects were the first 75 term infants born at the beginning of each season (April 2010, July 2010, October 2010, and January 2011) at Ben Taub General Hospital, a large county hospital in Houston. All newborns in this hospital have DB and total bilirubin (TB) measurements at 24 to 48 hours of life (HoL) regardless of clinical condition. No control subjects later developed liver disease.
Excluded subjects had BA splenic malformation (BASM) syndrome or were born prematurely. Subjects with BASM syndrome were defined as those with who had either absent, or >1 spleen on abdominal ultrasound and were excluded because they would be predicted to have congenital disease and elevated DB/CB levels at birth.5 Premature subjects were defined as those born before 37 weeks' gestation and were excluded because this population lacks well-accepted bilirubin ranges. In addition, premature subjects have a number of comorbidities that could elevate DB/CB levels shortly after birth, including sepsis, antibiotic use, and parenteral nutrition. No eligible subjects in this study were >42 weeks' gestation.
Data Acquisition
For subjects with BA and controls, bilirubin measurements from 0 to 96 HoL were obtained retrospectively from birth hospitals. In addition, birth times (or, if unavailable, times of cord-blood collection) were recorded to calculate time of bilirubin measurements, and laboratory methodologies were recorded to determine whether DB or CB measurements were made. For subjects with BA, demographic data and clinical course were also obtained retrospectively.
Subjects included in this study had either DB/TB or CB/unconjugated bilirubin measured. As previously reported, DB and CB levels are similar but not equivalent. DB assays are chemical reactions (diazo reactions) that detect some unconjugated bilirubin in addition to CB, whereas CB assays measure CB directly by using direct spectrophotometry.6 Seventy-one DB levels were obtained from 23 hospitals using Abbott (Abbott Park, IL), Beckman Coulter (Brea, CA), Roche (Indianapolis, IN), or Siemens (Deerfield, IL) machines. Most hospitals reported a normal DB range of 0.0 to 0.3 mg/dL; however, 1 hospital reported a DB range of 0.0 to 0.5 mg/dL, which was used in this study. Thirteen CB levels were obtained from 6 hospitals using Vitros machines (Ortho Clinical Diagnostics, Rochester, NY). A normal CB range of 0.0 to 0.3 mg/dL was used, consistent with findings from a recent large population study.7
Statistical Analysis
For analysis of demographic data, Fisher's exact test was used. For DB/TB analysis, levels were first grouped according to when they were collected (0–24, 24–48, 48–72, or 72–96 HoL). When more than 1 DB/TB level was recorded for a particular time interval, only the earliest measured level was used for calculations and graphs. Comparisons were then made by using the unpaired t test and reported as mean ± SD. For CB analysis, all levels for all subjects were plotted. Statistical operations were performed by using GraphPad Prism 5 software (GraphPad, La Jolla, CA).
RESULTS
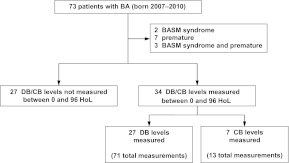
Of the 73 eligible subjects with BA seen at Texas Children's Hospital and born between 2007 and 2010, 61 met inclusion criteria (Fig 1). Fifty-six percent (34 of 61) had DB or CB measurements in the first 96 HoL. Although subjects with and without measurements differed in regard to newborn blood tests ordered, they were indistinguishable with respect to gender, race, ethnicity, and birth season. These infants also underwent the Kasai hepatoportoenterostomy at similar rates and progressed to end-stage liver disease similarly (as inferred by transplant status). Finally, the number of deceased subjects in each group was the same (Table 1).
FIGURE 1.
Patient selection. The 27 subjects with DB levels measured had 71 total measurements between 0 and 96 HoL, whereas the 7 subjects with CB levels measured had 13 total measurements. BASM indicates BA splenic malformation.
TABLE 1.
Characteristics of Subjects With BA
| Characteristic | DB/CB Not Measured (N = 27) | DB/CB Measured (N = 34) | P |
|---|---|---|---|
| Male gender, n (%) | 13 (48) | 14 (41) | .61 |
| Race, n (%) | |||
| White | 16 (59) | 24 (71) | .42 |
| Black | 5 (19) | 8 (24) | .76 |
| Asian | 6 (22) | 2 (6) | .12 |
| Hispanic, n (%) | 7 (26) | 16 (47) | .11 |
| Birth season, n (%) | |||
| Winter | 4 (15) | 5 (15) | 1.00 |
| Spring | 9 (33) | 7 (21) | .38 |
| Summer | 5 (19) | 7 (21) | 1.00 |
| Fall | 9 (33) | 15 (44) | .44 |
| Underwent Kasai hepatoportoenterostomy, n (%) | 21 (78) | 27 (79) | 1.00 |
| Listed/transplanted, n (%) | 18 (67) | 23 (68) | 1.00 |
| Died, n (%) | 2 (7) | 2 (6) | 1.00 |
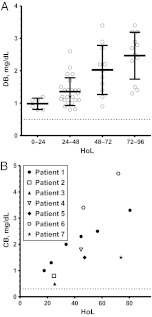
Serum DB/CB levels were elevated in all subjects with BA. DB levels were elevated at 0 to 24 HoL (0.98 ± 0.17 mg/dL); the earliest measurement was 1.1 mg/dL at 1 HoL (Fig 2A). Mean DB levels continued to increase, with a level of 2.5 ± 0.72 mg/dL at 72 to 96 HoL. Similarly, CB levels were elevated early; the earliest value was 1.0 mg/dL at 17 HoL (Fig 2B). CB levels trended upward with time; the latest value was 3.3 mg/dL at 80 HoL. It is important to note that no matter when recorded, every DB or CB value from subjects with BA exceeded the laboratory upper limit of normal.
FIGURE 2.
Patients with BA have elevated DB and CB levels immediately after birth. A, Mean DB levels at 0 to 24 HoL (n = 6), 24 to 48 HoL (n = 24), 48 to 72 HoL (n = 11), and 72 to 96 HoL (n = 12). Each subject's earliest measurement per interval was used. B, CB levels. The dashed lines indicate the upper limits of normal: 0.5 mg/dL (A) and 0.3 mg/dL (B).
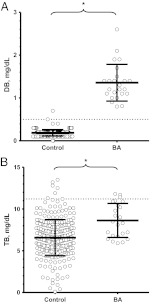
At 24 to 48 HoL, when newborn bilirubin measurements are typically measured, mean serum DB levels were higher in subjects with BA compared to controls (Fig 3A) (1.4 ± 0.43 vs 0.19 ± 0.075 mg/dL, respectively; P < .0001). There was no overlap, since the lowest DB value from a subject with BA (0.8 mg/dL) was greater than the highest DB value from a control subject (0.7 mg/dL). Even with elevated DB values, TB levels were not excessively high. TB levels from subjects with BA and controls, although different, were essentially superimposable and below phototherapy levels for healthy 37-week-gestation newborns (Fig 3B).8
FIGURE 3.
Patients with BA have elevated DB, but not TB, levels at 24 to 48 HoL. Shown are the mean DB (A) and TB (B) levels for controls (n = 300; collection time: 39 ± 5.6 HoL) versus patients with BA (n = 24; collection time: 34 ± 6.2 HoL). The dashed lines indicate the upper limits of normal (A, 0.5 mg/dL), or the approximate phototherapy level at 34 HoL (B, 11.2 mg/dL). a P < .0001.
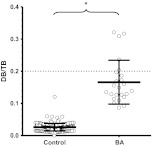
Despite their elevated DB/CB levels, only 53% (18 of 34) of the subjects with BA had directed follow-up after discharge from the nursery. One possible explanation is that the elevated DB/CB levels were considered normal by caregivers, because they were ≤20% of the TB levels.9 To investigate this further, we calculated the DB:TB ratio for all subjects with TB levels of >5 mg/dL at 24 to 48 HoL. The mean DB:TB ratio was ≤0.2 in both subjects with BA and controls (Fig 4) (0.17 ± 0.068 vs 0.026 ± .012, respectively; P < .0001). Seventy-nine percent (19 of 24) of the subjects with BA had a DB:TB ratio of ≤0.2, with the lowest ratio 0.087.
FIGURE 4.
The majority of patients with BA have a DB/TB ratio of ≤0.2. Shown are the mean DB/TB ratios for controls (n = 242) versus patients with BA (n = 24). Only subjects with a TB level of >5 mg/dL were used for analysis. The dashed line indicates the recommended normal limit (0.2). a P < .0001.
DISCUSSION
Although infants with BA who were diagnosed and treated earlier have better outcomes, it is still unknown when BA starts. The disease is thought to be acquired some time after birth in otherwise healthy infants. Our results, however, suggest that BA is already present in the immediate newborn period. All subjects with BA in this study had elevated DB/CB levels throughout the first 4 days of life and starting as early as 1 HoL. Furthermore, at 24 to 48 HoL, subjects with BA had significantly higher mean DB levels compared to controls, even though their mean TB level was below phototherapy limits and their mean DB:TB ratio was considered normal. Thus, rather than being unaffected at birth, and acquiring the disease later, it appears that newborns with BA have abnormalities that are readily detected by common laboratory tests. The findings raise the possibility of identifying infants who may have BA shortly after birth, which in turn has the potential to improve their outcomes.
Because these results disagree with expectations of BA as an acquired disease, we questioned whether subjects with DB/CB levels collected in the first 4 days were representative of the general population of infants with BA. Two sets of findings suggest that they are representative. First, subjects with and without measurements had similar courses and outcomes (Table 1). Both groups underwent the Kasai hepatoportoenterostomy and LT in the same proportions, and both had equivalent mortality rates. Second, subjects with DB/CB levels would have appeared healthy at birth, similar to patients with BA in general. They would not have been distinctively jaundiced, because they had TB levels below phototherapy limits (Fig 3B). Hence, given that our subjects with BA were indistinguishable from other patients with BA, the results from this study should be broadly applicable to other infants with the disease.
As the first study to examine newborn DB/CB levels in infants with BA, this study has limitations that can be addressed in future investigations. First, the study had only 73 subjects, although the subjects came from a broad geographical area spanning 9 states and Mexico. Second, the study included more DB levels than CB levels. DB measurements can differ between instruments, and their ranges may vary among hospitals.6,7 Third, we examined infants with BA rather than all infants) in a particular period and, as a result, provide information on sensitivity but not specificity. However, based upon previous studies, the specificity of elevated DB/CB levels for BA would likely be low, because more common conditions such as sepsis also cause elevated DB/CB levels in the newborn period.7
The findings from this study offer two possible ways of identifying patients with BA earlier. First, it appears that BA could be detected in the newborn period if all newborns are screened for elevated DB/CB levels. Currently, the American Academy of Pediatrics recommends screening only jaundiced infants with TB levels, primarily to plan for phototherapy and avoid kernicterus.10,11 There is no policy for infants who do not appear jaundiced, which might explain why only 56% of subjects with BA had DB/CB levels measured as newborns. Furthermore, if measurements of DB/CB are taken, the policy does not address those with normal TB levels but, only those with elevated DB/CB levels. This group constitutes the majority of subjects with BA in our study.
Universal screening for elevated DB/CB levels could address many of these issues and has a number of additional advantages. Universal testing is readily available, easily interpretable, and supported by experts in the field.12,13 DB/CB screening bypasses limitations of other proposed BA screening tests, such as measuring conjugated bile acids on newborn spot cards (overlap between BA and control values), or detecting acholic stools by using an “infant stool color card” (which requires parents to make subjective decisions on the basis of what they perceive as stool color).14–18 Most important is that universal DB/CB level screening is an extremely sensitive early test for BA. In this study, all 84 DB or CB levels measured between 0 and 96 HoL were elevated in subjects who were eventually diagnosed with BA.
Second, patients with BA could be detected earlier if all elevated DB/CB levels (no matter what the TB level) trigger further investigation. Currently, the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition recommends further testing only if the DB level exceeds 20% of the TB level in patients with TB levels >5 mg/dL.9 These guidelines acknowledge that elevated DB levels might sometimes reflect excessive unconjugated bilirubin because of the measurement techniques. However, the policy would detect only 21% of cases of BA with measurements in this study and may explain, in part, why only 53% of the subjects with elevated DB/CB levels received directed follow-up and the opportunity for earlier intervention.
One way to address these issues is by closely following all newborns with elevated DB/CB levels independent of their TB levels. Elevated DB levels would be determined on a hospital-by-hospital basis, because measurement technologies may vary; elevated CB levels, on the other hand, have been well defined in a recent large population study.7 In our group's practice, if infants have persistently elevated levels for the first 2 weeks, we ask that they be referred to a subspecialty center to distinguish BA from other liver diseases that cause elevated DB/CB levels shortly after birth. Prospective studies are needed to confirm whether this algorithm for detecting and treating BA early ultimately delays or reduces the need for LT.
Finally, on a more speculative note, the elevated DB/CB levels shortly after birth challenge the field's generally accepted BA pathogenesis model, which proposes that healthy infants acquire a viral infection that triggers an (auto)immune reaction against bile ducts.19,20 The acquired model is supported by several lines of evidence: (1) infants appear normal at birth; (2) no clear inheritance patterns exist; (3) viruses have been found in liver tissue from some patients; and (4) newborn mice develop BA-like disease when infected with rhesus rotavirus.21–25 However, the elevated DB/CB levels shortly after birth found in this study are more consistent with BA as a congenital or developmental disease. Future work to better the etiology and pathogenesis of BA should be able to reconcile these differences.
CONCLUSIONS
DB/CB levels are elevated in newborns ultimately diagnosed with BA. The elevated levels are often overlooked, however, in part because TB levels are normal and the DB:TB ratio may not exceed recommended abnormal limits. Our findings suggest 2 possibilities that might improve current practice: (1) screen all newborns for elevated DB/CB levels regardless of clinical appearance; and (2) follow elevated DB/CB levels regardless of TB levels. Taken together, these changes could transform BA management by identifying affected infants early even before clinically significant liver injury develops.
ACKNOWLEDGMENTS
Dr Harpavat receives funding from National Institutes of Health T32 training grant DK-007664 and the American Liver Foundation. Dr Karpen receives funding from National Institutes of Health grant DK-56239.
We thank Z. Apted, A. De La Torre, J. Economides, K. Pieplow, D. Samp, and A. Skelton for help with data acquisition and M. Dave for critically reviewing the manuscript.
Drs Harpavat, Finegold, and Karpen designed the study, analyzed and interpreted the data, revised the article, and approved the final version for publication; Dr Harpavat acquired the data and wrote the original draft; and Dr Karpen initiated the idea and provided overall supervision for the project.
A companion to this article can be found on page e1598 and online at www.pediatrics.org/cgi/doi/10.1542/peds.2011-2774
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
- BA
- biliary atresia
- LT
- liver transplantation
- DB
- direct bilirubin
- CB
- conjugated bilirubin
- HoL
- hour(s) of life
- TB
- total bilirubin
- BASM
- biliary atresia splenic malformation
REFERENCES
- 1. Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology. 2007;46(2):566–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mieli-Vergani G, Vergani D. Biliary atresia. Semin Immunopathol. 2009;31(3):371–381 [DOI] [PubMed] [Google Scholar]
- 3. Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet. 2009;374(9702):1704–1713 [DOI] [PubMed] [Google Scholar]
- 4. Serinet MO, Wildhaber BE, Broué P, et al. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics. 2009;123(5):1280–1286 [DOI] [PubMed] [Google Scholar]
- 5. Davenport M, Tizzard SA, Underhill J, Mieli-Vergani G, Portmann B, Hadzic N. The biliary atresia splenic malformation syndrome: a 28-year single-center retrospective study. J Pediatr. 2006;149(3):393–400 [DOI] [PubMed] [Google Scholar]
- 6. Westwood A. The analysis of bilirubin in serum. Ann Clin Biochem. 1991;28(pt 2):119–130 [DOI] [PubMed] [Google Scholar]
- 7. Davis AR, Rosenthal P, Escobar GJ, Newman TB. Interpreting conjugated bilirubin levels in newborns. J Pediatr. 2011;158(4):562.e1–565.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics. 1999;103(1):6–14 [DOI] [PubMed] [Google Scholar]
- 9. Moyer V, Freese DK, Whitington PF, et al. Guideline for the evaluation of cholestatic jaundice in infants: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2004;39(2):115–128 [DOI] [PubMed] [Google Scholar]
- 10. American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation [published correction appears in Pediatrics. 2004;114(4):1138]. Pediatrics. 2004;114(1):297–316 [DOI] [PubMed] [Google Scholar]
- 11. US Preventive Services Task Force Screening of infants for hyperbilirubinemia to prevent chronic bilirubin encephalopathy: US Preventive Services Task Force recommendation statement. Pediatrics. 2009;124(4):1172–1177 [DOI] [PubMed] [Google Scholar]
- 12. Powell JE, Keffler S, Kelly DA, Green A. Population screening for neonatal liver disease: potential for a community-based programme. J Med Screen. 2003;10(3):112–116 [DOI] [PubMed] [Google Scholar]
- 13. Trikalinos TA, Chung M, Lau J, Ip S. Systematic review of screening for bilirubin encephalopathy in neonates. Pediatrics. 2009;124(4):1162–1171 [DOI] [PubMed] [Google Scholar]
- 14. Mills KA, Mushtaq I, Johnson AW, Whitfield PD, Clayton PT. A method for the quantitation of conjugated bile acids in dried blood spots using electrospray ionization-mass spectrometry. Pediatr Res. 1998;43(3):361–368 [DOI] [PubMed] [Google Scholar]
- 15. Mushtaq I, Logan S, Morris M, et al. Screening of newborn infants for cholestatic hepatobiliary disease with tandem mass spectrometry. BMJ. 1999;319(7208):471–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen SM, Chang MH, Du JC, et al. ; Taiwan Infant Stool Color Card Study Group Screening for biliary atresia by infant stool color card in Taiwan. Pediatrics. 2006;117(4):1147–1154 [DOI] [PubMed] [Google Scholar]
- 17. Hsiao CH, Chang MH, Chen HL, et al. Universal screening for biliary atresia using an infant stool color card in Taiwan. Hepatology. 2008;47(4):1233–1240 [DOI] [PubMed] [Google Scholar]
- 18. Lien TH, Chang MH, Wu JF, et al. Effects of the infant stool color card screening program on 5-year outcome of biliary atresia in Taiwan. Hepatology. 2011;53(1):202–208 [DOI] [PubMed] [Google Scholar]
- 19. Bezerra JA. Potential etiologies of biliary atresia. Pediatr Transplant. 2005;9(5):646–651 [DOI] [PubMed] [Google Scholar]
- 20. Mack CL. The pathogenesis of biliary atresia: evidence for a virus-induced autoimmune disease. Semin Liver Dis. 2007;27(3):233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suchy FJ. Neonatal cholestasis. Pediatr Rev. 2004;25(11):388–396 [PubMed] [Google Scholar]
- 22. Smith BM, Laberge JM, Schreiber R, Weber AM, Blanchard H. Familial biliary atresia in three siblings including twins. J Pediatr Surg. 1991;26(11):1331–1333 [DOI] [PubMed] [Google Scholar]
- 23. Morecki R, Glaser JH, Cho S, Balistreri WF, Horwitz MS. Biliary atresia and reovirus type 3 infection. N Engl J Med. 1982;307(8):481–484 [DOI] [PubMed] [Google Scholar]
- 24. Rauschenfels S, Krassmann M, Al-Masri AN, et al. Incidence of hepatotropic viruses in biliary atresia. Eur J Pediatr. 2009;168(4):469–476 [DOI] [PubMed] [Google Scholar]
- 25. Riepenhoff-Talty M, Schaekel K, Clark HF, et al. Group A rotaviruses produce extrahepatic biliary obstruction in orally inoculated newborn mice. Pediatr Res. 1993;33(4 pt 1):394–399 [DOI] [PubMed] [Google Scholar]