Abstract
BACKGROUND:
The 2009 pandemic influenza A (H1N1) (pH1N1) virus continues to circulate worldwide. Determining the roles of chronic conditions and bacterial coinfection in mortality is difficult because of the limited data for children with pH1N1-related critical illness.
METHODS:
We identified children (<21 years old) with confirmed or probable pH1N1 admitted to 35 US PICUs from April 15, 2009, through April 15, 2010. We collected data on demographics, baseline health, laboratory results, treatments, and outcomes.
RESULTS:
Of 838 children with pH1N1 admitted to a PICU, the median age was 6 years, 58% were male, 70% had ≥1 chronic health condition, and 88.2% received oseltamivir (5.8% started before PICU admission). Most patients had respiratory failure with 564 (67.3%) receiving mechanical ventilation; 162 (19.3%) received vasopressors, and 75 (8.9%) died. Overall, 71 (8.5%) of the patients had a presumed diagnosis of early (within 72 hours after PICU admission) Staphylococcus aureus coinfection of the lung with 48% methicillin-resistant S aureus (MRSA). In multivariable analyses, preexisting neurologic conditions or immunosuppression, encephalitis (1.7% of cases), myocarditis (1.4% of cases), early presumed MRSA lung coinfection, and female gender were mortality risk factors. Among 251 previously healthy children, only early presumed MRSA coinfection of the lung (relative risk: 8 [95% confidence interval: 3.1–20.6]; P < .0001) remained a mortality risk factor.
CONCLUSIONS:
Children with preexisting neurologic conditions and immune compromise were at increased risk of pH1N1-associated death after PICU admission. Secondary complications of pH1N1, including myocarditis, encephalitis, and clinical diagnosis of early presumed MRSA coinfection of the lung, were mortality risk factors.
Keywords: influenza, intensive care unit, pediatric, mortality, pandemic, MRSA
WHAT'S KNOWN ON THIS SUBJECT:
The 2009 pandemic influenza A (H1N1) virus was associated with high rates of hospitalization and death in children worldwide. Limited data for children with pH1N1-related critical illness make it difficult to determine the role of chronic conditions and bacterial coinfection in mortality.
WHAT THIS STUDY ADDS:
Children with neurologic conditions and compromised immune function had an increased risk of mortality from 2009 pandemic H1N1. Coinfection with methicillin-resistant Staphylococcus aureus was a strong risk factor for mortality, increasing the risk of death in previously healthy children eightfold.
Although it is regarded as a mild-to-moderate pandemic,1 2009 pandemic influenza A (H1N1) [pH1N1] has been associated with school outbreaks,2–4 high incidence5 and illness complication rates,6 hospitalizations,7–11 and critical illness and death12 in pediatric populations worldwide. To date, clinical data are available for only limited numbers of critically ill children with pH1N1 virus infection-related complications13–16 or fatalities.17,18 Results have varied across studies: pH1N1-related PICU mortality rates ranged from 7% in 57 Canadian14 and 107 Australian-New Zealand19 children to 39% in 147 children in Argentina.13 Independent risk factors associated with fatal outcomes have not been well established because of limited sample sizes.
To assess critical illness among children during the 2009 influenza A (H1N1) pandemic, we established a large multicenter registry of patients admitted to PICUs in major US pediatric hospitals. In this report we describe the detailed clinical characteristics and hospital course of this large cohort of critically ill children with pH1N1 virus infection and discuss the role of chronic medical conditions and bacterial coinfections in mortality.
METHODS
Study Design and Eligibility Criteria
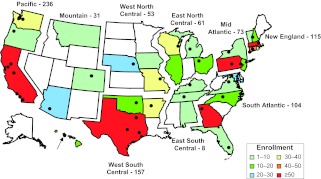
This 1-year multicenter retrospective and prospective observational cohort study identified patients with influenza virus infection admitted to 35 Pediatric Acute Lung Injury and Sepsis Investigators' (PALISI) network hospital PICUs (see Fig 1 for site locations) from April 15, 2009, through April 15, 2010. The study was initiated as a collaborative effort among the US National Institutes of Health, Centers for Disease Control and Prevention, the Assistant Secretary for Preparedness and Response, US Department of Health and Human Services, the National Heart, Lung, and Blood Institute's ARDSNet, and the PALISI network to track pH1N1-associated critical illness in adults (ARDSNet sites) and children (PALISI sites). The ARDSNet clinical coordinating center coordinated data management. After institutional review board approval, sites identified patients with any positive influenza A test result from April 15 through August 31, 2009, and any positive influenza test result or suspected case of influenza from September 1, 2009, through April 15, 2010. The first institutional review board approval was received November 15, 2009. Decisions regarding diagnostic testing were determined solely by clinicians. We excluded patients without laboratory confirmation of influenza and those with evidence of seasonal influenza A (H1N1 or H3N2) or B virus infections.
FIGURE 1.
The origin of patients enrolled in the 2009 pandemic influenza A (H1N1) PICU surveillance study according to state and region. The 35 participating hospitals (black circles) located throughout the United States serve as regional referral tertiary care centers with a median of 24 (IQR: 20–38) PICU beds. Of these hospitals, there were a total of 51 ICUs screened, including 38 medical or medical-surgical ICUs, 4 NICUs that admitted patients up to 6 months of age, and 9 other types of specialized ICUs (eg, cardiac, trauma). States are color-coded according to the number of patients enrolled from their zip code of residence in that state.
Data Collection
Data were recorded in a REDCap20 Web-based electronic case-report form and transmitted to the clinical coordinating center. PICU day 0 was the PICU admission day. Baseline values were the first recorded values in the PICU and, if unavailable, the values closest to admission (from transport, the emergency department, or referral hospital). Data on days 3, 7, and 14 were collected as close to 8:00 am as possible; unavailable data were considered missing. Final outcome (survival/death) was tracked to initial PICU discharge date for all patients and up to hospital day 90 for patients transferred to the ward. Cause of death was classified as primary respiratory, cardiovascular, multiorgan failure, severe brain injury and/or brain death, or other.
Definitions
Those who were considered to be previously healthy patients were healthy before the index illness, had no underlying medical conditions, and did not depend on medications or medical devices. The Pediatric Risk of Mortality III (PRISM III) acute physiology score21 was used to measure severity of illness within the first 24 hours of admission in children younger than 18 years, and the Acute Physiology and Chronic Health Evaluation II (APACHE II) score22 was used in those aged 18 to 20 years.
Noninvasive ventilation was defined as delivery of continuous or bilevel positive airway pressure from a mechanical ventilator via a mask. Shock requiring vasopressors was defined as receiving a dopamine infusion of >5 μg/kg per minute or any epinephrine, norepinephrine, or phenylephrine infusion to maintain blood pressure. Diagnosis of encephalitis was based on MRI findings, high cerebrospinal fluid protein level, and/or neurology consultation. Myocarditis was defined as the major clinical diagnosis underlying the reason for PICU admission. Mortality was defined as death that occurred anytime during the PICU stay and up to hospital day 90 for patients transferred to the ward. A confirmed case of pH1N1 was defined as that of a PICU patient with a respiratory specimen that tested positive for pH1N1 virus infection by real-time reverse transcription polymerase chain reaction assay using primers specific for pH1N1 virus or viral culture. A probable case of pH1N1 was defined as that of a PICU patient with a respiratory specimen that tested positive for influenza A virus infection by any influenza testing without further identification of subtype. Patients with early presumed bacterial coinfection of the lung had (1) a clinical diagnosis of bacterial pneumonia, (2) evidence of bacterial superinfection within 72 hours of the initial PICU admission, and (3) a bacterial pathogen identified in their respiratory secretions.
Statistical Analyses
With univariate analyses we examined the association between multiple factors and mortality by using χ2 and Fisher's exact tests. Unadjusted relationships between continuous variables were evaluated by using simple linear regression. Factors examined for their association with mortality were demographics; PICU admission clinical conditions including comorbidities and coinfections; treatments; and secondary influenza-related complications (eg, encephalitis, myocarditis). Factors possibly associated with mortality (P ≤ .10) were considered for inclusion in the multivariable model containing all independent predictors of mortality (P < .05). Missing data were not imputed. All analyses were completed by using SAS 9.2 (SAS Institute, Inc, Cary, NC). SAS's PROC GENMOD procedure produced adjusted relative risks (RRs) by using a generalized linear model. Adjusted risk estimates are reported with 95% confidence intervals (CIs).
RESULTS
Characteristics of the Study Patients
We enrolled 940 PICU patients and excluded 102 (14 aged ≥21 years, 9 with seasonal influenza A, 6 with influenza B, and 73 with no positive influenza test result [some met >1 criterion]). Of the 838 patients included, 545 (65%) had pH1N1 confirmed by real-time reverse transcription polymerase chain reaction or viral culture, and 293 (35%) were classified as having probable pH1N1 on the basis of a positive direct fluorescent antibody or rapid influenza diagnostic test result for influenza A without additional subtyping. Most children (553 [66%]) were prospectively identified; 62% were admitted to the PICU during September through November 2009, during the reported peak of influenza-related hospital admissions for the 2009–2010 season.
The characteristics of patients with confirmed or probable pH1N1 are listed in Table 1 and Supplemental Table 5. Most (60%) asthmatics were on weekly asthma-control medications. Reported influenza-related signs and symptoms in patients included fever (79%), cough (73%), shortness of breath (54%), vomiting (30%), diarrhea (12%), and seizures (10%) (Supplemental Table 5) (mean onset: 4 days [SD: 6.3]; median: 3 days [interquartile range (IQR): 1–5]; n = 694) before PICU admission. The majority of patients (579 [69.1%]) were transferred to the PICU from an emergency department, but 239 patients (28.5%) had been an inpatient at the same or another hospital, including 31 (3.7%) in another hospital's ICU, for a median of 1 day (IQR: 0.5–3.0) before PICU admission.
TABLE 1.
Characteristics of the 838 Children Admitted to a PICU With Confirmed or Probable pH1N1 in the United States (April 15, 2009, to April 15, 2010)
| Characteristic | n (%) |
|---|---|
| Female gender | 353 (42.1) |
| Age groupa | |
| <6 mo | 71 (8.5) |
| 6–23 mo | 113 (13.5) |
| 2–4 y | 131 (15.6) |
| 5–12 y | 336 (40.1) |
| 13–17 y | 157 (18.7) |
| 18–20 y | 30 (3.6) |
| Hispanic ethnicityb | 229 (27.3) |
| Raceb | |
| White | 458 (54.7) |
| Black | 168 (20.0) |
| Asian, Hawaiian, or Pacific Islander | 45 (5.4) |
| Native American | 8 (1.0) |
| Other or not reported | 159 (19.0) |
| Underlying health conditions | |
| Previously healthy (none) | 251 (30.0) |
| ≥1 underlying conditions | 587 (70.0) |
| Chronic respiratory | 356 (42.5) |
| Asthma | 258 (30.8) |
| Neurologic or neuromuscular | 263 (31.4) |
| Cardiovascular | 80 (9.6) |
| Gastrointestinal or hepatic | 80 (9.6) |
| Metabolic | 37 (4.4) |
| Immune compromise | 33 (3.9) |
| Current/active metastatic solid cancer | 6 (0.7) |
| Current/active hematologic malignancy | 9 (1.1) |
| Other immunosuppression (ie, transplant, HIV) | 18 (2.1) |
| Renal | 10 (1.2) |
| Hemoglobinopathy | 22 (2.6) |
| Other chronic condition | 126 (15.0) |
For an expansion of the data shown here, see Supplemental Table 5.
The median age of the patients was 6 years (range: 6 days to 20 years).
Race or ethnic group was reported in the clinical chart.
The median Pediatric Risk of Mortality III score was 5 (IQR: 2–10) for the patients younger than 18 years, and the median Acute Physiology and Chronic Health Evaluation II score was 14.5 (IQR: 8–20) for those aged 18 years or older. At presentation, 90 patients (10.7%) received vasopressors for shock, 11 (1.3%) were in cardiac arrest, and 29 (3.5%) had suspected central nervous system complications. Most patients (536 [64%]) presented with evidence of acute lower respiratory disease; 57.5% received assisted breathing on the day of PICU admission (excluding 27 patients on chronic mechanical ventilation). Chest radiographs were performed on 92.5% of the patients at or around PICU admission, and 51.4% had infiltrates in at least 2 of 4 quadrants. Selected laboratory testing parameters closest to admission are listed in Table 2.
TABLE 2.
Selected Laboratory Abnormalities Closest to PICU Admission in Patients With pH1N1 and Their Association With Mortality
| Laboratory Abnormality | n/N (%) | RR (95% CI) |
|---|---|---|
| Leukocytopenia (white cell count < 5000 per μL) | 161/753 (21.4) | 1.8 (1.2–2.9)a |
| Leukocytosis (white cell count > 11 000 per μL)b | 289/753 (38.4) | 0.7 (0.4–1.1) |
| Lymphocytopenia (<1000 per μL) | 365/719 (50.8) | 1.3 (0.8–2.1) |
| Neutropenia (<500 per μL) | 33/630 (5.2) | 2.8 (1.5–5.5)c |
| Thrombocytopenia (platelet count < 150 000 per μL) | 190/748 (25.4) | 2.9 (1.8–4.4)d |
| Elevated creatinine levele | 150/739 (20.3) | 1.8 (1.2–2.9)f |
| Elevated total bilirubin level (>1.2 mg/dL [21 μmol/L])b | 44/447 (9.8) | 0.5 (0.2–1.5) |
| Suspected rhabdomyolysis (CPK > 200 U/L)b | 51/115 (44.4) | 1.4 (0.6–3.7) |
| Severe hypoxiag | ||
| Pao2/Fio2 < 100 | 115/239 (48.1) | 3 (1.6–5.8)h |
| Pao2/Fio2 = 100–199 | 65/239 (27.2) | 1.1 (0.4–3.4) |
Laboratory values are based on work by Custer and Rau53 except for rhabdomyolysis. CPK indicates creatine phosphokinase; Fio2, fraction of inspired oxygen.
P = .009.
Newborns who were younger than 28 days were excluded from these analyses.
P = .003.
P < .0001.
Elevated creatinine level was defined as: >1 mg/dL for newborns younger than 28 days and for adolescents aged 13 to 20 years; >0.7 mg/dL for infants aged 29 to 364 days and for children aged 1 to 12 years.
P = .01.
Pao2 from arterial blood gas (when available) divided by delivered concentration of Fio2; the reference-group patients' Pao2/Fio2 was ≥200.
P = .0002.
Viral and Bacterial Coinfections
Viral coinfections were reported in 4.8% (40) of the patients, including respiratory syncytial virus (14) and adenovirus (4). Thirty-three percent (274 of 838) of the patients had a clinical diagnosis of bacterial pneumonia or other evidence of bacterial infection present within ≤72 hours of PICU admission. Pathogens identified in respiratory secretions included S aureus in 26% (71 of 274; 48% [34 of 71] were methicillin-resistant Staphylococcus aureus [MRSA]), Pseudomonas (all species) in 11% (30 of 274), Streptococcus pneumoniae in 5.5% (15 of 274), Haemophilus influenzae in 5% (13 of 274), Streptococcus pyogenes in 3% (7 of 274), and other bacteria in 20% (54 of 274) (Supplemental Table 6). No pathogens were identified in 33% (91 of 274) of the patients. Most of the patients presumed to be coinfected with Pseudomonas (87% [26 of 30]) had chronic lung disease (17 of 26 had tracheostomies). Empyema (with thoracostomy) was diagnosed in 3.5% (29 of 838) of the patients. Nearly 5% of the patients had bacteremia within 72 hours of PICU admission, and S aureus was the most common bacteria identified (11 of 38, 7 of which were MRSA and 4 of 7 also had MRSA in respiratory specimens ≤72 hours of admission).
Treatments and Clinical Outcomes
The majority of patients (67.3% [564 of 838]) received mechanical ventilator support (median duration: 5 days [IQR: 2–13]). The median PICU stay was 4 days (IQR: 2–9). Of the 75 (8.9%) children who died, death occurred in 31% on PICU days 0 to 2 and 35% on PICU days 3 to 14. All PICU deaths occurred within 90 days of admission except for 1 previously healthy teenager who died on PICU day 124 after lung transplant for pulmonary necrosis, presumably from MRSA coinfection. The primary cause of death reported was inability to ventilate or oxygenate the patient (46.7%), multiorgan failure (25.3%), brain death or severe brain injury (14.7%), refractory cardiovascular collapse (2.7%), and other causes (10.7%). Adjunctive treatments administered to patients are listed in Table 3. Extracorporeal membrane oxygenation (ECMO) was used in 33 patients (3.9%; median age: 8 years [IQR: 5–14]; n = 18 [54.5%] on day 0), for a mean of 18.9 days (SD: 21.4; range: 1–123) with 39% survival. A tracheostomy was performed on 3.1% (26) of the patients. The majority of the 763 survivors (745 [97.6%]) were discharged, but 18 (2.4%) remained hospitalized 90 days after PICU admission (7 were still in a PICU); 22 (2.9%) were transferred to a rehabilitation facility.
TABLE 3.
Adjunctive and Antiviral Treatments Received in the PICU and Survival (N = 838)
| Parameter | n (%) | RR Death (95% CI) | P |
|---|---|---|---|
| Mechanical ventilation | |||
| Via invasive route only | 259 (30.9) | 4.0 (2.5–6.2) | <.0001 |
| Noninvasive ventilation only | 136 (16.2) | 0.4 (0.2–0.9) | .02 |
| Both endotracheal and noninvasive ventilation | 169 (20.2) | 1.6 (1.0–2.6) | .04 |
| High-frequency ventilation | 115 (13.7) | 8.4 (5.6–12.8) | <.0001 |
| Inhaled nitric oxide | 76 (9.1) | 7.5 (5.0–11.0) | <.0001 |
| Prone positioning | 33 (3.9) | 3.8 (2.1–6.6) | <.0001 |
| Vasopressors for shocka | |||
| At time of PICU admission | 90 (10.7) | 4.4 (2.9–6.7) | <.0001 |
| During PICU course | 162 (19.3) | 7.9 (5.0–12.3) | <.0001 |
| Dialysis | 44 (5.3) | 4.5 (2.8–7.3) | <.0001 |
| ECMO | 33 (3.9) | 8.9 (6.1–12.9) | <.0001 |
| High-dose corticosteroidsb | 262 (31.3) | 3.5 (2.2–5.4) | <.0001 |
| Fresh-frozen plasma | 75 (9.0) | 6.8 (4.6–10.1) | <.0001 |
| Intravenous immunoglobulin | 26 (3.1) | 3.7 (2.0–6.9) | <.0001 |
| Influenza antiviral medications | |||
| Oseltamivir | 751 (89.6) | 0.8 (0.4–1.4) | NS |
| Peramivirc | 21 (2.5) | 6.0 (3.6–9.9) | <.0001 |
| Zanamivir | 12 (1.4) | 0.9 (0.1–6.2) | NS |
| Amantadine | 17 (2.0) | 0.7 (0.1–4.4) | NS |
| Ribavirin | 5 (0.6) | 2.3 (0.4–13.2) | NS |
| Rimantadine (none died) | 5 (0.6) | — | NS |
NS indicates not significant.
Vasopressors indicates intravenous dopamine >5 μg/kg per minute or any norepinephrine, epinephrine, or phenylephrine.
Greater than or equal to 2 mg/kg per d methylprednisolone or prednisone or stress-dose hydrocortisone at any time excluding for airway edema around extubation.
Median timing of initiation of peramivir was on PICU day 5 (IQR: 3.5–7.0).
Only 5.8% (49 of 838) of the patients had documentation of receiving influenza antiviral medications before PICU admission (initiation dates were unavailable). Most of the patients (88.2%) received enteral oseltamivir in a PICU for a median of 4 days (IQR: 2–5). Reported use of other influenza antiviral agents is indicated in Table 3.
Comparison of Survivors and Nonsurvivors
Nonsurvivors had higher Pediatric Risk of Mortality III scores (median: 17 [IQR: 9–29]) at PICU admission than survivors (median: 4 [IQR: 0–9]; P < .0001). Of the selected laboratory parameters documented close to PICU admission in patients who were tested, leukocytopenia, neutropenia, thrombocytopenia, elevated creatinine level, and severe hypoxia were associated with mortality (Table 2). Patients who received adjunctive therapies (eg, ECMO, dialysis) had a higher risk of death (see Table 3).
The associations of mortality with patient-related factors present before PICU admission (demographics, underlying health conditions, bacterial coinfections) and influenza-related clinical syndromes are listed in Table 4. The mortality was higher in patients transferred from another ICU (RR: 3.1 [95% CI: 1.6–5.9]; P = .0008). No females were pregnant. Height data were not routinely collected to determine the association of BMI with mortality. Encephalitis (14 [1.7%]) and myocarditis (12 [1.4%]) were uncommon but associated with mortality (see patient details in Supplemental Table 7 and Supplemental Table 8). Viral coinfection was not associated with mortality. MRSA was the only organism cultured from respiratory secretions within ≤72 hours of admission and presumed to be causing pneumonia or a bacterial infection that was associated with mortality (Table 4 and Supplemental Table 9). In a multivariable analysis of factors present at admission, female gender, having a preexisting neurologic condition or previous immune compromise, early presumed MRSA coinfection of the lung, encephalitis, and myocarditis remained significant independent predictors of mortality (Table 4).
TABLE 4.
Demographic Factors, Chronic Health Conditions, Clinical Conditions, and Secondary Infections Associated With Mortality in 838 Children Admitted to a PICU With pH1N1
| Parameter | n (%) | RR Death (95% CI) | P |
|---|---|---|---|
| Univariate analysis | |||
| Female gender | 353 (42.1) | 2.0 (1.3–3.0) | .002 |
| Age 5–20 y (vs 0–4 y) | 523 (62.4) | 1.5 (1.0–2.5) | .07 |
| Neurologic/neuromuscular condition | 263 (31.4) | 1.9 (1.2–2.9) | .003 |
| Immune compromise | 33 (3.9) | 2.5 (1.3–5.0) | .01 |
| Active hematologic malignancy | 9 (1.1) | 3.8 (1.5–9.9) | .04 |
| Metabolic disorder | 37 (4.4) | 2.2 (1.1–4.5) | .03 |
| Encephalitis diagnosed | 14 (1.7) | 3.3 (1.4–7.8) | .03 |
| Myocarditis diagnosed | 6 (0.7) | 3.8 (1.2–12.0) | .09 |
| Bacterial pneumonia/coinfectiona | 274 (32.7) | 1.8 (1.2–2.8) | .007 |
| Pathogen identified in respiratory secretions | |||
| Within ≤72 h of PICU admissionb | 183 (21.8) | 1.7 (0.8–3.5) | .2 |
| S aureus (lung) within ≤72 h | 71 (8.5) | 2.3 (1.3–3.9) | .004 |
| MSSA (lung) within ≤72 h | 37 (4.4) | 1.2 (0.5–3.2) | NS |
| MRSA (lung) within ≤72 h | 34 (4.1) | 3.2 (1.8–5.9) | .0003 |
| Multivariate analysisc | |||
| Female gender | 353 (42.1) | 1.9 (1.2–3.0) | .003 |
| Neurologic condition | 263 (31.4) | 1.8 (1.1–2.7) | .01 |
| Immune compromise | 33 (3.9) | 2.2 (1.1–4.5) | .02 |
| Encephalitis diagnosed | 14 (1.7) | 3.4 (1.6–7.5) | .002 |
| Myocarditis diagnosed | 6 (0.7) | 3.8 (1.2–12.0) | .03 |
| Presumed MRSA lung coinfection within ≤72 h | 34 (4.1) | 3.3 (1.7–6.4) | .0005 |
NS indicates not significant; MSSA, methicillin-susceptible S aureus.
Clinical diagnosis of bacterial pneumonia or superinfection at any time during PICU stay.
When the comparison group is not specified, patients with the factor were compared to patients without the factor for those patients for whom the factor was reported.
The results for all factors tested in the multivariate analysis are reported in Supplemental Table 9.
When the analysis was restricted to the 251 previously healthy patients, there were 26 (10.4%) with early presumed S aureus coinfection of the lung (Supplemental Table 10), and this infection was present in 8 of 18 (44%) of the previously healthy children who died. S aureus was cultured from endotracheal tube specimens of 25 of 26 and from empyema fluid of 1 of 26. Early presumed MRSA coinfection of the lung was the only previously identified factor that remained associated with mortality (RR: 8 [95% CI: 3.1–20.6]; P < .0001) (Supplemental Table 9). Of the 6 previously healthy children with MRSA coinfection of the lung who died, 5 were treated before or within 2 hours of PICU admission with intravenous vancomycin (Supplemental Table 10).
DISCUSSION
To our knowledge, this is the largest study to date of critically ill children with pH1N1 virus infection, which included 838 patients hospitalized at 35 US PICUs. Disease progression was rapid and often fulminant; 69% were admitted to a PICU from an emergency department, 58% received assisted ventilation at PICU admission, and 8.9% died. Sixty-two percent had critical illness from pH1N1 virus infection with no suspicion or evidence of secondary bacterial pneumonia or other bacterial infection at PICU admission. Independent risk factors for mortality were female gender, presence of a chronic neurologic condition or immune compromise, diagnosis of acute myocarditis or encephalitis, and early presumed MRSA coinfection of the lung. For the 30% of children who were previously healthy, only early presumed MRSA coinfection of the lung, which increased the RR of death eightfold, was strongly associated with mortality.
Although in previous decades invasive bacterial coinfection with MRSA was not identified as a major organism associated with pediatric influenza-related mortality, reports from 2003 onward suggest that its role in severe and fatal seasonal influenza complications among US children and adults might have increased.23–30 Although guidelines for empiric antimicrobial treatment of hospitalized adults with community-acquired pneumonia, including for suspected MRSA, exist,31 no guidance existed for hospitalized children with community-acquired pneumonia during the study period. Although our data support empiric antibiotic treatment of MRSA,32 in addition to treatment for other common coinfections (eg, S pneumoniae, S pyogenes), in the initial management of children with suspected or confirmed influenza and severe lower respiratory tract disease, there are not clear criteria for prospective identification of patients with bacterial coinfection.
The median age of children in our study was 6 years, similar to that reported for Canada14,33 and South Korea34 but higher than that for Argentina, where more than half of the children were infants.13 The proportion of children with chronic medical conditions and the overall mortality were similar to those reported for Canada.14 Our mortality and proportion of invasive mechanical ventilation were lower than those reported in 1 Argentina study.13 Although more male than female children were admitted to our PICUs with pH1N1-associated complications, female patients were at a significantly higher risk of death. This risk was not caused by pregnancy, which is a known risk factor.35 There were no gender differences in mortality rate among previously healthy children.
Neurologic and neuromuscular disorders have previously been identified as risk factors for influenza-related complications and hospitalization in children,36 and they were present in 31% of our patients. The rate of neurologic disorders is reportedly high in children who die from pH1N1-related complications17; ours is the first study to identify that presence of an underlying neurologic condition was an independent risk factor for death. Acute neurologic manifestations associated with pH1N1 also occurred; seizures occurred in 10% of the patients before admission, suspected central nervous system complications occurred in 3.5%, and acute encephalitis was diagnosed in 1.7%. Although there have been case reports of severe encephalopathy or encephalitis in patients with pH1N1,37–40 the size of our study allowed us to identify acute encephalitis with pH1N1 virus infection as an independent risk factor for mortality.
Confirming a previous report on Korean children,34 we found pH1N1-related acute myocarditis to be an independent risk factor for death. Seasonal influenza virus infection of the respiratory tract is associated with acute myocarditis,41 and myocarditis cases related to pH1N1 have been described.34,42–44 Current management of influenza-associated myocarditis is supportive care.
In a previous population-based report,45 11.6% of primarily adult patients with pH1N1 admitted to the ICU received ECMO in Australia and New Zealand compared with 3.9% of the patients in our study. We report 39% survival for pediatric patients with pH1N1 who received ECMO, which is much lower than the 71% survival previously reported for mostly adults.46 Other rescue therapies were also associated with high mortality. ECMO support was used in 7 of 10 of the patients who died; 4 had early presumed S aureus lung coinfection.
Most patients received oseltamivir treatment; antiviral medication use at any time during the PICU course was not associated with lower mortality. The authors of 1 study of PICU patients in Argentina reported that initiation of oseltamivir treatment within ≤24 hours of admission was associated with lower mortality.13 Authors of a study of adult ICU patients in Mexico also reported a survival benefit of neuraminidase inhibitor treatment.47 Others have reported the effectiveness of early antiviral treatment with oseltamivir to prevent progression of pH1N1 virus infection to severe and fatal illness.8,35,48 Data on the timing of oseltamivir initiation was not collected in our study. The vast majority of patients began receiving it after PICU admission; we cannot assess if earlier treatment could have improved outcome.
This study is subject to several limitations. First, although pH1N1 was confirmed in 65% of the cases, we believe that classifying 35% of the patients who tested positive for influenza A as having probable pH1N1 was valid, because US influenza surveillance identified 99% of influenza A viruses tested during the study period as pH1N1 viruses.49 It is likely that some patients with pH1N1 at participating PICUs were missed because of the use of less sensitive influenza tests.50 Although most clinical data were collected retrospectively, most data elements were easily captured from the patients' charts. It is unfortunate that bacterial isolates were not available for strain typing or identification of virulence factors such as Panton-Valentine leukocidin. A majority of presumed bacterial lung coinfections were detected by culture of endotracheal aspirate specimens; the sensitivity and specificity of such lower respiratory tract sampling is currently unknown. In addition, testing for other respiratory viruses or bacterial infections was performed as clinically indicated and was not done on a systematic basis or with more sensitive diagnostics, and administration of broad-spectrum antibiotics might have produced negative culture results for some bacterial pathogens. This might have underestimated certain coinfections that have been identified with greater frequency in other studies.51,52 We included children admitted to large PICUs in regional referral pediatric centers. Therefore, we could not determine pH1N1-associated risk factors for more severe illness. Children admitted to smaller PICUs were not represented.
CONCLUSIONS
The pH1N1 virus continues to circulate worldwide. In addition to influenza antiviral treatment, early empiric antimicrobial treatment of critically ill children with suspected or confirmed influenza and lower respiratory tract disease, shock, or sepsis should cover MRSA and other bacterial pathogens associated with influenza. It is important to note that most of the children coinfected with MRSA who died in our study received vancomycin promptly at or before PICU admission, which indicates that prevention and control strategies for increasing influenza-related immunity and optimizing antiviral treatment of influenza, new therapies for treating severe influenza, and new treatment strategies for MRSA pneumonia complicating influenza are urgently needed for children.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute, Department of Health and Human Services (contract N01 HR 56179).
The PALISI network participants include the following: Arkansas Children's Hospital (Little Rock, AR): Ronald C. Sanders, MD, and Glenda Hefley, MNsc, RN; Phoenix Children's Hospital (Phoenix, AZ): David W. Tellez, MD, and Velva Woodward, RN; Miller Children's Hospital Long Beach (Long Beach, CA): Christopher Babbitt, MD, and Rosalynn Gurrola, RN; Children's Hospital of Los Angeles (Los Angeles, CA): Barry Markovitz, MD, Jeff Terry, MBA, and Karen Waters, RN; Children's Hospital of Central California (Madera, CA): Ana Lia Graciano, MD, Melita Baldwin, CHCC, and Aurelia Ayala, RN; Children's Hospital and Research Center (Oakland, CA): Heidi Flori, MD, and Julie Simon, RN; Children's Hospital of Orange County (Orange, CA): Nick Anas, MD, and Stephanie Osborne, RN; UCSF Children's Medical Center (San Francisco, CA): Anil Sapru, MD, and Maureen Convery, RN; Yale-New Haven Children's Hospital (New Haven, CT): John S. Giuliano Jr, MD, and Kellie Martino; The Children's Hospital (Aurora, CO): Angela Czaja, MD, Peter Mourani, MD, and Susanna Burr; Connecticut Children's Medical Center (Hartford, CT): Christopher L. Carroll, MD, and Indira Sadhu, RN; Holtz Children's Hospital (Miami, FL): Gwenn McLaughlin, MD, and Yenis Mijares; Children's Healthcare of Atlanta at Egleston (Atlanta, GA): Matthew Paden, MD, and Rich Toney, RN; Kapi'olani Center for Women and Children (Honolulu, HI): Rupert Cheng, MD, Len Tanaka, MD, and Sara Murakami; Kosair Children's Hospital (Louisville, KY): Vicki Montgomery, MD, and Kara Richardson, RN; Children's Hospital Boston (Boston, MA): Adrienne Randolph, MD, MSc, Grace Yoon, RN, NNP, Ryan Sullivan RN, and Anna Agan, BA; Johns Hopkins Children's Center (Baltimore, MD): Melania Bembea, MD, MPH, and Beth White, RN; St Louis Children's Hospital (St Louis, MO): Allan Doctor, MD, Bertram Hicks, RN, and Regina Yu; Helen DeVos Children's Hospital (Grand Rapids, MI): Raja Surender, MD, and Jeni Wincek, RN, MSN; Duke Children's Hospital and Health Center (Durham, NC): David A. Turner, MD, and Samantha Tate; Children's Hospital of Nebraska (Omaha, NE): Edward Truemper, MD, and Machelle Zink, BSN, MEd; Children's Hospital at Dartmouth (Lebanon, NH): Daniel L. Levin, MD, and J. Dean Jarvis, RN; Golisano Children's Hospital (Rochester, NY): Kate Ackerman, MD, Jill Cholette, MD, and Eileen Tallie; Nationwide Children's Hospital (Columbus, OH): Mark W. Hall, MD, and Kristin Greathouse, BSN, MS; Children's Hospital at St Francis (Tulsa, OK): R. Philip Barton, MD, Kellie Brown, RN, and Christy Dallis, RN; Doernbecher Children's Hospital (Portland, OR): Aileen Kirby, MD; Penn State Hershey Children's Hospital (Hershey, PA): Neal Thomas, MD, Jill Raymond, RN, MSN, and Debra Spear, RN; Children's Hospital of Philadelphia (Philadelphia, PA): Mark Helfaer, MD, Mary Ann Diliberto, RN, and Ashleigh Clair, MS; Monroe Carell Jr Children's Hospital at Vanderbilt (Nashville, TN): Rick Barr, MD, MSc, and Pamela Berry, RN, CCRP; Dell Children's Medical Center of Central Texas (Austin, TX): Renee Higgerson, MD, LeeAnn Christie, RN, and Tamika Rhodes; University of Texas Southwestern Children's Medical Center at Dallas (Dallas, TX): Cindy Darnell, MD, and Julie Long, PhD; Texas Children's Hospital (Houston, TX): Laura L. Loftis, MD, Yvonne Kay Henry, BS, Nancy Jaimon, RN, Ursula Kyle, MS, RD/LD; University of Virginia Children's Medical Center (Charlottesville, VA): Douglas F. Willson, MD, and Christine Traul, MD; Seattle Children's Hospital (Seattle, WA): Jerry Zimmerman, MD, and Ruth Barker, RRT, NPS, CCRC; and Children's Hospital of Wisconsin (Milwaukee, WI): Rainer Gedeit, MD, Kathy Murkowski, RRT, and Kate Luther.
We thank Elizabeth Higgs, MD, and Andrea Harabin, PhD, for helpful comments on the manuscript. Anna Agan and Cathryn Oldmixon, RN, provided administrative coordination, and David Schoenfeld, PhD, provided statistical and data-management oversight. In addition, we thank all of the PALISI network participants.
Dr Randolph (principal investigator) conceived and designed the study, recruited the enrolling sites, designed the case-report forms, oversaw data acquisition and analysis, interpreted the data, drafted the manuscript, made multiple revisions, and approved the final version for submission for publication; Drs Uyeki, Rubinson, Thompson, and Rice made substantial contributions to the study conception and design, provided important input on data interpretation, revised the manuscript for important intellectual content, and approved the final version to be submitted for publication; Dr Vaughn (statistician) performed the main data analysis and interpretation, revised the manuscript for important intellectual content, and approved the final version to be submitted for publication; Mr Sullivan and Ms Yoon (study coordinators) made substantial contributions to the acquisition, analysis, and interpretation of the data, revised the manuscript for important intellectual content, and approved the final version to be submitted for publication; Ms Smoot (statistician) assisted with data acquisition and analysis, revised the manuscript for important intellectual content, and approved the final manuscript to be submitted for publication; and Drs Loftis, Helfaer, Doctor, Paden, Flori, Babbitt, Graciano, Gedeit, Sanders, Giuliano, and Zimmerman (site principal investigators) provided input on the design of the study, collected data at their sites, revised the manuscript critically for important intellectual content, and approved the final version of the manuscript before submission for publication.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the US Department of Health and Human Services.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
- pH1N1
- 2009 pandemic influenza A (H1N1)
- PALISI
- Pediatric Acute Lung Injury and Sepsis Investigators
- RR
- relative risk
- CI
- confidence interval
- IQR
- interquartile range
- MRSA
- methicillin-resistant Staphylococcus aureus
- ECMO
- extracorporeal membrane oxygenation
REFERENCES
- 1. Presanis AM, De AD, Hagy A, et al. The severity of pandemic H1N1 influenza in the United States, from April to July 2009: a Bayesian analysis. PLoS Med. 2009;6(12):e1000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Calatayud L, Kurkela S, Neave PE, et al. Pandemic (H1N1) 2009 virus outbreak in a school in London, April-May 2009: an observational study. Epidemiol Infect. 2010;138(2):183–191 [DOI] [PubMed] [Google Scholar]
- 3. Lessler J, Reich NG, Cummings DA, Nair HP, Jordan HT, Thompson N. Outbreak of 2009 pandemic influenza A (H1N1) at a New York City school. N Engl J Med. 2009;361(27):2628–2636 [DOI] [PubMed] [Google Scholar]
- 4. Shimada T, Gu Y, Kamiya H, et al. Epidemiology of influenza A(H1N1)v virus infection in Japan, May-June 2009. Euro Surveill. 2009;14(24):pii, 19244 [DOI] [PubMed] [Google Scholar]
- 5. Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet. 2010;375(9720):1100–1108 [DOI] [PubMed] [Google Scholar]
- 6. Reed C, Angulo FJ, Swerdlow DL, et al. Estimates of the prevalence of pandemic (H1N1) 2009, United States, April-July 2009. Emerg Infect Dis. 2009;15(12):2004–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hackett S, Hill L, Patel J, et al. Clinical characteristics of paediatric H1N1 admissions in Birmingham, UK. Lancet. 2009;374(9690):605. [DOI] [PubMed] [Google Scholar]
- 8. Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009;361(20):1935–1944 [DOI] [PubMed] [Google Scholar]
- 9. Larcombe PJ, Moloney SE, Schmidt PA. Pandemic (H1N1) 2009: a clinical spectrum in the general paediatric population. Arch Dis Child. 2011;96(1):96–98 [DOI] [PubMed] [Google Scholar]
- 10. Libster R, Bugna J, Coviello S, et al. Pediatric hospitalizations associated with 2009 pandemic influenza A (H1N1) in Argentina. N Engl J Med. 2010;362(1):45–55 [DOI] [PubMed] [Google Scholar]
- 11. Louie JK, Acosta M, Winter K, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302(17):1896–1902 [DOI] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention Surveillance for pediatric deaths associated with 2009 pandemic influenza A (H1N1) virus infection: United States, April-August 2009. MMWR Morb Mortal Wkly Rep. 2009;58(34):941–947 [PubMed] [Google Scholar]
- 13. Farias JA, Fernández A, Monteverde E, et al. Critically ill infants and children with influenza A (H1N1) in pediatric intensive care units in Argentina. Intensive Care Med. 2010;36(6):1015–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jouvet P, Hutchison J, Pinto R, et al. ; Canadian Critical Care Trials Group H1N1 Collaborative Critical illness in children with influenza A/pH1N1 2009 infection in Canada. Pediatr Crit Care Med. 2010;11(5):603–609 [DOI] [PubMed] [Google Scholar]
- 15. Lister P, Reynolds F, Parslow R, et al. Swine-origin influenza virus H1N1, seasonal influenza virus, and critical illness in children. Lancet. 2009;374(9690):605–607 [DOI] [PubMed] [Google Scholar]
- 16. Lockman JL, Fischer WA, Perl TM, Valsamakis A, Nichols DG. The critically ill child with novel H1N1 influenza A: a case series. Pediatr Crit Care Med. 2010;11(2):173–178 [DOI] [PubMed] [Google Scholar]
- 17. Sachedina N, Donaldson LJ. Paediatric mortality related to pandemic influenza A H1N1 infection in England: an observational population-based study. Lancet. 2010;376(9755):1846–1852 [DOI] [PubMed] [Google Scholar]
- 18. Cox CM, Blanton L, Dhara R, Brammer L, Finelli L. 2009 Pandemic influenza A (H1N1) deaths among children: United States, 2009–2010. Clin Infect Dis. 2011;52(suppl 1):S69–S74 [DOI] [PubMed] [Google Scholar]
- 19. Yung M, Slater A, Festa M, et al. ; Australia and New Zealand Intensive Care Influenza Investigators and the Paediatric Study Group and the Clinical Trials Group of the Australia New Zealand Intensive Care Society Pandemic H1N1 in children requiring intensive care in Australia and New Zealand during winter 2009. Pediatrics. 2011;127(1). Available at: www.pediatrics.org/cgi/content/full/127/1/e156 [DOI] [PubMed] [Google Scholar]
- 20. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24(5):743–752 [DOI] [PubMed] [Google Scholar]
- 22. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829 [PubMed] [Google Scholar]
- 23. Hageman JC, Uyeki TM, Francis JS, et al. Severe community-acquired pneumonia due to Staphylococcus aureus, 2003–04 influenza season. Emerg Infect Dis. 2006;12(6):894–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bhat N, Wright JG, Broder KR, et al. ; Influenza Special Investigations Team Influenza-associated deaths among children in the United States, 2003–2004. N Engl J Med. 2005;353(24):2559–2567 [DOI] [PubMed] [Google Scholar]
- 25. Dawood FS, Fiore A, Kamimoto L, et al. ; Emerging Infections Program (EIP) Network Influenza-associated pneumonia in children hospitalized with laboratory-confirmed influenza, 2003–2008. Pediatr Infect Dis J. 2010;29(7):585–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Finelli L, Fiore A, Dhara R, et al. Influenza-associated pediatric mortality in the United States: increase of Staphylococcus aureus coinfection. Pediatrics. 2008;122(4):805–811 [DOI] [PubMed] [Google Scholar]
- 27. Kallen AJ, Reed C, Patton M, Arnold KE, Finelli L, Hageman J. Staphylococcus aureus community-onset pneumonia in patients admitted to children's hospitals during autumn and winter of 2006–2007. Epidemiol Infect. 2010;138(5):666–672 [DOI] [PubMed] [Google Scholar]
- 28. Murray RJ, Robinson JO, White JN, et al. Community-acquired pneumonia due to pandemic A(H1N1)2009 influenzavirus and methicillin resistant Staphylococcus aureus co-infection. PLoS ONE. 2010;5(1):e8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reed C, Kallen AJ, Patton M, et al. Infection with community-onset Staphylococcus aureus and influenza virus in hospitalized children. Pediatr Infect Dis J. 2009;28(7):572–576 [DOI] [PubMed] [Google Scholar]
- 30. Shieh WJ, Blau DM, Denison AM, et al. 2009 pandemic influenza A (H1N1): pathology and pathogenesis of 100 fatal cases in the United States. Am J Pathol. 2010;177(1):166–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mandell LA, Wunderink RG, Anzueto A, et al. ; Infectious Diseases Society of America; American Thoracic Society Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, J Rybak M, Talan DA, Chambers HF; Infectious Diseases Society of America HYPERLINK http://www.ncbi.nlm.nih.gov/pubmed/21208910 Clinical Practice Guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011. February 1;52(3):e18–55 Epub 2011 Jan 4. Erratum in: Clin Infect Dis. 2011 Aug 1;53(3):319 [DOI] [PubMed] [Google Scholar]
- 33. Bettinger JA, Sauve LJ, Scheifele DW, et al. Pandemic influenza in Canadian children: a summary of hospitalized pediatric cases. Vaccine. 2010;28(18):3180–3184 [DOI] [PubMed] [Google Scholar]
- 34. Shin SY, Kim JH, Kim HS, et al. Clinical characteristics of Korean pediatric patients critically ill with influenza A (H1N1) virus. Pediatr Pulmonol. 2010;45(10):1014–1020 [DOI] [PubMed] [Google Scholar]
- 35. Siston AM, Rasmussen SA, Honein MA, et al. ; Pandemic H1N1 Influenza in Pregnancy Working Group Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303(15):1517–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Keren R, Zaoutis TE, Bridges CB, et al. Neurological and neuromuscular disease as a risk factor for respiratory failure in children hospitalized with influenza infection. JAMA. 2005;294(17):2188–2194 [DOI] [PubMed] [Google Scholar]
- 37. Baltagi SA, Shoykhet M, Felmet K, Kochanek PM, Bell MJ. Neurological sequelae of 2009 influenza A (H1N1) in children: a case series observed during a pandemic. Pediatr Crit Care Med. 2010;11(2):179–184 [DOI] [PubMed] [Google Scholar]
- 38. Davis LE. Neurologic and muscular complications of the 2009 influenza A (H1N1) pandemic. Curr Neurol Neurosci Rep. 2010;10(6):476–483 [DOI] [PubMed] [Google Scholar]
- 39. Ekstrand JJ, Herbener A, Rawlings J, et al. Heightened neurologic complications in children with pandemic H1N1 influenza. Ann Neurol. 2010;68(5):762–766 [DOI] [PubMed] [Google Scholar]
- 40. Lyon JB, Remigio C, Milligan T, Deline C. Acute necrotizing encephalopathy in a child with H1N1 influenza infection. Pediatr Radiol. 2010;40(2):200–205 [DOI] [PubMed] [Google Scholar]
- 41. Guarner J, Paddock CD, Shieh WJ, et al. Histopathologic and immunohistochemical features of fatal influenza virus infection in children during the 2003–2004 season. Clin Infect Dis. 2006;43(2):132–140 [DOI] [PubMed] [Google Scholar]
- 42. Baruteau AE, Boimond N, Ramful D. Myocarditis associated with 2009 influenza A (H1N1) virus in children. Cardiol Young. 2010;20(3):351–352 [DOI] [PubMed] [Google Scholar]
- 43. Haessler S, Paez A, Rothberg M, Higgins T. 2009 pandemic H1N1-associated myocarditis in a previously healthy adult. Clin Microbiol Infect. 2011;17(4):572–574 [DOI] [PubMed] [Google Scholar]
- 44. Bratincsák A, El-Said HG, Bradley JS, Shayan K, Grossfeld PD, Cannavino CR. Fulminant myocarditis associated with pandemic H1N1 influenza A virus in children. J Am Coll Cardiol. 2010;55(9):928–929 [DOI] [PubMed] [Google Scholar]
- 45. Webb SA, Pettila V, Seppelt I, et al. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009;361(20):1925–1934 [DOI] [PubMed] [Google Scholar]
- 46. Davies A, Jones D, Bailey M, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302(17):1888–1895 [DOI] [PubMed] [Google Scholar]
- 47. Domínguez-Cherit G, Lapinsky SE, Macias AE, et al. Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;302(17):1880–1887 [DOI] [PubMed] [Google Scholar]
- 48. Yu H, Liao Q, Yuan Y, et al. Effectiveness of oseltamivir on disease progression and viral RNA shedding in patients with mild pandemic 2009 influenza A H1N1: opportunistic retrospective study of medical charts in China. BMJ. 2010;341:c4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Centers for Disease Control and Prevention Update: influenza activity—United States, 2009–10 season. MMWR Morb Mortal Wkly Rep. 2010;59(29):901–908 [PubMed] [Google Scholar]
- 50. Uyeki T. Diagnostic testing for 2009 pandemic influenza A (H1N1) virus infection in hospitalized patients. N Engl J Med. 2009;361(25):e114. [DOI] [PubMed] [Google Scholar]
- 51. Ampofo K, Herbener A, Blaschke AJ, et al. Association of 2009 pandemic influenza A (H1N1) infection and increased hospitalization with parapneumonic empyema in children in Utah. Pediatr Infect Dis J. 2010;29(10):905–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Estenssoro E, Ríos FG, Apezteguía C, et al. ; Registry of the Argentinian Society of Intensive Care SATI Pandemic 2009 influenza A in Argentina: a study of 337 patients on mechanical ventilation. Am J Respir Crit Care Med. 2010;182(1):41–48 [DOI] [PubMed] [Google Scholar]
- 53. Custer JW, Rau RE. Harriett Lane Handbook: A Manual for Pediatric House Officers. 18th ed Philadelphia, PA: Elsevier Mosby; 2009 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.