Abstract
A major subtype of glutamate receptors, AMPA receptors (AMPARs), are generally thought to mediate excitation at mammalian central synapses via the ionotropic action of ligand-gated channel opening. It has recently emerged, however, that synaptic activation of AMPARs by glutamate released from the climbing fibre input elicits not only postsynaptic excitation but also presynaptic inhibition of GABAergic transmission onto Purkinje cells in the cerebellar cortex. Although presynaptic inhibition is critical for information processing at central synapses, the molecular mechanisms by which AMPARs take part in such actions are not known. This study therefore aimed at further examining the properties of AMPAR-mediated presynaptic inhibition at GABAergic synapses in the rat cerebellum. Our data provide evidence that the climbing fibre-induced inhibition of GABA release from interneurons depends on AMPAR-mediated activation of GTP-binding proteins coupled with down-regulation of presynaptic voltage-dependent Ca2+ channels. A Gi/o-protein inhibitor, N-ethylmaleimide, selectively abolished the AMPAR-mediated presynaptic inhibition at cerebellar GABAergic synapses but did not affect AMPAR-mediated excitatory actions on Purkinje cells. Furthermore, both Gi/o-coupled receptor agonists, baclofen and DCG-IV, and the P/Q-type calcium channel blocker ω-agatoxin IVA markedly occluded the AMPAR-mediated inhibition of GABAergic transmission. Conversely, AMPAR activation inhibited action potential-triggered Ca2+ influx into individual axonal boutons of cerebellar GABAergic interneurons. By suppressing the inhibitory inputs to Purkinje cells, the AMPAR-mediated presynaptic inhibition could thus provide a feed-forward mechanism for the information flow from the cerebellar cortex.
Keywords: AMPA-type glutamate receptor, cerebellum, GABAergic inhibitory synapse, presynaptic inhibition, rat
Introduction
Glutamate is a major excitatory neurotransmitter in the mammalian CNS acting through activation of ionotropic (ion-permeable) glutamate receptors (GluRs). GluRs are generally classified into the three subtypes: AMPA (α-amino-3-hydroxy-5-methylisoxasole-4-propionic acid)-, NMDA (N-methyl-d-aspartate)- and KA (kainic acid)-type GluRs (for reviews, see Ozawa et al., 1998; Dingledine et al., 1999). The main function of ionotropic GluRs is therefore to mediate postsynaptic excitation leading to rapid point-to-point signalling at central synapses. It has emerged, however, that all subtypes of ionotropic GluRs couldexert presynaptic inhibition of neurotransmitter release at many central synapses, consequently modulating synaptic strength. Activation of presynaptic KA-type GluRs in the hippocampus inhibits the release of GABA (γ-aminobutyric acid) from interneurons (Chittajallu et al., 1996; Rodríguez-Moreno et al., 1997; Kullmann, 2001). In the cerebellum, activation of presynaptic NMDA receptors in interneurons has been associated with changes in action potential-independent GABA release (Glitsch & Marty, 1999). We have recently found that activation of AMPA-type GluRs (AMPARs) by glutamate released from the climbing fibre (CF) input to the cerebellar cortex elicits not only postsynaptic excitation in Purkinje cells (PCs) but also presynaptic inhibition of GABAergic transmission from cerebellar interneurons, basket cells (BCs), that converge on PCs (Satake et al., 2000). We therefore proposed that the AMPAR-mediated dual action of direct excitation at CF–PC synapses and inhibition at BC–PC synapses serves as a feed-forward mechanism boosting the activity of PCs, the sole output from the cerebellar cortex.
While the excitatory actions of postsynaptic AMPARs have been extensively studied, little is known about mechanisms that underlie their presynaptic inhibitory actions. Determining the receptor mechanism is crucial for understanding the physiological implications of this unique action of AMPARs in the chemical signalling at central synapses. In the present study, we therefore aimed at examining how the activation of AMPARs leads to presynaptic inhibition of GABAergic transmission at BC–PC synapses in the rat cerebellum. We find that, in addition to mediating direct postsynaptic excitation in PCs, the CF transmitter induces presynaptic inhibition of GABAergic transmission through activation of AMPARs that depends on the Gi/o protein-mediated down-regulation of presynaptic voltage-gated Ca2+ channels, leading to inhibition of GABA release from cerebellar BCs. This study therefore reveals the basic molecular mechanism by which glutamate acting on AMPARs exerts direct excitation as well as disinhibition of PCs, enabling the accurate signal transfer from the CF input.
Materials and methods
Recording of inhibitory postsynaptic currents (IPSCs) from PCs, and conditioning the CF input
All experiments were carried out according with the institute guideline for animal experiments. Sagittal slices (250–300 μm thick) were prepared from the cerebellum of Wistar rats (12–18-day-old, anaesthetized with halothane) as previously described (Saitow et al., 2000). After incubation with oxygenated saline at room temperature for at least 1 h, the slices were transferred to a recording chamber mounted on the microscope stage and continuously superfused with an artificial cerebrospinal fluid (ACSF) that contained(in mm): NaCl, 138.6; KCl, 3.35; CaCl2, 2.5; MgCl2, 1.0; NaHCO3, 21.0; NaH2PO4, 0.6; and glucose, 10.0 (bubbled with 95% O2 and 5% CO2 and kept at pH 7.4). Flow rate was 0.5–1.0 mL/min, and all experiments were performed at room temperature. Membrane currents of PCs held at −40 to −20 mV were recorded in the whole-cell mode using a voltage-clamp amplifier (EPC-8, List Electronic). Patch-clamp electrodes (resistance 3–6 MΩ) were filled with an internal solution that contained (in mm): caesium methanesulphonate, 150.0; KCl, 5.0; K-EGTA, 0.1; Na-HEPES, 5.0; Mg-ATP, 3.0; and Na-GTP, 0.4 (pH adjusted to 7.4). Cerebellar interneuron-mediated IPSCs were evoked by stimulation (30 V for 100 μs) through glass electrodes filled with ACSF and placed in the molecular layer, 100–300 μm from the recorded cells. IPSCs thus recorded were completely abolished by bicuculline (10 μm), indicating that they were mediated by GABAA receptors; therefore, they were referred to as GABAA-IPSCs. Miniature IPSCs were recorded in an ACSF containing 0.5 μm tetrodotoxin (TTX) and analysed based on the detection threshold as described previously (Satake et al., 2000). Spontaneously occurring and miniature IPSCs (mIPSCs) were recorded during a constant period of 5–20 s before and after drug treatments, and detected after visual inspection of recorded traces to analyse their frequency and amplitude distributions. In some experiments, the CF input was conditioned by repetitive stimulation (5–50 Hz for 0.8–1 s) to examine CF-induced inhibition of GABAAIPSCs (Satake et al., 2000). To examine the GABAA receptor sensitivity in PCs, GABA (100 mm) was applied by iontophoresis from microelectrodes placed in the vicinity of PCs.
Monitoring intracellular Ca2+ at BC terminals
Slices were viewed through a water-immersion objective (60×, NA 0.9) of an upright microscope with a confocal laser scan head attached (Olympus, Fluo view FV300). BCs were identified using differential interference contrast (DIC) microscopy and heldin whole-cell mode. Recordings were made using the patch electrode filled with an internal solution that contained (in mm): potassium methanesulphonate, 150.0; MgCl2, 4.6; Na-HEPES, 10.0; Mg-ATP, 3.0; and Na-GTP, 0.4; supplemented with 200 μm of the Ca2+ indicator Oregon Green 488 BAPTA-1 (Molecular Probes). Approximately 20 min after loading with the indicator, fluorescence (frame mode) as well as DIC images were inspected to identify BC terminals located in the layer of PC soma. To monitor rapid Ca2+ influx into the indicator-filled terminals, the fluorescence time course was recorded in line-scan mode: the scan line was positioned across the identified locations of BC–PC synaptic contacts, and fluorescence signals were sampled at a rate of 2.5 ms per line in 1.5-s-long sweeps. BCs were held at −60 mV and stimulated by a train of three pulses (3 ms to 0 mV) to induce action potentials (escape action currents) 150 ms after the imaging onset. The Ca2+ transient amplitude was expressed as the percentage fractional change in fluorescence ΔF/F0 where F0 is the baseline fluorescence before action potential generation and ΔF = F − F0 is the incremental change in fluorescence induced by action potentials following BC stimulation.
Drug application
Drugs were applied by superfusion unless otherwise stated. The sources of drugs were as follows: (RS)-AMPA, α-methyl-4-carboxyphenylglycine (MCPG), α-cyclopropyl-4-phosphonophenylglycine (CPPG), d-(–)-2-amino-5-phosphonopentanoic acid (d-AP5), 1-aminoindan-1,5-dicarboxylic acid (AIDA), 2S,2′R,3′R-2-(2′,3′-dicar-boxycyclopropyl)glycine (DCG-IV), SCH50911, ZM241385, WIN55212-2 and AM251 (Tockris Cookson); GABA, KA, kynurenate, for skol in and ryanodine (Sigma); cyclothiazide, H-7 and bisindolylmaleimide I (Research Biochemical International); BAPTA and N-ethylmaleimide (Nacalai Tesque); TTX (Sankyo); ω-agatoxin IVA (AgTX) and ω-conotoxin GVIA (CgTX; Peptide Institute Inc). GYKI53655 was kindly provided by Eli Lilly and Company and CGP55845A by Novart is Pharma. GYKI53655, ZM241385, WIN55212-2 and AM251 dissolved in dimethyl sulfoxide (DMSO) were stored at −20 °C and diluted before experiments, at concentrations of DMSO <0.1% (v/v).
Data analysis
The paired-pulse ratio of IPSC amplitudes was calculated from the amplitude ratio of the second to the first IPSCs (interstimulus interval 80 ms) as previously described (Saitow et al., 2000; Satake et al., 2000). All the plotted data and columns in graphs represent mean ± SEM. The statistical significance of differences was examined by one-way analysis of variance or Tukey–Kramer’s parametric multiple comparison test. Differences giving P-values <0.05 were judged significant.
Results
Pharmacological profile of AMPAR-mediated disinhibition
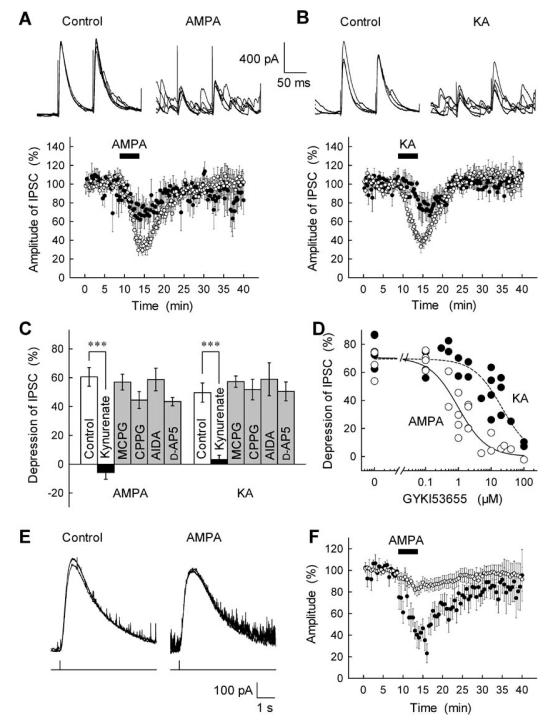
Presynaptic inhibition at hippocampal interneuron–pyramidal cell synapses has been associated with the ionotropic action of KA-type GluRs (Kullmann, 2001). Therefore, we first attempted to determine the pharmacological profile of AMPAR-dependent inhibition of GABAergic transmission at cerebellar BC–PC synapses and to compare it with the actions of KA. Application of either of the non-NMDA-type GluR agonists AMPA or KA, at concentrations of 0.3–3 μm, markedly reduced the amplitude of GABAA receptor-mediated IPSCs (GABAA-IPSCs) in PCs (Fig. 1A and B) as reported previously (Satake et al., 2000): e.g. AMPA (0.5 μm) and KA (3 μm) inhibited IPSCs by 61 ± 7% (n = 16) and 54 ± 7% (n = 16), respectively, with no changes in the IPSC kinetics [the decay time constants in control, AMPA and KA were 22 ± 1.6 (n = 20), 24 ± 2.2 (n = 10) and 22 ± 1.6 ms (n = 10), respectively]. Both AMPA- and KA-induced inhibition of GABA-IPSCs were completely abolished by the nonselective antagonist of ionotropic GluRs, kynurenate (3 mm; Fig. 1C), whereas the nonselective metabotropic GluR (mGluR) antagonist MCPG (0.5 mm), the group II/III mGluR-selective antagonist CPPG (0.5 mm), the group I mGluR antagonist AIDA (0.3 mm) and the NMDA receptor antagonist d-AP5 (50 μm) were all without significant effect (Fig. 1C). Furthermore, the AMPA-induced inhibition of IPSCs was not affected by 20 μm forskolin (Fig. 2F), which could suppress the IPSC inhibition induced by the group II/III mGluR agonist DCG-IV (Konishi & Mitoma, 1997). These observations rule out the involvement of group I and II/III mGluRs in the AMPA-induced IPSC inhibition.
Fig. 1.
Pharmacological profile of the inhibitory effects of GluR activation on GABAA-IPSCs at cerebellar BC–PC synapses. (A,B) Inhibitory actions of AMPA and KA on stimulation-evoked GABAA-IPSCs recorded from PCs. Upper traces represent superimposed successive IPSCs evoked in a PC every 15 s by paired-pulse stimulation before (control) and after bath application of AMPA (0.5 μm in A) and KA (3 μm in B). Lower graphs represent average time course and concentration-dependency of GluR agonist-induced IPSC inhibition. AMPA, 0.5 μm (○) and 0.3 μm (●) in A and KA, 3 μm (○) and 1 μm (●) in B were applied during the periods indicated by horizontal bars, and the amplitude of IPSCs was expressed as a percentage of the control determined before agonist application (n = 5–16). (C) Summary of the effects of GluR antagonists on the AMPA- and KA-induced inhibition of IPSCs. The effect of 5-min application of AMPA (0.5 μm) or KA(3 μm) on IPSCs was tested in the control solution and after each drug (kynurenate, 3 mm; MCPG, 0.5 mm; CPPG, 0.5 mm; AIDA, 0.3 mm; and d-AP5, 50 μm) was applied for at least 15 min (n = 5–10). ***P < 0.001. (D) Dose-response curves for actions of GYKI53655 (applied for at least 15 min) on the AMPA- and KA-induced IPSC inhibition. Each point on the corresponding dose of GYKI53655 represents the extent of IPSC inhibition induced by the 5-min application of AMPA (0.5 μm, open circles) or KA (3 μm, closed circles), as determined in separate experiments. (E) Effects of AMPA on GABA-induced currents in a PC. GABA was iontophoretically applied to the vicinity of the recorded PC via constant current pulses (lower trace) before (control) and after AMPA (0.5 μm) superfusion. Traces represent sample recordings from data shown in F. (F) Comparison of the effects of AMPA on the GABAA receptor sensitivity of PCs and stimulation-evoked GABAA-IPSCs. GABA currents were repeatedly induced by iontophoretic application of GABA (100 mm) at a constant interval, and IPSCs were evoked as in A. AMPA (0.5 μm) was applied by superfusion during the period indicated by a horizontal bar. The amplitudes of GABA currents (○) and stimulation-evoked IPSCs (●) were expressed as a percentage of the control determined before AMPA application (n = 10).
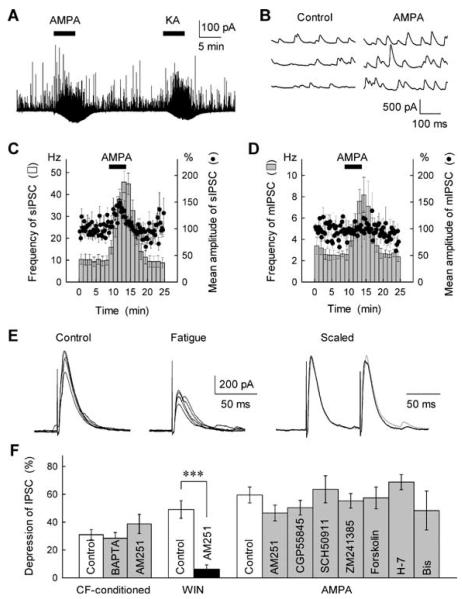
Fig. 2.
The CF stimulation- and AMPA-induced disinhibition at BC–PC synapses was independent of indirect actions of releasing retrograde inhibitory messengers or modulators. (A) Small inward currents (downward deflections) and an increase in the frequency of spontaneous IPSCs (upward deflections) in a PC following application of AMPA (0.5 μm) and KA (3 μm). (B) Successive traces of spontaneous IPSCs recorded from a PC in the control solution (left) and after 0.5 μm AMPA application (right). (C,D) Effects of AMPA on spontaneous IPSCs (sIPSCs) and mIPSCs recorded in PCs. Changes in the frequency (hatched column) and the mean amplitude (●) of sIPSCs (C, n = 14) and mIPSCs (D, n = 5) were determined before and after bath application of AMPA (0.5 μm). Recordings in D were made in the presence of TTX (0.5 μm). (E) Sample records of first IPSCs evoked by paired-pulse stimuli before (control, left) and 500 ms after repetitive stimulation of presynaptic inputs at 50 Hz for 2 s (fatigue, middle). After averaging five successive traces, the depressed first IPSC (black trace) was scaled to the control IPSC (grey trace) and superimposed, showing no significant change in the PPR after the repetitive stimulation (scaled, right). (F) Tests of possible involvements of intracellular Ca2+ increase and retrograde inhibitory messengers or modulators in the CF- and AMPA-induced disinhibition. The extents of IPSC depression induced by CF-conditioning stimulation (5 Hz for 1 s as in Fig. 3B), 1 μm WIN55212-2 (WIN, a CB1 agonist) and 0.5 μm AMPA were determined in the control solution (open columns) and after each treatment (closed columns). BAPTA (40 mm) infused into PCs from the recording electrode was without effect on the CF-dependent disinhibition. AM251 treatment (2 μm, > 20 min) selectively suppressed the effect of the CB1 agonist without significantly affecting the CF- and AMPA-induced disinhibition. The GABAB receptor antagonists CGP55845A (1 μm) and SCH50911 (50 μm) and the adenosineA2A receptor antagonist ZM241385 (10 μm) caused no discernible effects on the GluR-agonist-induced disinhibition (n = 5–13). Treatments with forskolin (20 μm), H-7 (30 μm) and bisindolylmaleimide I (Bis; 1 μm) were without effects on the AMPA-induced disinhibition (n = 5–19). ***P < 0.001.
We further determined subtypes of ionotropic non-NMDA GluRs involved in the GABAA-IPSC inhibition using two pharmacological tools. First, the AMPAR-specific antagonist GYKI53655 (Paternain et al., 1995) suppressed the AMPA-induced, but not KA-induced, inhibition of IPSCs in the concentration range 1–10 μm (Fig. 1D); the selectivity of GYKI53655 was, however, concentration-dependent, with the IC50 values of 1.0 and 21.4 μm for AMPA and KA, respectively. Second, as we reported previously, cyclothiazide, an inhibitor of AMPA receptor desensitization (Partin et al., 1993; Wong & Mayer, 1993), selectively enhanced the AMPA-induced inhibition without affecting the KA-induced inhibition (Satake et al., 2000). Taken together, these results indicate that the AMPA-induced inhibition of GABA release at BC–PC synapses is selectively mediated by activation of AMPA-type GluRs, which can be pharmacologically distinguished from the KA receptor-mediated action.
Presynaptic locus of AMPAR-mediated disinhibition
We then asked whether the GluR-mediated disinhibition involves the presynaptic, rather than postsynaptic, site of action. Both AMPA and KA, in the concentration range eliciting the inhibition of GABAA-IPSCs, produced a slight inward current (Fig. 2A) accompanied by a decrease in the input resistance, consequently resulting in a small reduction in the postsynaptic GABAA receptor sensitivity in PCs (Fig. 1E and F, open circles). However, the extent of the AMPA-induced reduction in the IPSC amplitude was far greater than that of the postsynaptic changes (Fig. 1F, closed circles), indicating that the postsynaptic actions of AMPA cannot account for the observed GluR-mediated GABAA-IPSC inhibition. To test this further, we analysed the effects of GluR agonists on the paired-pulse ratio (PPR) of stimulation-evoked IPSCs. This ratio was significantly increased by 0.5 μm AMPA (from 1.04 ± 0.04 to 1.57 ± 0.16; n = 16, P < 0.01) or 3 μm KA (from 1.05 ± 0.04 to 1.35 ± 0.12; n = 13, P < 0.05; see also sample records in Fig. 1A and B). The GluR agonists increased both the frequency and the mean amplitude of spontaneous (action potential-driven) IPSCs in PCs (Fig. 2A–C). Furthermore, neither AMPA nor KA altered the mean amplitude of TTX-insensitive mIPSCs recorded from PCs, although both agonists increased the frequency of mIPSCs (see Fig. 2D). The change is consistent with a previous report showing age-dependent up-regulation of GABA release (an increase in the mIPSC frequency) following activation of AMPARs in cerebellar interneurons (Bureau & Mulle, 1998). These observations suggest that a presynaptic mechanism for inhibiting GABA release (namely, inhibition of evoked IPSCs) mainly contributes to the AMPA- and KA-induced inhibition of GABAergic transmission at BC–PC synapses.
We next examined whether the presynaptic inhibitory action of AMPA can be explained by the fatigue of the GABA release machinery resulting from the AMPAR-induced increase in BC firing rates. To address this, we first compared the effect of AMPA application with that of high-frequency stimulation of presynaptic inputs (which would mimic the increased firing rate) on the PPR of evoked IPSCs. We found that electrical stimulation at 50 Hz for 2s in the presence of the GABAB receptor antagonist CGP55845A (1 μm) reduced the mean amplitude of GABAA-IPSCs to an extent which exceeded the effect of 0.5 μm AMPA application (decrease by 71 ± 4 vs. 61 ± 7%, n = 19 and 16, respectively; see Figs 1A and 2E). The repetitive stimulation, however, produced only a slight change in the PPR (an increase by 16 ± 10%, from 0.88 ± 0.04 before to 1.00 ± 0.08 after the repetitive stimulation, n = 19; P > 0.1, Fig. 2E), whereas the AMPA application resulted in a substantial increase in this parameter by 51 ± 13%; different from the baseline PPR at P < 0.01 (see above) and different from the effect of repetitive stimulation at P < 0.05. This finding indicates that the synaptic fatigue associated with the increased firing rate is unlikely to explain the observed changes in the GABA release probability following application of AMPA. Furthermore, when a brief pressure pulse (10 psi, 50 ms) of AMPA (2–5 μm) was focally applied through a patch pipette in the somatic vicinity of the recorded PC (the area of BC terminals), spontaneous GABAA-IPSCs in PCs were transiently depressed (by 30 ± 6%, n = 24, P < 0.001) for a period of up to 200 ms postpulse, which was followed by a slowly developing facilitation lasting several seconds (consistent with the diffusive spreading of AMPA). This depression could not be explained by the synaptic fatigue, suggesting instead a direct effect of AMPAR activation on the GABA release machinery.
AMPAR action was independent of diffusible second messengers
Next, we sought to examine whether the GluR-mediated disinhibition is elicited by a direct or indirect second messenger-mediated action of AMPA or KA: it has recently been reported that the retrograde messengers released from postsynaptic neurons, in particular endocannabinoids, suppress GABAergic inhibitory transmission via activation of presynaptic CB1 receptors at cerebellar and hippocampal synapses (Ohno-Shosaku et al., 2001; Wilson & Nicoll, 2001; Yoshida et al., 2002). Previously it has been shown that buffering free Ca2+ in the postsynaptic cell with 40 mm BAPTA abolishes this retrograde signalling, whereas 10 mm BAPTA was almost ineffective (Glitsch et al., 2000). Because we previously tested the effect of 10 mm BAPTA on the GluR-mediated disinhibition (Satake et al., 2000), we loaded cells with 40 mm BAPTA in the present study. This, however, failed to alter the CF stimulation-induced inhibition of GABAA-IPSCs (Fig. 2F, see also Fig. 3D), thus ruling out the involvement of postsynaptic Ca2+-dependent retrograde messengers. To address the involvement of presynaptic cannabinoid receptors more directly, we incubated slices with the CB1 antagonist AM251 (2 μm) (Wilson & Nicoll, 2001); this treatment had no effect on the CF- and AMPA-induced inhibition of IPSCs (Fig. 2F). This negative result could not be explained simply by an incomplete action of the drug in slice preparations, because AM251 virtually abolished the inhibitory action of WIN55212-2 (1 μm), an exogenous CB1 agonist (Ohno-Shosaku et al., 2001; Wilson & Nicoll, 2001; Yoshida et al., 2002), on GABAA-IPSCs (Fig. 2F).
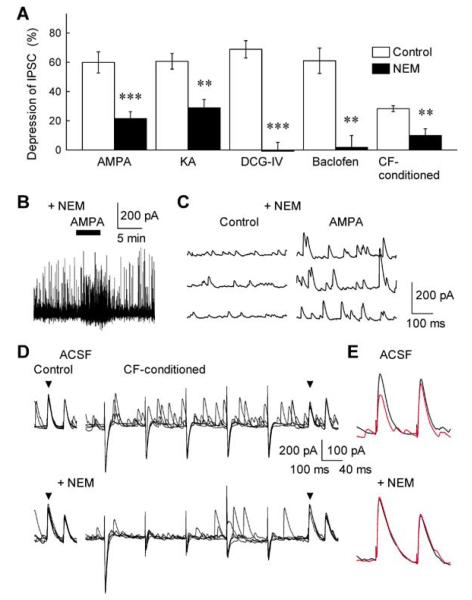
Fig. 3.
Possible involvement of Gi/o proteins in the GluR- and CF stimulation-induced inhibition of cerebellar GABAergic transmission. (A) Effects of NEM, a Gi/o protein inhibitor, on GABAA-IPSC depression induced by the GluR agonist AMPA and KA, the Gi/o protein-coupled receptor agonist DCG-IV and baclofen, and CF conditioning-stimulation. The extents of IPSC depression during 5-min applications of AMPA (0.5 μm), KA (3 μm), DCG-IV (0.5 μm) and baclofen (5 μm) were determined in the control ACSF (open column) and after treatment with 250 μm NEM (closed column) (n = 5–8). The CF-dependent IPSC depression was induced by conditioning stimulation of CF at 5–50 Hz for 0.8–1 s (n = 10). ***P < 0.001, **P < 0.01. (B) AMPA-induced inward current in a PC and enhancement of sIPSCs after the treatment with NEM. AMPA (0.5 μm) produced inward currents in PCs: mean amplitude, 108 ± 31 before and 109 ± 36 pA after the NEM treatment, respectively (n = 10, P > 0.9). (C) sIPSCs recorded in a PC before (control) and during 0.5 μm AMPA application after the treatment with NEM. Note that both the AMPA-induced inward current and increase in sIPSCs remained unaltered after the NEM treatment in B and C. (D,E) Sample records of successive control IPSCs (left) and CF-conditioned (5–50 Hz for 0.8–1 s) IPSCs (right) recorded in a PC in the control ACSF (upper traces) and after treatment with 250 μm NEM (lower traces). Each arrowhead indicates the peak of first IPSCs evoked by test paired-pulse stimuli. In E, averaged control (black trace) and CF-conditioned GABAA-IPSCs (red trace) were superimposed with an expanded trace scale. The NEM treatment suppressed the CF-dependent disinhibition without significantly affecting IPSCs and CF-induced EPSCs (downward responses).
Previously, it has been reported that KA receptor activation inhibits GABAergic transmission between hippocampal CA1 interneurons and pyramidal neurons through an indirect action of releasing GABA from interneurons that results in activation of presynaptic GABAB auto-receptors (Frerking et al., 1999). However, this possibility was unlikely to contribute significantly to the phenomenon studied here because application of AMPA or KA still inhibited stimulus-evoked GABAA-IPSCs recorded in PCs after treatments with GABAB receptor antagonists CGP55845A, 1 μm, (Davies et al., 1993; Yamada et al., 1999) and SCH50911, 50 μm, (Frerking et al., 1999) (see Fig. 2F). Moreover, AMPA and KA inhibited the IPSCs in the presence of an adenosineA2A receptor antagonist, ZM241385, 10 μm,(Poucher et al., 1995) (Fig. 2F) and mGluR antagonists including MCPG, CPPG and AIDA (Fig. 1C). Therefore, these data strongly suggest that the GluR agonists trigger a direct receptor-mediated mechanism of presynaptic inhibition of GABAergic transmission at BC–PC synapses rather than acting indirectly through a secondary diffusible modulator.
AMPAR-mediated disinhibition depended on G-protein-coupled signalling
We then examined the mechanisms by which GluR activation inhibits GABA release from cerebellar interneurons. Although it has been suggested that AMPA/KA-type GluRs in cortical and hippocampal neurons activate G-protein-dependent intracellular signalling cascades (Wang et al., 1997; Rodríguez-Moreno & Lerma, 1998; Frerking et al., 2001; Marin et al., 2001), the precise mechanisms are not fully understood (see Kullmann, 2001 for a review). We therefore attempted to investigate the signalling mechanism that underlies the GluR-dependent presynaptic inhibition at cerebellar inhibitory synapses. To address whether the AMPA/KA receptor-mediated disinhibition at BC–PC synapses also involves G-protein activation, we first tested the effects of N-ethylmaleimide (NEM), a Gi/o protein blocker (Asano & Ogasawara, 1986). When cerebellar slices were treated with NEM (250 μm) for 5–10 min, the AMPA/KA-induced inhibition of GABAA-IPSCs was markedly suppressed (Fig. 3A), with no effect on the inward current responses produced in PCs by the GluR agonists (see Figs 2A and 3B; n = 10, P > 0.5). The observation points to the involvement of Gi/o proteins in the GluR-mediated inhibition of IPSCs at BC–PC synapses. This notion was further supported by the finding that the NEM treatment also abolished GABAA-IPSC inhibition induced by the two authentic Gi/o-coupled receptor activators, DCG-IV, an mGluR2/3 agonist, and baclofen, a GABAB receptor agonist (Fig. 3A). While the NEM treatment virtually abolished the actions of DCG-IV and baclofen on IPSCs, its effect on the AMPA-induced disinhibition was incomplete. The remaining inhibitory action of AMPA after the NEM treatment (≈ 20%) was probably due to the postsynaptic action on GABA receptor sensitivity (see Fig. 1F). In contrast, NEM had no significant effects on IPSC inhibition induced by the Gq-coupled mGluR1/5 agonist (RS)-3,5-dihydroxyphenylglycine (DHPG), confirming the specificity of NEM treatment on Gi/o-dependent synaptic processes (H. Kubota, unpublished observations).
In addition to the inhibition of stimulation-evoked GABAA-IPSCs by AMPA or KA, the GluR agonists increased the frequency of spontaneous IPSCs recorded in PCs (see Fig. 2A and B). However, not only the AMPA-induced facilitatory action but also the AMPA-evoked in ward currents were unaffected by the NEM treatment (Fig. 3B and C), although NEM by itself increased the occurrence of synaptic responses: AMPA (0.5 μm) increased the frequency of spontaneous IPSCs, both in the control solution and after treatment of NEM, by 576 ± 94 (n = 14) and 135 ± 18%, respectively (n = 8, see Figs 2C and 3B), and there was no significant change in the AMPA (0.5 μm)-induced inward currents in PCs (the mean amplitudes of 108 ± 31 before and 109 ± 36 pA after the NEM treatment, respectively; n = 10, P > 0.9). Thus, the exogenous GluR agonists appear to elicit two distinct actions on GABAergic transmission at BC–PC synapses: (i) the NEM-sensitive G-protein-coupled inhibition of GABA release and (ii) the NEM-insensitive (and therefore ionotropic) excitation in cerebellar interneurons, resulting in the increase in spontaneous IPSCs in PCs.
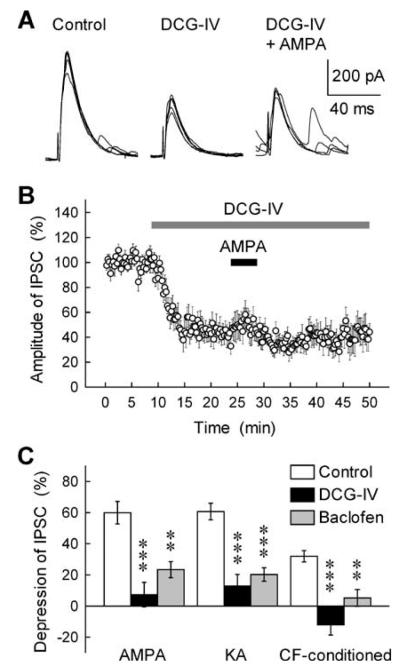
Although manipulations with exogenous GluR agonists indicated a link from AMPA/KA-type receptors to the G-protein-dependent signalling cascade, it was important to establish whether a similar mechanism would be evoked by synaptic release of glutamate. Figure 3D and E shows that the NEM treatment did suppress the CF stimulation-induced inhibition of GABAA-IPSCs, with no significant effect on either stimulus-evoked IPSCs or CF-induced excitatory postsynaptic currents (EPSCs) per se. These results suggest that both endogenous and exogenous GluR agonists act on GABA release by activating the Gi/o protein-coupled intracellular signalling pathway. This prompted us to test the hypothesis that the AMPA/KA-type GluRs and typical G-protein-coupled receptors would share the same mechanisms downstream receptor activation. This hypothesis was examined in the experiment illustrated in Fig. 4. The Gi/o-coupled receptor agonist DCG-IV (0.1 μm) and baclofen (3 μm) not only suppressed the GABAA-IPSC (see Figs 3A and 4A) but also resulted in almost complete occlusion of the AMPA/KA- and CF stimulation-induced disinhibition (Fig. 4A–C). This result provides independent evidence for the possible involvement of G-protein-dependent pathway in the AMPA/KA receptor-mediated disinhibition.
Fig. 4.
Occlusion of the GluR- and CF stimulation-induced disinhibition by the mGluR2/3 agonist DCG-IV and the GABAB receptor agonist baclofen. (A) Successive traces of GABAA-IPSCs evoked every 15 s in a PC were super-imposed in control solution (left), and in the presence of DCG-IV (0.1 μm) (middle), and both DCG-IV (0.1 μm) and AMPA (0.5 μm) (right). (B) DCG-IV (0.1 μm) and AMPA (0.5 μm) were applied during the periods indicated by horizontal bars, and the amplitude of IPSCs was expressed as a percentage of the control determined before the DCG-IV application (n = 7). (C) The inhibitory effects of AMPA (0.5 μm), KA (3 μm) and CF-conditioning stimulation (5–50 Hz for 0.8–1 s) on GABAA-IPSCs were determined in control solution (open column), and in the presence of DCG-IV (0.1 μm) (closed column) and baclofen (3 μm) (stippled column) (n = 5–11). ***P < 0.001, **P < 0.01.
AMPAR activation inhibited presynaptic Ca2+ influx into individual BC terminals
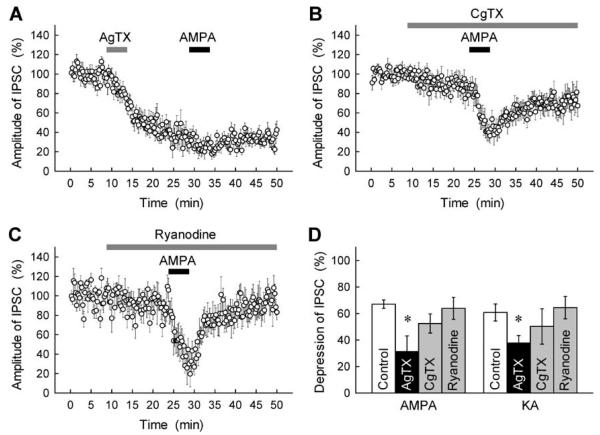
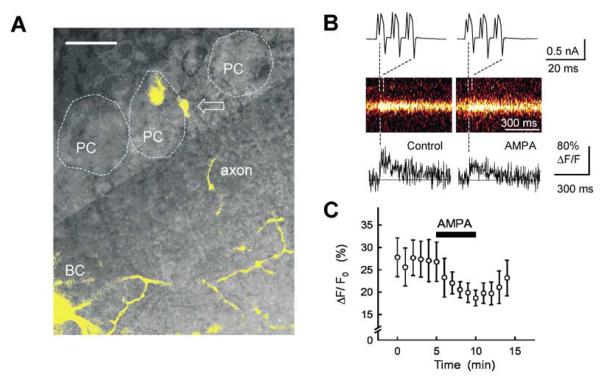
We next sought to determine which signalling pathways are linked to the GluR-mediated G-protein activation. One potential candidate is the pathway that involves voltage-dependent Ca2+ channels (VDCCs), because neurotransmitter-activated G-proteins have been shown to modulate the functional state of VDCCs (Zamponi & Snutch, 1998; Ikeda & Dunlap, 1999). To address this, we examined whether Ca2+ channel antagonists would affect the GluR-mediated inhibition of GABAergic transmission. The P/Q-type VDCC antagonist AgTX (0.1 μm) effectively suppressed GABAA-IPSCs at BC–PC synapses (Fig. 5A), whereas the N-type VDCC antagonist CgTX (1 μm) showed only a slight blocking action (Fig. 5B) (see also Forti et al., 2000; Stephens et al., 2001). The latter was not due to incomplete action of CgTX, because this blocker depressed EPSCs evoked in PCs by activation of parallel fibres (inhibition by 45 ± 5%, n = 7). with AgTX markedly abolished the AMPA-induced Treatment inhibition of GABAA-IPSCs (Fig. 5A and D). In contrast, the inhibitory action of AMPA remained almost unaltered after treatment with CgTX (Fig. 5B and D). This result suggests that AMPAR-mediated G-protein signalling is negatively coupled to P/Q-type VDCCs, thereby inhibiting GABA release from presynaptic terminals of BCs. This hypothesis was further supported by the experiments in which we imaged action potential-evoked Ca2+ transients in individual axonal boutons of BCs (Fig. 6A and B; also Forti et al., 2000): presynaptic Ca2+ entry triggered in response to BC action potentials was significantly reduced by application of 0.5 μm AMPA (Fig. 6B and C). The time-course and spatial localization of axonal Ca2+ transients evoked by BC action potentials were similar to those shown in the previous study by Forti et al. (2000).
Fig. 5.
Involvement of inhibition of P/Q-type VDCCs in the AMPA/KA-induced inhibition at BC–PC synapses. (A–C) Effects of VDCC antagonists and ryanodine on GABAA-IPSCs and the GluR agonist-induced disinhibition. The amplitude of IPSCs evoked as in Fig. 1A was expressed as percentage of the control determined before application of each drug. AgTX (0.1 μm, A), CgTX (1 μm, B), ryanodine (100 μm, C) and AMPA (0.5 μm) were applied during the periods indicated by horizontal bars. (D) Summary data for the effects of VDCC antagonists and ryanodine on the IPSC inhibition induced by 5-min application of AMPA (0.5 μm) and KA (3 μm)(n = 5–10). *P < 0.05.
Fig. 6.
Inhibitory effect of AMPA on action potential-induced fast Ca2+ influx in presynaptic BC terminals. (A) A confocal fluorescence image of BC superimposed on a differential inference contrast image. A single BC (in yellow) was visualized after loading with the Ca2+ indicator Oregon Green 488 BAPTA-1 (0.2 mm) via the patch electrode. Gray shade separates the molecular layer from the PC layer; dotted profiles indicate three PC bodies and an arrow shows one axonal varicosity adjacent to a PC body across which a scanning line was positioned to monitor Ca2+ transients. (B) Action potential-triggered Ca2+ transients in a single axonal bouton of the BC. Three depolarization pulses in a BC elicited action potentials (escape action currents, upper panel), which evoked Ca2+-dependent fluorescence transients at the imaged varicosity (original scans in middle panel, and scan profiles in lower panel); indicative examples for control (left panels) and in the presence of 0.5 μm AMPA (right panels). (C) The average time course of changes in evoked-Ca2+ transients (measured as an incremental signal normalized by the baseline level, ΔF/F0). AMPA (0.5 μm) was applied during the period indicated by the horizontal bar. Average ΔF/F0 at the end of AMPA application was 78 ± 10% of baseline ΔF/F0 immediately before the initiation of application (n = 8, P < 0.05). Scale bar, 20 μm.
The AMPA-induced inhibition of IPSCs was not affected by the Ca2+ store modulator ryanodine (100 μm; Fig. 5C and D), the adenylyl cyclase activator forskolin (20 μm) or the cell-permeable protein kinase inhibitor H-7 (30 μm) and bisindolylmaleimide I (1 μm) (Fig. 2F; also Rodríguez-Moreno et al., 2000), ruling out the involvements of intracellular Ca2+ stores (Llano et al., 2000; Fill & Copello, 2002) and protein kinase-dependent cascades (Rodríguez-Moreno & Lerma, 1998; Rodríguez-Moreno et al., 2000) in the GluR-mediated disinhibition at BC–PC synapses.
Discussion
The present results suggest that, in addition to acting on ionotropic GluRs to produce the postsynaptic excitation, the excitatory transmitter released by the CF elicits presynaptic inhibition of GABAergic transmission via activation of AMPA-type GluRs that involves, either directly or indirectly, G-protein-mediated metabotropic activity. Our data are consistent with the previous biochemical evidence showing that the GluR1 subunit of AMPA receptors could interact with Gαi1 and activate Gi proteins, leading to inhibition of the forskolin-stimulated activity of adenylyl cyclase in cortical neurons (Wang et al., 1997). More recently, it has also been reported that KA-type GluRs exhibit metabotropic actions at hippocampal inhibitory synapses where their activation depresses GABAergic transmission through a G-protein-dependent phospholipase C–protein kinase C cascade (Rodríguez-Moreno & Lerma, 1998). In the present study, however, the AMPA/KA-induced inhibition of GABAA-IPSC at the BC–PC synapse was not affected by treatments with protein kinase inhibitors H-7 and bisindolylmaleimide I, or the adenylyl cyclase activator forskolin (Fig. 2F). This is in parallel with the previous study showing that KA receptor activation at hippocampal excitatory synapses inhibits glutamate release via a mechanism that depends on G-proteins but does not require protein kinases (Frerking et al., 2001). Thus, it appears that GluR-activated G-proteins are coupled with distinct and multiple signalling cascades at excitatory and inhibitory synapses.
GABAergic transmission has been shown to undergo modulation through the actions of multiple neurotransmitters and modulators. Among them is the retrograde messenger endocannabinoid, which diffuses from postsynaptic neurons acting on presynaptic terminals to inhibit GABA release in the hippocampus (Ohno-Shosaku et al., 2001; Wilson & Nicoll, 2001) and the cerebellar cortex (Diana et al., 2002; Yoshida et al., 2002). Moreover, GABA released following KA receptor activation from hippocampal interneurons depresses GABAergic transmission through an indirect action on presynaptic GABAB auto receptors (Frerking et al., 1999). However, the AMPA/KA- and CF-stimulation induced IPSC inhibition at cerebellar BC–PC synapses was unaffected by BAPTA infusion into the postsynaptic neuron PCs or by pharmacological manipulations with the antagonists for CB1, GABAB, adenosine receptors and mGluRs (Figs 1C and 2F; Satake et al., 2000). Furthermore, it has recently been reported that stimulation of Bergmann glia induces a long-lasting depression of GABAergic transmission via activation of ionotropic GluRs (Brockhaus & Deitmer, 2002). The onset and time course of this glia-mediated modulation of GABAergic synapses are clearly different from those of the CF- and AMPAR-mediated inhibition of GABAA-IPSCs described here. Therefore, our findings suggest that the activation of AMPARs by synaptically released glutamate as well as exogenous GluR agonists directly inhibits GABA release at cerebellar inhibitory synapses without recruiting secondary inhibitory modulators.
One alternative explanation for the observed IPSC depression is an increase in the ambient level of GABA following the AMPA-induced enhancement of interneuron activity. The latter could in principle inhibit the GABA release machinery by activating presynaptic GABAB receptors (see, e.g., Frerking et al., 1999). However, this mechanism would imply a positive correlation between the network activity of interneurons and the extent of IPSC depression, which was not the case as indicated by our data from the test of high frequency stimulation in presynaptic inputs (Fig. 2E). Furthermore, the AMPA-dependent increase in ambient GABA could not explain the rapid transient depression of IPSCs following a local application of AMPA.
As for the role of G-proteins in GluR-mediated presynaptic inhibition, it remains to be determined precisely whether the inhibition of GABA release is a direct consequence of AMPAR-mediated G-protein activation or associated with another mechanism that is under the tonic control of G-proteins and affected by AMPAR activation. The marked occlusion of actions mediated by AMPARs and G-protein-coupled receptors, which we observed at BC–PC synapses (Fig. 4), makes the latter possibility less likely. A candidate mechanism underlying the GluR-mediated inhibition of GABA release at the BC–PC synapse is that the βγ subunits of G-proteins (Gβγ) dissociated by AMPA/KA receptor activation may inhibit the activity of P/Q-type Ca2+ channels in nerve terminals of cerebellar interneurons. Modulation of presynaptic VDCCs by Gβγ may serve as a dominant mechanism of presynaptic inhibition at various central synapses (Zamponi & Snutch, 1998; Ikeda & Dunlap, 1999).
Our data from the experiments using the subtype-specific toxins showed that: (i) the P/Q-type Ca2+ channel mainly contributes to stimulation-evoked GABA release from cerebellar BC terminals (Forti et al., 2000; Stephens et al., 2001), and (ii) the AMPA/KA-induced inhibition of GABA release was occluded by the P/Q-type-specific blocker AgTX (Fig. 5). Therefore, the observation that AMPA inhibited the Ca2+ transients elicited by action potentials in GABAergic interneuron terminals (Fig. 6) strongly supports the notion that P/Q-type Ca2+ channels (which normally occur in axons rather than dendrites) serve as a target of the GluR-mediated G-protein activation. It is also possible that AMPAR activation could inhibit presynaptic Na+ spikes and consequently suppress Ca2+ influx into BC terminals. In any case, the suppression by AMPAR activation of evoked Ca2+ influx into presynaptic axonal varicosities would explain the inhibition of GABA release observed in this study. To further substantiate this hypothesis, it would be important to investigate whether GluRs occur in nerve terminals of cerebellar GABAergic interneurons and whether their inhibitory actions are specific to their axonal, rather than dendritic, location.
What is the physiological significance of the CF- and AMPAR-mediated disinhibition in the cerebellar function? The CF stimulation mimicked every aspect of the exogenous AMPA application-induced disinhibitory action on GABAergic transmission at BC–PC synapses in the cerebellar cortex (the present study and Satake et al., 2000). Thus, we propose that glutamate released from the CF input activates ‘ionotropic’ AMPARs eliciting postsynaptic excitation at CF–PC synapses and‘metabotropic’ AMPARs mediating presynaptic inhibition at BC–PC inhibitory synapses, this consonant action ensuring the cerebellar output from the PC. In hippocampal cell culture, AMPAR subunits GluR1 and GluR2 are expressed and organized in functional receptors in axonal terminals of hippocampal neurons (Schenk et al., 2003). Recent studies have also shown that functional AMPARs that occur in central terminals of the dorsal root ganglion neurons could inhibit glutamate release from primary afferent terminals in the rat spinal cord (Lee et al., 2002; Lu et al., 2002). It is therefore conceivable that the GluR-mediated presynaptic inhibition depending on G-protein-coupled VDCC regulation represents a mode of the gain control of neurotransmission which is widely distributed to excitatory and inhibitory synapses in the mammalian CNS. This possibility awaits further scrutiny.
Acknowledgements
We thank D. M. Kullmann, T. Murakoshi and H. Suzuki for comments on the manuscript, I. Nishizaki for technical assistance, and Eli Lilly and Company and Novartis Pharma for the generous gifts of GYKI53655 and CGP55845A. S.K. is a research director of CREST, JST (Core Research for Evolutional Science and Technology, Japan Science and Technology Corporation).
Abbreviations
- ACSF
artificial cerebrospinal fluid
- AgTX
ω-agatoxin IVA
- AIDA
1-aminoindan-1,5-dicarboxylic acid
- AMPA
α-amino-3-hydroxy-5-methylisoxa-sole-4-propionic acid
- AMPAR
AMPA receptor
- BC
basket cell
- CF
climbing fibre
- CgTX
ω-conotoxin GVIA
- CPPG
α-cyclopropyl-4-phosphonophenylglycine
- d-AP5
d-(–)-2-amino-5-phosphonopentanoic acid
- DCG-IV
(2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine
- DHPG
(RS)-3,5-dihydroxyphenylglycine
- DMSO
dimethyl sulfoxide
- EPSC
excitatory postsynaptic current
- GABA
γ-aminobutyric acid
- Gβγ, βγ subunit of G-protein
GluR
- glutamate receptor
IPSC
- inhibitory postsynaptic current
KA
- kainic acid
MCPG, α-methyl-4-carboxyphenylglycine
- mGluR
metabotropic glutamate receptor
- mIPSC
miniature IPSC
- NEM
N-ethylmaleimide
- NMDA
N-methyl-d-aspartate
- PC
Purkinje cell
- PPR
paired-pulse ratio
- TTX
tetrodotoxin
- VDCC
voltage-dependent Ca2+ channel.
References
- Asano T, Ogasawara N. Uncoupling of γ-aminobutyric acid B receptors from GTP-binding proteins by N-ethylmaleimide: Effect of N-ethylmaleimide on purified GTP-binding proteins. Mol. Pharmacol. 1986;29:244–249. [PubMed] [Google Scholar]
- Brockhaus J, Deitmer JW. Long-lasting modulation of synaptic input to Purkinje neurons by Bergmann glia stimulation in rat brain slices. J. Physiol. (Lond.) 2002;545:581–593. doi: 10.1113/jphysiol.2002.028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau I, Mulle C. Potentiation of GABAergic synaptic transmission by AMPA receptors in mouse cerebellar stellate cells: changes during development. J. Physiol. (Lond.) 1998;509:817–831. doi: 10.1111/j.1469-7793.1998.817bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Vignes M, Dev KK, Barnes JM, Collingridge GL, Henley JM. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature. 1996;379:78–81. doi: 10.1038/379078a0. [DOI] [PubMed] [Google Scholar]
- Davies CH, Pozza MF, Collingridge GL. CGP55845A: a potent antagonist of GABAB receptors in the CA1 region of rat hippocampus. Neuropharmacology. 1993;32:1071–1073. doi: 10.1016/0028-3908(93)90073-c. [DOI] [PubMed] [Google Scholar]
- Diana MA, Levenes C, Mackie K, Marty A. Short-term retrograde inhibition of GABAergic synaptic currents in rat Purkinje cells is mediated by endogenous cannabinoids. J. Neurosci. 2002;22:200–208. doi: 10.1523/JNEUROSCI.22-01-00200.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol. Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol. Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- Forti L, Pouzat C, Llano I. Action poteintial-evoked Ca2+ signals and calcium channels in axons of developing rat cerebellar interneurones. J. Physiol. (Lond.) 2000;527:33–48. doi: 10.1111/j.1469-7793.2000.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerking M, Petersen CCH, Nicoll RA. Mechanisms underlying kainate receptor-mediated disinhibition in the hippocampus. Proc. Natl Acad. Sci. USA. 1999;96:12917–12922. doi: 10.1073/pnas.96.22.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerking M, Schmitz D, Zhou Q, Johansen J, Nicoll RA. Kainate receptors depress excitatory synaptic transmission at CA3-CA1 synapses in the hippocampus via a direct presynaptic action. J. Neurosci. 2001;21:2958–2966. doi: 10.1523/JNEUROSCI.21-09-02958.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch M, Marty A. Presynaptic effects of NMDA in cerebellar Purkinje cells and interneurons. J. Neurosci. 1999;19:511–519. doi: 10.1523/JNEUROSCI.19-02-00511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch M, Parra P, Llano I. The retrograde inhibition of IPSCs in rat cerebellar Purkinje cells is highly sensitive to intracellular Ca2+ Eur. J. Neurosci. 2000;12:987–993. doi: 10.1046/j.1460-9568.2000.00994.x. [DOI] [PubMed] [Google Scholar]
- Ikeda SR, Dunlap K. Voltage-dependent modulation of N-type calcium channels: role of G protein subunits. Adv. Second Messenger Phosphoprotein Res. 1999;33:131–151. doi: 10.1016/s1040-7952(99)80008-1. [DOI] [PubMed] [Google Scholar]
- Konishi S, Mitoma H. Cyclic AMP-dependent and independent mechanisms for presynaptic modulation of GABAergic transmission in the cerebellar cortex. In: Okada Y, editor. The Role of Adenosine in the Nervous System. Elsevier Science BV; Amsterdam: 1997. pp. 89–95. [Google Scholar]
- Kullmann DM. Presynaptic kainate receptors in the hippocampus: slowly emerging from obscurity. Neuron. 2001;32:561–564. doi: 10.1016/s0896-6273(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Lee CJ, Bardoni R, Tong C-K, Engelman HS, Joseph DJ, Magherini PC, MacDermott AB. Functional expression of AMPA receptors on central terminals of rat dorsal root ganglion neurons and presynaptic inhibition of glutamate release. Neuron. 2002;35:135–146. doi: 10.1016/s0896-6273(02)00729-8. [DOI] [PubMed] [Google Scholar]
- Llano I, Gonzalez J, Caputo C, Lai FA, Blayney LM, Tan YP, Marty A. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat. Neurosci. 2000;3:1256–1265. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- Lu C-R, Hwang SJ, Phend KD, Rustioni A, Valtschanoff JG. Primary afferent terminals in spinal cord express presynaptic AMPA receptors. J. Neurosci. 2002;22:9522–9529. doi: 10.1523/JNEUROSCI.22-21-09522.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin P, Fagni L, Torrens Y, Alcaraz G, Couraud F, Bockaert J, Glowinski J, Prémont J. AMPA receptor activation induces association of G-beta protein with alpha subunit of the sodium channel in neurons. Eur. J. Neurosci. 2001;14:1953–1960. doi: 10.1046/j.0953-816x.2001.01827.x. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Prog. Neurobiol. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Partin KM, Patneau DK, Winters CA, Mayer ML, Buonanno A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron. 1993;11:1069–1082. doi: 10.1016/0896-6273(93)90220-l. [DOI] [PubMed] [Google Scholar]
- Paternain AV, Morales M, Lerma J. Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron. 1995;14:185–189. doi: 10.1016/0896-6273(95)90253-8. [DOI] [PubMed] [Google Scholar]
- Poucher SM, Keddie JR, Singh P, Stoggall SM, Caulkett PWR, Jones G, Collis MG. The in vitro pharmacology of ZM 241385, a potent, non-xanthine, A2a selective adenosine receptor antagonist. Br. J. Pharmacol. 1995;115:1096–1102. doi: 10.1111/j.1476-5381.1995.tb15923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Moreno A, Herreras O, Lerma J. Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron. 1997;19:893–901. doi: 10.1016/s0896-6273(00)80970-8. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Moreno A, Lerma J. Kainate receptor modulation of GABA release involves a metabotropic function. Neuron. 1998;20:1211–1218. doi: 10.1016/s0896-6273(00)80501-2. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Moreno A, López-García JC, Lerma J. Two populations of kainate receptors with separate signaling mechanisms in hippocampal interneurons. Proc. Natl Acad. Sci. USA. 2000;97:1293–1298. doi: 10.1073/pnas.97.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitow F, Satake S, Yamada J, Konishi S. β-Adrenergic receptor-mediated presynaptic facilitation of inhibitory GABAergic transmission at cerebellar interneuron-Purkinje cell synapses. J. Neurophysiol. 2000;84:2016–2025. doi: 10.1152/jn.2000.84.4.2016. [DOI] [PubMed] [Google Scholar]
- Satake S, Saitow F, Yamada J, Konishi S. Synaptic activation of AMPA receptors inhibits GABA release from cerebellar interneurons. Nat. Neurosci. 2000;3:551–558. doi: 10.1038/75718. [DOI] [PubMed] [Google Scholar]
- Schenk U, Verderio C, Benfenati F, Matteoli M. Regulated delivery of AMPA receptor subunits to the presynaptic membrane. EMBO J. 2003;22:558–568. doi: 10.1093/emboj/cdg059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens GJ, Morris NP, Fyffe REW, Robertson B. The Cav2.1/α1A (P/Q-type) voltage-dependent calcium channel mediates inhibitory neurotransmission onto mouse cerebellar Purkinje cells. Eur. J. Neurosci. 2001;13:1902–1912. doi: 10.1046/j.0953-816x.2001.01566.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Small DL, Stanimirovic DB, Morley P, Durkin JP. AMPA receptor-mediated regulation of a Gi-protein in cortical neurons. Nature. 1997;389:502–504. doi: 10.1038/39062. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Wong LA, Mayer ML. Differential modulation by cyclothiazide and concanavallin A of desensitization at native α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid- and kainate-preferring glutamate receptors. Mol. Pharmacol. 1993;44:504–510. [PubMed] [Google Scholar]
- Yamada J, Saitow F, Satake S, Kiyohara T, Konishi S. GABAB receptor-mediated presynaptic inhibition of glutamatergic and GABAergic transmission in the basolateral amygdala. Neuropharmacology. 1999;38:1743–1753. doi: 10.1016/s0028-3908(99)00126-4. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Hashimoto K, Zimmer A, Maejima T, Araishi K, Kano M. The cannabinoid CB1 receptor mediates retrograde signals for depolarization-induced suppression of inhibition in cerebellar Purkinje cells. J. Neurosci. 2002;22:1690–1697. doi: 10.1523/JNEUROSCI.22-05-01690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi GW, Snutch TP. Modulation of voltage-dependent calcium channels by G proteins. Curr. Opin. Neurobiol. 1998;8:351–356. doi: 10.1016/s0959-4388(98)80060-3. [DOI] [PubMed] [Google Scholar]