Abstract
BACKGROUND:
Inhaled nitric oxide (iNO) is an effective therapy for pulmonary hypertension and hypoxic respiratory failure in term infants. Fourteen randomized controlled trials (n = 3430 infants) have been conducted on preterm infants at risk for chronic lung disease (CLD). The study results seem contradictory.
DESIGN/METHODS:
Individual-patient data meta-analysis included randomized controlled trials of preterm infants (<37 weeks' gestation). Outcomes were adjusted for trial differences and correlation between siblings.
RESULTS:
Data from 3298 infants in 12 trials (96%) were analyzed. There was no statistically significant effect of iNO on death or CLD (59% vs 61%: relative risk [RR]: 0.96 [95% confidence interval (CI): 0.92–1.01]; P = .11) or severe neurologic events on imaging (25% vs 23%: RR: 1.12 [95% CI: 0.98–1.28]; P = .09). There were no statistically significant differences in iNO effect according to any of the patient-level characteristics tested. In trials that used a starting iNO dose of >5 vs ≤5 ppm there was evidence of improved outcome (interaction P = .02); however, these differences were not observed at other levels of exposure to iNO. This result was driven primarily by 1 trial, which also differed according to overall dose, duration, timing, and indication for treatment; a significant reduction in death or CLD (RR: 0.85 [95% CI: 0.74–0.98]) was found.
CONCLUSIONS:
Routine use of iNO for treatment of respiratory failure in preterm infants cannot be recommended. The use of a higher starting dose might be associated with improved outcome, but because there were differences in the designs of these trials, it requires further examination.
Keywords: inhaled nitric oxide, chronic lung disease, respiratory disease, preterm infants, individual-patient data meta-analysis
Approximately 8% to 13% of infants in developed countries are born prematurely. Preterm delivery accounts for 75% to 80% of all neonatal morbidity and mortality.1,2 Although survival rates have improved markedly in recent decades, preterm infants who require assisted ventilation are still at significant risk of both pulmonary and cerebral injury.
An estimated 63% of infants with a birth weight of <1000 g develop respiratory distress syndrome, and nearly 40% are still oxygen-dependent at a postmenstrual age of 36 weeks.3 The most common definition of chronic lung disease (CLD) is a condition that requires the continued receipt of supplemental oxygen at 36 weeks' postmenstrual age. Infants with severe CLD remain at high risk for pulmonary morbidity and mortality during their first 2 years of life.4 In addition, long-term neurodevelopmental impairments associated with cerebral palsy, mental retardation, sensorineural hearing loss, and visual impairment are more frequently observed in infants with CLD than in preterm infants without this complication.5,6 The incidence of these neurodevelopmental impairments increases with decreasing birth weight. Neonates with birth weights of 1501 to 2500 g have an 8% incidence, compared with a 25% rate in infants who are born at <1000 g.7
Inhaled nitric oxide (iNO) has been hypothesized as a treatment for preventing lung injury in preterm infants. Although initially investigated for its pulmonary vasodilating effect, it has become clear that the potential pulmonary effects of iNO are multiple and complex. Studies of a variety of animal models have addressed the effects and mechanisms of iNO on lung development and injury related to bronchopulmonary dysplasia (BPD). These effects include a decrease in airway resistance (in piglet and lamb models), which translates into a decreased need for supplemental oxygen and ventilatory support and presumably results in less oxidative stress8–10 and more normal development and alveolarization in iNO-treated premature lambs. iNO treatment also attenuates hyperoxic injury in lambs.11 In a preterm baboon model of BPD, iNO therapy from birth improves endogenous surfactant function as well as lung growth, angiogenesis, and alveolarization.12,13 Endothelial NO synthase–deficient mice have very deficient lung growth under conditions of hypobaric hypoxia, and inhaled NO treatment can completely restore normal lung structure in this model.14 In infant rats and premature baboons, hyperoxic exposure impairs microvascular development and reduces vascular endothelial growth factor (VEGF) expression.15,16 Lung growth in rats is also impaired by administration of an inhibitor of VEGF receptor, an effect that is attenuated by iNO treatment, which supports the concept that VEGF regulates alveolar growth, in part, via NO.17 Prematurity in the baboon,18 and presumably in the human, is associated with developmentally deficient endogenous NO production; accordingly, iNO in this situation could be viewed as replacement therapy. Thus, animal data from a number of models indicate that NO is required for normal lung development and suggest that replacement iNO therapy, over a period of weeks, is beneficial in the injured lung, particularly for vascular and air-space development.
Fourteen randomized controlled trials (total N = 3430 infants) have been conducted on preterm infants to determine if iNO reduces the rates of death and/or CLD.19–32 The most recent Cochrane review, published in 2010, included the same studies.33 These 14 studies differed not only in their design, intervention, and indications but also in the eligible patient populations. The latest version of the Cochrane review revealed no effect on death or CLD at 36 weeks (relative risk [RR]: 0.93 [95% confidence interval (CI): 0.86–1.01]) in the subset of studies with routine use of iNO in intubated preterm infants with some heterogeneity. The trials of early treatment of infants that were based on oxygenation criteria or of later enrollment based on the risk of CLD did not reveal significant benefit of iNO for the primary end point of death or CLD at 36 weeks when analyzed according to standard aggregate data meta-analytic techniques. However, there is significant heterogeneity in the results; some trials have reported benefit, and others have revealed no effect.
One way in which to confirm or refute these results and determine if certain patient or treatment characteristics might predict benefit from iNO in premature infants is by undertaking an individual-patient data (IPD) meta-analysis. Such analysis involves the central collection and reanalysis of line-by-line raw data from each randomly assigned participant in each of the included trials. The advantages of an IPD meta-analysis over meta-analysis based on aggregate data include ensuring uniformity in defining patient characteristics and outcome measures including subgroup definitions; the ability to adjust the analyses for the nonindependence of siblings within the data set; and the opportunity to collect information on longer-term outcomes. To date, this methodology has been underused for addressing neonatal questions.34
Thus, the objectives of this IPD meta-analysis were to determine if iNO in preterm infants who receive assisted ventilation improves survival rates without morbidity, specifically without CLD or major neurologic injury, and if the effects of iNO differ according to patient- or intervention-related factors including gestational age at birth, birth weight, multiplicity, race, antenatal steroid use, postnatal age at the time of randomization, severity of illness (in terms of oxygenation index [OI]), patent ductus arteriosus, pulmonary hypertension, postnatal steroids, ventilation mode at randomization, administration of exogenous surfactant, iNO dosage, and duration of administration.
METHODS
Studies were considered eligible for this IPD meta-analysis if they randomly assigned preterm infants (<37 weeks' gestation) who were receiving respiratory support (either mechanical ventilation or continuous positive airway pressure) to either an iNO or control group. Bibliographic databases (including Medline, Embase, Cochrane Controlled Trials Register, and PAS abstracts) were searched to identify potentially eligible trials up to December 2009.
The investigators of each identified eligible trial were contacted and invited to join the collaborative group. The collaborative group agreed on a prespecified protocol that outlined the data items to be collected, the outcomes to be assessed, and the data-analysis plans, including those of primary, secondary, additional, subgroup, and sensitivity analyses.35 Trialists who agreed to participate supplied line-by-line raw data for each individual randomly assigned patient, and these data were checked for missing information, errors, and inconsistencies with published reports. Main outcomes were calculated for each patient to align with the prespecified and agreed-on definitions as indicated in the protocol.35 To date, only short-term outcome data have been sought from the collaborators.
Binary outcomes were analyzed by using log-binomial regression with trial as a fixed effect in the model to account for the variation across trials. The log-binomial model has an advantage in that the inverse log of the parameter estimate for treatment effect is an RR; thus, all results are presented as RRs with 95% CIs.
All data available for each end point were included when possible and analyzed according to the intention-to-treat principle. Data from entire trials were excluded from analyses when zero cell counts resulted in model instability/nonconvergence. For subgroup analyses, the breakdown of data within small trials into further subgroups resulted in greater instability when events were few. In these situations, the modified Poisson regression framework with robust error variances was used.36
The possibility of correlation in outcomes between siblings from multiple births was accounted for by using the multiple-outputations method on 1000 repetitions.37 As a sensitivity analysis, generalized estimating equations were used to adjust for sibling correlation on the main outcomes and provided almost identical results to those of the multiple-outputations method. Differences in treatment effect across predefined subgroups of patients were tested by examining the treatment-by-subgroup interaction effect. Additional details of the planned analyses have been published elsewhere.35
There were 2 primary end points: death or CLD and severe adverse neurologic events after randomization. Death or CLD was defined as death during the trial or CLD (receipt of supplemental oxygen at 36 weeks' postmenstrual age). If CLD at 36 weeks was unable to be calculated, the trialists' own definition of CLD was used. Severe adverse neurologic events (assessed by imaging) comprised intraventricular hemorrhage grade III or IV, cystic periventricular leukomalacia, or other pathologies such as periventricular echodensity, periventricular cysts, ventriculomegaly, or hydrocephalus, if such events first occurred after randomization.
Two sided P values of <.05 were considered statistically significant. No adjustments were made for multiple comparisons. Results were considered for groups of related outcomes and individually such that no single result was considered in isolation. Any result that showed a significant effect when related outcomes did not was interpreted cautiously. All analyses were completed by using SAS 9.2 (SAS Institute, Inc, Cary, NC).
RESULTS
Ninety-six percent of published worldwide data were made available to the collaboration by the trialists, which resulted in 3298 infants from 11 trials being available for the analysis. Details of the included trials are listed in Tables 1 and 2. Not all trials were able to supply data for all end points analyzed. However, the first primary end point of death or CLD was calculable for all patients in all trials.
TABLE 1.
Protocol Specifications of Included Trials
| Study (Year) | Enrolled Patients, N | Protocol Specifications |
|||||
|---|---|---|---|---|---|---|---|
| Gestational Age, wk | Postnatal Age at Randomization | iNO Dose, ppm | Weaning Protocol, ppma | Planned Duration of Exposure | Gas Continued After Extubation? | ||
| Subhedar et al28 (1997) | 42 | <32 | >96 h | 20→5b | — | 72 h | iNO not continued after extubation |
| Kinsella et al25 (1999) | 80 | <34 | <7 d | 5b | — | 7–14 d | iNO not continued after extubation |
| Srisuparp et al27 (2002) | 34 | <32 | <72 h | 20→0b | — | 72 h to 7 d | iNO not continued after extubation |
| Schreiber et al26 (2003) | 207 | <34 | <72 h | 10→5a | 10 (1 d), 5 (6 d) | 7 d | In infants who were extubated within 7 d, gas was stopped 1 h before extubation |
| Field et al (INNOVO)23 (2005) | 126 | <34 | <28 d | 5→40b | — | 48 h to 3 d | iNO stopped before extubation |
| Van Meurs et al29,30 (2005 and 2007) | 449 | <34 | >4 h | 5→10b | — | <14 d | iNO not continued after extubation |
| Hascoet et al22 (2005) | 145 | <32 | 6–48 h | 5→10b | — | Up to the time when a/Ao2 increases to >0.22 (median: 28 h) | iNO not continued after extubation |
| Dani et al21 (2006) | 40 | <30 | <7 d | 10→6a | 10 (4 h), 6 (until extubation) | 81 h | iNO not continued after extubation |
| Kinsella et al24 (2006) | 793 | <34 | <48 h | 5a | No change | 21 d | iNO stopped before extubation |
| Ballard et al19 (2006) | 582 | <32 | 7–21 d | 20→2a | 20 (48–96 h), 10, 5, 2 at weekly intervals | 24 d minimum | iNO continued after extubation and after discontinuation of nasal CPAP ± oxygen |
| Mercier et al (EUNO)31 (2010) | 800 | <29 | <72 h | 5a | No change | 7–21 d | iNO continued for 7 d or until extubation, whichever came later |
| Total | 3298 | — | — | — | — | — | — |
a-Ao2 indicates alveolar-arterial oxygen gradient; CPAP, continuous positive airway pressure.
No dose change or change based on prespecified weaning protocol with fixed time points.
Dose changes based on measured biological response in patient.
TABLE 2.
Additional Details of Included Trials
| Trial | Control-Group Therapy | Randomization Method | Intervention Masking | Enrollment Criteria for Preterm Infants | Exclusion Criteria |
|---|---|---|---|---|---|
| Subhedar et al28 (1997) | Conventional management ± dexamethasone (2 × 2 factorial design) | Sealed envelopes | Unmasked | Mechanically ventilated; received surfactant therapy; considered high risk for developing BPD as defined by a modified prediction score | Major congenital anomaly, structural cardiac defect, or significant ductal shunting; culture-positive sepsis, IVH with parenchymal involvement; pulmonary or gastrointestinal hemorrhage; disordered coagulation or thrombocytopenia (platelets < 50) |
| Kinsella et al25 (1999) | No gas delivered (sham monitoring) | Sealed opaque envelopes | Masked | Severe hypoxemia defined as arterial to alveolar Po2 ratio of <0.1 on 2 successive blood gas measurements in the first 7 d of life despite mechanical ventilation and surfactant treatment | Fatal congenital anomalies or congenital heart disease (except atrial and ventricular septal defects) |
| Srisuparp et al27 (2002) | No treatment | Card-picking scheme | Unmasked | Birth weight between 500 and 2000 g; received surfactant; had clinical RDS that required mechanical ventilation; were <72 h of age with OIs that exceeded birth weight–specific criteria and had a systemic arterial catheter | Major congenital abnormalities (except PDA and/or foramen ovale) or hydrops fetalis |
| Schreiber et al26 (2003) | Oxygen | Masked | Masked | Birth weight of <2000 g; receiving ventilation for RDS | Major congenital malformations or hydrops fetalis |
| Field et al (INNOVO)23 (2005) | No treatment | Telephone | Masked | Severe respiratory failure that required assisted ventilation if the responsible physician was uncertain about whether an infant might benefit from iNO | Uncorrectable bleeding disorders; cerebral ultrasound evidence of intraparenchymal lesions (Papille grade IV); contraindication to continuation of intensive care (eg, severe congenital abnormalities or lethal chromosomal anomaly) |
| Van Meurs et al29,30 (2005 and 2007) | Placebo (simulated flow) | Telephone | Masked | Diagnosis of RDS, sepsis, or pneumonia, aspiration syndrome, idiopathic persistent pulmonary hypertension, or suspected pulmonary hypoplasia; birth weight between 401 and 1500 g29 or >1500 g30; received assisted ventilation at least 4 h after surfactant therapy and considered at high risk of death or BPD according to OI | Congenital heart disease other than ventricular septal defect, atrial level shunt, or PDA; any major congenital abnormality that involved the respiratory system, thrombocytopenia, or bleeding diathesis or a decision not to provide full treatment |
| Hascoet et al22 (2005) | Placebo | Call-in telephone system | Unmasked | Intubated with hypoxic respiratory failure criteria, defined as the need for mechanical ventilation; Fio2 > 0.4 and a/Ao2 ratio < 0.22 | Refractory hypoxemia, defined as Po2 < 50 and Pco2 < 50 mm Hg for Fio2 = 1.0, thrombocytopenia or major fetal abnormality |
| Dani et al21 (2006) | No treatment | Sealed opaque envelopes | Unmasked | Ventilated with severe respiratory distress; an Fio2 of >0.5 and an a/Ao2 ratio of <0.15 despite surfactant treatment | Major congenital anomaly; hydrops fetalis; thrombocytopenia; bleeding disorder |
| Kinsella et al24 (2006) | Nitrogen | Masked | Masked | Respiratory failure that required mechanical ventilation and birth weight between 500 and 1250 g | Lethal congenital abnormalities or congenital heart disease; active pulmonary hemorrhage; unevacuated pneumothorax; expected duration of ventilation of <48 h |
| Ballard et al19 (2006) | Nitrogen | Central randomization | Masked | Birth weight 500 to 1250 g; receiving mechanical ventilation for lung disease (not apnea) between 7 and 21 d of age; infants with a birth weight of 500–799 g who were being treated with nasal CPAP were also eligible | Life-threatening conditions such as complex congenital abnormalities, bilateral grade IV IVH, or previous iNO treatment |
| Mercier et al (EUNO)31 (2010) | Placebo | Centralized interactive Web-based randomization system | Masked | Birth weight ≥ 500 g and required surfactant within 24 h of birth or CPAP (Fio2 of ≥0.3, on mean airway pressure of at least 4 cm H2O) within 24 h of birth to maintain an oxygen saturation of ≥85% | Required Fio2 of >0.5 to maintain oxygen saturation at >85% on a sufficient mean airway pressure (eg, >8 cm H2O on intermittent mandatory ventilation) to achieve adequate lung expansion 2 h after administration of exogenous surfactant; had substantial congenital heart disease (other than PDA), lung hypoplasia, or abnormal hemostasis; had substantial congenital disorders such that full treatment was not indicated |
IVH indicates intraventricular hemorrhage; RDS, respiratory distress syndrome; PDA, patent ductus arteriosus; Fio2, fraction of inspired oxygen; a/Ao2, alveolar-arterial oxygen gradient; CPAP, continuous positive airway pressure.
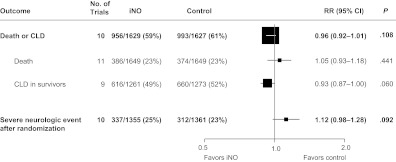
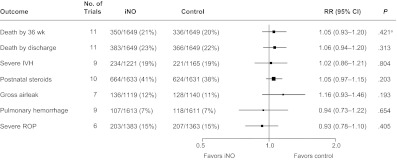
Overall, death or CLD occurred in 59% of iNO-treated infants versus 61% of control infants (RR: 0.96 [95% CI: 0.92–1.01]; P = .11). Severe neurologic events revealed by imaging occurred after random assignment in 25% of infants in the iNO group compared with 23% of infants in the control group (RR: 1.12 [95% CI: 0.98–1.28]; P = .09) (Fig 1). There were no statistically significant differences between iNO and control for any of the secondary outcomes (Fig 2).
FIGURE 1.
Primary outcomes. All P > .05 (χ2 test for heterogeneity). RRs, CIs, and P values were derived from 1000 iterations of a log-binomial model using the multiple-outputation method.
FIGURE 2.
Secondary outcomes. RRs, CIs, and P values were derived from 1000 iterations of a log-binomial model using the multiple-outputation method. a χ2 test for heterogeneity: P = .04; all other P > .05. IVH indicates intraventricular hemorrhage; ROP, retinopathy of prematurity.
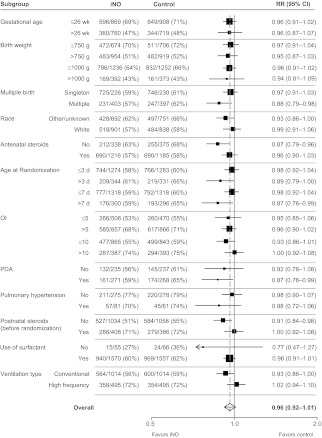
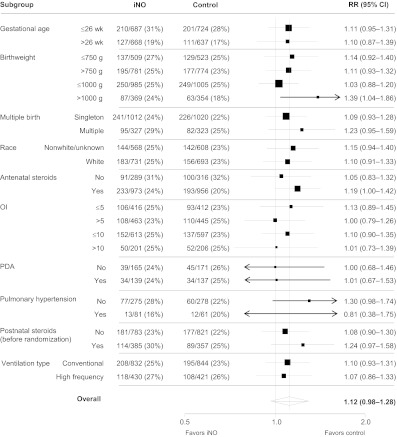
There were no statistically significant differences in iNO effect for either of the primary end points according to any of the patient-level characteristics tested in subgroup analyses, as can be seen in Figs 3 and 4; all P values for the subgroup-by-treatment interaction tests were >.05. Posthoc analyses were undertaken to assess any treatment effect modification based on grouping infants according to both birth weight (≤750 or >750 g and ≤1000 or >1000 g) and OI (≤5 or >5 and ≤10 or >10) categories for the primary end points. The interaction term for the treatment-by-birth weight–OI categories was not significant in any of these analyses (P values ranged from .08 to .85, data not shown). Hence, we did not find evidence that the effect of iNO differed significantly between infants in different birth weight/illness-severity categories.
FIGURE 3.
Death or CLD according to subgroup. All P > .05 for subgroup-by-treatment interaction effects. Estimates were derived from 1000 iterations of a Poisson regression model with robust error variance using the multiple-outputation method. PDA indicates patent ductus arteriosus.
FIGURE 4.
Severe neurologic events according to subgroup. All P > .05 for subgroup-by-treatment interaction effects. Estimates were derived from 1000 iterations of a Poisson regression model with robust error variance using the multiple-outputation method. PDA indicates patent ductus arteriosus.
Study protocols varied between trials for increasing or decreasing concentration of the study drug, and the trialists were interested in investigating the effect of increasing dose. For some trials22,23,25,27–29 (Table 1), the dose of iNO received depended on some measured response variable in the infant. For these trials the dose effect could not be compared on an individual-patient level, because dose was the result of individual response and not the cause. Trials also differed according to the planned length of iNO duration and whether the gas was stopped or continued after extubation (Table 1).
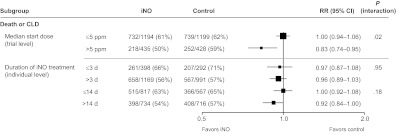
Analysis of the dose effect was based on trial classifications as well as classifying individual patients using a limited number of trials (Fig 5). Trials were classified on the basis of starting dose, categorized as >5 ppm or not. The RR of treatment effect on death or CLD in the lower-starting-dose group was significantly different than the RR in the higher-starting-dose group (RR: 1.00 vs 0.83, respectively; P = .02 for interaction).
FIGURE 5.
Death or CLD according to dosage. Estimates were derived from 1000 iterations of a Poisson regression model with robust error variance using the multiple-outputation method.
The actual duration of treatment received was calculated for individual patients, and 2 cut points for classification of shorter or longer duration were used (≤3 vs >3 days and ≤14 vs >14 days). There was no significant difference in the effect of treatment according to duration of treatment received (Fig 5).
For the end point of severe neurologic events as assessed in the short-term, fewer data were available because the major high-dose trial (Ballard [2006]19) did not supply this end point. This was because one of the entry criterion for infants in this trial was an age older than 7 days when the effect of iNO on intraventricular hemorrhage would be expected to be less relevant.
Trials differed in many respects. Although all trials were randomized, not all of them concealed the treatment allocation after randomization. Inclusion criteria for entry into the trials also differed greatly, which resulted in some trials with a population at higher risk than others. For specific information on trial differences, including inclusion/exclusion criteria, see Tables 1 and 2 (see also Table 2 in the published protocol35).
DISCUSSION
The results of this IPD meta-analysis revealed no benefit for the routine early use of iNO in preterm infants receiving respiratory support (either mechanical ventilation or continuous positive airway pressure). Although within some individual subgroups there were suggestions of significant benefits, this result was likely mainly due to selection of particular trials with the relevant information. On the basis of treatment-by-subgroup interaction tests for differences between subgroups, there was no clear evidence that iNO was more or less effective for any particular subgroup of preterm patients. For example, infants born at lower gestational ages or with a higher OI were no more or less likely to benefit from iNO than other infants.
We used IPD meta-analysis to determine if the age at which iNO was commenced and the dose and duration given made a difference for the effect of the treatment. Subgroup analyses based on age at random assignment (as a surrogate for age when iNO was commenced) did not show a statistically significant difference in infants who started the study gas earlier versus later (using either a 3- or 7-day cut point). However, both results were in the same direction and of similar magnitude, which suggests a possible trend to greater benefit when iNO was used later in the neonatal course (Fig 3).
The effect of iNO dosage was more difficult to explore. Because this study was not a prospective IPD meta-analysis,38 the included studies varied widely in their intended iNO dose, including starting dose, response criteria, and weaning protocols. For trials in which the dose of iNO received depended on some measured response variable in the infant22,23,25,27–29 (Table 1), the dose effect could not be compared on an individual-patient level.
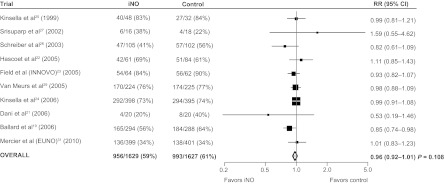
Results of the analysis of the planned trial start dose did suggest more benefit in the higher-dose subgroup when a >5-ppm cut point was used (interaction P = .02) for both death and CLD (Fig 5) and severe adverse neurologic events (data not shown). However, it is difficult to draw firm conclusions from these results, because trials that started with a low dose tended to use protocols that increased dose on the basis of response, whereas trials that started on a higher dose used protocols that reduced the dose over time. The 1 trial that specifically enrolled infants who remained at high risk of BPD after 7 days of age and used a high iNO starting dose of 20 ppm (Ballard et al19) did find a statistically significant reduction in the instance of death or CLD (RR: 0.85 [95% CI: 0.74–0.98]) (Fig 6). This trial also differed in ways other than dose, so it is possible that the difference in effect seen was a result of a combination of dose, timing, and patient selection. Further study is required to properly assess the effect of iNO exposure (dose and duration) in this population, and such a study should include appropriately powered trials that specifically test different dosing regimes.39,40 Three such trials,41–43 conducted by members of the Meta-analysis of Preterm Patients on Inhaled Nitric Oxide (MAPPiNO) collaboration, are known to be underway, and there is the possibility of updating our current results with further information from these trials when available.
FIGURE 6.
Death or CLD according to trial. All P > .05 (χ2 test for heterogeneity). Estimates were derived from 1000 iterations of a log-binomial model using the multiple-outputation method.
Although the planned analyses reported here were prespecified in an agreed-on protocol, the availability of the IPD meant that many comparisons were possible and, with that, the increased possibility of a type 1 error. Hence, these results should be interpreted in their totality rather than focusing on isolated findings within the data set.
We accounted for the possible correlation of outcomes between siblings from multiple births and the significant proportion of siblings within the data set (13%) by using the multiple-outputations method and conducted sensitivity analyses by using generalized estimating equations as well as no adjustment. All 3 methods obtained almost identical results. The best method for both randomly assigning siblings and accounting for the possible correlation in their outcomes remains unresolved. We hope to use the MAPPiNO data set to investigate this issue further in the future.
There were some differences between this IPD meta-analysis (12 trials, N = 3298) and the results reported in the corresponding latest Cochrane review33 (14 trials, N = 3430) and another recent meta-analysis on this topic.44 The investigators of a small trial (N = 65) by Su and Chen (2008)32 could not be contacted by the MAPPiNO group; thus, their trial was not included in the IPD meta-analysis, but aggregate data were included in the 2010 Cochrane review. The 1999 Franco-Belgium Collaborative NO Trial Group (N = 85)20 were unable to supply IPD for inclusion in our meta-analysis, but aggregate data from this trial were included in the Cochrane review. In the published trials available to the Cochrane review authors, subgroups were defined in variable ways depending on the study, often either according to birth weight or gestational age, and with varying cut points for these subgroups. The only way to accurately classify infants within particular subgroups was by sourcing the actual data for each individual participant, as was done in our IPD meta-analysis. Because analyses undertaken within the Cochrane review software cannot allow for regression modeling or adjustment for sibling correlation, results for some trials were different between the 2 analyses. For these reasons we believe that our IPD meta-analysis should be considered the more robust analysis.
The formation of a collaborative group, which was required to undertake an IPD meta-analysis, resulted in benefits in itself. The opportunity for all trialists interested in iNO treatment to meet and discuss the differences between trials led to a greater understanding by all the collaborators of the potential mechanisms of effect and reasons for variation in trial designs and results.
CONCLUSIONS
The results of this IPD meta-analysis of all available worldwide data indicate that routine use of iNO for treatment of respiratory failure in preterm infants cannot be recommended. The use of a higher starting dose might be associated with improved outcome, but because there were differences in the designs of the trials included in the analyses, it requires further examination. Further planned research by the MAPPiNO Collaborative group includes collection and analysis of longer-term follow-up outcome data, predictive modeling, and methodologic work regarding the best methods for accounting for multiples within neonatal trials and meta-analyses.
IPD meta-analysis offers considerable benefits when addressing neonatal treatments and should be used more widely.
ACKNOWLEDGMENTS
Ikaria Inc provided funding for the project via an unrestricted grant administered through the University of California, San Diego. The company had no input into the design, conduct, analysis, or publication decisions related to this project. Dr Kinsella was supported by NIH/NCRR Colorado CTSI grant UL1 RR025780 and NHLBI grant U01HL064857.
In addition to the named MAPPiNO authors, the following people have contributed to the success of the collaboration: Angela E. Carberry (NHMRC Clinical Trials Centre, University of Sydney, Sydney, Australia), Wei Lei (NHMRC Clinical Trials Centre), Kylie Hunter (NHMRC Clinical Trials Centre), Polly Hardy (London School of Hygiene and Tropical Medicine, London, United Kingdom), James Carpenter (London School of Hygiene and Tropical Medicine), Elizabeth Williamson (Murdoch Childrens Research Institute, Melbourne, Australia), Gabriel-Bennewitz Raquel (University of Chicago, Chicago, IL), Alessandra Cecchi (Department of Surgical and Medical Critical Care, Section of Neonatology, Careggi University Hospital of Florence, Florence, Italy), Ivana Brajkovic (University of Chicago), Kitty Perritt (Research Triangle Institute International, Research Triangle Park, NC), Abhik Das (Research Triangle Institute International), James S. Baldassarre (Ikaria Inc, Hampton, NJ), Brahm Goldstein (Ikaria Inc), Olek Czepla (Ikaria Inc), Joe Young (Ikaria Inc), Dezheng Z. Huo (Health Studies, University of Chicago), William Truog (University of Missouri, Kansas City School of Medicine, Kansas City, MO), and Dennis Black (University of California, San Francisco, CA).
This meta-analysis has been registered at www.anzctr.org.au (registration No. ACTRN12609000859280).
FINANCIAL DISCLOSURE: Dr Ballard received an unrestricted grant from Ikaria Inc for studies on bronchopulmonary dysplasia but received no salary support; Dr Kinsella served as a consultant for Ikaria Inc in the past 3 years; Prof Mercier received minor fees and traveling support for 3 Ikaria Inc/iNO Therapeutics advisory boards in the United States and a research grant from iNO Therapeutics, which completed the research funds obtained from the French Ministry of Health to study the effect of iNO on the developing brain in rat pups; Dr Schreiber received research grant support from Ikaria Inc for investigator-initiated research studies; Dr Subhedar was a steering-committee member of the European Inhaled Nitric Oxide Registry, which is funded through an educational grant from Linde Gas (Sweden); and Dr VanMeurs is the site principal investigator for the Ikaria Inc trial entitled “(IK-3001-BPD-301) Inhaled Nitric Oxide for the Prevention of Bronchopulmonary Dysplasia (BPD) in Preterm Infants Requiring Mechanical Ventilation or Positive Pressure Support on Days 5–14 after birth.” The other authors have indicated they have no financial relationships relevant to this article to disclose.
COMPANION PAPER: A companion to this article can be found on page 775, and online at www.pediatrics.org/cgi/doi/10.1542/peds.2011-2190.
- NO
- nitric oxide
- CLD
- chronic lung disease
- iNO
- inhaled nitric oxide
- BPD
- bronchopulmonary dysplasia
- RR
- relative risk
- CI
- confidence interval
- IPD
- individual-patient data
- OI
- oxygenation index
- MAPPiNO
- Meta-analysis of Preterm Patients on Inhaled Nitric Oxide
REFERENCES
- 1. Australian and New Zealand Neonatal Network Report of the Australian and New Zealand Neonatal Network 2006. Sydney, Australia: Australian and New Zealand Neonatal Network; 2009 [Google Scholar]
- 2. Furdon SA, Clark DA. Prematurity. Available at: http://emedicine.medscape.com/article/975909-overview Accessed March 16, 2010
- 3. Fanaroff AA, Stoll BJ, Wright LL, et al. ; NICHD Neonatal Research Network Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196(2):147.e1–147.e8 [DOI] [PubMed] [Google Scholar]
- 4. Bhandari A, Bhandari V. Bronchopulmonary dysplasia: an update. Indian J Pediatr. 2007;74(1):73–77 [DOI] [PubMed] [Google Scholar]
- 5. Schmidt B, Asztalos E, Roberts R, Robertson C, Sauve R, Whitfield M. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the Trial of Indomethacin Prophylaxis in Preterms. JAMA. 2003;289(9):1124–1129 [DOI] [PubMed] [Google Scholar]
- 6. Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR. The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed. 2005;90(2):F134–F140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bennett FC, Scott DT. Long-term perspective on premature infant outcome and contemporary intervention issues. Semin Perinatol. 1997;21(3):190–201 [DOI] [PubMed] [Google Scholar]
- 8. Bland R, Albertine K, Carlton D, MacRitchie A. Inhaled nitric oxide effects on lung structure and function in chronically ventilated preterm lambs. Am J Respir Crit Care Med. 2005;172(7):899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamon I, Fresson J, Nicolas MB, Buchweiller MC, Franck P, Hascoet JM. Early inhaled nitric oxide improves oxidative balance in very preterm infants. Pediatr Res. 2005;57(5 pt 1):637–643 [DOI] [PubMed] [Google Scholar]
- 10. Martin RJ, Mhanna MJ, Haxhiu MA. The role of endogenous and exogenous nitric oxide on airway function. Semin Perinatol. 2002;26(6):432–438 [DOI] [PubMed] [Google Scholar]
- 11. Cotton RB, Sundell HW, Zeldin DC, et al. Inhaled nitric oxide attenuates hyperoxic lung injury in lambs. Pediatr Res. 2006;59(1):142–146 [DOI] [PubMed] [Google Scholar]
- 12. McCurnin DC, Pierce RA, Chang LY, et al. Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. Am J Physiol Lung Cell Mol Physiol. 2005;288(3):L450–L459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ballard PL, Gonzales LW, Godinez RI, et al. Surfactant composition and function in a primate model of infant chronic lung disease: effects of inhaled nitric oxide. Pediatr Res. 2006;59(1):157–162 [DOI] [PubMed] [Google Scholar]
- 14. Balasubramaniam V, Maxey AM, Morgan DB, Markham NE, Abman SH. Inhaled NO restores lung structure in eNOS-deficient mice recovering from neonatal hypoxia. Am J Physiol Lung Cell Mol Physiol. 2006;291(1):L119–L127 [DOI] [PubMed] [Google Scholar]
- 15. Lin YJ, Markham NE, Balasubramaniam V, et al. Inhaled nitric oxide enhances distal lung growth after exposure to hyperoxia in neonatal rats. Pediatr Res. 2005;58(1):22–29 [DOI] [PubMed] [Google Scholar]
- 16. Maniscalco WM, Watkins RH, Pryhuber GS, Bhatt A, Shea C, Huyck H. Angiogenic factors and alveolar vasculature: development and alterations by injury in very premature baboons. Am J Physiol Lung Cell Mol Physiol. 2002;282(4):L811–L823 [DOI] [PubMed] [Google Scholar]
- 17. Tang JR, Markham NE, Lin YJ, et al. Inhaled nitric oxide attenuates pulmonary hypertension and improves lung growth in infant rats after neonatal treatment with a VEGF receptor inhibitor. Am J Physiol Lung Cell Mol Physiol. 2004;287(2):L344–L351 [DOI] [PubMed] [Google Scholar]
- 18. Shaul PW, Afshar S, Gibson LL, et al. Developmental changes in nitric oxide synthase isoform expression and nitric oxide production in fetal baboon lung. Am J Physiol Lung Cell Mol Physiol. 2002;283(6):L1192–L1199 [DOI] [PubMed] [Google Scholar]
- 19. Ballard RA, Truog WE, Cnaan A, et al. ; NO CLD Study Group Inhaled nitric oxide in preterm infants undergoing mechanical ventilation [published correction appears in N Engl J Med. 2007;357(14):1444–1445]. N Engl J Med. 2006;355(4):343–353 [DOI] [PubMed] [Google Scholar]
- 20. The Franco-Belgium Collaborative NO Trial Group Early compared with delayed inhaled nitric oxide in moderately hypoxaemic neonates with respiratory failure: a randomized controlled trial [published correction appears in Lancet. 1999;354(9192):1826]. Lancet. 1999;354(9184):1066–1071 [PubMed] [Google Scholar]
- 21. Dani C, Bertini G, Pezzati M, Filippi L, Cecchi A, Rubaltelli FF. Inhaled nitric oxide in very preterm infants with severe respiratory distress syndrome. Acta Paediatr. 2006;95(9):1116–1123 [DOI] [PubMed] [Google Scholar]
- 22. Hascoet JM, Fresson J, Claris O, et al. The safety and efficacy of nitric oxide therapy in premature infants. J Pediatr. 2005;146(3):318–323 [DOI] [PubMed] [Google Scholar]
- 23. Field D, Elbourne D, Truesdale A, et al. ; INNOVO Trial Collaborating Group Neonatal ventilation with inhaled nitric oxide versus ventilatory support without inhaled nitric oxide for preterm infants with severe respiratory failure: the INNOVO multicentre randomised controlled trial. Pediatrics. 2005;115(4):926–936 [DOI] [PubMed] [Google Scholar]
- 24. Kinsella JP, Cutter GR, Walsh WF, et al. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med. 2006;355(4):354–364 [DOI] [PubMed] [Google Scholar]
- 25. Kinsella JP, Walsh WF, Bose CL, et al. Inhaled nitric oxide in premature neonates with severe hypoxaemic respiratory failure: a randomised controlled trial. Lancet. 1999;354(9184):1061–1065 [DOI] [PubMed] [Google Scholar]
- 26. Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med. 2003;349(22):2099–2107 [DOI] [PubMed] [Google Scholar]
- 27. Srisuparp P, Heitschmidt M, Schreiber MD. Inhaled nitric oxide therapy in premature infants with mild to moderate respiratory distress syndrome. J Med Assoc Thai. 2002;85(suppl 2):S469–S478 [PubMed] [Google Scholar]
- 28. Subhedar N, Ryan S, Shaw N. Open randomized controlled trial of inhaled nitric oxide and early dexamethasone in high risk preterm infants. Arch Dis Child Fetal Neonatal Ed. 1997;77(3):F185–F190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Van Meurs K, Wright L, Ehrenkranz R, et al. ; Preemie Inhaled Nitric Oxide Study Inhaled nitric oxide for premature infants with severe respiratory failure. N Engl J Med. 2005;353(1):13–22 [DOI] [PubMed] [Google Scholar]
- 30. Van Meurs KP, Hintz SR, Ehrenkranz RA, et al. Inhaled nitric oxide in infants >1500 g and <34 weeks gestation with severe respiratory failure. J Perinatol. 2007;27(6):347–352 [DOI] [PubMed] [Google Scholar]
- 31. Mercier JC, Hummler H, Durrmeyer X, et al. Inhaled nitric oxide for prevention of bronchopulmonary dysplasia in premature babies (EUNO): a randomised controlled trial. Lancet. 2010;376(9738):346–354 [DOI] [PubMed] [Google Scholar]
- 32. Su PH, Chen JY. Inhaled nitric oxide in the management of preterm infants with severe respiratory failure. J Perinatol. 2008;28(2):112–116 [DOI] [PubMed] [Google Scholar]
- 33. Barrington KJ, Finer N. Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database Syst Rev. 2010;(12):CD000509. [DOI] [PubMed] [Google Scholar]
- 34. Cools F, Askie LM, Offringa M, et al. Elective high-frequency oscillatory versus conventional ventilation in preterm infants: a systematic review and meta-analysis of individual patients' data. Lancet. 2010;375(9731):2082–2091 [DOI] [PubMed] [Google Scholar]
- 35. Askie LM, Ballard RA, Cutter G, et al. ; Meta-analysis of Preterm Patients on Inhaled Nitric Oxide (MAPPiNO) Collaboration Inhaled nitric oxide in preterm infants: a systematic review and individual patient data meta-analysis. BMC Pediatr. 2010;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zou GA. Modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706 [DOI] [PubMed] [Google Scholar]
- 37. Follmann D, Proschan M, Leifer E. Multiple outputation: inference for complex clustered data by averaging analyses from independent data. Biometrics. 2003;59(2):420–429 [DOI] [PubMed] [Google Scholar]
- 38. Ghersi D, Berlin J, Askie LM. Prospective meta-analysis. In: Higgins JPT, Green S. eds. Cochrane Handbook for Systematic Reviews of Interventions. Vol 5.0.1 Available at: www.cochrane-handbook.org Accessed September 9, 2010
- 39. Ahluwalia J, Tooley J, Cheema I, et al. A dose response study of inhaled nitric oxide in hypoxic respiratory failure in preterm infants. Early Hum Dev. 2006;82(7):477–483 [DOI] [PubMed] [Google Scholar]
- 40. Cole FS, Alleyne C, Barks JDE, et al. NIH Consensus Development Conference statement: inhaled nitric-oxide therapy for premature infants. Pediatrics. 2011;127(2):363–369 [DOI] [PubMed] [Google Scholar]
- 41. National Heart, Lung, and Blood Institute Examining the use of non-invasive inhaled nitric oxide to reduce chronic lung disease in premature newborns. Available at: http://clinicaltrials.gov/ct2/results?term=NCT00955487 Accessed September 9, 2010
- 42. INO Therapeutics Inhaled nitric oxide (INO) for the prevention of bronchopulmonary dysplasia (BPD) in preterm infants. Available at: http://clinicaltrials.gov/ct2/results?term=NCT00931632 Accessed September 9, 2010
- 43. University of Chicago Inhaled nitric oxide and neuroprotection in premature infants (NOVA2). Available at: http://clinicaltrials.gov/ct2/results?term=NCT00515281 Accessed September 9, 2010
- 44. Donohue PK, Gilmore MM, Cristofalo E, et al. Inhaled nitric oxide in preterm infants: a systematic review. Pediatrics. 2011;127(2). Available at: www.pediatrics.org/cgi/content/full/127/2/e414 [DOI] [PubMed] [Google Scholar]