Abstract
OBJECTIVE:
To estimate the prevalence of asthma among youth with types 1 and 2 diabetes and examine associations between asthma and glycemic control.
METHODS:
This was a cross-sectional analysis of data from the SEARCH for Diabetes in Youth study, which included youth diagnosed with type 1 (n = 1683) and type 2 (n = 311) diabetes from 2002 through 2005. Asthma status and medications were ascertained from medical records and self-administered questionnaires, and glycemic control was assessed from hemoglobin A1c measured at the study visit.
RESULTS:
Prevalence of asthma among all youth with diabetes was 10.9% (95% confidence interval [CI]: 9.6%–12.3%). The prevalence was 10.0% (95% CI: 8.6%–11.4%) among youth with type 1 and 16.1% (95% CI: 12.0%–20.2%) among youth with type 2 diabetes and differed according to race/ethnicity. Among youth with type 1 diabetes, those with asthma had higher mean A1c levels than those without asthma, after adjustment for age, gender, race/ethnicity, and BMI (7.77% vs 7.49%; P = .034). Youth with asthma were more likely to have poor glycemic control, particularly those with type 1 diabetes whose asthma was not treated with pharmacotherapy, although this association was attenuated by adjustment for race/ethnicity.
CONCLUSIONS:
Prevalence of asthma may be elevated among youth with diabetes relative to the general US population. Among youth with type 1 diabetes, asthma is associated with poor glycemic control, especially if asthma is untreated. Specific asthma medications may decrease systemic inflammation, which underlies the complex relationship between pulmonary function, BMI, and glycemic control among youth with diabetes.
Keywords: asthma, diabetes mellitus, diabetes type 1, diabetes type 2, obesity
WHAT'S KNOWN ON THIS SUBJECT:
Asthma, obesity, and diabetes are common, complex conditions among youth that have increased in prevalence over the last 2 decades. Poor glycemic control increases the risk of diabetes-related complications, but the contribution of asthma to glycemic control is not well understood.
WHAT THIS STUDY ADDS:
Described is the prevalence of asthma among a diverse cohort of youth with type 1 and type 2 diabetes, and the association between asthma and glycemic control is examined. Asthma was associated with poor glycemic control among these youth with diabetes.
Asthma, diabetes, and obesity are common, complex disorders for which the prevalence among youth has increased since the 1990s.1–3 Although several epidemiologic studies have reported their co-occurrence,4–9 the relationship between these 3 conditions is not well understood. Because obesity has recently been recognized as a condition of systemic inflammation, investigators have proposed a relationship between the proinflammatory state in obesity and the increasing prevalence of asthma.9–12 The inflammation underlying obesity may further exacerbate the immune dysregulation inherent in both asthma and type 1 diabetes.12,13 In addition, upregulation of inflammatory processes in the obese state is associated with insulin resistance, and it is well established that insulin resistance plays a primary role in the pathogenesis of type 2 diabetes.14
Moreover, several studies have observed associations between impaired lung function and elevated glucose levels, as well as diabetes diagnoses. These studies demonstrated poorer pulmonary function among persons with diabetes compared with nondiabetic controls.15,16 Longitudinal studies in persons with diabetes found that those with poorly controlled diabetes, or high hemoglobin A1c levels, have steeper declines in lung function over time than those with good glycemic control.17,18 Although good glycemic control prevents or delays the onset of diabetic complications in children,19 adolescents,20 and adults21,22 with type 1 and type 2 diabetes, the relationship between asthma and glycemic control among youth with type 1 and type 2 diabetes has not been thoroughly investigated.
The SEARCH for Diabetes in Youth Study (SEARCH) is a large observational study among a highly diverse population of children and adolescents with type 1 and type 2 diabetes. We describe the prevalence of asthma among these youth with type 1 and type 2 diabetes, and explore its association with the demographic and clinical characteristics of the youth in the study. We also examine the association between asthma and categories of glycemic control, as measured by hemoglobin A1c, as well as the relationship between asthma treatment and glycemic control, among youth with type 1 diabetes and asthma.
METHODS
Population and Data Sources
SEARCH is a multicenter study that began conducting population-based ascertainment of youth with clinically diagnosed diabetes who were younger than 20 years in 2001 and continues to enroll youth with newly diagnosed (incident) diabetes.23 SEARCH recruited youth from 4 geographically defined populations, Indian Health Service beneficiaries from 4 Native American populations, and enrollees in several managed health care plans. Institutional review boards for each site approved the study protocol.
SEARCH participants who completed an initial survey were asked to participate in an in-person visit. At the time of the study visit, informed consent and assent (when applicable) was obtained, health questionnaires were administered, and physical measurements and blood samples were obtained from metabolically stable participants (no episodes of diabetic ketoacidosis during the previous month) after a minimum 8-hour overnight fast. All measures were conducted by trained, certified staff in accordance with standardized study protocols. Information was abstracted from the medical record for the period from 2 months before to 6 months after diagnosis for inpatient and outpatient clinical encounters and prescribed medications.
Glycemic Control
Hemoglobin A1c was measured in blood drawn at the study visit, by ion exchange, high-performance liquid chromatography (Tosoh Bioscience, Montgomeryville, PA). Hemoglobin A1c was used to categorize glycemic control in accordance with the American Diabetes Association recommended age-specific cutoff points for good control (7.5%–8.5% at younger than 6 years, <8.0% at age 6–12 years, <7.5% at age 13–18 years, and <7.0% at age 19 years and older).24,25 Values of hemoglobin A1c >9.5% at any age were classified as “poor” control; “intermediate” control includes values between the definition of “good” and “poor” control.
Asthma Status
Study participants were classified as having asthma if (1) a diagnosis of asthma was present in their medical record or (2) if asthma was reported on the health questionnaire completed at the time of the study visit and a prescription for at least 1 asthma-specific medication was noted in the medical record or reported in the medication inventory section of the health questionnaire. All other participants, including those who reported on the health questionnaire having asthma but had no corresponding diagnosis or prescription for asthma medications in the medical record, nor any indication of asthma-specific medications on the health questionnaire, were classified as not having asthma.
Asthma Medication
Asthma medications listed in the medical record or reported on the health questionnaire were categorized as inhaled corticosteroids (Beclomethasone Dipropionate, Budesonide, Fluticasone, Fluticasone-Salmeterol, and Triamcinolone Acetonide), leukotriene modifiers (Montelukast), rescue inhalers (Albuterol, Albuterol Sulfate, Levalbuterol, Metaproterenol Sulfate, and Pirbuterol Sulfate), and other inhaled medications (Cromolyn, Ipratropium Bromide, and Salmeterol). For analytic purposes, asthma medications were grouped hierarchically into the following categories: (1) inhaled corticosteroids, alone or in combination with any other medication; (2) leukotriene modifiers, alone or in combination with any other medication except inhaled corticosteroids; and (3) rescue inhalers, alone or in combination with any other medication except inhaled corticosteroids or leukotriene modifiers.
Clinical and Demographic Characteristics
Diabetes type was based on physician diagnosis. Height and weight were used to calculate BMI. Age- and gender-specific BMI z scores were derived from the Centers for Disease Control and Prevention national standards, and used to define BMI categories: underweight or normal weight if <85th percentile, overweight if between 85th and 95th percentiles, and obese if ≥95th percentile.
Race/ethnicity was obtained through self-report using standard census questions.26 Youth who reported Hispanic ethnicity were categorized as Hispanic; non-Hispanic youth who reported >1 race were categorized by using the plurality approach of the National Center for Health Statistics.27 Parental education, household income, family composition, and insurance status were obtained at the time of the study visit. Participants were categorized as living in a 2-parent household, 1-parent household, or other living situation. Parental education was defined as the highest educational level attained by either parent. Insurance status was classified as private (provided by an employer or purchased by the participant or his or her family), state-funded (including Medicaid, Medicare, and other state-funded sources), other forms (including Indian Health Service, student health clinics, military, or other/unknown sources), and no insurance.
Statistical Analysis
χ2 tests were used to determine significant differences in proportions of clinical and demographic characteristics according to asthma status, among all youth with diabetes and according to diabetes type. Fisher's exact test was used for variables with expected counts <10. We examined associations between asthma (yes/no) and categories of glycemic control using multinomial logistic regression models, and used multiple logistic regression to test for significant association between asthma (yes/no) and poor glycemic control (yes/no). We transformed continuous hemoglobin A1c to approximate univariate normality and examined its association with asthma. Models were adjusted for age at examination, gender, race/ethnicity, and BMI status. Lastly, we examined association between categories of glycemic control and asthma medication use, among youth with type 1 diabetes and asthma. Analyses were performed with SAS 9.1 (SAS Institute, Cary, NC).
RESULTS
The study sample consisted of 1994 youth with diabetes ranging in age from 3 to 21 years; 1683 had type 1 and 311 had type 2 diabetes (Table 1). Among all participants, asthma was present in 218 youth (10.9% [95% confidence interval (CI): 9.6%–12.3%]), including 168 youth with type 1 (10.0% [95% CI: 8.6%–11.4%]) and 50 youth with type 2 diabetes (16.1% [95% CI: 12.0%–20.2%]). Overall, compared with those without asthma, a higher proportion of participants with asthma were older; male; black, Hispanic, or Asian/Pacific Islander; obese, and from single-parent households. Youth with asthma were also more likely to have poor glycemic control (P = .025). The majority of participants (57.3%) with asthma were treated with rescue inhalers alone or in combination with other inhaled medications. Approximately 11% were treated with inhaled corticosteroids and 13% with leukotriene modifiers, while 18% had no indication of asthma pharmacotherapy in the medical record or on the health questionnaire.
TABLE 1.
Characteristics of Youth With Type 1 or 2 Diabetes According to Asthma Status: SEARCH 2002–2005 Incident Cohorts
| Characteristic | All Participants (N = 1994), n (%) |
Pa | Type 1 Diabetes (n = 1683), n (%) |
Pa | Type 2 Diabetes (n = 311), n (%) |
Pa | |||
|---|---|---|---|---|---|---|---|---|---|
| No Asthma (n = 1776) | Asthma (n = 218) | No Asthma (n = 1515) | Asthma (n = 168) | No Asthma (n = 261) | Asthma (n = 50) | ||||
| Age at examination | .039 | .329 | .913 | ||||||
| 3–5 y | 206 (11.6) | 18 (8.3) | 206 (13.6) | 18 (10.7) | 0 (0.0) | 0 (0.0) | |||
| 6–10 y | 555 (31.3) | 57 (26.2) | 543 (35.8) | 55 (32.7) | 12 (4.6) | 2 (4.0) | |||
| 11–14 y | 612 (34.5) | 77 (35.3) | 506 (33.4) | 58 (34.5) | 106 (40.6) | 19 (38.0) | |||
| 15–21 y | 403 (22.7) | 66 (30.3) | 260 (17.2) | 37 (22.0) | 143 (54.8) | 29 (58.0) | |||
| Female | 914 (51.5) | 122 (56.0) | .038 | 746 (49.2) | 97 (57.7) | .086 | 168 (64.4) | 25 (50.0) | .055 |
| Race/ethnicity | <.001 | <.001 | .139 | ||||||
| Non-Hispanic white | 1207 (67.9) | 116 (53.2) | 1155 (76.2) | 102 (60.7) | 52 (19.9) | 14 (28.0) | |||
| Black | 234 (13.2) | 46 (21.1) | 143 (9.4) | 30 (17.9) | 91 (34.9) | 16 (32.0) | |||
| Hispanic | 230 (12.9) | 21 (14.2) | 163 (10.8) | 24 (14.3) | 67 (25.7) | 7 (14.0) | |||
| Asian/Pacific Islander | 60 (3.4) | 12 (5.5) | 38 (2.5) | 9 (5.4) | 22 (8.4) | 3 (6.0) | |||
| Native American | 33 (1.9) | 12 (5.5) | 7 (0.5) | 2 (1.2) | 26 (10.0) | 10 (20.0) | |||
| Other | 12 (0.7) | 1 (0.5) | 9 (0.6) | 1 (0.6) | 3 (1.1) | 0 (0.0) | |||
| BMI status | <.001 | .007 | .695 | ||||||
| Less than normal weight (<85th percentile) | 1025 (57.7) | 96 (44.1) | 1007 (66.5) | 92 (54.8) | 18 (6.9) | 4 (8.0) | |||
| Overweight (85th–95th percentile) | 312 (17.6) | 41 (18.8) | 288 (19.0) | 36 (21.4) | 24 (9.2) | 5 (10.0) | |||
| Obese (>95th percentile) | 412 (23.2) | 77 (35.3) | 200 (13.2) | 36 (21.4) | 212 (81.2) | 41 (82.0) | |||
| Unknown | 27 (1.5) | 4 (1.8) | 20 (1.3) | 4 (2.4) | 7 (2.7) | 0 (0.0) | |||
| Family structure | .003 | .073 | .294 | ||||||
| 2-parent household | 1176 (66.2) | 125 (57.3) | 1062 (70.1) | 107 (63.7) | 114 (43.7) | 18 (36.0) | |||
| 1-parent household | 525 (29.6) | 74 (33.9) | 406 (26.8) | 51 (30.4) | 119 (45.6) | 23 (46.0) | |||
| Other/unknown household structure | 75 (4.2) | 19 (8.7) | 47 (3.1) | 10 (5.9) | 28 (10.7) | 9 (18.0) | |||
| Household income | .475 | .282 | .438 | ||||||
| Less than $25 000 | 293 (16.5) | 45 (20.6) | 191 (12.6) | 30 (17.9) | 102 (39.1) | 15 (30.0) | |||
| $25 000–49 000 | 399 (22.5) | 51 (23.4) | 342 (22.6) | 34 (20.2) | 57 (21.8) | 17 (34.0) | |||
| $50 000–74 000 | 329 (18.5) | 33 (15.1) | 299 (19.7) | 28 (16.7) | 30 (11.5) | 5 (10.0) | |||
| $75 000 | 590 (33.2) | 71 (32.6) | 566 (37.4) | 66 (39.3) | 24 (9.2) | 5 (10.0) | |||
| Unknown/refused | 165 (9.3) | 18 (8.3) | 117 (7.7) | 10 (5.9) | 48 (18.4) | 8 (16.0) | |||
| Parental education (%) | .181 | .363 | .090 | ||||||
| Less than high school | 124 (7.0) | 11 (5.1) | 75 (5.0) | 7 (4.2) | 49 (18.8) | 4 (8.0) | |||
| High school graduate or GED | 312 (17.6) | 38 (17.4) | 229 (15.1) | 26 (15.5) | 83 (31.8) | 12 (24.0) | |||
| Some college | 570 (32.1) | 85 (39.0) | 493 (32.5) | 65 (38.7) | 77 (29.5) | 20 (40.0) | |||
| Bachelor's degree or more | 744 (41.9) | 79 (36.2) | 703 (46.4) | 70 (41.7) | 41 (15.7) | 9 (18.0) | |||
| Unknown | 26 (1.4) | 5 (2.3) | 15 (1.0) | 0 (0.0) | 11 (4.2) | 5 (10.0) | |||
| Insurance | .186 | .264 | .330 | ||||||
| None | 31 (1.8) | 3 (1.4) | 21 (1.4) | 2 (1.2) | 10 (3.8) | 1 (2.0) | |||
| Private | 1311 (73.8) | 147 (67.4) | 1177 (77.7) | 123 (73.2) | 134 (51.3) | 24 (48.0) | |||
| Medicaid/Medicare | 368 (20.7) | 57 (26.1) | 272 (18.0) | 40 (23.8) | 96 (36.8) | 17 (34.0) | |||
| Other/unknown | 66 (3.7) | 11 (5.1) | 45 (3.0) | 3 (1.8) | 21 (8.0) | 8 (16.0) | |||
| Age at diabetes diagnosis | .095 | .362 | .909 | ||||||
| 0–5 y | 273 (15.4) | 25 (11.4) | 273 (18.0) | 25 (14.9) | 0 (0.0) | 0 (0.0) | |||
| 6–10 y | 607 (34.2) | 66 (30.3) | 584 (38.6) | 62 (36.9) | 23 (8.8) | 4 (8.0) | |||
| 11–14 y | 606 (34.1) | 80 (36.7) | 479 (31.6) | 54 (32.1) | 127 (48.7) | 26 (52.0) | |||
| 15–20 y | 290 (16.3) | 47 (21.6) | 179 (11.8) | 27 (16.1) | 111 (42.5) | 20 (40.0) | |||
| Diabetes duration | .193 | .434 | .455 | ||||||
| <5 mo | 525 (29.5) | 60 (27.5) | 464 (30.6) | 47 (28.0) | 61 (23.4) | 13 (26.0) | |||
| 6–10 mo | 470 (26.5) | 57 (26.1) | 395 (26.1) | 43 (25.6) | 75 (28.7) | 14 (28.0) | |||
| 11–14 mo | 348 (19.6) | 33 (15.1) | 302 (19.9) | 28 (16.7) | 46 (17.6) | 5 (10.0) | |||
| 15–19 mo | 269 (15.2) | 44 (20.2) | 224 (14.8) | 31 (18.5) | 45 (17.2) | 13 (26.0) | |||
| >20 mo | 164 (9.2) | 24 (11.0) | 130 (8.6) | 19 (11.3) | 34 (13.0) | 5 (10.0) | |||
| Glycemic controlb | .025 | .025 | .885 | ||||||
| Good | 1105 (62.2) | 129 (59.2) | 936 (61.8) | 96 (57.1) | 169 (64.7) | 33 (66.0) | |||
| Intermediate | 492 (27.7) | 54 (24.8) | 443 (29.2) | 46 (27.4) | 49 (18.8) | 8 (16.0) | |||
| Poor | 179 (10.1) | 35 (16.0) | 136 (9.0) | 26 (15.5) | 43 (16.5) | 9 (18.0) | |||
| Asthma treatmentc | |||||||||
| Inhaled corticosteroid | — | 24 (11.0) | — | 18 (10.7) | — | 6 (12.0) | |||
| Leukotriene modifiers | — | 29 (13.3) | — | 25 (14.9) | — | 4 (8.0) | |||
| Rescue inhalers | — | 125 (57.3) | — | 99 (58.9) | — | 26 (52.0) | |||
| No medication | — | 40 (18.4) | — | 26 (15.5) | — | 14 (28.0) | |||
GED indicates general equivalency diploma.
P value based on χ2 test or Fisher's exact test for variables with expected cell counts of <10.
Glycemic control defined as good (hemoglobin A1c between 7.5% and 8.5% at younger than 6 years of age, <8.0% at 6–12 years of age, <7.5% at 13–18 years of age, and <7.0% at 19 years of age and older); and poor (hemoglobin A1c >9.5% at any age); and intermediate (values between the definition of good and poor control).
Treatment was defined as (1) inhaled corticosteroids alone or in combination with any other medication, (2) leukotriene modifiers alone or in combination with any medication except inhaled corticosteroids, and (3) rescue inhalers alone or in combination with any medication except inhaled corticosteroid or leukotriene modifiers.
Associations between demographic characteristics and asthma status were predominantly driven by youth with type 1 diabetes (Table 1). In addition, asthma was significantly associated with glycemic control among youth with type 1 diabetes but not among youth with type 2 diabetes. Less than 15% of the entire sample had type 2 diabetes; only 50 of those had asthma. The demographic characteristics of youth with type 2 were substantially different from those with type 1 diabetes. Therefore, we conducted the remaining analyses of asthma among youth with type 1 diabetes.
Among participants with type 1 diabetes, the unadjusted risk for intermediate versus good glycemic control was not significantly elevated for youth with asthma compared with those without asthma (Table 2). However, those with asthma were 1.37 times as likely to have poor glycemic control than good control (95% CI: 1.08–1.73) and 1.91 times as likely to have poor glycemic control than either intermediate or good control (95% CI: 1.21–3.02) compared with youth without asthma. Although risk for poor glycemic control remained significantly elevated after adjustment for gender and age at examination, adjustment for race/ethnicity substantially attenuated odds ratios and their significance. Further adjustment for BMI had little impact on the estimates of risk for poor glycemic control.
TABLE 2.
Unadjusted and Adjusted Odds Ratios and 95% CIs for the Association Between Asthma and Categories of Glycemic Control
| Variable | All Participants (N = 1994) |
Type 1 Diabetes Only (n = 1683) |
||||
|---|---|---|---|---|---|---|
| Intermediate (n = 546) vs Good (n = 1234)a | Poor (n = 214) vs Good (n = 1234)a | Poor (n = 214) vs Intermediate/Good (n = 1780)b | Intermediate (n = 489) vs Good (n = 1032)b | Poor (n = 162) vs Good (n = 1032)a | Poor(n = 162) vs Intermediate/Good (n = 1521)b | |
| Asthma, unadjusted | ||||||
| No | Referent | Referent | Referent | Referent | Referent | Referent |
| Yes | 0.97 (0.82–1.15) | 1.29 (1.06–1.58) | 1.76 (1.19–2.61) | 1.01 (0.84–1.08) | 1.37 (1.08–1.73) | 1.91 (1.21–3.02) |
| Asthma, adjustedc | ||||||
| No | Referent | Referent | Referent | Referent | Referent | Referent |
| Yes | 0.96 (0.81–1.14) | 1.25 (1.02–1.54) | 1.65 (1.10–2.46) | 1.00 (0.83–1.20) | 1.33 (1.05–1.69) | 1.83 (1.15–2.90) |
| Asthma, adjustedd | ||||||
| No | Referent | Referent | Referent | Referent | Referent | Referent |
| Yes | 0.97 (0.82–1.15) | 1.16 (0.94–1.43) | 1.40 (0.92–2.11) | 1.00 (0.83–1.20) | 1.17 (0.91–1.50) | 1.41 (0.87–2.30) |
| Asthma, adjustede | ||||||
| No | Referent | Referent | Referent | Referent | Referent | Referent |
| Yes | 1.00 (0.84–1.18) | 1.19 (0.96–1.47) | 1.41 (0.93–2.14) | 1.02 (0.85–1.24) | 1.20 (0.93–1.54) | 1.41 (0.87–2.30) |
Glycemic control defined as good (hemoglobin A1c between 7.5% and 8.5% at younger than 6 years of age, <8.0% at 6–12 years of age, <7.5% at 13–18 years of age, and <7.0% at 19 years of age and older); and poor (hemoglobin A1c >9.5% at any age); and intermediate (values between the definition of good and poor control).
Estimates and CIs from multinomial logistic regression model.
Estimates and CIs from standard logistic regression model.
Effect of asthma adjusted for age at examination and gender.
Effect of asthma adjusted for age at examination, gender, and race/ethnicity.
Effect of asthma adjusted for age at examination, gender, race/ethnicity, and BMI.
In addition to categorical glycemic control, we also assessed the difference in mean hemoglobin A1c for youth with and without asthma. After adjustment for age at examination, gender, race/ethnicity, and BMI, youth with asthma had higher mean hemoglobin A1c levels than those without asthma (mean ± SD: 7.77% ± 0.26% vs 7.49% ± 0.23%; P = .034).
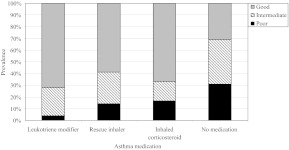
Given that asthma treatment may influence glycemic control, we examined the proportion of youth with type 1 diabetes and asthma (n = 168), with good, intermediate, and poor glycemic control according to medication use (Fig 1). Among these study participants, glycemic control was significantly associated with medication use (P = .045). Approximately 31% of those who did not receive pharmacologic treatment for asthma had poor glycemic control, as did 17% of youth treated with inhaled corticosteroids and 14% of those treated with rescue inhalers. However, when analyses were restricted to youth with prescribed asthma medications (n = 142), of whom 56 (39%) had medications listed only in the medical record, 31 (22%) only on the health questionnaire, and 55 (39%) in both sources, there was no significant difference in the proportion of participants with poor glycemic control among the 3 medication categories (P = .32). The significant association between asthma medication use and glycemic control is primarily due to the high proportion of youth with poor control observed among those with untreated asthma. Youth with untreated asthma had 3.29 greater odds (95% CI: 1.09–9.95) of having poor glycemic control than those receiving asthma pharmacotherapy, after adjustment for age, gender, and race/ethnicity (P = .035).
FIGURE 1.
Proportion of good, intermediate, and poor glycemic control among youth with type 1 diabetes and asthma. Level of glycemic control varied significantly according to asthma medication (P = .045). The P value was determined by using Fisher's exact test.
DISCUSSION
In this sample of 1994 youth with diabetes, the prevalence of asthma was 10.9%. This estimate is slightly higher than population-based prevalence estimates based on self-report, published by the Centers for Disease Control and Prevention in 2001, that 8.7% of all youth younger than 18 years in the United States had asthma.28 When we restricted our sample to youth younger than 18 years at the time of the study visit for comparison purposes (n = 1891), 10.8% (95% CI: 9.4%–12.2%) had asthma. Consistent with reports from other studies, the higher prevalence of asthma observed in the SEARCH study population may be indicative of an increasing incidence of asthma among youth with diabetes compared with the general population.4,5
We observed a higher prevalence of asthma among youth with type 2 (16.1% [95% CI: 12.0%–20.2%]), than those with type 1 diabetes (10.0% [95% CI: 8.6%–11.4%]). Nonoverlapping CIs around the estimates in each group indicate a significantly higher prevalence of asthma among youth with type 2 diabetes; however, this may be due to the fact that youth receiving medical care for asthma may be more likely to have previously undiagnosed type 2 diabetes recognized. Youth with type 1 diabetes, however, are typically diagnosed at younger ages, irrespective of other comorbidities.
When we examined demographic characteristics associated with asthma, we observed a strong association between race/ethnicity and asthma among youth with type 1 diabetes. The prevalence of asthma was highest among Native American (22.2% [95% CI: 0%–49%]), Asian/Pacific Islander (19.2% [95% CI: 7.9%–30.4%]), and black (17.3% [95% CI: 11.7%–23.0%]) youth and lowest among non-Hispanic white (8.1% [95% CI: 6.6%–9.6%]) youth. Although rates of asthma observed in non-Hispanic white and black subjects were relatively similar to those observed in other large cross-sectional studies conducted among children and adolescents in the United States,29,30 asthma prevalence among Asian/Pacific Islanders was higher. These findings may suggest that rates of asthma among individuals with type 1 diabetes vary according to specific racial/ethnic groups but may also be indicative of higher asthma prevalence reported in specific Asian/Pacific Islander subgroups.31
BMI status was also associated with asthma among youth with type 1 diabetes; nearly 43% of those with asthma were overweight or obese, compared with 32% of those without asthma. The association between BMI and asthma observed in our study is consistent with the findings of several population-based studies of asthma and obesity in children and adolescents.6–8 As expected, a high proportion of youth with type 2 diabetes were overweight or obese (90.6%); although there were marginally more youth who were overweight or obese among those with asthma than those without asthma, the difference was not statistically significant. This is likely due to the strong correlation between childhood obesity and development of type 2 diabetes, which limited the variability of BMI in this group. However, we also observed a significant association between BMI and asthma among youth with type 1 diabetes, which may suggest a role for the inflammatory processes underlying obesity and the development of asthma, among those with autoimmune-related diabetes.
Although the absolute difference was small, hemoglobin A1c was significantly higher in participants with asthma than those without asthma, with and without adjustment for demographic and clinical covariates. We also observed an association between asthma and poor glycemic control among youth with type 1 diabetes. This relationship was significant after adjustment for age and gender but was attenuated after controlling for race/ethnicity and BMI. The SEARCH study previously reported a strong association between glycemic control and race/ethnicity as well as BMI.32
The relationship between use of asthma medications and glycemic control was significant. Youth with type 1 diabetes who had a diagnosis of asthma in the medical record but did not have asthma pharmacotherapy indicated in the medical record or self-reported at the time of the study visit were more likely to have poor glycemic control than those treated for asthma. It is interesting to note that those whose asthma was treated with leukotriene modifiers, alone or in combination with rescue inhalers or other inhaled medications, had the lowest prevalence of poor glycemic control. In fact, 72% of these participants had good glycemic control, representing the highest proportion of good glycemic control among all groups of medication users. A possible explanation for this finding is that these medications block leukotriene synthesis or interfere with leukotriene-receptor binding, thereby reducing airway inflammation and mucus secretion, and improving mucociliary clearance and lung function.33 The anti-inflammatory action of these medications may also ameliorate systemic inflammation present in both obesity and diabetes.
We acknowledge several limitations of this study. The cross-sectional and observational nature of these data did not allow us to assess changes in asthma severity, which may be associated with glycemic control. The medical record abstraction and health questionnaire did not query medication dosages or dispense dates. This lack of information precluded us from evaluating medication adherence or treatment duration, which may have affected glycemic control. In addition, although oral steroids may negatively impact glycemic control, only 6 youth with type 1 diabetes and asthma had oral steroids noted in the medical record; none reported taking oral steroids at the time of the study visit when hemoglobin A1c was measured. As such, it is likely that the impact of oral steroid use on glycemic control was minimal.
Despite extensive efforts to optimize recruitment, only 62% of eligible youth from the 2002–2005 incident cohorts completed the SEARCH study visit. To determine if our prevalence estimates may have been biased by selection into the study, we examined medical records of youth in the 2002–2005 cohorts that are available for all youth regardless of whether they completed a study visit, and identified those with asthma diagnoses and medications. Youth who did not complete a study visit (n = 1307) had an asthma prevalence of 9.3% (95% CI: 7.8%–10.9%), which is comparable to the 9.7% (95% CI: 8.4%–11.0%) prevalence observed among the youth included in these analyses (n = 1994). The proportion of youth with asthma based solely on medical record diagnoses and medication prescriptions is slightly lower than the proportion identified by combination of medical record data and self-report. This is likely due to the time difference between medical record abstraction and the in-person study visit; youth who reported asthma and concomitant asthma medication use on the health questionnaire who did not have a diagnosis of asthma in the medical record, may have developed asthma >6 months after diabetes diagnosis.
The strengths of our study include its large sample of racially/ethnically diverse youth with type 1 and type 2 diabetes, and the ability to assess glycemic control based on hemoglobin A1c values collected using a common protocol and tested at a central laboratory. Unlike many studies of asthma that rely on self-report alone,1,6,7,9,28–31 we were able to ascertain asthma diagnoses and pharmacologic treatment from medical records, supplemented by self-report, to estimate its prevalence and examine its relation to glycemic control. Of those participants who completed the study visit, 99.2% completed the health questionnaire, of which 95% also had medical record data available.
CONCLUSIONS
Our findings suggest that asthma may contribute to poor glycemic control in youth with diabetes, especially if asthma is left untreated. It is possible that treatment of asthma, particularly with leukotriene modifiers alone or in conjunction with rescue inhalers, ameliorates the inflammatory processes underlying asthma, obesity, and diabetes and/or prevents the decline in lung function that is associated with poor glycemic control. Further investigation of the effect of asthma-specific medications on systemic inflammation is necessary to understand the complex relationships between pulmonary function, BMI, and glycemic control.
ACKNOWLEDGMENTS
SEARCH is funded by the Centers for Disease Control and Prevention (PA 00097, DP-05-069, and DP-10-001) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Site contract numbers are as follows: Kaiser Permanente Southern California, U01 DP000246; University of Colorado Denver, U01 DP000247; Kuakini Medical Center, U01 DP000245; Children's Hospital Medical Center (Cincinnati), U01 DP000248; University of North Carolina at Chapel Hill, U01 DP000254; University of Washington School of Medicine, U01 DP000244; and Wake Forest University School of Medicine, U01 DP000250.
We acknowledge the involvement of General Clinical Research Centers at the Medical University of South Carolina (grant M01 RR01070); Seattle Children's and the University of Washington School of Medicine (grant M01RR00037 and M01RR001271); Colorado Pediatric General Clinical Research Center (grant M01 RR00069); and the Institutional Clinical and Translational Science Award and National Institutes of Health/National Center for Research Resources at the University of Cincinnati (grant 1UL1RR026314-01).
SEARCH is indebted to the many youth and their families, and their health care providers, whose participation made this study possible.
Each author participated in the concept and design, analysis and interpretation of data, or drafting or revising of the manuscript, and each author has approved the manuscript as submitted. All authors, as well as the SEARCH Publication and Presentation Committee and the SEARCH Steering Committee, approved the manuscript. Dr Black participated in the concept and design and analysis and interpretation of the data, drafted and revised the manuscript, and contributed to the discussion; Ms Anderson analyzed the data and reviewed/edited the manuscript; Drs Bell, Dabelea, Pihoker, Saydah, and Seid, Ms Standiford, and Drs Waitzfelder and Marcovina reviewed/edited the manuscript and contributed to the discussion; and Dr Lawrence participated in the concept and design and interpretation of the data, reviewed/edited the manuscript, and contributed to the discussion.
The views in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the National Institute of Diabetes and Digestive and Kidney Diseases.
SEARCH is funded by the Centers for Disease Control and Prevention (PA 00097, DP-05-069, and DP-10-001) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
- SEARCH
- SEARCH for Diabetes in Youth Study
- CI
- confidence interval
REFERENCES
- 1. Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980-2007. Pediatrics. 2009;123(suppl 3):S131–S145 [DOI] [PubMed] [Google Scholar]
- 2. Writing Group for the SEARCH for Diabetes in Youth Study Group; Dabelea D, Bell RA, D'Agostin RB, JR, et al. Incidence of diabetes in youth in the United States. JAMA. 2007;297(24):2716–2724 [DOI] [PubMed] [Google Scholar]
- 3. Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295(13):1549–1555 [DOI] [PubMed] [Google Scholar]
- 4. Stene LC, Nafstad P. Relation between occurrence of type 1 diabetes and asthma. Lancet. 2001;357(9256):607–608 [DOI] [PubMed] [Google Scholar]
- 5. Kero J, Gissler M, Hemminki E, Isolauri E. Could TH1 and TH2 diseases coexist? Evaluation of asthma incidence in children with celiac disease, type 1 diabetes, or rheumatoid arthritis: a register study. J Allergy Clin Immunol. 2001;108(5):781–783 [DOI] [PubMed] [Google Scholar]
- 6. Gilliland FD, Berhane K, Islam T, et al. Obesity and the risk of newly diagnosed asthma in school-age children. Am J Epidemiol. 2003;158(5):406–415 [DOI] [PubMed] [Google Scholar]
- 7. Ahmad N, Biswas S, Bae S, Meador KE, Huang R, Singh KP. Association between obesity and asthma in US children and adolescents. J Asthma. 2009;46(7):642–646 [DOI] [PubMed] [Google Scholar]
- 8. Enfield K, Shim M, Sharma G. Asthma, obesity and type 2 diabetes: mechanisms, management and prevention. Diabetes Voice. 2009;54(2):30–33 [Google Scholar]
- 9. Visness CM, London SJ, Daniels JL, et al. Association of childhood obesity with atopic and nonatopic asthma: results from the National Health and Nutrition Examination Survey 1999-2006. J Asthma. 2010;47(7):822–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beuther DA, Weiss ST, Sutherland ER. Obesity and asthma. Am J Respir Crit Care Med. 2006;174(2):112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Al-Shawwa BA, Al-Huniti NH, DeMattia L, Gershan W. Asthma and insulin resistance in morbidly obese children and adolescents. J Asthma. 2007;44(6):469–473 [DOI] [PubMed] [Google Scholar]
- 12. Michelson PH, Williams LW, Benjamin DK, Barnato AE. Obesity, inflammation, and asthma severity in childhood: data from the National Health and Nutrition Examination Survey 2001-2004. Ann Allergy Asthma Immunol. 2009;103(5):381–385 [DOI] [PubMed] [Google Scholar]
- 13. Wilkin TJ. The accelerator hypothesis: weight gain as the missing link between type 1 and type 2 diabetes. Diabetologia. 2001;44(7):914–922 [DOI] [PubMed] [Google Scholar]
- 14. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607 [DOI] [PubMed] [Google Scholar]
- 15. Lange P, Groth S, Kastrup J, et al. Diabetes mellitus, plasma glucose and lung function in a cross-sectional population study. Eur Respir J. 1989;2(1):14–19 [PubMed] [Google Scholar]
- 16. Walter RE, Beiser A, Givelber RJ, O'Connor GT, Gottlieb DJ. Association between glycemic state and lung function: the Framingham Heart Study. Am J Respir Crit Care Med. 2003;167(6):911–916 [DOI] [PubMed] [Google Scholar]
- 17. Lange P, Groth S, Mortensen J, et al. Diabetes mellitus and ventilatory capacity: a five year follow-up study. Eur Respir J. 1990;3(3):288–292 [PubMed] [Google Scholar]
- 18. Davis WA, Knuiman M, Kendall P, Grange V, Davis TM. Fremantle Diabetes Study Glycemic exposure is associated with reduced pulmonary function in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care. 2004;27(3):752–757 [DOI] [PubMed] [Google Scholar]
- 19. Levine BS, Anderson BJ, Butler DA, Antisdel JE, Brackett J, Laffel LM. Predictors of glycemic control and short-term adverse outcomes in youth with type 1 diabetes. J Pediatr. 2001;139(2):197–203 [DOI] [PubMed] [Google Scholar]
- 20. Diabetes Control and Complications Trial Research Group Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr. 1994;125(2):177–188 [DOI] [PubMed] [Google Scholar]
- 21. Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986 [DOI] [PubMed] [Google Scholar]
- 22. UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). [published correction in Lancet. 1999;354(9178):602]. Lancet. 1998;352(9131):837–853 [PubMed] [Google Scholar]
- 23. SEARCH Study Group SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials. 2004;25(5):458–471 [DOI] [PubMed] [Google Scholar]
- 24. Silverstein J, Klingensmith G, Copeland K, et al. ; American Diabetes Association Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care. 2005;28(1):186–212 [DOI] [PubMed] [Google Scholar]
- 25. Type 2 diabetes in children and adolescents. American Diabetes Association. Diabetes Care. 2000;23(3):381–389 [DOI] [PubMed] [Google Scholar]
- 26. US Census Bureau U.S. Census 2000 Basics, 2002. Available at: http://www.census.gov/prod/2001pubs/c2kbr01-1.pdf Accessed August 29, 2011
- 27. Ingram DD, Parker JD, Schenker N, et al. United States Census 2000 population with bridged race categories. Vital Health Stat 2. 2003;(135):1–55 [PubMed] [Google Scholar]
- 28.National Health Interview Survey 2001, National Center for Health Statistics, Centers for Disease Control and Prevention. [Accessed August 29, 2011]. Available at: http://www.cdc.gov/asthma/nhis/01/table4-1.htm.
- 29. Litonjua AA, Carey VJ, Weiss ST, Gold DR. Race, socioeconomic factors, and area of residence are associated with asthma prevalence. Pediatric Pulmonol. 1999;28(6):394–401 [DOI] [PubMed] [Google Scholar]
- 30. Brim SN, Rudd RA, Funk RH, Callahan DB. Asthma prevalence among US children in underrepresented minority populations: American Indian/Alaska Native, Chinese, Filipino, and Asian Indian. Pediatrics. 2008;122(1). Available at: www.pediatrics.org/cgi/content/full/122/1/e217 [DOI] [PubMed] [Google Scholar]
- 31. Davis AM, Kreutzer R, Lipsett M, King G, Shaikh N. Asthma prevalence in Hispanic and Asian American ethnic subgroups: results from the California Healthy Kids Survey. Pediatrics. 2006;118(2). Available at: www.pediatrics.org/cgi/content/full/118/2/e363 [DOI] [PubMed] [Google Scholar]
- 32. Petitti DB, Klingensmith GJ, Bell RA, et al. ; SEARCH for Diabetes in Youth Study Group Glycemic control in youth with diabetes: the SEARCH for diabetes in Youth Study. J Pediatr. 2009;155(5):668–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Horwitz RJ, McGill KA, Busse WW. The role of leukotriene modifiers in the treatment of asthma. Am J Respir Crit Care Med. 1998;157(5 pt 1):1363–1371 [DOI] [PubMed] [Google Scholar]