Abstract
Children with medical complexity (CMC) have medical fragility and intensive care needs that are not easily met by existing health care models. CMC may have a congenital or acquired multisystem disease, a severe neurologic condition with marked functional impairment, and/or technology dependence for activities of daily living. Although these children are at risk of poor health and family outcomes, there are few well-characterized clinical initiatives and research efforts devoted to improving their care. In this article, we present a definitional framework of CMC that consists of substantial family-identified service needs, characteristic chronic and severe conditions, functional limitations, and high health care use. We explore the diversity of existing care models and apply the principles of the chronic care model to address the clinical needs of CMC. Finally, we suggest a research agenda that uses a uniform definition to accurately describe the population and to evaluate outcomes from the perspectives of the child, the family, and the broader health care system.
Keywords: medical complexity, special needs, chronic illness/conditions, delivery of care, definitions
Since 1998, the Maternal and Child Health Bureau has defined children with special health care needs (CSHCN) as those children who have or are at increased risk of a chronic physical, developmental, behavioral, or emotional condition and require health care and related services of a type or amount beyond that required by children generally.1 An extensive process informed the development of an intentionally broad and inclusive CSHCN definition for the definition to be meaningful for broad program planning and development. Although 13% to 18% of children are considered to have special needs (excluding those who are “at risk” for special needs),2 there is considerable variation in medical complexity, functional limitations, and resource need among CSHCN.3,4 One important subgroup is the children who are the most medically fragile and have the most intensive health care needs. Examples vary and include children who have a congenital or acquired multisystem disease, a severe neurologic condition with marked functional impairment, or patients with cancer/cancer survivors with ongoing disability in multiple areas. Terms traditionally used to describe this subgroup include a combination of children with 1 or more of the following terms: complex, chronic, medical, conditions, and/or needs (eg, complex chronic conditions [CCCs],5 complex medical needs,6 complex medical conditions,7 and complex health conditions8), as well as medically complex children.9,10 In this article, we use the term “children with medical complexity” (CMC). The rationales are that it uses “person-first” terminology and refers to the extra time, expertise, and resources necessary to achieve optimal health outcomes for these children.
CMC are likely increasing in prevalence because of increased survival rates of infants born prematurely,11 those born with various congenital anomalies,12,13 and/or those with chronic conditions, as well as improved treatments for acute illnesses in fields such as intensive care14 and oncology.15 These medical successes in survivorship have likely also resulted in rising rates of complications and childhood disability,16,17 with subsequent increases in intensive medical technology use,18 medical and nursing care,19 and coordination needs.20 Regardless of underlying diagnoses, all CMC share similar functional and resource-use consequences, including (1) intensive hospital- and/or community-based service need, (2) reliance on technology, polypharmacy, and/or home care or congregate care to maintain a basic quality of life, (3) risk of frequent and prolonged hospitalizations, which leads to high health-resource utilization, and (4) an elevated need for care coordination.21 The need to coordinate care for CMC has been underscored by the recent emergence of numerous novel complex care programs across North America that address the service needs of CMC in a variety of inpatient, outpatient, and community-based settings.10,22–24 However, meaningful evaluation of the outcomes of these and other programs is limited by a lack of agreement on a definition of CMC and the absence of a clinical and research agenda. The objectives of this article are to (1) present a definitional framework for describing CMC and (2) propose a clinical and research agenda for a model of service delivery that is aimed at improving the quality of health care for CMC and their families.
DEFINITIONAL FRAMEWORK OF CMC
Despite recent attention focused on CMC in the clinical setting, there are considerable inconsistencies in the way these children are defined in the research literature. Clinical and research initiatives would benefit from the use of a uniform definition that is clear, reproducible, and comparable across studies. This definition should be able to consistently identify children whose health and quality of life depend on integrating health care between a primary care medical home, tertiary care services, and other important loci of care such as transitional care facilities, rehabilitation units, the home, the school, and other community-based settings. The threshold for definition of CMC may be context-specific; definitions used in epidemiologic studies may differ from enrollment criteria for inclusion in clinical programs in different locales depending on resource availability, alternative models of care, and the specific family situation. Additional issues to address in operationalizing a definition for CMC include the periodicity of assessments, because complexity of care needs may change over time, as well as the mechanism to identify such patients (eg, individual providers, systems of care, parental reports, etc).
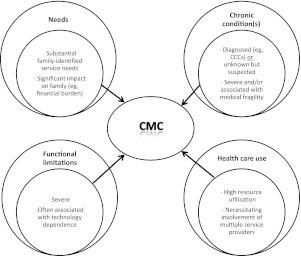
Our definitional framework adapts recommendations from a recent systematic review of chronic disease of childhood (Fig 1).25 In this review, we propose a framework of 4 broad domains to characterize chronic conditions of childhood: needs; chronic conditions; functional limitations; and health care use. Conceptually, the combination of specific manifestations of each of these 4 domains encompasses the collective features of CMC.
FIGURE 1.
Definitional framework for CMC among other definitions of chronic conditions of childhood.25 In this framework, CMC are defined as children with characteristic patterns of needs, chronic conditions, functional limitations, and health care use. CCCs are as defined by Feudtner et al.26
Needs: CMC are characterized by substantial family-identified health care service needs such as medical care, specialized therapy, and educational needs. The service needs have a significant impact on the family unit, specifically time devoted to direct care, frequent provider visits, care coordination, and financial burden. The type, intensity, and consistency of these manifestations may change dynamically over the life of the child depending on a variety of medical, psychosocial, and community factors.
Chronic condition(s): CMC have 1 or more chronic clinical condition(s), either diagnosed or unknown, that are severe and/or associated with medical fragility (eg, high morbidity and mortality rates). The condition and/or its sequelae should be expected to be potentially lifelong, although some children may improve with optimal care or with time. Examples may include a known condition identified among an established list of CCCs.26 An unknown but suspected complex and chronic condition, such as a child born with multiple congenital anomalies but lacking a unifying diagnosis, would be included.
Functional limitations: Functioning is typically classified by using key dimensions of body structure and function, performance of activities, and participation in communal life.27,28 For CMC, the limitations are typically severe and may require assistance from technology such as a tracheostomy tube, feeding tube, or a wheelchair. The type, consistency, and severity of functional limitations may vary over the life of the child in the context of environmental and personal factors.
Health care use: CMC typically have high projected utilization of health resources that may include frequent or prolonged hospitalization, multiple surgeries, or the ongoing involvement of multiple subspecialty services and providers. The intensity of health care use may also vary over time but is anticipated to be substantial when compared with other populations of CSHCN.
Clinical examples of CMC are described in the Appendix.
Our 4 domains complement the existing literature on CMC. Previous definitions lacked at least 1 domain, in part because of the data source or a particular focus such as family-identified needs, diagnostic and procedural codes, functional limitations, and resource use.
The National Survey of Children With Special Health Care Needs (NS-CSHCN) assesses family-reported need and enables state-level prevalence estimates and descriptions of CSHCN.29 One report described rising complexity by an increasing number of affirmative answers to 5 screening questions that identify special needs; there was a resultant upward trend in costs and service needs.3 The NS-CSHCN inquires about 16 common health conditions, the presence of 14 different functional limitations, and the use of numerous specific types of health services that may capture CMC. Although the condition list may detect many of the comorbidities among CSHCN, the NS-CSHCN lacks information about less common primary medical conditions that may be important contributors to the child's complexity, the duration, frequency, and severity of underlying chronic health issues, and specific details on functional limitations (such as technology dependence) and intensive service use, which may better capture and describe this key population.
In 2000, Feudtner et al26 compiled a list of International Classification of Diseases, Ninth Revision–coded “complex chronic conditions” (CCCs) based on an operational definition of a medical condition that lasts for >12 months and involves several different organ systems or 1 organ system requiring a high level of specialty care and hospitalization. Examples of CCCs include brain and spinal cord malformations, metabolic disorders, cardiac and respiratory malformations, and malignancies. CCCs account for 2.3% of all newborn discharges from hospitals in Ontario, Canada,30 as well as an increasing proportion of childhood deaths26 and pediatric hospital care.31 There are limitations to approaches that rely exclusively on diagnostic codes. CCCs were not designed to describe the interaction of the condition with needs, functional limitations, and health care use. Thus, a particular CCC (eg, cystic fibrosis) can include children ranging from those who are virtually asymptomatic, and therefore unlikely to meet criteria to be considered CMC, to those with severe functional impairment who spend large portions of their lives interfacing with the health care system and would fulfill criteria for CMC. CCCs may also exclude children whose condition(s) is not defined by a particular coded diagnosis.
Other definitions of complexity focus on descriptions of functional domains and fragility in keeping with the World Health Organization's framework for classifying impairments, disabilities, and handicaps. These definitions include terms such as (1) children with multiple impairments (those with “significant physical disabilities combined with sensory and/or cognitive deficits”),1 (2) the technology-dependent child (“a child who requires both a medical device to compensate for the loss of a vital body function and significant and sustained care to avert death or further disability”),32 (3) the medically fragile child (who is either technology dependent or “requires substantial ongoing nursing care to avert death or further disability”),33 and (4) children with complex needs (“children with multiple health/developmental needs that require multiple services from multiple sectors, in multiple locations”).33 Of these definitions, medical technology dependence on devices such as a tracheostomy or enteral feeding tube has been most frequently used but by itself may only identify a smaller subpopulation of CMC.34
Lastly, administrative data have been used to profile resource use by classifying people into a health-status group and a severity level. Neff et al35,36 identified medically extreme “catastrophic” patients as having chronic conditions that are expected to be lifelong and progressive and to require extensive services. Examples of catastrophic conditions included quadriplegia, cystic fibrosis, and spina bifida. This “catastrophic” category comprised only 0.4% of the children but was responsible for 11% of health care charges and 24% of all pediatric hospital charges. However, in a follow-up article, Neff et al37 noted that only half of “catastrophic” children in a given year were identified because of the varying utilization between different years.
In sum, although a variety of definitions exist that capture 1 or more of the domains of CMC, each has its limitations. A definitional framework that captures needs, chronic conditions, functional limitations, and health care use is necessary to accurately describe this population of children and to develop interventions to improve their outcomes.
CLINICAL AGENDA
Despite the relatively small numbers of CMC, the impact of suboptimal care for these children on their health, their family's well-being, and the health care system is substantial. It is not surprising that in 2003 the Institute of Medicine identified CSHCN as a priority for national action and emphasized the impact of those with substantial medical problems.38
The evidence from examinations of clinical models of effective and efficient provision of health care for CMC remains remarkably limited. Existing care models include medical homes, comanagement, and hospital-based and hybrid models that focus on care coordination. Reports from several mainly uncontrolled studies have described the medical home,39–42 hospital-based programs,10,24,43 hospital-to-medical home transitions,22 home care,44–48 tele-home care,59 and disease-specific specialty clinics (eg, cystic fibrosis,50 epilepsy,51 and sickle cell disease52). Adult providers are also developing adult complex care models.53 The care models all typically provide enhanced care coordination and/or condition-specific expertise.
The medical home model, with the primary care physician as the central hub for care coordination, has been espoused as ideal for the care of CSHCN. However, implementation for CMC has been limited,54 likely because of time restrictions, inadequate payment, and lack of decision-making support for primary care providers. Many subspecialty care clinics are restricted to patients who fit a specific diagnosis; tertiary care complex care models provide enhanced decision-making support and care coordination at the hospital but can subsume care away from the community-based medical home.
A clinical agenda focused on an optimal model of care for CMC can be informed by the principles of the chronic care model (CCM) created by Wagner et al.55,56 The CCM has received considerable recent support in the literature as a framework for system-based reform.57 The CCM espouses health system redesign and emphasizes family/provider partnerships, improved self-management, and decision-making support by primary care providers. In accordance with the CCM, the ideal care model for CMC addresses each of the elements that define CMC. Specific components within the 4 domains of the definitional framework include:
Needs: a family-centered system of care that provides accessible health care services as well as information to families and empowers families in self-management. Provider uptake of the principles of family-centered care and inclusion of families as advisors to system redesign are crucial.
Chronic conditions: sufficient knowledge, understanding, and decision-making support across the entire continuum of care including both the community and tertiary care levels. In particular, ongoing education or support to primary care providers on the care requirements of the child or population may be necessary.
Functional limitations: ensuring availability of supports for the family and community, including necessary medical technology for maximization of functioning within the key dimensions of body structure and function, performance of activities, and participation in communal life.27
Health care use: a care-delivery system that prioritizes high-quality and efficient care through enhanced care coordination and clearly defined provider roles across different settings.
Creating care models for CMC requires champions across a variety of settings to implement a quality-improvement agenda. Initial efforts can be focused on single or bundled quality-improvement implementation tools. Examples include care plans, dedicated care coordinators, targeted patient-safety initiatives (eg, enhanced medical reconciliation), and/or provider education (eg, best practices for specific procedures or complications). Shared decision-making aids are another potentially useful tool that can help patients and families make value-based informed choices among relevant health care options.58 The frequent interface that these children have with the medical system also provides an opportunity to target patient-safety enhancements such as reduction of medical59 and medication60 errors. New services dedicated to CMC need to take into account the local facilities, expertise, existing care access, and community resources for CMC. Specific issues to address include the roles of complex care services and referring providers; the technical expertise, knowledge, and after-hours availability required of such services, particularly in the primary care setting; and communication tools, including referral forms, integrated electronic medical records, and communication between providers, needed to create a seamless and effective plan of care.
Providers for and families of CMC should advocate to hospitals, payers, and policy leaders for the priority of CMC as a target population for system reform. However, advocacy for CMC should not preclude advocacy for all CSHCN, because suboptimal systems performance is common for CSHCN. Such advocacy may include funding for dedicated clinical services; community provider information support; communication tools; and increased advisory roles and partnership by families. Care for complex and chronic illness is complicated by health care payment, which provides incentives for volume and procedures among physicians at the expense of care coordination, expanded nursing roles, and home care.61 Excessive hospital-based resource utilization has been used to justify development of complex care services.62 Although there has been no known controlled trial of dedicated complex care service provision for children, results of preliminary studies have shown potential for substantial cost savings by decreasing utilization of hospital days through care-coordination interventions.10,24,63–65 A key clinical and research priority that has yet to be addressed is the use of alternative payment models to reduce costs and improve quality for CMC.
RESEARCH AGENDA
The Institute of Medicine has identified the comparative effectiveness of programmatic models in severe chronic disease as a priority area of research.66
To date, the definitions, designs, and outcomes of evaluation studies for CMC vary markedly. A focused research agenda on CMC must operationalize the definitional framework found in Fig 1 into tools that are reliable, valid, and feasible. Most administrative data sets cannot fully capture CMC and will almost certainly be limited in scope if they cannot incorporate family-identified needs and functional limitations. However, adaptation of existing instruments such as adding questions that capture elements of our definitional framework to the NS-CSHCN may help achieve this goal, particularly if it is augmented by linked data from health administrative data sets and/or instruments completed by providers to create a complexity scale.
Improvement science that addresses the care of CMC needs to link the clinical interventions being assessed (eg, models of care, care plans, reimbursement strategies) with important and measurable outcomes. Examples of such outcomes should reflect the definitional framework of CMC, specifically meeting family-identified needs, reducing condition-specific health complications, addressing functional limitations, and reducing unnecessary health service use, including emergency department visits, hospitalization days, readmissions, and cost of total care. Outcomes should discriminate between whether the intervention is condition-specific or condition-independent. Some existing literature focuses on condition-specific interventions and outcomes common to many CMC. Examples include comparative effectiveness of treatments for particular subgroups of children (eg, gastroesophageal reflux disease and comparative antireflux procedures for children with neurologic impairment).67,68 It is unfortunate that CMC are often excluded from condition-specific studies because of the rarity of underlying conditions, disease severity, and/or multiple comorbidities.69
Studies that focus on condition-independent outcomes could include measures of perceptions of health care quality or health-related quality of life, both of which are particularly important for a group of children who interface so frequently with the medical system. Mental health should also be measured as salient outcomes, because psychiatric issues are common in many CMC.70 Because of the potent and pervasive impact of complex conditions on families and parental caregivers, including enhanced stress,71 poor health,72,73 marital discord,74 and employment and financial consequences,75 families should be actively involved in the research process and help inform the selection of important family-based outcomes. Examples of family-based outcomes include caregiver health and quality of life, family functioning and resiliency, the effects on siblings, and/or financial impact. Other condition-independent outcomes include integration and participation in the community76 and family knowledge about and utilization of their child's medical care and community resources. Careful consideration should be given in all studies of condition-independent outcomes in CMC to appropriate comparison groups, including both children with and without special health care needs. Outcome measures should encompass the holistic International Classification of Functioning, Disability and Health definition of functioning, disability, and health,27,28 which includes domains that may (eg, body structure and function) or may not (eg, activities and participation) be permanently impaired among some CMC. For the many CMC with life-limiting conditions, key processes of care such as the availability of palliative care, end-of-life care, advanced directives, and accessibility to hospice care should be addressed as well.
Finally, critical goals for all CSHCN include seamless health care transitions for adolescents and young adults to the adult medical care system.77,78 Examples of successful transitions to adult care exist for specific complex and chronic medical conditions.79–81 Such models need to be applied and evaluated in a broader group of youth regardless of their underlying diagnostic condition.
CONCLUSIONS
CMC pose important challenges to families, providers, and our health care system that are qualitatively different from those of other populations of CSHCN. The development of novel complex care programs at multiple institutions suggests that traditional systems of care may not be meeting the substantial needs of such patients and their families. Although all CSHCN have unique and important needs, we believe that the coexistence of multiple family-identified service needs, chronic conditions, functional limitations, and extraordinarily high health care use that characterize these children demands a focused approach for this important subset of CSHCN. The creation of sustainable evidence-based models of care, using providers who are adequately trained and resourced to serve the needs of CMC, is essential for enhancing the quality of life and outcomes for these children.
ACKNOWLEDGMENTS
Dr Cohen is supported by the Norman Saunders Complex Care Initiative at the Hospital for Sick Children. Dr Kuo is supported by the Arkansas Biosciences Institute, the Marion B. Lyon New Scientist Award, and the National Center for Research Resources (grant KL2RR029883). Dr Berry is supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) grant K23 HD058092-01. Dr Simon is supported by National Institute of Neurological Disorders and Stroke Award K23 NS062900. Dr Srivastava is supported by NICHD career development award K23 HD052553 and in part by the Children's Health Research Center at the University of Utah and Primary Children's Medical Center Foundation.
We acknowledge the contributions of John Gordon, MD, Hema Patel, MD, David Hall, MD, Carl Tapia, MD, Allison Ballantine, MD, Linda Lindeke, RN, PhD, Sanjay Mahant, MD, and Nancy Murphy, MD, to the development of ideas described in and/or for critical review of this article.
APPENDIX.
Clinical Examples of CMC
| Domain | Type of Complexity | Subtype | Child With Severe Neurologic Impairments | Child With Multiple Congenital Anomalies | Child With a Complex Cardiac Condition | Child With Severe Autism |
|---|---|---|---|---|---|---|
| Needs | Medical problems | Organ system | Brain (seizures, global developmental delay, abnormal tone, behavioral problems, visual/hearing impairment); respiratory (chronic and recurrent pneumonia, reactive airways disease); ear, nose, and throat (sialorrhea, upper airway obstruction); gastrointestinal (gastroesophageal reflux, oromotor feeding problems, constipation); musculoskeletal (scoliosis/contractures) | Condition- and phenotype-specific | Brain (eg, at risk of developmental delay); respiratory (chronic lung disease); gastrointestinal (gastroesophageal reflux disease, failure to thrive) | Brain (seizures, global developmental delay, abnormal tone, behavioral problems) |
| Medications | Antiepileptic drugs; bronchodilators; inhaled corticosteroids; gastric acid suppressants; promotility agents; antispasticity agents; antibiotics; laxatives | Condition- and phenotype-specific | Gastric acid suppressants; promotility agents; diuretics; cardiac medications (eg, anti-arrhythmic agent) | Antiepileptic medication; other neuropsychiatric drugs (eg, antipsychotic medications, mood stabilizers, etc) | ||
| Surgeries | Nissen fundoplication; scoliosis repair; salivary gland ligation; ventriculoperitoneal shunt insertion | Condition- and phenotype-specific | ≥1 complex cardiac surgery; Nissen fundoplication | |||
| Other, family-identified problems | Financial burden; respite; education; support | Financial burden; respite; education; support | Financial burden; respite; education; support | Financial burden; respite; education; support | ||
| Conditions | CCCs that are severe and/or associated with fragility | Known: congenital; acquired; unknown (eg, undiagnosed or undescribed syndrome) | Brain tumors; congenital brain anomalies; chromosomal anomaly (eg, trisomy 21); other genetic syndrome (eg, Rett syndrome, tuberous sclerosis, etc); acquired brain injury; neurodegenerative metabolic disease (eg, Krabbe disease); hypoxic ischemic encephalopathy; cerebral palsy with global developmental delay; etc | Chromosomal anomalies; skeletal dysplasias; overgrowth syndromes; storage disorders; congenital connective tissue disorders; sequences; associations; teratogens; unknown; etc | Hypoplastic left heart syndrome; double-inlet left ventricle, other congenital heart diseases; heart transplantation; etc | May be associated with other conditions (eg, tuberous sclerosis, other neurologic conditions) |
| Functional limitations | Developmental disability | Motor domain | Unable to sit and/or walk and require seating and ambulatory assistive devices | Condition- and phenotype-specific | Condition- and child-specific but global developmental disability common in some (eg, hypoplastic left heart); exercise limitation common | Variable |
| Speech domain | Unable to verbally and/or nonverbally communicate; require assistive communicative devices | Condition- and phenotype-specific | Variable | Wide range of issues in both verbal and nonverbal domains characteristic | ||
| Feeding domain | Unsafe and/or unable to feed normal child foods/liquids, which requires alterations of food textures and type | Condition- and phenotype-specific | Need for supplemental nutrition/hypercaloric intake common | Variable | ||
| Social domain | Variable; integration and participation in community-based activities can be challenging | Variable; integration and participation in community-based activities can be challenging | Variable; integration and participation in community-based activities can be challenging | Impairments in social activities (eg, reciprocal interactions) characteristic | ||
| Technology dependence | Enterostomy tubes; suction machines; tracheostomy tubes; ventriculoperitoneal shunts; feeding pumps; home oxygen | Condition- and phenotype-specific | Variable but could include one or more of the following: enterostomy tubes, tracheostomy tubes, home oxygen, implantable cardiac devices, etc | Variable but can be completely reliant on others for activities of daily living | ||
| Health care utilization | Providers | Medical | Primary care provider (eg, pediatrician); neurologist; gastroenterologist; pulmonologist; otolaryngologist; developmental pediatrician; general surgeon; orthopedic surgeon; neurosurgeon; hospitalist | Primary care provider (eg, pediatrician); large number of subspecialists characteristic | Primary care provider (eg, pediatrician); cardiologist; cardiac surgeon; pulmonologist; large number of other; specialists common | Primary care provider (eg, pediatrician); neurologist; developmental pediatrician; psychiatrist |
| Other health professionals | Physiotherapist; occupational therapist; social worker; speech/language pathologist; dietician; nurses; pharmacist | Physiotherapist; occupational therapist; social worker; speech/language pathologist; dietician; nurses; pharmacist | Physiotherapist; occupational therapist; social worker; speech/language pathologist; dietician; nurses; pharmacist | Social worker; physiotherapist; occupational therapist; speech/language pathologist; nurses; pharmacist; mental health professionals | ||
| Health services | Hospital | Emergency department; outpatient clinics; inpatient stays; laboratory services; diagnostic imaging | Emergency department; outpatient clinics; inpatient stays; laboratory services; diagnostic imaging | Emergency department; outpatient clinics; inpatient stays; laboratory services; diagnostic imaging | Hospital use less likely to be inpatient-based but still common; use of extensive psychiatric services common both in hospital and, in particular, in the community | |
| Community | School; home/congregate care; respite care; rehabilitation services; transitional care | School; home/congregate care; respite care; rehabilitation services; transitional care | School; home/congregate care; respite care; rehabilitation services; transitional care | School; home/congregate care; respite care; rehabilitation services; transitional care | ||
| Interrelated Services | Specifics may be jurisdiction-specific, but cross-sectorial care is almost universal | Family/social support; mental and behavioral health; housing; transportation; faith-based/spiritual | Family/social support; mental and behavioral health; housing; transportation; faith-based/spiritual | Family/social support; mental and behavioral health; housing; transportation; faith-based/spiritual | Family/social support; mental and behavioral health; housing; transportation; faith-based/spiritual |
These examples are for illustrative purposes to emphasize some of the types of children and the spectrum of clinical and systems issues that characterize CMC.
The funders and sponsors were not involved in the design or conduct of the study or preparation, review, or approval of the manuscript.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
- CSHCN
- children with special health care needs
- CCC
- complex chronic condition
- CMC
- children with medical complexity
- NS-CSHCN
- National Survey of Children With Special Health Care Needs
REFERENCES
- 1. McPherson M, Arango P, Fox H, et al. A new definition of children with special health care needs. Pediatrics. 1998;102(1 pt 1):137–140 [DOI] [PubMed] [Google Scholar]
- 2. Bethell CD, Read D, Blumberg SJ, Newacheck PW. What is the prevalence of children with special health care needs? Toward an understanding of variations in findings and methods across three national surveys. Matern Child Health J. 2008;12(1):1–14 [DOI] [PubMed] [Google Scholar]
- 3. Bramlett MD, Read D, Bethell C, Blumberg SJ. Differentiating subgroups of children with special health care needs by health status and complexity of health care needs. Matern Child Health J. 2009;13(2):151–163 [DOI] [PubMed] [Google Scholar]
- 4. Davis MM, Brosco JP. Being specific about being special: defining children's conditions and special health care needs. Arch Pediatr Adolesc Med. 2007;161(10):1003–1005 [DOI] [PubMed] [Google Scholar]
- 5. Feudtner C, Feinstein JA, Satchell M, Zhao H, Kang TI. Shifting place of death among children with complex chronic conditions in the United States, 1989–2003. JAMA. 2007;297(24):2725–2732 [DOI] [PubMed] [Google Scholar]
- 6. Lauver LS. Parenting foster children with chronic illness and complex medical needs. J Fam Nurs. 2008;14(1):74–96 [DOI] [PubMed] [Google Scholar]
- 7. Palfrey JS. Amber, Katie, and Ryan: lessons from children with complex medical conditions. J Sch Health. 1995;65(7):265–267 [DOI] [PubMed] [Google Scholar]
- 8. Cady R, Finkelstein S, Kelly A. A telehealth nursing intervention reduces hospitalizations in children with complex health conditions. J Telemed Telecare. 2009;15(6):317–320 [DOI] [PubMed] [Google Scholar]
- 9. Hochstadt NJ, Yost DM. The health care-child welfare partnership: transitioning medically complex children to the community. Child Health Care. 1989;18(1):4–11 [DOI] [PubMed] [Google Scholar]
- 10. Gordon JB, Colby HH, Bartelt T, Jablonski D, Krauthoefer ML, Havens P. A tertiary care-primary care partnership model for medically complex and fragile children and youth with special health care needs. Arch Pediatr Adolesc Med. 2007;161(10):937–944 [DOI] [PubMed] [Google Scholar]
- 11. Msall ME, Tremont MR. Measuring functional outcomes after prematurity: developmental impact of very low birth weight and extremely low birth weight status on childhood disability. Ment Retard Dev Disabil Res Rev. 2002;8(4):258–272 [DOI] [PubMed] [Google Scholar]
- 12. Tennant PW, Pearce MS, Bythell M, Rankin J. 20-year survival of children born with congenital anomalies: a population-based study. Lancet. 2010;375(9715):649–656 [DOI] [PubMed] [Google Scholar]
- 13. Wong LY, Paulozzi LJ. Survival of infants with spina bifida: a population study, 1979–94. Paediatr Perinat Epidemiol. 2001;15(4):374–378 [DOI] [PubMed] [Google Scholar]
- 14. Hallahan AR, Shaw PJ, Rowell G, O'Connell A, Schell D, Gillis J. Improved outcomes of children with malignancy admitted to a pediatric intensive care unit. Crit Care Med. 2000;28(11):3718–3721 [DOI] [PubMed] [Google Scholar]
- 15. Magnani C, Pastore G, Coebergh JW, Viscomi S, Spix C, Steliarova-Foucher E. Trends in survival after childhood cancer in Europe, 1978–1997: report from the Automated Childhood Cancer Information System project (ACCIS). Eur J Cancer. 2006;42(13):1981–2005 [DOI] [PubMed] [Google Scholar]
- 16. Hjern A, Lindblad F, Boman KK. Disability in adult survivors of childhood cancer: a Swedish national cohort study. J Clin Oncol. 2007;25(33):5262–5266 [DOI] [PubMed] [Google Scholar]
- 17. Thompson JR, Carter RL, Edwards AR, et al. A population-based study of the effects of birth weight on early developmental delay or disability in children. Am J Perinatol. 2003;20(6):321–332 [DOI] [PubMed] [Google Scholar]
- 18. Glendinning C, Kirk S, Guiffrida A, Lawton D. Technology-dependent children in the community: definitions, numbers and costs. Child Care Health Dev. 2001;27(4):321–334 [DOI] [PubMed] [Google Scholar]
- 19. Betz CL. Transition of adolescents with special health care needs: review and analysis of the literature. Issues Compr Pediatr Nurs. 2004;27(3):179–241 [DOI] [PubMed] [Google Scholar]
- 20. Stille CJ, Antonelli RC. Coordination of care for children with special health care needs. Curr Opin Pediatr. 2004;16(6):700–705 [DOI] [PubMed] [Google Scholar]
- 21. Srivastava R, Stone BL, Murphy NA. Hospitalist care of the medically complex child. Pediatr Clin North Am. 2005;52(4):1165–1187, x [DOI] [PubMed] [Google Scholar]
- 22. Kelly A, Golnik A, Cady R. A medical home center: specializing in the care of children with special health care needs of high intensity. Matern Child Health J. 2008;12(5):633–640 [DOI] [PubMed] [Google Scholar]
- 23. Cohen E, Friedman JN, Mahant S, Adams S, Jovcevska V, Rosenbaum P. The impact of a complex care clinic in a children's hospital. Child Care Health Dev. 2010;36(4):574–582 [DOI] [PubMed] [Google Scholar]
- 24. Berman S, Rannie M, Moore L, Elias E, Dryer LJ, Jones MD., Jr Utilization and costs for children who have special health care needs and are enrolled in a hospital-based comprehensive primary care clinic. Pediatrics. 2005;115(6). Available at: www.pediatrics.org/cgi/content/full/115/6/e637 [DOI] [PubMed] [Google Scholar]
- 25. van der Lee J, Mokking L, Grooetnhuis M, Heymans H, Offringa M. Definitions and measurement of chronic health conditions in childhood. JAMA. 2007;297(24):2741–2751 [DOI] [PubMed] [Google Scholar]
- 26. Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980–1997. Pediatrics. 2000;106(1 pt 2):205–209 [PubMed] [Google Scholar]
- 27. World Health Organization International Classification of Impairments, Disabilities and Handicaps: A Manual of Classification Relating to the Consequences of Diseases. Geneva, Switzerland: World Health Organization; 1980 [Google Scholar]
- 28. World Health Organization International Classification of Functioning, Disability and Health: Children and Youth Version (ICF-CY). Geneva, Switzerland: World Health Organization; 2007 [Google Scholar]
- 29. Blumberg SJ, Welch EM, Chowdhury SR, Upchurch HL, Parker EK, Skalland BJ. Design and operation of the National Survey of Children With Special Health Care Needs, 2005–2006. Vital Health Stat 1. 2008;(45):1–188 [PubMed] [Google Scholar]
- 30. Wang C, Guttmann A, To T, Dick PT. Neighborhood income and health outcomes in infants: how do those with complex chronic conditions fare? Arch Pediatr Adolesc Med. 2009;163(7):608–615 [DOI] [PubMed] [Google Scholar]
- 31. Simon TD, Berry J, Feudtner C, et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126(4):647–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stein R, Jessop D. A noncategorical approach to chronic childhood illness. Public Health Rep. 1982;97(4):354–362 [PMC free article] [PubMed] [Google Scholar]
- 33. Law M, Rosenbaum P. Service Coordination for Children and Youth With Complex Needs. Hamilton, ON, Canada: CanChild Centre for Childhood Disability Research, McMaster University; 2004 [Google Scholar]
- 34. Buescher PA, Whitmire JT, Brunssen S, Kluttz-Hile CE. Children who are medically fragile in North Carolina: using Medicaid data to estimate prevalence and medical care costs in 2004. Matern Child Health J. 2006;10(5):461–466 [DOI] [PubMed] [Google Scholar]
- 35. Neff JM, Sharp VL, Muldoon J, Graham J, Myers K. Profile of medical charges for children by health status group and severity level in a Washington State Health Plan. Health Serv Res. 2004;39(1):73–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Neff JM, Sharp VL, Muldoon J, Graham J, Popalisky J, Gay JC. Identifying and classifying children with chronic conditions using administrative data with the clinical risk group classification system. Ambul Pediatr. 2002;2(1):71–79 [DOI] [PubMed] [Google Scholar]
- 37. Neff JM, Sharp VL, Popalisky J, Fitzgibbon T. Using medical billing data to evaluate chronically ill children over time. J Ambul Care Manage. 2006;29(4):283–290 [DOI] [PubMed] [Google Scholar]
- 38. Institute of Medicine Priority areas for national action: transforming health care quality. Available at: http://iom.edu/Reports/2003/Priority-Areas-for-National-Action-Transforming-Health-Care-Quality.aspx Accessed June 17, 2010
- 39. Palfrey JS, Sofis LA, Davidson EJ, Liu J, Freeman L, Ganz ML; Pediatric Alliance for Coordinated Care The Pediatric Alliance for Coordinated Care: evaluation of a medical home model. Pediatrics. 2004;113(5 suppl):1507–1516 [PubMed] [Google Scholar]
- 40. Cooley WC; American Academy of Pediatrics, Committee on Children With Disabilities Providing a primary care medical home for children and youth with cerebral palsy. Pediatrics. 2004;114(4):1106–1113 [DOI] [PubMed] [Google Scholar]
- 41. Cooley WC, McAllister JW. Building medical homes: improvement strategies in primary care for children with special health care needs. Pediatrics. 2004;113(5 suppl):1499–1506 [PubMed] [Google Scholar]
- 42. Cooley WC, McAllister JW, Sherrieb K, Kuhlthau K. Improved outcomes associated with medical home implementation in pediatric primary care. Pediatrics. 2009;124(1):358–364 [DOI] [PubMed] [Google Scholar]
- 43. Gillette Y, Hansen NB, Robinson JL, Kirkpatrick K, Grywalski R. Hospital-based case management for medically fragile infants: results of a randomized trial. Patient Educ Couns. 1991;17(1):59–70 [DOI] [PubMed] [Google Scholar]
- 44. Stein RE, Jessop DJ. Does pediatric home care make a difference for children with chronic illness? Findings from the Pediatric Ambulatory Care Treatment Study. Pediatrics. 1984;73(6):845–853 [PubMed] [Google Scholar]
- 45. Jessop DJ, Stein RE. Providing comprehensive health care to children with chronic illness. Pediatrics. 1994;93(4):602–607 [PubMed] [Google Scholar]
- 46. Jessop DJ, Stein RE. Who benefits from a pediatric home care program? Pediatrics. 1991;88(3):497–505 [PubMed] [Google Scholar]
- 47. Stein R. A home care program for children with chronic illness. Child Health Care. 1983;12(2):90–92 [DOI] [PubMed] [Google Scholar]
- 48. Bergius H, Eng A, Fagerberg M, et al. Hospital-managed advanced care of children in their homes. J Telemed Telecare. 2001;7(suppl 1):32–34 [DOI] [PubMed] [Google Scholar]
- 49. Liddy C, Dusseault JJ, Dahrouge S, Hogg W, Lemelin J, Humbert J. Telehomecare for patients with multiple chronic illnesses: pilot study. Can Fam Physician. 2008;54(1):58–65 [PMC free article] [PubMed] [Google Scholar]
- 50. Thomas CL, O'Rourke PK, Wainwright CE. Clinical outcomes of Queensland children with cystic fibrosis: a comparison between tertiary centre and outreach services. Med J Aust. 2008;188(3):135–139 [DOI] [PubMed] [Google Scholar]
- 51. Williams J, Sharp GB, Griebel ML, et al. Outcome findings from a multidisciplinary clinic for children with epilepsy. Children's Health Care. 1995;24(4):235–244 [DOI] [PubMed] [Google Scholar]
- 52. Rahimy MC, Gangbo A, Ahouignan G, et al. Effect of a comprehensive clinical care program on disease course in severely ill children with sickle cell anemia in a sub-Saharan African setting. Blood. 2003;102(3):834–838 [DOI] [PubMed] [Google Scholar]
- 53. Weiss KB. Managing complexity in chronic care: an overview of the VA state-of-the-art (SOTA) conference. J Gen Intern Med. 2007;22(suppl 3):374–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Strickland B, McPherson M, Weissman G, van Dyck P, Huang ZJ, Newacheck P. Access to the medical home: results of the National Survey of Children With Special Health Care Needs. Pediatrics. 2004;113(5 suppl):1485–1492 [PubMed] [Google Scholar]
- 55. Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood). 2001;20(6):64–78 [DOI] [PubMed] [Google Scholar]
- 56. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288(14):1775–1779 [DOI] [PubMed] [Google Scholar]
- 57. Coleman K, Austin BT, Brach C, Wagner EH. Evidence on the chronic care model in the new millennium. Health Aff (Millwood). 2009;28(1):75–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Edwards A, Elwyn G. Shared Decision-Making in Health Care: Achieving Evidence-Based Patient Choice. New York, NY: Oxford University Press; 2009 [Google Scholar]
- 59. Sacchetti A, Sacchetti C, Carraccio C, Gerardi M. The potential for errors in children with special health care needs. Acad Emerg Med. 2000;7(11):1330–1333 [DOI] [PubMed] [Google Scholar]
- 60. Stone BL, Boehme S, Mundorff MB, Maloney CG, Srivastava R. Hospital admission medication reconciliation in medically complex children: an observational study. Arch Dis Child. 2010;95(4):250–255 [DOI] [PubMed] [Google Scholar]
- 61. Rappo PD. The care of children with chronic illness in primary care practice: implications for the pediatric generalist. Pediatr Ann. 1997;26(11):687–695 [DOI] [PubMed] [Google Scholar]
- 62. Srivastava R, Stone BL, Patel R, et al. Delays in discharge in a tertiary care pediatric hospital. J Hosp Med. 2009;4(8):481–485 [DOI] [PubMed] [Google Scholar]
- 63. Balaban RB, Weissman JS, Samuel PA, Woolhandler S. Redefining and redesigning hospital discharge to enhance patient care: a randomized controlled study. J Gen Intern Med. 2008;23(8):1228–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Criscione T, Walsh KK, Kastner TA. An evaluation of care coordination in controlling inpatient hospital utilization of people with developmental disabilities. Ment Retard. 1995;33(6):364–373 [PubMed] [Google Scholar]
- 65. Liptak GS, Burns CM, Davidson PW, McAnarney ER. Effects of providing comprehensive ambulatory services to children with chronic conditions. Arch Pediatr Adolesc Med. 1998;152(10):1003–1008 [DOI] [PubMed] [Google Scholar]
- 66. Institute of Medicine Initial national priorities for comparative effectiveness research. Available at: www.iom.edu/Reports/2009/ComparativeEffectivenessvResearchPriorities.aspx Accessed July 19, 2010
- 67. Wales PW, Diamond IR, Dutta S, et al. Fundoplication and gastrostomy versus image-guided gastrojejunal tube for enteral feeding in neurologically impaired children with gastroesophageal reflux. J Pediatr Surg. 2002;37(3):407–412 [DOI] [PubMed] [Google Scholar]
- 68. Srivastava R, Downey EC, O'Gorman M, et al. Impact of fundoplication versus gastrojejunal feeding tubes on mortality and in preventing aspiration pneumonia in young children with neurologic impairment who have gastroesophageal reflux disease. Pediatrics. 2009;123(1):338–345 [DOI] [PubMed] [Google Scholar]
- 69. Cohen E, Zlotnik Shaul R. Beyond the therapeutic orphan: children and clinical trials. Ped Health. 2008;2(2):151–159 [Google Scholar]
- 70. Witt WP, Kasper JD, Riley AW. Mental health services use among school-aged children with disabilities: the role of sociodemographics, functional limitations, family burdens, and care coordination. Health Serv Res. 2003;38(6 pt 1):1441–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wallander JL, Varni JW. Effects of pediatric chronic physical disorders on child and family adjustment. J Child Psychol Psychiatry. 1998;39(1):29–46 [PubMed] [Google Scholar]
- 72. Brehaut JC, Kohen DE, Raina P, et al. The health of primary caregivers of children with cerebral palsy: how does it compare with that of other Canadian caregivers? Pediatrics. 2004;114(2). Available at: www.pediatrics.org/cgi/content/full/114/2/e182 [DOI] [PubMed] [Google Scholar]
- 73. Thorne SE, Radford MJ, Armstrong EA. Long-term gastrostomy in children: caregiver coping. Gastroenterol Nurs. 1997;20(2):46–53 [DOI] [PubMed] [Google Scholar]
- 74. Sabbeth BF, Leventhal JM. Marital adjustment to chronic childhood illness: a critique of the literature. Pediatrics. 1984;73(6):762–768 [PubMed] [Google Scholar]
- 75. Ratliffe CE, Harrigan RC, Haley J, Tse A, Olson T. Stress in families with medically fragile children. Issues Compr Pediatr Nurs. 2002;25(3):167–188 [DOI] [PubMed] [Google Scholar]
- 76. Rosenbaum P, Stewart D. The World Health Organization International Classification of Functioning, Disability, and Health: a model to guide clinical thinking, practice and research in the field of cerebral palsy. Semin Pediatr Neurol. 2004;11(1):5–10 [DOI] [PubMed] [Google Scholar]
- 77. American Academy of Pediatrics; American Academy of Family Physicians; American College of Physicians-American Society of Internal Medicine A consensus statement on health care transitions for young adults with special health care needs. Pediatrics. 2002;110(6 pt 2):1304–1306 [PubMed] [Google Scholar]
- 78. Reiss J, Gibson R. Health care transition: destinations unknown. Pediatrics. 2002;110(6 pt 2):1307–1314 [PubMed] [Google Scholar]
- 79. Parker HW. Transition and transfer of patients who have cystic fibrosis to adult care. Clin Chest Med. 2007;28(2):423–432 [DOI] [PubMed] [Google Scholar]
- 80. Reid GJ, Irvine MJ, McCrindle BW, et al. Prevalence and correlates of successful transfer from pediatric to adult health care among a cohort of young adults with complex congenital heart defects. Pediatrics. 2004;113(3 pt 1). Available at: www.pediatrics.org/cgi/content/full/113/3/e197 [DOI] [PubMed] [Google Scholar]
- 81. Simon TD, Lamb S, Murphy NA, Hom B, Walker ML, Clark EB. Who will care for me next? Transitioning to adulthood with hydrocephalus. Pediatrics. 2009;124(5):1431–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]