Abstract
The purpose of this review is to highlight existing literature on the epidemiology, pathophysiology, and treatments of stroke sleep disorders. Stroke sleep disorders are associated with many intermediary vascular risk factors leading to stroke, but they may also influence these risk factors through direct or indirect mechanisms. Sleep disturbances may be further exacerbated by stroke or caused by stroke. Unrecognized and untreated sleep disorders may influence rehabilitation efforts and poor functional outcomes following stroke and increase risk for stroke recurrence. Increasing awareness and improving screening for sleep disorders is paramount in the primary and secondary prevention of stroke and in improving stroke outcomes. Many vital questions about the relationship of sleep disorders and stroke are still unanswered and await future well-designed studies.
Keywords: insomnia, sleep apnea, sleep disorders, stroke
Introduction
Sleep disorders continue to be the most unrecognized modifiable risk factor for stroke (1). One third of US adults report sleeping less than seven-hours per night and 50–70 million have a sleep disorder (1,2). The relationship between sleep disorders and vascular risk factors and stroke has been well-documented but not fully understood. Sleep disorders may contribute to stroke vascular pathology through multiple direct or indirect mechanisms. In addition, they may also be caused or exacerbated by stroke. Furthermore, the consequences of untreated sleep disorders (cognitive dysfunction, altered mood, sleepiness, and fatigue) may impede stroke rehabilitation, lengthen hospital stay, and influence stroke outcomes and stroke recurrence (1,3). The following review article will provide the current state of epidemiology, pathophysiology, treatment, and prevention strategies for sleep-disordered breathing (SDB), insomnia, circadian rhythms, and sleep-related movement disorders in relation to stroke.
SDB
SDB is a broad term that describes breathing and ventilatory problems during sleep (4). SDB encompasses habitual snoring, obstructive sleep apnea (OSA), and central sleep apnea (CSA) (Table 1).
Table 1.
Sleep terms and definitions
| Term | Definition |
|---|---|
| Apnea | Cessation of airflow for at least 10 s, usually accompanied by oxygen desaturation |
| Hypopnea | Reduction in airflow by at least 30–50% of baseline, usually accompanied by oxygen desaturation or an arousal |
| Arousal | Awakening from sleep lasting three-seconds to 15 s |
| Apnea–hypopnea index | Number of apneas and hypopneas per hour of sleep, a marker of sleep apnea severity |
| Obstructive sleep apnea and hypopnea | Apneas or hypopneas resulting from complete or partial collapse of the upper airway collapse during sleep |
| Central sleep apnea and hypopnea | Apneas or hypopneas resulting from absence or insufficient central respiratory drive to respiratory muscles |
| Sleep apnea syndrome | At least five apneas and hypopneas per hour of sleep, associated with complaints of snoring, breathing pauses, insomnia, unrefreshing sleep, excessive daytime sleepiness, or fatigue |
| Epworth Sleepiness Scale | Questionnaire measuring sleepiness (0–3) in eight daytime situations. A score of 10 or higher is associated with significant sleepiness |
| Polysomnography | Multichannel physiological recording of electroencephalographic, electrooculographic, electromyoghaphic, electrocardiogram, and respiratory activity with audiovisual correlation to detect sleeping disturbances |
| Insomnia | Difficulty with sleep initiation or maintenance or unintended early awakenings |
| Circadian rhythm | Biological process with periodicity approximating 24 h |
| Actigraphy | 24-h motion recording detected by wearing a watch-like device (accelerometer) used to infer sleep-wake patterns for prolonged periods |
| Restless legs syndrome | Sensorimotor disorder composed of uncomfortable sensations with an urge to move the legs, worsened by inactivity, and in the evening, relieved by movement of the legs, often causing insomnia |
| Periodic limb movements | Rhythmic movements of the extremities (most commonly the legs) occurring during sleep or wakefulness, sometimes interrupting sleep onset or continuity |
Habitual snoring affects up to 40% of the adult population and along with breathing pauses in sleep and daytime sleepiness are cardinal symptoms of OSA (5). The characteristics of OSA are partial or complete closure of the upper airway, leading to blood oxygen desaturation and sleep fragmentation (5). Epidemiological studies estimate that up to 17% of the adult population has OSA with increased prevalence and severity in the elderly (6–8). OSA is present in up to 25% of patients over 65 years (6–8). There are race–ethnic differences in the prevalence of sleep symptoms and OSA. In the population-based Sleep Heart Health Study (SHHS), a higher prevalence of snoring symptoms was reported in Hispanics and African Americans than in Caucasians (9). Clinical risk factors for OSA include obesity, male gender, increased neck size (≥17 in. in men; ≥16 in. in women), and craniofacial features (e.g. retrognathia) that reduce upper airway size (5,7). CSA may be more prevalent in men (about 8%) than in women (less than 1%) (1,4). The prevalence of central apnea is influenced by several factors, including age, gender, the presence of heart failure, and certain metabolic disorders.
Symptoms of OSA and screening methods
Daytime sleepiness is one of the cardinal symptoms of OSA. One of the most common methods used to evaluate for daytime sleepiness is the Epworth Sleepiness Scale (ESS) (10). The ESS rates self-reports of dozing off unintentionally during the day in eight sedentary situations:
sitting and reading
watching television
sitting inactive in a public place
as a passenger in a car, train, or bus
lying down to rest in the afternoon when circumstances permit
sitting and talking to someone
sitting quietly after a lunch without alcohol
in a car while stopped for a few minutes in traffic.
Responses ranged from 0 = rarely or never dozing to 3 = most or all of the time dozing with a maximum score of 24. Daytime sleepiness is considered pathological when ESS score is ≥10.
Risk predictive models have been proposed to identify individuals at high risk for OSA. The Berlin Questionnaire is commonly used and combines risk factors such as snoring, daytime sleepiness, obesity, and hypertension to reliably predict OSA from polysomnography (PSG) (11). These responses are used to stratify patients into low- or high-risk categories for sleep apnea. A subject is considered to be at high risk if two of the three following categories are met:
report of snoring symptoms (volume and frequency) at least three times per week
daytime sleepiness exceeding three times per week, or history of falling asleep while driving
the presence of hypertension or a body mass index greater than 30 kg/m2.
The presence of two out of the four factors in the Berlin Questionnaire predicts OSA with a sensitivity of 86%, specificity of 77%, and positive predictive value of 89%. Present and strong evidence supports high-risk patients undergoing confirmatory PSG in an expedited manner in order to initiate treatment (Tables 2 and 4).
Table 2.
Population at high risk for sleep disordered breathing
| Populations at high risk for sleep-disordered breathing and obstructive sleep apnea | History and symptoms prompting sleep evaluation by polysomnography |
|---|---|
| History of Stroke | Daytime sleepiness (≥10 Epworth Sleepiness Scale)* |
| Obesity (body mass index > 30)* | Loud and habitual snoring* |
| Congestive heart failure | History of hypertension* |
| Atrial fibrillation | Gasping/choking at night |
| Refractory hypertension | Witnessed apneas |
| Type 2 diabetes | Short-lasting morning headaches |
| Nocturnal dysrhythmias | Frequent awakenings |
| Nondipping blood pressure | Memory loss |
| Pulmonary hypertension | Decreased concentration |
| Shift workers | Frequent nocturia |
Berlin Questionnaire items.
Table 4.
Sleep disorders, cardiovascular disease, and stroke in epidemiological studies
| Study | Design | n | Outcome | OR/HR (95% CI) |
|---|---|---|---|---|
| OSA | ||||
| SHHS; Shahar et al. 2001 | Cross-sectional | 6424 | Heart failure; | 2·4 (1·2–4·6) |
| CAD; | 1·3 (1·0–1·6) | |||
| Stroke | 1·6 (1·0–2·5) | |||
| WSC; Arzt et al. 2005 | Cross-sectional | 1475 | Stroke | 3·8 (1·2–12·5) |
| Yaggi et al. 2005 | Prospective cohort | 1022 | Stroke or death | 2·0 (1·1–3·5) |
| Munoz et al. 2006 | Prospective cohort | 394 | Stroke | 2·5 (1·0–6·0) |
| SHHS | Prospective cohort | 5422 | Stroke | Men, 2·9 (1·1–7·4) |
| Redline et al. 2010 | Women, 1·2 (0·7–2·2) | |||
| Insomnia | ||||
| ARIC | Prospective cohort | 11 863 | HTN; | 1·2 (1·0–1·3) |
| Phillips and Mannino, 2007 | CVD | 1·5 (1·1–2·0) | ||
| Vgontzas et al. 2009 | Cross-sectional | 1741 | HTN <5 vs. >six-hours of sleep | 5·1 (2·2–11·8) |
| Chien et al. 2010 | Prospective cohort | 3430 | CAD/stroke; | 1·8 (1·0–3·1) |
| Death | 1·7 (1·2–2·5) | |||
| RLS | ||||
| SHHS; Winkelman et al. 2008 | Cross-sectional | 3433 | HTN; | 1·3 (0·9–1·8) |
| CAD; | 2·2 (1·4–3·5) | |||
| CAD/stroke | 2·4 (1·6–3·7) | |||
| WSC; Winkelman et al. 2006 | Cross-sectional | 2821 | HTN; | 1·3 (0·9–1·9) |
| CAD/stroke | 2·6 (1·4–4·8) | |||
| Ulfberg et al. 2001 | Cross-sectional | 2608 | CVD; | 2·5 (1·4–4·3) |
| HTN | 1·5 (0·9–2·4) |
ARIC, Atherosclerosis Risk In Communities; CAD, coronary artery disease; CI, confidence interval; CVD, cardiovascular disease; HR, hazards ratio; HTN, hypertension; OR, odds ratio; OSA, obstructive sleep apnea; PSG, polysomnography; RLS, restless legs syndrome SHHS, Sleep Heart Health Study; WSC, Wisconsin Sleep Cohort.
OSA and vascular risk factors
The association between OSA and vascular disease is in part mediated by the presence of major vascular risk factors. OSA is closely associated with hypertension, diabetes, and obesity. OSA increases the risk of hypertension in a dose-response pattern, the most prominent independent risk factor for ischemic stroke. In the SHHS, adjusted odds of hypertension (>140/90 mmHg) increased steadily with apnea–hypopnea index (AHI) > 15/h of sleep (12). A high prevalence of OSA is also described in treatment resistant hypertension (13). OSA is linked to type 2 diabetes by the mechanisms of increased insulin resistance and cortisol levels (14). Also, OSA is related to altered levels of leptin, a hormone secreted by adipocytes, which promotes the sensation of feeling full and increases the metabolic rate (15). Untreated OSA causes resistance to the metabolic effects of leptin, promoting weight gain and obesity in this population.
In addition, two risk factors for cardioembolism have been linked to OSA, patent foramen ovale (PFO) and cardiac arrhythmias. It is suggested that patients with OSA have an increased risk of cardioembolism through paradoxical emboli (16). This may occur through increased intrathoracic pressure during apneas, but further studies are needed to clarify this relationship (17). OSA can cause or exacerbate cardiac arrhythmias during sleep and potentially lead to lethal tachyarrhythmias. The most common arrhythmias in relation to OSA are sinus pauses, heart block, and premature ventricular contractions (2). A significantly higher prevalence of moderate or severe OSA (AHI > 15/h) has been observed in younger patients (mean age 50 ± 12 years) with paroxysmal atrial fibrillation (AF) or sustained AF with normal left ventricular function as compared with age- and gender-matched subjects (62% vs. 38% controls, P = 0·01) (18). More frequent episodes of AF have been noted in subjects with worse OSA severity (18). Similarly, older subjects (mean age 61 ± 10 years) with sustained AF also have an increased prevalence of OSA (AHI > 10) when compared with controls (82% vs. 60% controls, P = 0·03) (19). These cardiac rhythm abnormalities are worse during rapid eye movement (REM) sleep, when autonomic system deregulation is expected. There is also an increased prevalence of OSA in patients with AF compared with patients with established cardiovascular risk factors (49% vs. 32%, P = 0·0004) (20). Up to 40% of symptomatic AF episodes occur between midnight and 8 am (21). Observational studies show an improvement or resolution of cardiac arrhythmias and AF with OSA treatment (22).
OSA symptoms and stroke
Habitual snoring and OSA are independent risk factors for stroke in middle-aged adults and in the elderly (23,24). The strong association between snoring and stroke is similar to that of traditional risk factors (25,26). Also, habitual snoring was more strongly associated with stroke during sleep (25). Excessive daytime sleepiness has been associated with stroke in the longitudinal Northern Manhattan Study (26). Excessive daytime sleepiness has also been described as a complication after acute stroke, especially with involvement of the thalamomesencephalic structures (1).
Population-based studies of OSA and stroke
With the use of PSG, the association between OSA and stroke has been established in multiple observational cohort studies (Table 4) (23,24,27–29). In a cross-sectional analysis of 6000 subjects from SHHS, prevalent stroke (odds ratio (OR): 1·6; 95% confidence interval (CI) 1·02–2·5) was greater among subjects with OSA (27). A cross-sectional analysis from the Wisconsin Sleep Cohort study of 1475 individuals showed an increased risk of stroke in subjects with an AHI ≥ 20 (OR 3·8; 95% CI: 1·2–12·6) after controlling for covariates (28). In another observational cohort study of patients referred to an academic sleep center for the evaluation of SDB, OSA was associated with an increased risk of stroke or death (hazard ratio (HR) 3·3; 95% CI: 1·7–6·3) independently of demographics and vascular risk factors (23). In the recent prospective analyses from the SHHS with a median follow up of 8·7 years (29), OSA at all levels of severity was associated with an increased risk of stroke in men (for the top AHI quartile of >19, an adjusted HR 2·9; 95% CI: 1·1–7·4) but not in women. There was an increased risk of stroke in women only with an AHI of more than 25 (29). A recent meta-analysis of 29 studies has reported that up to 72% of stroke patients have OSA as defined by an AHI greater than 5 (30). The patients with `cryptogenic stroke' had the highest incidence of OSA, raising the possibility that OSA may be an important cause of ischemic stroke of unknown etiology (30). Several mechanisms were suggested to explain this association including paradoxical embolism through a PFO, increased platelet activation, and apnea-induced reductions in cerebral blood flow (CBF) and oxygen saturation (30).
OSA and sub-clinical vascular disease
The effect of OSA on sub-clinical vascular disease has been reported. In a cross-sectional study of Japanese men, brain magnetic resonance imaging revealed silent brain infarcts in 25% of patients with moderate to severe OSA but in only 8% of patients with mild OSA and in 6% of control subjects, suggesting that OSA may elicit early and asymptomatic cerebrovascular damage (31). Sub-clinical carotid atherosclerosis (carotid intima–media thickness (IMT)) has been proposed as an intermediary between OSA and stroke, but this relationship remains controversial. In the two large population-based studies, Northern Manhattan Study and SHHS, snoring and insomnia were not found to be associated with carotid IMT (32,33). However, another study reported a significant association between severe OSA and carotid atherosclerosis (34). This study, however, involved a relatively small number of subjects referred to a tertiary care center.
Mechanisms linking OSA to stroke
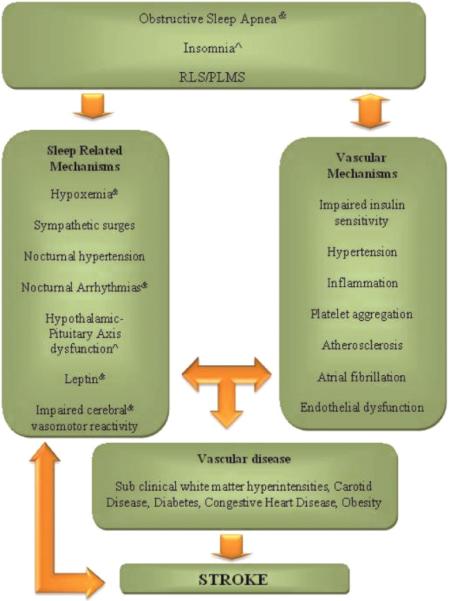
It is proposed that several OSA mechanisms increase the risk of stroke (Fig. 1). Hypoxemia, sympathetic surges, and nocturnal hypertension have been postulated as contributors of OSA in vascular disease. Damage to endothelial cells may occur by oxidative stress during hypoxemia related to apneas (35). In addition, large fluctuations in blood pressure (BP) during the obstructive events and sustained hypertension during sleep can cause increased turbulence and shear stress to the blood vessel walls (36). Arousals from sleep are associated to increased sympathetic surges, which may play a significant role in the amount of `nondipping' of BP in sleep leading to nocturnal hypertension (37). Possible intermediaries between OSA and stroke are alterations in cerebral hemodynamic flow. Obstructive events can lead to reductions in CBF and impaired cerebral autoregulation (38). Decreased CBF velocities in the middle cerebral artery territory during apneas have been demonstrated with transcranial Doppler (38). Changes in intrathoracic pressures during obstructive events may reduce CBF increasing the risk of stroke in vulnerable patients (38). Also, decreased CBF and impaired vasomotor reactivity have been observed during wakefulness in subjects with OSA (38). These mechanisms may cause cerebral small vessel disease as well as cerebral white matter disease and lead to sub-clinical cerebrovascular damage and permanent structural changes to the brain.
Fig. 1.
The association between sleep disorders, vascular disease and stroke. Mechanisms specific to sleep disorders are represented in the left column and established vascular mechanisms are represented in the right column. Sleep disorders potentiate vascular mechanisms, and vascular disease, in turn, contributes to sleep disorders (or exacerbate sleep disorders). The sleep-related mechanisms can lead to the development of risk factors (i.e. hypertension) and to sub-clinical vascular disease (brain white matter hyperintesitiens, carotid disease) and stroke; or sleep-related mechanisms are directly linked to stroke.
Another important mechanism that may cause clinical deterioration during an acute ischemic stroke is the Reversed Robin Hood Syndrome. This is an intracranial steal phenomenon associated with neurological deterioration in up to 7% of acute stroke patients (39). Oxygen desaturations coupled with hypercapnia during obstructions in OSA can trigger this cerebral blood steal phenomenon. Clinicians should be aware of OSA as a potential etiology of neurological worsening in acute stroke.
Treatment of OSA
Continuous positive airway pressure (CPAP) is the first-line treatment for patients with moderate to severe OSA. CPAP is administrated by a small-motorized unit that pushes pressurized air through a hose attached to a mask strapped on the patient's face. CPAP works as a`pneumatic splint' by delivering a positive intraluminal pressure, alleviating the repetitive episodes of upper airway collapse characteristic of sleep apnea, and completely or partially reversing its daytime consequences. Aside from improving sleep quality and daytime symptoms related to OSA, CPAP has been shown to decrease risk for cardiovascular events and have modest effects on BP (40,41) (Table 3). Its benefits are directly related to its adherence with improvements in subjective sleepiness achieved with >four-hours of nightly use. Mild decreases in BP (1·89 mmHg in systolic BP and 2·19 mmHg in diastolic BP) require a minimum of 5·6 h of CPAP nightly use (41).
Table 3.
Recommendations on diagnosis and treatment of sleep disorders and stroke (levels of evidence)
| Recommendation | Level of evidence |
|---|---|
| OSA | |
| Consider OSA treatment in men with any degree of OSA or in women with AHI > 25/h as a potential strategy for primary stroke prevention | B |
| Every stroke patient should be risk-stratified for OSA | A |
| Acute stroke patients at high risk for OSA should have overnight PSG >two-weeks after event | B |
| Positional therapy may be an alternative treatment for acute stroke patients with suspected or confirmed OSA | B |
| Stroke patients with moderate to severe OSA should be encouraged to use CPAP on a daily basis as a potential secondary stroke prevention strategy | B |
| Insomnia | |
| Short-term hypnotic medications and cognitive behavioral therapy are first-line treatments for patients chronic insomnia complaints (but trials in stroke populations are lacking) | C |
| Circadian disorders | |
| Consider hypertensive chronotherapy in patients with aberrant BP dipping pattern to decrease future cardiovascular and stroke risk | B |
| RLS/PLMs | |
| Patients with strokes involving the basal ganglia, corona radiate, or pyramidal tract should be clinically screened for RLS | B |
| Poststroke RLS complaints (>two times/week) with or without PLMs resulting in insomnia, disrupted sleep, and daytime consequences should be treated to enhance stroke recovery | C |
A, present and strong; AHI, apnea–hypopnea index; B, present but inconclusive or inconsistent; BP, blood pressure; C, lacking; CPAP, continuous positive airway pressure; OSA, obstructive sleep apnea; PLM, periodic limb movement; PSG, polysomnography; RLS, restless legs syndrome.
The benefits and timing of CPAP therapy during an acute stroke, however, have not been established. The effects of CPAP therapy on BP during an acute stroke are not well-known. CPAP treatment therefore may be problematic in acute stroke patients that are vulnerable to changes in CBF. In addition, the severity and type of sleep apnea may evolve from the acute to the chronic stroke phase (1). Acute stroke patients may show poor CPAP adherence due to cognitive and motor impairment that hinders appropriate mask fit and placement. An alternative option for inpatient treatment for OSA may be positional therapy (avoiding supine positioning), which has showed modest decreases in the AHI of about 20% compared with sleeping at lib (42). A five-year prospective observational study of patients with OSA two-months after stroke showed that those with moderate or severe OSA who were nonadherent to CPAP had increased mortality (HR 1·6; 95% CI: 1·0–2·5) from cardiovascular disease or stroke as compared with CPAP adherent patients (43). Randomized clinical trials studying the effects of CPAP therapy on stroke outcomes and recurrence are currently ongoing; however, given its high benefit-to-risk ratio, stroke patients with OSA should be encouraged to use CPAP regularly as part of routine stroke care (Table 4). Larger studies are needed to determine the optimal timing of CPAP therapy after stroke, determinants of CPAP adherence in stroke patients, and the effects of CPAP use on stroke risk reduction and mortality.
CSA and stroke
CSA is characterized by repetitive cessation of ventilation during sleep resulting from loss of ventilatory drive. By definition, a central apnea is a 10-s pause in ventilation without associated respiratory effort. Cheyne–Stokes respiration (CSR) is characterized by recurrent cyclic fluctuations of central apneas and hyperpneas (increased ventilation) during which tidal volume waxes and wanes in a gradual crescendo–decrescendo pattern. CSR is commonly seen in congestive heart disease (2).
CSA may be a consequence rather than a cause of stroke and can be a heralding symptom of vascular disease. During an acute stroke, there is an increase frequency of central apneas that improves during the sub-acute phase to completely resolve within months after the stroke (1). Also, CSA, such as CSR, is associated with congestive heart disease, a risk factor for cardioembolic strokes (2). In a cross-sectional analysis of the SHHS, the association between sleep parameters (e.g. AHI, arousals, desaturations) and sub-clinical cerebral white matter disease by magnetic resonance imaging showed that central apneas were the only sleep factor associated with increase sub-clinical white matter hyperintensities (44). A relation between CSA and asymptomatic carotid artery disease has also been observed (45). An increased hypercapnic response with subsequent ventilator instability may also be a consequence of increased sub-clinical brain disease in the respiratory centers of the brain and/or damaged baroreceptors in the carotid bulb as seen in carotid stenosis.
Treatment of CSA
Treatment of CSA and CSR encompasses the use of CPAP and/or oxygen supplementation that requires following patients over time to monitor effectiveness of treatment. A newer ventilator device, adaptive servo-ventilation, provides ventilatory support that adapts to the phase of the cycle in CSR allowing for maximum support during the apneic phase and minimum support during the hyperpnea, allowing for normalization of breathing pattern during the first night of use.
Insomnia, sleep duration, and stroke
Insomnia is the most common sleep disorder with up to 22% of middle-aged and older Americans suffering from chronic insomnia (46). Insomnia is characterized by a complaint of difficulty falling asleep, staying asleep, early morning awakening, and/or nonrestorative sleep, given sufficient opportunity for sleeping (4). The diagnosis of insomnia requires at least one associated daytime functional impairment (fatigue, daytime sleepiness, irritability, memory, or concentration difficulties among other complaints) (4). Multiple studies, including a large cohort from the Atherosclerosis Risk in Communities Study, have found that self-reported complaints of insomnia are associated with an increased risk of coronary heart disease, myocardial infarction (MI), or death (47). Insomnia complaints combined with short sleep duration (≤five-hours of sleep) are also associated with incident hypertension (OR 5·1; 95% CI: 2·2–11·8) and type 2 diabetes (OR 2·95; 95% CI: 1·2–7·0) (48,49). Insomnia was associated with all-cause mortality (OR 1·7; 95% CI: 1·2–2·5) and cardiovascular death (OR 1·8; 95% CI: 1·0–3·1) (50). Increased sympathetic activity and hypothalamic–pituitary–adrenal axis activation leading to increased cortisol levels are proposed mechanisms for the effect of insomnia on vascular disease (48).
Insomnia with short sleep is associated with adverse health outcomes. Epidemiological studies have demonstrated the lowest mortality in those sleeping seven-hours per night, and increased mortality in those sleeping eight-hours or more, or six-hours or less, independent of other sleep symptoms (51,52). A U-shaped relation between sleep duration and vascular outcomes has been described, with short sleep (less than six-hours of sleep) associated with diabetes, obesity, incident hypertension, and coronary artery disease, and long sleep (nine≥ more hours of sleep) with stroke and coronary artery disease (51–56). A recent meta-analysis of 15 prospective population-based studies has shown that short sleep duration (HR 1·2; 95% CI: 1·0–1·3) and long sleep duration (HR 1·7; 95% CI: 1·5–1·9) are predictors of ischemic stroke (52). Short sleep has been shown to impair insulin sensitivity, increase sympathetic tone and cortisol levels, and alter inflammatory markers (55,56). However, the mechanisms by which long sleep is associated to cardiovascular comorbidities are not well-understood. There is a continuing controversy on whether long sleep duration is a risk factor for cardiovascular disease or it is a marker of underlying poor health. Long sleep may be a consequence, rather than a cause, of unrecognized comorbidities leading to increased mortality (51,52,54).
Treatment of insomnia
The treatment of insomnia encompasses pharmacologic and nonpharmacologic/behavioral interventions. Among the pharmacologic agents available, benzodiazepine agonists (i.e. zolpidem) are the first line of treatment. Newer agents include ramelteon, targeting melatonin receptors, and low-dose doxepin, which are both US Food and Drug Administration (FDA)-approved for the treatment of primary insomnia. Other treatment agents include sedating antidepressants (mirtazapine, trazodone), antihistamines, and benzodiazepines. The mainstay of nonpharmacologic intervention is cognitive behavioral therapy. Cognitive behavioral therapy evaluates patients' sleep habits and dysfunctional sleep beliefs. Then, strategies tailored to patients' sleep difficulties are recommended including maintenance of regular sleep–wake schedules, stimulus control therapy, and sleep time-in-bed restriction. Cognitive behavioral therapy has been shown to be as effective as pharmacological interventions in the treatment of insomnia, although convincing evidence in stroke patients is not available (Table 4).
Circadian rhythms
Circadian rhythms are biological and physiological processes with a periodicity approximating 24 h. They are primarily driven by internal circadian pacemaker (the suprachiasmatic nucleus of the hypothalamus) and entrained by exogenous cues (light exposure, meal times). The best characterized circadian processes are the sleep–wake cycle, body temperature, BP, and the release of neuroendocrine hormones. Circadian rhythm disorders occur when there is intermittent or chronic misalignment between the patient's sleep pattern and the pattern that is desired or regarded as the societal norm (57). The most common complaint is that the patient cannot sleep when sleep is desired or expected; however, wakefulness may also occur at undesired times as a result of sleeping at inappropriate times (i.e. while at work, while driving) (57). Thus, patients may complain of excessive sleepiness or insomnia. Diagnosis of circadian rhythm abnormalities requires correlation of sleep complaints (insomnia, excessive sleepiness) with circadian rhythm misalignment (endogenous circadian rhythm is out of sync with exogenous factors that affect timing or duration of sleep) documented with sleep diaries or wrist actigraphy (4).
Circadian rhythm disorders and stroke
The circadian rhythm abnormality associated with the most morbidity in adults is shift work sleep disorder. According to the 2004 data from the US Bureau of Labor statistics,15 million Americans work full time on evening shift, night shift, rotating shift, or other irregular schedule (58). Shift workers attempting to sleep during the day often have curtailed sleep duration due to environmental disruptors (i.e. noise, sunlight), circadian misalignment, and unrecognized comorbid sleep disorders (59). Night shift work is associated with significant stress and interferes with endogenous nocturnal BP decline. This results in elevated BP during the shift,which persists into the following day (60). Thus, prolonged shift work is known to have multiple deleterious health consequences including increased risk of obesity, hypertension, diabetes, cardiovascular disease, and all-cause mortality, but it is controversial whether it also increases stroke risk (61–65). Although some small case-control studies did not find an association with shift work and incident stroke (66), the Nurses' Health Study, an ongoing cohort study of over 80 000 registered female nurses, reported that rotating night shift work was associated with a 4% increased ischemic stroke risk for every five-years of shift work after controlling for vascular risk factors (67).
Sleep, ambulatory BP and risk of stroke
BP exhibits a prominent 24 h rhythm, with two daytime peaks, the first around 9 am and the second around 7 pm, and a nadir during sleep (68). Normally, BP decrease by 10–20% during non-rapid eye movement (NREM) sleep as compared with daytime levels (68). A variety of abnormal BP variation patterns have been described in which the nocturnal fall of BP may be >20% (extreme dippers), <10% (nondippers), or <0% (reversed dippers, with nocturnal BP exceeding daytime BP) (69). Prospective studies have shown that patients who do not experience typical BP `dipping' during sleep are at increased risk for end organ damage, including left ventricular hypertrophy, myocardial infarction, congestive heart failure, vascular dementia, and stroke (68–72). Extreme dipper, nondipper, or reverse dipper pattern have all been associated with intracranial hemorrhages, ischemic strokes, silent brain infarcts, and stroke deaths (69–71,73,74). Excessive morning BP surge have been associated with a 2·7-fold increase risk of stroke in the elderly (75). The Ohsama study, a population-based study of the prognostic value of ambulatory BP monitoring in over 1500 Japanese subjects, found that after an average follow-up of 9·2 years, a 5% decrease in the nocturnal systolic BP in hypertensive patients was associated with approximately a 20% increased risk of cardiovascular mortality (72). In addition, `dipper hypertensives' had a risk of cardiovascular mortality (HR 2·4; 95% CI: 1·5–3·8) similar to that of `nondipper hypertensives' (HR 2·2; 95% CI: 1·3–3·6). These results suggest that loss of nocturnal BP dipping is a significant risk factor of cardiovascular mortality, independent of 24-hour mean BP. Hence, altered diurnal BP pattern with absence of nocturnal BP dipping represents an important risk factor for stroke and cardiovascular events.
Circadian rhythm abnormalities after stroke
Several studies employing BP monitoring have documented reduction or loss of BP and other circadian rhythms after acute stroke of various etiologies (76–79). Continuous ambulatory BP monitoring studies in acute stroke found rates of BP nondipping and reverse dipping patterns of 30% and 58%, respectively (77). This lack of nocturnal BP dipping in acute stroke may have several possible etiologies. These include stress associated with hospitalization, activation of neuroendocrine systems (corticotropic, sympathetic, renin–aldosterone–angiotensin), injury to central centers responsible modulation of autonomic BP control (i.e. insular cortex), alterations in cardiac output, and physiological response to reduced perfusion of the ischemic penumbra (68,76–79). Acute stroke patients with preserved nocturnal BP decline in the first 24 h from onset have been shown to have improved one-week neurological outcomes compared with patients with nondipping BP pattern (76). Using 24-h BP recordings, each 10-mmHg difference between day and nighttime diastolic BP within 24 h of stroke onset was associated with the fourfold odds for complete functional recovery, whereas each 10-mmHg difference between day and night systolic BP was associated with the twofold odds for an improvement in neurological status (76). Circadian rhythms abnormalities in the acute stroke phase may serve as prognostic indicators of poor long-term functional recovery.
Treatment of circadian rhythm abnormalities in stroke
Awareness of circadian rhythms and their abnormalities as a risk factor for stroke and in acute stroke setting may influence acute stroke care and stroke prevention.Knowledge of aberrant circadian BP patterns after acute ischemic stroke may prevent use of antihypertensive medication prematurely, resulting in deleterious consequences from relative hypotension. In the outpatient setting, altered circadian BP pattern is a recognized risk factor for stroke and cardiovascular disease. Thus, optimization of antihypertensive therapy requires identification of individual patient circadian BP patterns by ambulatory BP monitoring and tailoring medication regimen according to its abnormality. There is some prospective evidence that such hypertensive chronotherapy (with conventional antihypertensives taken at bedtime) improves ambulatory (including nocturnal) BP control and normalizes BP dipping pattern and significantly reduces vascular deaths, cardiovascular events, and strokes as compared with conventional (morning, once-aday dosing) hypertensive therapy (70). Hence, bedtime administration of antihypertensive medications should be considered in patients with nondipping or reverse-dipping BP pattern as opposed to ingesting all medications upon awakening.Another circadian-based therapeutic option is using chronotherapeutic formulations of antihypertensives, medications designed to proportion medication concentration synchronously with known variations in day–night BP, delivering peak concentrations in the morning (Table 4). Finally, assessment of patients' work shift history, screening and treatment of coexisting sleep disorders, and education concerning maladaptive sleep habits may help reduce their vascular risk.
Sleep-related movement disorders
Sleep-related movement disorders and their impact upon incident stroke have received much less attention than sleep duration and SDB; however, there is mounting evidence that these disorders may also contribute to elevated stroke risk. Restless legs syndrome (RLS) is a sensorimotor disorder clinically diagnosed by four minimal criteria:
uncomfortable/unpleasant sensations with an urge to move the legs
symptom exacerbation by periods of rest or inactivity
full or partial relief by movements (walking/stretching), and
diurnal fluctuation of symptoms with worsening at night or in the evening (80).
RLS is one of the most common sleep disorders, with population-based surveys estimating its frequency between 5% and 10 % of the general population in North America and Europe (81–85). The prevalence increases with age until after age 60 to 70, after which it decreases. The prevalence is about two times more common in women than in men (82,83). The pathophysiology of RLS implicates deficiencies in central dopaminergic transmission, which is vital in modulating spinal excitability (80).
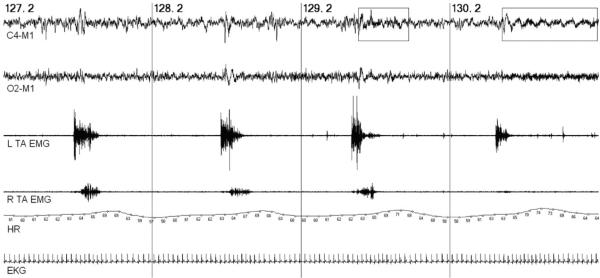
Periodic limb movements (PLMs) occur in up to 80% of patients with RLS and are the cause of sleep fragmentation (80). These repetitive, stereotypical limb movements are often described as `jerks' by bed partners and can cause brief arousals or full awakenings from sleep. They are most commonly composed of periodic flexions and extensions involving the toes, ankles, and occasionally the knee and hip. PLMs require PSG for diagnosis and must occur in a series of at least four consecutive movements lasting from 0·5 to 10 s with intermovement intervals of four-seconds to 90 s (see Fig. 2) (80). There is only one population-based study of PLMs employing PSG, estimating a prevalence of nearly 8% in the tri-county Detroit area, with noted racial differences (86). They are more common in the elderly, found in up to 45% of patients over the age of 65 (87). They have variable electroencephalography (EEG)-defined arousals and consistent autonomic arousals, with surges in sympathetic activity resulting in BP and heart rate increases lasting for five-seconds to 10 s following individual leg movements (88). These changes in BP and heart rate are comparable with those observed following apneic episodes of OSA. An example of heart rate variability associated with PLMs is shown in Fig. 2.
Fig. 2.
Periodic limb movements in sleep. Two-minutes of stage 2 NREM sleep with periodic limb movements (PLMs) in bilateral tibialis anterior (TA) muscles. PLMs with cortical (EEG-defined) arousals (rectangular boxes) show greater autonomic activation (heart rate surges) than those without cortical arousals. Vertical lines represent 30 s sleep epochs. Top to bottom tracings: unilateral right central and occipital EEG leads; left (L) and right (R) tibialis anterior electromyography (TA EMG), heart rate (HR), and electrocardiogram (EKG).
Sleep-related movement disorders and stroke
Evidence of causative roles for RLS and PLMs in ischemic stroke is lacking; however, RLS and PLMs have been associated with increased risk of hypertension and cardiovascular disease in some epidemiological studies after controlling for wellestablished vascular risk factors (81,85). Several studies, however, have found only a significant association between frequent RLS and cardiovascular disease and stroke but not with hypertension (Table 3) (82,84,89). Frequency and severity of symptoms has been shown to strengthen the association between RLS and cardiovascular disease (84,89). In the SHHS, the association of RLS with coronary artery disease was primarily seen in those patients reporting RLS symptoms on most days of the month (89). The main postulated mechanism for this association involves repeated sympathetic arousals and associated BP and heart rates surges associated with PLMs. The association of PLMs with hypertension severity has been reported (90). In a sample of 861 RLS patients, those with PLMs > 30/h had a twofold increased risk of hypertension (OR 2·3, 95% CI 1·3–4·0) (91). Thus, RLS and associated PLMs may mediate risk for cardiovascular disease, hypertension, and incident stroke through repeated nocturnal autonomic arousals similar to that observed with OSA (92).
Sleep-related movement disorders after stroke
There are several case reports of new onset RLS and PLMs or exacerbation of preexisting RLS symptoms following acute ischemic stroke (93). In a small retrospective study, PLMs were found to be more prevalent in patients with history of stroke (48%) as compared with matched subjects without history of stroke (13%); however, it was unclear whether PLMs preceded incident strokes or were one of its consequences (94). In one prospective study, poststroke RLS symptoms were found in 12% of patients within a month of ischemic stroke (95). The most frequent stroke locations associated with RLS were subcortical locations (basal ganglia/corona radiata) and brainstem areas (pyramidal tract/pons), which are prominent in coordinating motor system output and sleep–wake transitions.
Treatment of sleep-related movement disorders
The gold standard treatment for significant RLS and PLMs is dopamine agonist therapy (80). There are currently three FDA-approved medications for treatment of moderate-to-severe RLS. Two nonergotamine dopamine agonists, ropirinole (0·25–2·0 mg) and pramexipole (0·25–1 mg), are taken about one-hour before bedtime (1) and the recently approved gabapentin enacarbil, a gabapentin prodrug (600–1200 mg) with alpha-2 delta activity. In Europe, another dopamine agonist, rotigotine, is approved as a transdermal patch (1). Other RLS and PLMs treatments include bedtime gabapentin (300–1200 mg), pregabalin (100–500 mg), clonazepam (0·25–1 mg), and levodopa/carbidopa (125–250 mg) (1). Caffeine, alcohol, and certain medications (dopamine blockers, selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors, and tricyclic antidepressants) known to exacerbate RLS and PLMs should be avoided (80). There is no evidence showing treatment of RLS/PLMS prevents incident hypertension, cardiovascular events or stroke (Table 4).
Consequences on sleep disorders on stroke rehabilitation
Although there are few data to directly link the consequences of altered hospital sleep pattern to increased patient morbidity and mortality, the consequences of poor sleep may affect inpatient stroke rehabilitation efforts. Poor sleep quality or duration, regardless of etiology, significantly impairs patient's rehabilitation for a variety of reasons. Sleep disorders impede the restorative processes, which occur during undisturbed sleep (96). OSA, insomnia, and RLS are associated with depressive symptoms, daytime sleepiness, fatigue, and executive dysfunction, all of which limit a patient's rehabilitation regimen (1,5,31,46,85). Stroke patients with untreated sleep disorders may lack the motivation, energy, and concentration necessary to participate in intensive rehabilitation therapy. Patients with preexisting OSA and RLS have been shown to have poorer functional stroke outcomes than stroke patients without sleep disorders (96,97). In a recent small-randomized trial in 22 patients with OSA, CPAP treatment after stroke has been shown to improve motor functional recovery and depressive symptoms, but not cognitive impairment (98). Present but inconclusive or inconsistent evidence suggests (Table 4) that treatment of sleep disorders in patients with stroke may help maximize stroke recovery but more rigorous studies are needed.
Future directions
There are many unanswered questions regarding the impact of sleep disorders on incident and recurrent stroke. There are limited data regarding race–ethnic prevalence of sleep disorders, which may be of importance in stroke prevention as Hispanics have a twofold increased risk for stroke in comparison with Whites (99). A large multicenter epidemiological study, the Hispanics Community Health Study with 16 000 participants, will evaluate the effect of sleep disorders on adverse cardiovascular outcomes and will help clarify the effect of sleep disorders on sociodemographic and vascular disease in US Hispanic population (100). Longitudinal clinical trials are needed to determine if treatments of sleep disorders in the general population may be effective in primary prevention of stroke.Thirteen active trials (clinicaltrials.gov) currently investigate the effects of OSA on vascular disease and the effects of CPAP on future cardiovascular events. One large ongoing trial (the Sleep Apnea Cardiovascular Endpoints Study, with 5000 participants) is investigating whether CPAP treatment for moderate to severe OSA is effective for prevention of cardiovascular disease and stroke in patients with established coronary or cerebrovascular disease; and the results from this trial will be available in 2015. Currently, there are no active studies evaluating the effects of treating sleep disorders other than OSA on reduction of stroke risk. Furthermore, defining the patho-physiological mechanisms through which other sleep disorders mediate increased risk of stroke warrants further investigations. In addition, OSA and other SDB may play a role in long-term cognitive impairment and dementia and more studies are needed to investigate these relations. More studies are also needed to determine if treatment of poststroke sleep disorders improve stroke rehabilitation and stroke outcomes including mood and cognitive function. Given the emerging evidence that sleep disorders may be modifiable stroke risk factors, increased awareness of their effects, management, and prevention are paramount among health-care professionals and the public. Incorporating efficient sleep disorders screening as part of routine stroke care is an important first step. Educating neurologists-in-training regarding these novel therapeutic targets should help modify stroke risk and improve outcome and quality of life after stroke. Finally, educating stroke patients and their families regarding the impact of sleep on stroke recovery and risk of subsequent stroke may help improve adherence with sleep therapies.
Conclusion
Sleep disorders are highly prevalent in patients with stroke and in those at risk for stroke. Thus, sleep disorders screening through questionnaires such as the ESS and the Berlin Questionnaire should become standard of care in stroke clinics. Further clinical and research collaborations between stroke and sleep specialists should be encouraged to improve the knowledge, the prevention strategies, and subsequently, the clinical outcomes for stroke patients. Finally, newer strategies to educate future health-care professionals and the public about the importance of sleep and its impact on stroke and cardiovascular disease are vital.
Footnotes
Conflict of interest: None declared.
References
- 1.Hermann DM, Bassetti CL. Sleep-related breathing and sleep-wake disturbances in ischemic stroke. Neurology. 2009;73:1313–22. doi: 10.1212/WNL.0b013e3181bd137c. [DOI] [PubMed] [Google Scholar]
- 2.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118:1080–111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 3.Culebras A. Sleep and stroke. Semin Neurol. 2009;29:438–45. doi: 10.1055/s-0029-1237121. [DOI] [PubMed] [Google Scholar]
- 4.American Academy of Sleep Medicine . The International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd edn American Academy of Sleep Medicine; Westchester, IL: 2005. [Google Scholar]
- 5.Guilleminault C, Ramar K. Neurologic aspects of sleep apnea: is obstructive sleep apnea a neurologic disorder? Semin Neurol. 2009;29:368–71. doi: 10.1055/s-0029-1237122. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin CM, Ervin AM, Mays MZ, et al. Sleep disturbances, quality of life, and ethnicity: the Sleep Heart Health Study. J Clin Sleep Med. 2010;6:176–83. [PMC free article] [PubMed] [Google Scholar]
- 7.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 8.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–95. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Connor GT, Lind BK, Lee ET, et al. Variation in symptoms of sleep-disordered breathing with race and ethnicity: the Sleep Heart Health Study. Sleep. 2003;26:74–9. [PubMed] [Google Scholar]
- 10.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 11.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 12.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 13.Williams SK, Ravenell J, Jean-Louis G, et al. Resistant hypertension and sleep apnea: pathophysiologic insights and strategic management. Curr Diab Rep. 2011;11:64–9. doi: 10.1007/s11892-010-0161-z. [DOI] [PubMed] [Google Scholar]
- 14.Rasche K, Keller T, Tautz B, et al. Obstructive sleep apnea and type 2 diabetes. Eur J Med Res. 2010;15(Suppl. 2):152–6. doi: 10.1186/2047-783X-15-S2-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tokuda F, Sando Y, Matsui H, Koike H, Yokoyama T. Serum levels of adipocytokines, adiponectin and leptin, in patients with obstructive sleep apnea syndrome. Intern Med. 2008;47:1843–9. doi: 10.2169/internalmedicine.47.1035. [DOI] [PubMed] [Google Scholar]
- 16.Beelke M, Angeli S, Del Sette M, et al. Prevalence of patent foramen ovale in subjects with obstructive sleep apnea: a transcranial Doppler ultrasound study. Sleep Med. 2003;4:219–23. doi: 10.1016/s1389-9457(02)00256-3. [DOI] [PubMed] [Google Scholar]
- 17.Lau EM, Yee BJ, Grunstein RR, Celermajer DS. Patent foramen ovale and obstructive sleep apnea: a new association? Sleep Med Rev. 2010;14:391–5. doi: 10.1016/j.smrv.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Stevenson IH, Teichtahl H, Cunnington D, Ciavarella S, Gordon I, Kalman JM. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J. 2008;29:1662–9. doi: 10.1093/eurheartj/ehn214. [DOI] [PubMed] [Google Scholar]
- 19.Braga B, Poyares D, Cintra F, et al. Sleep-disordered breathing and chronic atrial fibrillation. Sleep Med. 2009;10:212–6. doi: 10.1016/j.sleep.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–7. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita T, Murakawa Y, Sezaki K, et al. Circadian variation of paroxysmal atrial fibrillation. Circulation. 1997;96:1537–41. doi: 10.1161/01.cir.96.5.1537. [DOI] [PubMed] [Google Scholar]
- 22.Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol. 1983;52:490–4. doi: 10.1016/0002-9149(83)90013-9. [DOI] [PubMed] [Google Scholar]
- 23.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 24.Munoz R, Duran-Cantolla J, Martinez-Vila E, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37:2317–21. doi: 10.1161/01.STR.0000236560.15735.0f. [DOI] [PubMed] [Google Scholar]
- 25.Palomaki H. Snoring and the risk of ischemic brain infarction. Stroke. 1991;22:1021–5. doi: 10.1161/01.str.22.8.1021. [DOI] [PubMed] [Google Scholar]
- 26.Boden-Albala B, Bazil C, Moon Y, et al. Daytime sleepiness and risk of stroke and vascular disease: findings from the Northern Manhattan Study. Stroke. 2008;39:94. doi: 10.1161/CIRCOUTCOMES.111.963801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 28.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–51. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–77. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med. 2010;6:131–7. [PMC free article] [PubMed] [Google Scholar]
- 31.Nishibayashi M, Miyamoto M, Miyamoto T, Suzuki K, Hirata K. Correlation between severity of obstructive sleep apnea and prevalence of silent cerebrovascular lesions. J Clin Sleep Med. 2008;4:242–7. [PMC free article] [PubMed] [Google Scholar]
- 32.Ramos-Sepulveda A, Wohlgemuth W, Gardener H, et al. Snoring and insomnia are not associated with subclinical atherosclerosis in the Northern Manhattan Study. Int J Stroke. 2010;5:264–8. doi: 10.1111/j.1747-4949.2010.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wattanakit K, Boland L, Punjabi NM, Shahar E. Relation of sleep-disordered breathing to carotid plaque and intima–media thickness. Atherosclerosis. 2008;197:125–31. doi: 10.1016/j.atherosclerosis.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 34.Minoguchi K, Yokoe T, Tazaki T, et al. Increased carotid intima- media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:625–30. doi: 10.1164/rccm.200412-1652OC. [DOI] [PubMed] [Google Scholar]
- 35.Baguet JP, Hammer L, Levy P, et al. The severity of oxygen desaturation is predictive of carotid wall thickening and plaque occurrence. Chest. 2005;128:3407–12. doi: 10.1378/chest.128.5.3407. [DOI] [PubMed] [Google Scholar]
- 36.Kaynak D, Goksan B, Kaynak H, Degirmenci N, Daglioglu S. Is there a link between the severity of sleep-disordered breathing and atherosclerotic disease of the carotid arteries? Eur J Neurol. 2003;10:487–93. doi: 10.1046/j.1468-1331.2003.00658.x. [DOI] [PubMed] [Google Scholar]
- 37.Ancoli-Israel S, Stepnowsky C, Dimsdale J, Marler M, Cohen-Zion M, Johnson S. The effect of race and sleep-disordered breathing on nocturnal BP `dipping': analysis in an older population. Chest. 2002;122:1148–55. doi: 10.1378/chest.122.4.1148. [DOI] [PubMed] [Google Scholar]
- 38.Urbano F, Roux F, Schindler J, Mohsenin V. Impaired cerebral autoregulation in obstructive sleep apnea. J Appl Physiol. 2008;105:1852–7. doi: 10.1152/japplphysiol.90900.2008. [DOI] [PubMed] [Google Scholar]
- 39.Alexandrov AV, Sharma VK, Lao AY, Tsivgoulis G, Malkoff MD, Alexandrov AW. Reversed Robin Hood syndrome in acute ischemic stroke patients. Stroke. 2007;38:3045–8. doi: 10.1161/STROKEAHA.107.482810. [DOI] [PubMed] [Google Scholar]
- 40.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea–hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 41.Barbe F, Duran-Cantolla J, Capote F, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718–26. doi: 10.1164/rccm.200901-0050OC. [DOI] [PubMed] [Google Scholar]
- 42.Svatikova A, Chervin RD, Wing JJ, Sanchez BN, Migda EM, Brown DL. Positional therapy in ischemic stroke patients with obstructive sleep apnea. Sleep Med. 2011;12:262–6. doi: 10.1016/j.sleep.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Martinez-Garcia MA, Soler-Cataluna JJ, Ejarque-Martinez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180:36–41. doi: 10.1164/rccm.200808-1341OC. [DOI] [PubMed] [Google Scholar]
- 44.Robbins J, Redline S, Ervin A, Walsleben JA, Ding J, Nieto FJ. Associations of sleep-disordered breathing and cerebral changes on MRI. J Clin Sleep Med. 2005;1:159–65. [PubMed] [Google Scholar]
- 45.Rupprecht S, Hoyer D, Hagemann G, Witte OW, Schwab M. Central sleep apnea indicates autonomic dysfunction in asymptomatic carotid stenosis: a potential marker of cerebrovascular and cardiovascular risk. Sleep. 2010;33:327–33. doi: 10.1093/sleep/33.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phillips B, Mannino D. Correlates of sleep complaints in adults: the ARIC study. J Clin Sleep Med. 2005;1:277–83. [PubMed] [Google Scholar]
- 47.Phillips B, Mannino DM. Do insomnia complaints cause hypertension or cardiovascular disease? J Clin Sleep Med. 2007;3:489–94. [PMC free article] [PubMed] [Google Scholar]
- 48.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–7. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care. 2009;32:1980–5. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chien KL, Chen PC, Hsu HC, et al. Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: report from a community-based cohort. Sleep. 2010;33:177–84. doi: 10.1093/sleep/33.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 52.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18:148–58. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 53.Qureshi AI, Giles WH, Croft JB, Bliwise DL. Habitual sleep patterns and risk for stroke and coronary heart disease: a 10-year follow-up from NHANES I. Neurology. 1997;48:904–11. doi: 10.1212/wnl.48.4.904. [DOI] [PubMed] [Google Scholar]
- 54.Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB. Correlates of long sleep duration. Sleep. 2006;29:881–9. doi: 10.1093/sleep/29.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 56.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30:1667–73. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klerman EB. Clinical aspects of human circadian rhythms. J Biol Rhythms. 2005;20:375–86. doi: 10.1177/0748730405278353. [DOI] [PubMed] [Google Scholar]
- 58.United States Department of Labor [(accessed 11 May 2011)];Workers on Flexible and Shift Schedules in 2004 Summary. 2005 Available at http://www.bls.gov/news.release/flex.nr0.htm.
- 59.Monk T. Shift work: basic principles. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th edn Elsevier; Philadelphia, PA: 2005. pp. 673–80. [Google Scholar]
- 60.Lo SH, Lin LY, Hwang JS, Chang YY, Liau CS, Wang JD. Working the night shift causes increased vascular stress and delayed recovery in young women. Chronobiol Int. 2010;27:1454–68. doi: 10.3109/07420528.2010.498067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hannerz H, Albertsen K, Nielsen ML, Tuchsen F, Burr H. Occupational factors and 5-year weight change among men in a Danish national cohort. Health Psychol. 2004;23:283–8. doi: 10.1037/0278-6133.23.3.283. [DOI] [PubMed] [Google Scholar]
- 62.Ellingsen T, Bener A, Gehani AA. Study of shift work and risk of coronary events. J R Soc Promot Health. 2007;127:265–7. doi: 10.1177/1466424007083702. [DOI] [PubMed] [Google Scholar]
- 63.Suwazono Y, Dochi M, Sakata K, et al. A longitudinal study on the effect of shift work on weight gain in male Japanese workers. Obesity. 2008;16:1887–93. doi: 10.1038/oby.2008.298. [DOI] [PubMed] [Google Scholar]
- 64.Suwazono Y, Dochi M, Oishi M, Tanaka K, Kobayashi E, Sakata K. Shiftwork and impaired glucose metabolism: a 14-year cohort study on 7104 male workers. Chronobiol Int. 2009;26:926–41. doi: 10.1080/07420520903044422. [DOI] [PubMed] [Google Scholar]
- 65.Kawachi I, Colditz GA, Stampfer MJ, et al. Prospective study of shift work and risk of coronary heart disease in women. Circulation. 1995;92:3178–82. doi: 10.1161/01.cir.92.11.3178. [DOI] [PubMed] [Google Scholar]
- 66.Hermansson J, Gillander Gadin K, et al. Ischemic stroke and shift work. Scand J Work Environ Health. 2007;33:435–9. doi: 10.5271/sjweh.1165. [DOI] [PubMed] [Google Scholar]
- 67.Brown DL, Feskanich D, Sanchez BN, Rexrode KM, Schernhammer ES, Lisabeth LD. Rotating night shift work and the risk of ischemic stroke. Am J Epidemiol. 2009;169:1370–7. doi: 10.1093/aje/kwp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hermida RC, Ayala DE, Smolensky MH, Portaluppi F. Chronotherapy in hypertensive patients: administration-time dependent effects of treatment on blood pressure regulation. Expert Rev Cardiovasc Ther. 2007;5:463–75. doi: 10.1586/14779072.5.3.463. [DOI] [PubMed] [Google Scholar]
- 69.Routledge FS, McFetridge-Durdle JA, Dean CR. Night-time blood pressure patterns and target organ damage: a review. Can J Cardiol. 2007;23:132–8. doi: 10.1016/s0828-282x(07)70733-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629–51. doi: 10.3109/07420528.2010.510230. [DOI] [PubMed] [Google Scholar]
- 71.Izzedine H, Launay-Vacher V, Deray G. Abnormal blood pressure circadian rhythm: a target organ damage? Int J Cardiol. 2006;107:343–9. doi: 10.1016/j.ijcard.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 72.Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. 2002;20:2183–9. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 73.Kario K, Shimada K, Pickering TG. Abnormal nocturnal blood pressure falls in elderly hypertension: clinical significance and determinants. J Cardiovasc Pharmacol. 2003;41(Suppl. 1):S61–6. [PubMed] [Google Scholar]
- 74.Kario K, Shimada K, Schwartz JE, Matsuo T, Hoshide S, Pickering TG. Silent and clinically overt stroke in older Japanese subjects with white-coat and sustained hypertension. J Am Coll Cardiol. 2001;38:238–45. doi: 10.1016/s0735-1097(01)01325-0. [DOI] [PubMed] [Google Scholar]
- 75.Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–6. doi: 10.1161/01.cir.0000056521.67546.aa. [DOI] [PubMed] [Google Scholar]
- 76.Bhalla A, Wolfe CD, Rudd AG. The effect of 24 h blood pressure levels on early neurological recovery after stroke. J Intern Med. 2001;250:121–30. doi: 10.1046/j.1365-2796.2001.00858.x. [DOI] [PubMed] [Google Scholar]
- 77.Jain S, Namboodri KK, Kumari S, Prabhakar S. Loss of circadian rhythm of blood pressure following acute stroke. BMC Neurol. 2004;4:1–6. doi: 10.1186/1471-2377-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dawson SL, Evans SN, Manktelow BN, Fotherby MD, Robinson TG, Potter JF. Diurnal blood pressure change varies with stroke subtype in the acute phase. Stroke. 1998;29:1519–24. doi: 10.1161/01.str.29.8.1519. [DOI] [PubMed] [Google Scholar]
- 79.Pandian JD, Wong AA, Lincoln DJ, et al. Circadian blood pressure variation after acute stroke. J Clin Neurosci. 2006;13:558–62. doi: 10.1016/j.jocn.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 80.Trenkwalder C, Paulus W. Restless legs syndrome: pathophysiology, clinical presentation and management. Nat Rev Neurol. 2010;6:337–46. doi: 10.1038/nrneurol.2010.55. [DOI] [PubMed] [Google Scholar]
- 81.Ulfberg J, Nystrom B, Carter N, Edling C. Prevalence of restless legs syndrome among men aged 18 to 64 years: an association with somatic disease and neuropsychiatric symptoms. Mov Disord. 2001;16:1159–63. doi: 10.1002/mds.1209. [DOI] [PubMed] [Google Scholar]
- 82.Hogl B, Kiechl S, Willeit J, et al. Restless legs syndrome: a community-based study of prevalence, severity, and risk factors. Neurology. 2005;64:1920–4. doi: 10.1212/01.WNL.0000163996.64461.A3. [DOI] [PubMed] [Google Scholar]
- 83.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: rest general population study. Arch Intern Med. 2005;165:1286–92. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 84.Winkelman JW, Finn L, Young T. Prevalence and correlates of restless legs syndrome symptoms in the Wisconsin Sleep Cohort. Sleep Med. 2006;7:545–52. doi: 10.1016/j.sleep.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 85.Phillips B, Hening W, Britz P, Mannino D. Prevalence and correlates of restless legs syndrome: results from the 2005 National Sleep Foundation Poll. Chest. 2006;129:76–80. doi: 10.1378/chest.129.1.76. [DOI] [PubMed] [Google Scholar]
- 86.Scofield H, Roth T, Drake C. Periodic limb movements during sleep: population prevalence, clinical correlates, and racial differences. Sleep. 2008;31:1221–7. [PMC free article] [PubMed] [Google Scholar]
- 87.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Periodic limb movements in sleep in community-dwelling elderly. Sleep. 1991;14:496–500. doi: 10.1093/sleep/14.6.496. [DOI] [PubMed] [Google Scholar]
- 88.Pennestri MH, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68:1213–8. doi: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 89.Winkelman JW, Shahar E, Sharief I, Gottlieb DJ. Association of restless legs syndrome and cardiovascular disease in the Sleep Heart Health Study. Neurology. 2008;70:35–42. doi: 10.1212/01.wnl.0000287072.93277.c9. [DOI] [PubMed] [Google Scholar]
- 90.Espinar-Sierra J, Vela-Bueno A, Luque-Otero M. Periodic leg movements in sleep in essential hypertension. Psychiatry Clin Neurosci. 1997;51:103–7. doi: 10.1111/j.1440-1819.1997.tb02370.x. [DOI] [PubMed] [Google Scholar]
- 91.Billars L, Hicks A, Bliwise D, et al. Hypertension risk and PLMS in restless legs syndrome. Sleep. 2007;30:A297–8. [Google Scholar]
- 92.Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009;32:589–97. doi: 10.1093/sleep/32.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sechi G, Agnetti V, Galistu P, et al. Restless legs syndrome and periodic limb movements after ischemic stroke in the right lenticulostriate region. Parkinsonism Relat Disord. 2008;14:157–60. doi: 10.1016/j.parkreldis.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 94.Coelho FM, Georgsson H, Narayansingh M, Swartz RH, Murray BJ. Higher prevalence of periodic limb movements of sleep in patients with history of stroke. J Clin Sleep Med. 2010;6:428–30. [PMC free article] [PubMed] [Google Scholar]
- 95.Lee SJ, Kim JS, Song IU, An JY, Kim YI, Lee KS. Poststroke restless legs syndrome and lesion location: anatomical considerations. Mov Disord. 2009;24:77–84. doi: 10.1002/mds.22303. [DOI] [PubMed] [Google Scholar]
- 96.Mansukhani MP, Bellolio MF, Kolla BP, Enduri S, Somers VK, Stead LG. Worse outcome after stroke in patients with obstructive sleep apnea: an observational cohort study. J Stroke Cerebrovasc Dis. 2010;20:401–5. doi: 10.1016/j.jstrokecerebrovasdis.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 97.Medeiros CA, de Bruin PF, Paiva TR, Coutinho WM, Ponte RP, de Bruin VM. Clinical outcome after acute ischaemic stroke: the influence of restless legs syndrome. Eur J Neurol. 2011;18:144–9. doi: 10.1111/j.1468-1331.2010.03099.x. [DOI] [PubMed] [Google Scholar]
- 98.Ryan CM, Bayley M, Green R, Murray BJ, Bradley TD. Influence of continuous positive airway pressure on outcomes of rehabilitation in stroke patients with obstructive sleep apnea. Stroke. 2011;42:1062–7. doi: 10.1161/STROKEAHA.110.597468. [DOI] [PubMed] [Google Scholar]
- 99.Sacco RL, Boden-Albala B, Abel G, et al. Race-ethnic disparities in the impact of stroke risk factors: the Northern Manhattan Stroke Study. Stroke. 2001;32:1725–31. doi: 10.1161/01.str.32.8.1725. [DOI] [PubMed] [Google Scholar]
- 100.Sorlie PD, Aviles-Santa LM, Wassertheil-Smoller S, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:629–41. doi: 10.1016/j.annepidem.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]