Abstract
Allogeneic islet cell transplantation is a promising treatment for human type 1 diabetes. Currently, human islets are transplanted via intra-portal infusions. While successful, it leads to significant early islet attrition from instant blood-mediated inflammatory reaction. An extra-hepatic site was established by transplanting islet-loaded microporous poly(lactide-co-glycolide) (PLG) scaffolds into the epididymal fat pad in syngeneic islet transplant models. This study examined this technology in allogeneic islet transplantation and determined whether transplant tolerance could be effectively induced to protect PLG scaffold transplanted allogeneic islets. The efficacy of an established tolerance induction strategy using donor splenocytes treated with ethylcarbodiimide(ECDI) was tested. ECDI-fixed donor splenocytes were infused 7 days before and 1 day after islet transplantation. Immediate normoglycemia was restored, and treated mice maintained indefinite normoglycemia whereas untreated mice rejected islet grafts within 20 days of transplantation. Interestingly, efficacy of tolerance induction was superior in PLG scaffold compared with intra-portal transplanted islets. Protection of PLG scaffold islet allografts was associated with several mechanisms of immune regulation. In summary, PLG scaffolds can serve as an alternative delivery system for islet transplantation that does not impair tolerance induction. This approach of combining tolerance induction with scaffold islet transplantation has potential therapeutic implications for human islet transplantation.
Keywords: Islet, Transplantation, Scaffold, Poly(lactide-co-glycolide) (PLG), Allogeneic cell, Tolerance, Immunomodulation
1. Introduction
Cell transplantation is being increasingly employed in therapeutic strategies, and biomaterials are being explored to enhance engraftment and function. Islet transplantation is currently in clinical trials for treatment of type 1 diabetes[1, 2]. In human clinical studies, islets are infused intra-portally into the liver for engraftment. This route of implantation, while successful, has been associated with high degrees of initial islet damage, largely due to the instant blood-mediated inflammatory reaction (IBMIR) triggered by complement activation and consumption of the coagulation system[3, 4]. This decreases the efficiency of the transplant in the short-term and also impairs long-term islet graft function [5, 6] due in part to continuing local intra-hepatic inflammation in addition to ongoing alloimmunity and possibly recurrent autoimmunity. Therefore, alternative implantation sites for human islet cell transplantation are urgently needed.
Biomaterials have been increasingly investigated as a vehicle for cell transplantation, as they can be engineered for control of local micro-environment of the transplanted cells, and for effective delivery of supportive factors for cell adhesion, cell growth, angiogenesis, and immunomodulation[7]. We have previously reported that a synthetic polymer scaffold fabricated from copolymers of lactide and glycolide (PLG) can serve as a platform for extra-hepatic islet cell transplantation[8], and can be modified with extracellular matrix proteins to further enhance the engraftment and function of the transplanted islets[9].
The source of islets for transplantation in type 1 diabetic patients is allogeneic, therefore life-long immunosuppressive medications must be given in order to prevent rejection of the islet graft. However, systemic immunosuppression has multiple undesirable side effects, including an increased vulnerability to infection and malignancy[10], as well as direct cytotoxicity to the transplanted islet cells themselves[11]. Therefore, strategies for inducing and maintaining donor-specific tolerance are highly desirable. We have previously shown that intravenous injection of donor splenocytes treated with ethylcarbodiimide (ECDI) prior to allogeneic islet transplantation induces donor-specific T cell hypo-responsiveness in vivo and provides indefinite islet allograft protection in the treated recipients in a murine islet transplant model wherein the islets are implanted in the renal sub-capsular space[12]. The mechanism with which ECDI-treated donor cell infusions induce antigen-specific tolerance is not completely understood, but involves targeting of recipient antigen presenting cells (APCs) and inducing expression of co-inhibitory molecules (Kheradmand and Luo, unpublished data).
Synthetic biomaterials such as PLG scaffolds for islet transplantation may elicit non-specific immune responses to the biomaterials themselves, which may interfere with transplant tolerance induction. In this report, we investigated whether ECDI-fixed donor cell infusions was effective for tolerance induction in the setting of PLG scaffold transplanted allogeneic islet cells in a functional murine model. Islet allografts were transplanted via PLG scaffolds implanted in the intra-peritoneal fat pad in the abdomen. Long-term islet allograft survival and mechanisms of allograft protection were examined. This strategy of combining donor-specific tolerance induction with scaffold islet transplantation has significant potential for clinical application in human islet transplantation. It additionally provides the foundation to broadly apply this approach to biomaterial-based allogeneic cell transplantation.
2. Materials and methods
2.1. Scaffold preparation and characterization
PLG microspheres were used as the building block to make the scaffolds using a gas foaming technique. Microspheres were prepared as previously described [13, 14]. PLG (75% D,L-lactide/25% glycolide, i.v. 0.76 dL/g) (Lakeshore Biomaterials, Birmingham, AL) was dissolved in dichloromethane to make a 6% (w/w) solution, which was then emulsified in 1% poly(vinyl alcohol) to create microspheres. The microspheres were collected by centrifugation, washed 3 times with deionized water to remove residual poly (vinyl alcohol), and lyophilized overnight.
The scaffold was constructed by mixing 2.5 mg of 6% PLG microspheres with 75 mg of NaCl (250 mm< particle diameter <425 mm) and then compressing the mixture in a 5 mm die at 1500 psi using a Carver press. The construct was then equilibrated with high pressure CO2 gas (800 psi) for 16 h in a custom-made pressure vessel. Afterwards, the pressure was released over a period of 25 min, which serves to fuse adjacent microspheres creating a continuous polymer structure. To remove the salt, each scaffold was leached in 2 mL of water for 3 hrs while shaking.
2.2. Animals and induction of diabetes
Male BALB/c and C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) between 8 and 12 weeks of age were used as islet donors and transplant recipients respectively. Clinical diabetes was induced by intraperitoneal injection of 190 mg/kg of streptozotocin (Sigma, St. Louis, MO)[15]. Diabetes was confirmed by blood glucose measurements greater than 250 mg/dL on two consecutive days prior to transplantation. Foxp3GFP mice on C57BL/6 background were purchased from the Jackson Laboratory. All studies were approved by the Northwestern University Animal Care and Use Committee.
2.3. ECDI Cell coupling and tolerance induction
Tolerance was induced by i.v. injections of ECDI treated donor splenocytes as described previously[12]. Briefly, spleens from BALB/c mice were processed into single cell suspensions. Red blood cells were lysed and splenocytes were incubated with ECDI (Calbiochem, 150 mg/ml for every 3.2×108 cells) on ice for 1 hour with agitation followed by washing. 1×108 ECDI-treated splenocytes in 200 ìl of PBS were injected on day −7 and day +1, with day 0 being the day of islet transplantation.
2.4. Islet isolation, scaffold seeding and transplantation
Islet isolation and scaffold seeding were performed as previously described[8, 12]. Briefly, islets were isolated from donor pancreata by a mechanically-enhanced enzymatic digestion using collagenase (type XI; Sigma). After filtration through a mesh screen, the filtrate was applied to a discontinuous ficoll (Sigma) gradient. Islets were hand-picked from the gradient, washed, and counted. Scaffolds were immersed in 70% ethanol for 30 sec and then washed in serum-containing media. After air-drying for 10 min, each scaffold was seeded with 300 islets in a minimal volume of media by applying them to the scaffold and allowing them to filter into the microporous structure. Examination of the tissue culture media following removal of the scaffolds demonstrated that greater than 98% of the islets were retained within the scaffolds. Scaffolds were then incubated at 37°C in 5% CO2 and 95% air for 30 min. An additional 20 μL of serum containing media was added to the scaffold followed by incubation for 30 min.
Recipient mice were anesthetized with an intraperitoneal injection of Avertin (250 mg/kg) and the abdominal region was shaved and prepped in a sterile manner. For scaffold islet transplant, the right epididymal fat pad was identified following a short, midline lower abdominal incision, and spread on the shaved and sterilized exterior abdominal surface. Scaffolds pre-seeded with islets were then placed on and wrapped by the epididymal fat pad and returned to the intraperitoneal cavity. For intra-portal islet transplantation, approximately 500 mouse islets in 200 μl of PBS were injected into the liver via the portal vein with a 27-gauge butterfly needle using a previously described method[16]. The wound was closed in two layers.
2.5. Assessment of graft function
Following transplantation, non-fasting blood glucose measurements were taken between 12:00 and 17:00 using the following schedule: everyday during the first post-operative week, every other day during weeks 2 – 4, once per week from week 5 until the conclusion of the study. Grafts rejection was confirmed if blood glucose level was more than 250 mg/dL on two consecutive days. For some recipients, grafts were removed at post transplant day 60 and blood glucose levels monitored daily for 3 days to confirm return of hyperglycemia, after which the mice were euthanized.
2.6. Graft histology
Immunohistochemistry was performed on tissue sections to analyze degrees of cellular infiltration and the presence of multiple cell types. At 7, 14, and 28 days post transplantation, scaffold grafts were retrieved and frozen in optimum cutting temperature (OCT) compound. The tissue was sectioned in 14-μm thick sections using a cryostat (Microm HM 525 N; Microm International, Walldorf, Germany) and stored at −20°C until staining. Prior to all staining, tissue sections were fixed in 4% PFA and blocked with 10% goat serum (Sigma-Aldrich); antibodies were diluted in buffer containing 1% goat serum. Detection of insulin was accomplished by staining with anti-insulin (1:300, Guinea pig polyclonal, Dako) and biotinylated anti-rabbit (1:200, Goat IgG, Vector) antibodies. Visualization for insulin was accomplished with Vectastain ABC kit (Vector Laboratories) and DAB substrate kit (BD Biosciences). Tissue was also examined for CD4 and CD8 T cells, macrophages, dendritic cells, and Foxp3+ regulatory cells. Representative sections were stained from each time point with rat anti-mouse CD4 mAb (1:750, rat (DA) IgG2a, κ clone RM4-5, Novus Biologicals), rat anti-mouse CD8 (1:750, rat IgG2a, κ clone 53-6.7, Novus Biologicals), rat anti-mouse F4/80 (1:200, rat IgG2b clone CI:A3-1, AbD Serotec) and hamster anti-mouse CD11c (1:100, hamster IgG clone AP-MAB0814, Novus Biologicals). For visualization of CD4, CD8 and F4/80, goat anti-rat Alexa Fluor 546 (1:500, Invitrogen) was used. For visualization of CD11c, goat anti-hamster Texas Red (1:500, Novus Biologicals) was used. A Hoechst stain was concurrently performed with these stains to identify cell nuclei. For Foxp3 staining, the frozen sections were additionally blocked with Avidin/ Biotin blocking kit (Vector Laboratories). Staining for Foxp3 was accomplished with anti-mouse Foxp3 mAb (1:400, rat IgG2a, κ clone FJK-16s; eBioscience) and biotinylated goat anti-rat Ig (1:200, goat Ig clone polyclonal; BD Biosciences). Visualization of Foxp3 was accomplished with Vectastain ABC kit (Vector Laboratories) and DAB substrate kit (BD Biosciences). Sections were counterstained with hematoxylin (Fisher Scientific Company). Negative controls were performed by eliminating the primary antibodies during the staining process.
2.7. Quantification of CD4+CD25+Foxp3+ regulatory T cells
We used Foxp3-GFP knock-in mice[17] as graft recipients. At indicated time points, recipient mice were sacrificed and single cell suspension prepared from the spleen and the graft draining lymph nodes (peri-aortic lymph nodes). Cells were stained with PerCP-conjugated anti-CD4 (GK1.5; BD Biosciences) and APC-conjugated anti-CD25 (PC61, BD Biosciences), and analyzed by fluorescence-activated cell sorting (FACS). Percentages of CD4+CD25+Foxp3GFP+ cells among all live cells (used as an estimation of total number of CD4+CD25+Foxp3GFP+ cells) were compared between control mice and mice treated with ECDI-fixed donor cell infusions.
2.8. Proliferation and cytokine production during in vitro re-stimulation
CD4+ T cells were purified from spleens of recipient C57BL/6 mice using CD4+ negative isolation kit (Miltenyi) and used as responders in in vitro mixed lymphocyte reactions (MLR). T cell-depleted splenocytes (by negative selection using anti-thy1.2 microbeads (Miltenyi)) from donor BALB/c mice were irradiated at 25 Gy and used as stimulators. Responders and stimulators were co-cultured in 96-well plates at 1:5 ratios. Cultures were pulsed with 1 μCi of [3H]thymidine (PerkinElmer) per well during the last 18 hours of the 96 hour culture. Supernatants from identical cultures without [3H]thymidine pulse were analyzed with Liquichip Mouse 22-cytokine assay kit (Millipore) for cytokine productions.
2.9. Statistical analysis
Graft survival was calculated by Kaplan Meier analysis. Log Rank test was used to compare survival between groups. Student’s t test was used for comparisons of in vitro cell phenotyping analysis. P values < 0.05 were considered to be statistically significant.
3. Results
3.1 ECDI-fixed donor splenocyte infusions for protection of PLG scaffold transplanted islet allografts
We first examined whether allogeneic islets transplanted via PLG scaffold could be protected by treatment with ECDI-fixed donor splenocyte (ECDI-SP) infusions. As shown in Figure 1, diabetic C57BL/6 recipients were treated on day −7 and day +1 with 1×108 ECDI-fixed BALB/c splenocytes. Islets transplanted via PLG scaffolds led to immediate reversal of diabetes in 100% of the recipients, confirming the effectiveness of this alternative site for islet transplantation[8]. However, in the absence of treatment with ECDI-SP, all grafts rejected by post-transplant day 20. On the other hand, with treatment using ECDI-SP, 80% of the islet grafts were permanently protected (>150 days post transplantation). To confirm that long-term euglycemia was indeed due to persistent function of the transplant islet grafts, grafts were removed from long-term euglycemic mice and prompt hyperglycemia (within 24 to 48 hours after graft removal) was observed (data not shown). Therefore, tolerance efficacy of ECDI-SP in PLG scaffold transplanted islets is similar to that observed in kidney capsule transplanted islets as we previously reported (P = 0.4, +tol kidney capsule vs. +tol scaffold, Figure 1)[12]. We also compared tolerance efficacy in PLG scaffold transplanted islets to that in intra-portal transplanted islets, which is the current route for islet transplantation in humans. The initial engraftment of transplanted islets was comparable in both models (100% reversal of hyperglycemia in both models). However, ECDI-fixed donor cell infusions conferred superior protection to PLG scaffold transplant islets than to intra-portal transplanted islets (P = 0.014, +tol scaffold vs. +tol intra-portal).
Figure 1. Allogeneic islets transplanted via PLG scaffold are permanently protected by treatment with ECDI-fixed donor cell infusions.
(A) Schematic treatment plan. 1×108 ECDI-fixed BALB/c splenocytes (ECDI-SP) were infused to diabetic C57BL/6 recipients on day −7 and day +1. BALB/c islets were transplanted on day 0. Blood glucose levels were followed until rejection occurred or 150 days post transplantation. (B) Islet allograft survival. ****P = 0.4, +tol kidney capsule vs. +tol scaffold; ***P = 0.014, +tol scaffold vs. +tol intra-portal; **P = 0.003, +tol scaffold vs. −tol scaffold; and *P = 0.03, +tol intra-portal vs. −tol intra-portal. “+tol”: with ECDI-SP infusions; “−tol”: without ECDI-SP infusions.
3.2 Histological examination of PLG scaffold transplanted islet allografts
We next examined the histology of the PLG scaffold transplanted islets in tolerized versus non-tolerized recipients. The PLG scaffold islet grafts were removed on day 7, 14 and 21 post transplantation from tolerized ((+)tol) and non-tolerized ((−)tol) recipients. We first examined the presence of insulin producing cells in the PLG scaffold islet grafts. In (−)tol recipients, insulin producing cells were detected at day 7 and but were completely absent by day 14 (Figure 2). Consistent with that, at day 7, islet structure was still discernable in (−)tol recipients, but by day 14, the islet structure was completely obliterated. In contrast, in (+)tol recipients, robust insulin producing cells were persistently observed in islet grafts examined on day 7, 14, and 21, with well preserved islet architecture at all of these time points (Figure 2).
Figure 2. Protection of PLG scaffold transplanted islets by ECDI-SP is associated with robust allograft insulin production.
Grafts from days 7, 14 and 21 post transplant were sectioned and stained for insulin. Left panels: graft sections from −tol recipients; right panels: graft sections from +tol recipients. Arrows point to individual islets positive for insulin. “+tol”: with ECDI-SP infusions; “−tol”: without ECDI-SP infusions. Magnification x 5.
We next examined the presence of immune cells in the PLG-islet grafts. Sections shown in Figures 3, 4, and 5 were all taken at the edge of the scaffolds where the islet grafts were resident (i.e., in (−)tol on day 7, and in (+)tol on day 7, 14, or 21), or where the islet grafts had been before they were destroyed (in (−)tol on day 14 or 21). We initially stained with antibodies to CD11c and F4/80 to identify dendritic cells (DCs) and macrophages (Mφs) that are professional phagocytes and antigen presenting cells (APCs). As shown in Figure 3, at day 7, both CD11c+ DCs and F4/80+ Mφs infiltrated the PLG scaffold. Both DCs and Mφs appeared to attach to the PLG material that lined the pores of the scaffold, with few DCs or Mφs seen among the cells filling the pore interior or the islet grafts themselves. No obvious differences were seen in infiltrating DCs or Mφs between grafts from (−)tol versus (+)tol recipients. The distribution of DCs and Mφs did not change over time in the (+)tol grafts examined on day 21 compared to day 7.
Figure 3. PLG scaffold transplanted islets are infiltrated with DCs and Mφs.
Immunofluorescent images of PLG scaffold transplanted islet grafts stained with CD11c (left panels) and F4/80 (right panels) antibodies. Grafts from days 7 (from +tol or −tol recipients) and 21 (from +tol recipients only) post transplant were sectioned and stained with indicated antibodies. “+tol”: with ECDI-SP infusions; “−tol”: without ECDI-SP infusions. Red: CD11c (left panels) or F4/80 (right panels); blue: Hoechst stain of nuclei. Magnification x 20.
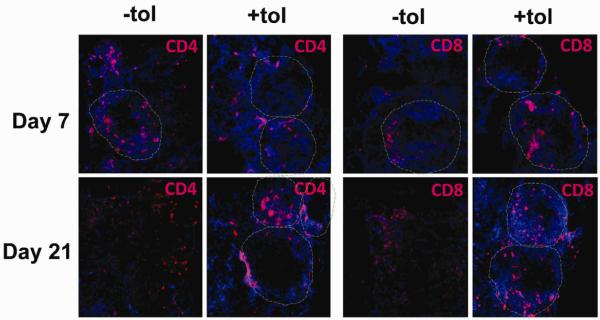
Figure 4. Protection of PLG scaffold transplanted islets by ECDI-SP is associated with less invasive infiltrations of CD4 and CD8 T cells around the islets.
Immunofluorescent images of PLG scaffold transplanted islet grafts stained with CD4 (left panels) and CD8 (right panels) antibodies. Grafts from days 7 and 21 post transplant were sectioned and stained with indicated antibodies. “+tol”: with ECDI-SP infusions; “−tol”: without ECDI-SP infusions. Red: CD4 (left panels) or CD8 (right panels); blue: Hoechst stain of nuclei. Dashed circles outline individual islets as determined by insulin staining of adjacent serial sections. Magnification x 20.
Figure 5. Protection of PLG scaffold transplanted islets by ECDI-SP is associated with accumulation of Foxp3+ Treg cells among peri-islet infiltrating cells.
Grafts from days 7, 14 and 21 post transplant were sectioned, stained with Foxp3 antibody and counterstained with hematoxylin. “+tol”: with ECDI-SP infusions; “−tol”: without ECDI-SP infusions. Arrows point to representative Foxp3+ cells. Magnification as indicated.
Next, we examined the infiltrating CD4 and CD8 T cells, which are normally responsible for rejection of transplanted islets. As shown in Figure 4, in (−)tol grafts, both CD4 and CD8 T cells infiltrated the islet graft, initially peripherally (peri-isletitis, day 7), but gradually destroyed and replaced the entire islet structure (isletitis, day 21). In contrast, in (+)tol recipients, both CD4 and CD8 T cells infiltrated the islet graft, but remained relatively peripheral to the islets (peri-isletitis, day 7 and 21), therefore allowing persistence of the integrity of islet architecture.
3.3 Immune mechanisms in the protection of PLG scaffold transplanted islet allografts by ECDI-SP
First, we examined the presence or absence of regulatory T cell population in the islet grafts, as these cells function to modulate the activity of CD4 and CD8 T cells to prevent rejection[18]. Tissue sections of scaffold islet graft were stained for the transcription factor Foxhead box P3 (Foxp3) that marks a CD4 regulatory T cell population (Tregs). As shown in Figure 5, in the (−)tol grafts at all time points, very few to no Foxp3+ cells were observed among the peri-islet infiltrating cells at day 7 or later among destructive infiltrating cells (day 14 and 21). In contrast, in (+)tol grafts, a progressive increase of Foxp3+ cells was detected among the peri-islet infiltrating cells from day 7 to day 14 and 21. These findings suggest that the Foxp3+ Treg population may play a critical role in the observed graft protection seen in (+)tol recipients.
We next examined whether T cells in tolerized mice exhibited altered immune responses upon in vitro re-stimulation with donor antigens. Splenic T cells were isolated on post-transplant day 21 from PLG scaffold islet transplant recipients with or without treatment with ECDI-SP, and stimulated with donor (BALB/c) or third party (SJL) APCs. Proliferation in response to donor stimulation measured by thymidine uptake was decreased by ~60% in T cells from ECDI-SP treated mice compared with those from untreated mice (Figure 6A). In contrast, proliferation to third party stimulation (SJL APCs) was not statistically significantly different between T cells from ECDI-SP treated vs. untreated mice. Cytokine production was measured in culture supernatant from the above donor-specific (BALB/c APCs) proliferation assays. A significant increase in IL-10 as well as IL-13 production by T cells from ECDI-SP treated mice was observed compared with those from untreated mice (Figure 6B). Furthermore, there was a small but significant decrease in IFN-γ production by T cells from ECDI-SP treated mice. No difference in IL-17 production was observed.
Figure 6. Splenic T cells from tolerized recipients are less prone to be activated during in vitro re-stimulation and exhibit altered profile of cytokine production.
In vitro MLR was set up as described in Materials and Methods using T cells isolated from spleens of +tol and −tol recipients on post transplant day 21. (A) Proliferation was measured by 3H thymidine update and expressed as CPM (counts per minute). P = 0.005, +tol vs. −tol for BALB/c APCs; P = NS +tol vs. −tol for SJL APCs. (B) Culture supernatants were collected from the above in vitro re-stimulation assay and analyzed for cytokine production. P values are as indicated. “+tol”: with ECDI-SP infusions; “−tol”: without ECDI-SP infusions. Data shown are representative of 2 individual experiments.
One of the reasons for diminished proliferation and increased IL-10 production during in vitro donor-specific re-stimulation could be the presence of increased numbers of CD4+CD25+Foxp3+ Tregs among the responding T cells as the CD4+CD25+Foxp3+ Tregs suppress naïve T cell proliferation. In addition, they have been shown to produce IL-10 as a means for exerting their regulatory function[19]. We therefore quantified the CD4+CD25+Foxp3+ Tregs post transplant in the spleen and the graft draining lymph nodes (dLNs) in mice with or without ECDI-SP treatment. For this experiment, Foxp3-GFP knock-in mice were used, in which all Foxp3+ cells are genetically tagged with the green fluorescent protein (GFP) to allow in vivo tracking. On post transplant day 7, a significant increase of CD4+CD25+Foxp3GFP+ cells, both in the percentage among CD4+ cells (Figure 7A) and in the percentage among total live cells (Figure 7B, used to estimate the total number of CD4+CD25+Foxp3GFP+ cells), was observed in the spleen of ECDI-SP treated mice compared with that of untreated mice. A similar increase of these cells in the dLN of ECDI-SP treated mice was also seen. This increase persisted at a later time point on post transplant day 21 (data not shown). More importantly, in the dLN of untreated mice, a distinct population of CD4+CD25+Foxp3GFP− cells representing activated effector T cells (Teff) was present (14%) that was almost completely absent (2%) in the dLN of ECDI-SP treated mice. The latter finding suggests compromised activation of donor-specific Teff cells in the dLN of ECDI-SP treated mice.
Figure 7. Protection of PLG scaffold transplanted islets by ECDI-SP is associated with peripheral up-regulation of the Treg population and down-regulation of the Teff population.
Foxp3-GFP knock-in mice were transplanted with PLG scaffold islet allografts with or without ECDI-SP. Foxp3GFP+ Treg cells were assessed in the spleen and the graft draining lymph node (dLN) on day 7 post transplantation. (A) Percentages of CD25+Foxp3GFP+(Treg) and CD25+Foxp3GFP−(Teff) T cells among all CD4+ cells of the spleen and the dLN are shown. Dot plots were gated on CD4+ T cells. (B) Percentages of CD4+CD25+Foxp3GFP+ cells among all live cells were used to estimate the total number of CD4+CD25+Foxp3GFP+ cells in the spleen and the dLN. Comparisons were made between +tol and −tol samples. “+tol”: with ECDI-SP infusions; “−tol”: without ECDI-SP infusions. Data shown are representative of 3 individual experiments.
4. Discussion
In current clinical islet cell transplantation, islets are delivered to the liver via intra-portal infusion. Extravasations within the liver sinusoids combined with the instant blood-mediated inflammatory response can have significant negative influences on islet engraftment, leading to islet loss estimated to exceed 70% during the first few days following transplant. Porous scaffolds have been employed as a means for engineering an extra-hepatic space for islet cell transplantation by promoting islet survival and engraftment. Most biomaterial-based approaches involve islet encapsulation in order to isolate islets from immune attack[20]; however, porous scaffolds encourage cell infiltration and thus integration with the host[8, 9, 21-23]. Islets can be readily seeded onto microporous PLG scaffolds and upon implantation, the scaffold creates and maintains a space for the transplanted islets[8, 9]. A high degree of porosity allows nutrient diffusion while enabling rapid host cell infiltration and revascularization of the transplanted islets. Additionally, modification of the scaffold with collagen IV can further enhance the functionality and viability of the transplanted islets, exemplifying the potentials for further scaffold engineering with biological signals to enhance its performance[9].
As a proof of concept, PLG scaffolds have been demonstrated to be a feasible alternative site for islet cell transplantation using syngeneic islet transplant models[8]. However, in allogeneic transplantation, it is unclear whether initial non-specific inflammatory responses to the PLG scaffold material would augment donor-specific allo-immune responses thereby impairing islet engraftment or tolerance induction. It has been previously shown that donor-specific tolerance can be impaired in setting of inflammation[24, 25]. Therefore, it is not surprising that ECDI-fixed donor cell infusions are not as protective to intra-portal transplanted allogeneic islets compared with kidney sub-capsular transplanted islets (Figure 1). Consistent with this notion, therapies that diminish IBMIR have been shown to improve initial islet engraftment[26-28] and may allow more efficient tolerance induction with intra-portal islet transplantation.
Biomaterial scaffolds used for allogeneic islet transplantation may elicit a foreign body inflammatory response[29, 30]. Therefore, as a potential alternative site for allogeneic islet cell transplantation, it would be highly relevant to determine efficacy of tolerance in setting of PLG scaffold transplanted allogeneic islets. The process of implantation and the biomaterial can recruit host APCs, such as Mφs and DCs, which can in turn induce secretion of inflammatory cytokines at the injury site. This inflammatory environment can lead to further activation of APCs to allow effective donor antigen presentation to naïve T cells infiltrating the graft; or alternatively, these APCs can travel to the graft draining LN to present antigens to recipient T cells there. Additionally, donor APCs that are carried to the recipient via the islet allograft can also be activated to prime recipient T cells in the graft or in secondary lymphoid organs. Interestingly, Figure 3 shows that the infiltrating DCs and Mφs are mainly associated with the PLG material lining the pores of the scaffold, a pattern similar to that seen when scaffolds are implanted without islets[14]. There are also no significant differences in the presence or infiltrative patterns of these cells in the scaffold islet grafts from control mice or mice treated with ECDI-SP (Figure 3). More importantly, ECDI-SP therapy is highly effective in protecting PLG scaffold transplanted allogeneic islets despite the presence of these cells in the scaffold (Figure 1). Therefore, distinct from the IBMIR seen in intra-portal islet transplantation, the initial infiltrating innate cells at the scaffold graft site do not seem to subvert tolerance induction by ECDI-SP, demonstrating an additional advantage using PLG scaffold as an extra-hepatic site for islet transplantation.
Tolerance induction by ECDI-SP in PLG scaffold transplanted islet allografts is associated with both local and systemic regulatory mechanisms. At the graft level, there is a robust accumulation of CD4+Foxp3+ Tregs in the tolerant scaffold allograft (Figure 5). It is not clear if these cells are induced or expanded locally in the graft, or they travel to the graft after being induced or expanded elsewhere (such as in the graft draining LN). Nonetheless, the PLG scaffold microenvironment is conducive for the accumulation of these cells in the graft, and does not provide the inflammatory signals that would allow phenotypic or functional re-programming of these cells, a characteristic of Tregs inherent to their known plasticity[31, 32]. More importantly, presence of the Treg population in the graft prevents destructive insulitis by the remaining infiltrating CD4 and CD8 T cells (Figure 4), which may be mediated by Tregs via production of soluble factors such as IL-10 or TGF-β[33, 34]. Tregs can further suppress immune responses via controlling maturation and activation of DCs and limiting their migration to draining LN for priming[19, 33]. This mechanism is consistent with our observation of a markedly dimished effector popluation in the draining LN of tolerant mice treated with ECDI-SP (Figure 7A). Systemically, T cells from the spleens of tolerized mice exhibit enhanced production of regulatory cytokines IL-10 and IL-13 upon in vitro donor-specific re-stimulation (Figure 6B). The enhanced IL-10 production is consistent with induction and/or expansion of various regulatory T cell populations as discussed above. The enhanced IL-13 production is indicative of a tendency of T cells from tolerized mice to polarize towards a Th2 phenotype upon donor stimulation. This phenotype has been associated with down-regulation of Th1 immunity[35] and prolongation of allogeneic graft survival by work from others[36-38]. The role of this cytokine in our model is currently being investigated.
5. Conclusions
In summary, this report demonstrates effective tolerance induction using ECDI-SP for allogeneic islet cells transplanted via PLG microporous scaffolds into the peritoneal fat pad. PLG scaffold transplanted allogeneic islets with this tolerance regimen restore euglycemia indefinitely in diabetic mice without further need for immunosuppression. Tolerance by ECDI-SP in this model is associated with both local Treg accumulation, down-regulation of the effector population as well as production of regulatory cytokines. Therefore, this strategy has significant therapeutic potential for human islet transplantation. It may also be more broadly applicable as a means to promote tolerance to allogeneic cells transplanted on biomaterial scaffolds, which could have numerous applications in regenerative medicine.
Acknowledgments
This work is supported by grants from the Juvenile Diabetes Research Foundation (JDRF) Postdoctoral Fellowship Grant 3-2010-447 (T.K.), NRSA F30DK084649 (R.F.G.), JDRF Regular Research Grant 1-2007-1055 (S.D.M and X.L.), NIH R01EB009910 and R21EB009502 (L.D.S.), NIH R01NS026543 (S.D.M.), and NIH Directors New Innovator Award DP2 DK083099 (X.L.).
Footnotes
Conflict of interest The authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Robertson RP. Islet transplantation a decade later and strategies for filling a half-full glass. Diabetes. 2010;59(6):1285–91. doi: 10.2337/db09-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo X, Herold KC, Miller SD. Immunotherapy of type 1 diabetes: where are we and where should we be going? Immunity. 2010;32(4):488–99. doi: 10.1016/j.immuni.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennet W, Groth CG, Larsson R, Nilsson B, Korsgren O. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups J Med Sci. 2000;105(2):125–33. doi: 10.1517/03009734000000059. [DOI] [PubMed] [Google Scholar]
- 4.Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes. 2002;51(6):1779–84. doi: 10.2337/diabetes.51.6.1779. [DOI] [PubMed] [Google Scholar]
- 5.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54(7):2060–9. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–30. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 7.Huebsch N, Mooney DJ. Inspiration and application in the evolution of biomaterials. Nature. 2009;462(7272):426–32. doi: 10.1038/nature08601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blomeier H, Zhang X, Rives C, Brissova M, Hughes E, Baker M, et al. Polymer scaffolds as synthetic microenvironments for extrahepatic islet transplantation. Transplantation. 2006;82(4):452–9. doi: 10.1097/01.tp.0000231708.19937.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salvay DM, Rives CB, Zhang X, Chen F, Kaufman DB, Lowe WL, Jr., et al. Extracellular matrix protein-coated scaffolds promote the reversal of diabetes after extrahepatic islet transplantation. Transplantation. 2008;85(10):1456–64. doi: 10.1097/TP.0b013e31816fc0ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutierrez-Dalmau A, Campistol JM. Immunosuppressive therapy and malignancy in organ transplant recipients: a systematic review. Drugs. 2007;67(8):1167–98. doi: 10.2165/00003495-200767080-00006. [DOI] [PubMed] [Google Scholar]
- 11.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest. 2007;117(9):2553–61. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo X, Pothoven KL, McCarthy D, DeGutes M, Martin A, Getts DR, et al. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc Natl Acad Sci U S A. 2008;105(38):14527–32. doi: 10.1073/pnas.0805204105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang JH, Rives CB, Shea LD. Plasmid delivery in vivo from porous tissue-engineering scaffolds: Transgene expression and cellular Transfection. Mol Ther. [Article] 2005;12(3):475–83. doi: 10.1016/j.ymthe.2005.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rives CB, Rieux Ad, Zelivyanskaya M, Stock SR, Lowe WL, Jr, Shea LD. Layered PLG scaffolds for in vivo plasmid delivery. Biomaterials. 2009;30(3):394–401. doi: 10.1016/j.biomaterials.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dufrane D, van Steenberghe M, Guiot Y, Goebbels RM, Saliez A, Gianello P. Streptozotocin-induced diabetes in large animals (pigs/primates): role of GLUT2 transporter and beta-cell plasticity. Transplantation. 2006;81(1):36–45. doi: 10.1097/01.tp.0000189712.74495.82. [DOI] [PubMed] [Google Scholar]
- 16.Goto M, Groth CG, Nilsson B, Korsgren O. Intraportal pig islet xenotransplantation into athymic mice as an in vivo model for the study of the instant blood-mediated inflammatory reaction. Xenotransplantation. 2004;11(2):195–202. doi: 10.1046/j.1399-3089.2003.00107.x. [DOI] [PubMed] [Google Scholar]
- 17.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6(11):1142–51. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 18.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3(3):199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 19.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30(5):636–45. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Narang AS, Mahato RI. Biological and biomaterial approaches for improved islet transplantation. Pharmacol Rev. 2006;58(2):194–243. doi: 10.1124/pr.58.2.6. [DOI] [PubMed] [Google Scholar]
- 21.Dufour JM, Rajotte RV, Zimmerman M, Rezania A, Kin T, Dixon DE, et al. Development of an ectopic site for islet transplantation, using biodegradable scaffolds. Tissue Eng. 2005;11(9-10):1323–31. doi: 10.1089/ten.2005.11.1323. [DOI] [PubMed] [Google Scholar]
- 22.Chun S, Huang Y, Xie WJ, Hou Y, Huang RP, Song YM, et al. Adhesive growth of pancreatic islet cells on a polyglycolic acid fibrous scaffold. Transplant Proc. 2008;40(5):1658–63. doi: 10.1016/j.transproceed.2008.02.088. [DOI] [PubMed] [Google Scholar]
- 23.Berman DM, O’Neil JJ, Coffey LC, Chaffanjon PC, Kenyon NM, Ruiz P, Jr., et al. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant. 2009 Jan;9(1):91–104. doi: 10.1111/j.1600-6143.2008.02489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang T, Chen L, Ahmed E, Ma L, Yin D, Zhou P, et al. Prevention of allograft tolerance by bacterial infection with Listeria monocytogenes. J Immunol. 2008;180(9):5991–9. doi: 10.4049/jimmunol.180.9.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang T, Ahmed EB, Chen L, Xu J, Tao J, Wang CR, et al. Infection with the intracellular bacterium, Listeria monocytogenes, overrides established tolerance in a mouse cardiac allograft model. Am J Transplant. 2010;10(7):1524–33. doi: 10.1111/j.1600-6143.2010.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Contreras JL, Eckstein C, Smyth CA, Bilbao G, Vilatoba M, Ringland SE, et al. Activated protein C preserves functional islet mass after intraportal transplantation: a novel link between endothelial cell activation, thrombosis, inflammation, and islet cell death. Diabetes. 2004;53(11):2804–14. doi: 10.2337/diabetes.53.11.2804. [DOI] [PubMed] [Google Scholar]
- 27.Cui W, Wilson JT, Wen J, Angsana J, Qu Z, Haller CA, et al. Thrombomodulin improves early outcomes after intraportal islet transplantation. Am J Transplant. 2009;9(6):1308–16. doi: 10.1111/j.1600-6143.2009.02652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tokodai K, Goto M, Inagaki A, Nakanishi W, Ogawa N, Satoh K, et al. Attenuation of cross-talk between the complement and coagulation cascades by C5a blockade improves early outcomes after intraportal islet transplantation. Transplantation. 2010;90(12):1358–65. doi: 10.1097/tp.0b013e3181ffb9f5. [DOI] [PubMed] [Google Scholar]
- 29.Rucker M, Laschke MW, Junker D, Carvalho C, Schramm A, Mulhaupt R, et al. Angiogenic and inflammatory response to biodegradable scaffolds in dorsal skinfold chambers of mice. Biomaterials. 2006;27(29):5027–38. doi: 10.1016/j.biomaterials.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 30.Thevenot PT, Nair AM, Shen J, Lotfi P, Ko CY, Tang L. The effect of incorporation of SDF-1alpha into PLGA scaffolds on stem cell recruitment and the inflammatory response. Biomaterials. 2010;31(14):3997–4008. doi: 10.1016/j.biomaterials.2010.01.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lal G, Yin N, Xu J, Lin M, Schroppel S, Ding Y, et al. Distinct inflammatory signals have physiologically divergent effects on epigenetic regulation of foxp3 expression and treg function. Am J Transplant. 2011;11(2):203–14. doi: 10.1111/j.1600-6143.2010.03389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hori S. Developmental plasticity of Foxp3+ regulatory T cells. Curr Opin Immunol. 2010;22(5):575–82. doi: 10.1016/j.coi.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol. 2007;7(11):875–88. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194(5):629–44. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deepak P, Kumar S, Jr., Kishore D, Acharya A. IL-13 from Th2-type cells suppresses induction of antigen-specific Th1 immunity in a T-cell lymphoma. Int Immunol. 2010;22(1):53–63. doi: 10.1093/intimm/dxp114. [DOI] [PubMed] [Google Scholar]
- 36.Davidson C, Verma ND, Robinson CM, Plain KM, Tran GT, Hodgkinson SJ, et al. IL-13 prolongs allograft survival: association with inhibition of macrophage cytokine activation. Transpl Immunol. 2007;17(3):178–86. doi: 10.1016/j.trim.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 37.Ke B, Shen XD, Zhai Y, Gao F, Busuttil RW, Volk HD, et al. Heme oxygenase 1 mediates the immunomodulatory and antiapoptotic effects of interleukin 13 gene therapy in vivo and in vitro. Hum Gene Ther. 2002;13(15):1845–57. doi: 10.1089/104303402760372945. [DOI] [PubMed] [Google Scholar]
- 38.Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R, et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116(25):5738–47. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]