Abstract
The cAMP-regulatory element (CRE) binding protein (CREB) functions as a trans-acting regulator of genes containing the CRE sequence in their promoter. These include a number of critical genes, such as CRF, involved in the hypothalamic response to stressful stimuli in the adult. The ability of the developing rat (during the first 2 postnatal weeks) to mount the full complement of this stress response has been questioned. We have previously demonstrated the stress-induced up-regulation of the transcription of hypothalamic CRF during the second postnatal week in the rat. The focus of the current study was to explore the mechanism of transcriptional regulation in response to stress through the physiological induction of transcriptional trans-activators that bind to the CRE in the developing rat brain. CRE-binding activity was detected via gel shift analysis in extracts from both the hypothalamus and the cerebral cortex of the developing rat. CREB was identified in these extracts by Western blot analysis and was shown to be the major contributor to the CRE-binding activity by gel shift analysis with two specific antibodies directed against CREB. After acute hypothermic stress, the abundance of CRE-binding activity (but not of total immunoreactive CREB), increased in hypothalamic extracts. This enhanced CRE-binding activity was blocked by an antiserum directed against CREB and was accompanied by an apparent increase in CREB phosphorylation. These results indicate that posttranslational enhancement of CRE-binding activity is likely to constitute an important mechanism for up-regulation of genes possessing the CRE sequence in the developing rat hypothalamus by adverse external signals.
INTRODUCTION
The complement of cellular gene expression is determined by complex interactions of trans-acting transcriptional regulatory factors with specific DNA sequences. The ability to rapidly and precisely alter the expression of individual genes in response to environmental stimuli is believed to result from modulating the interactions of these transcription factors with their corresponding DNA-regulatory elements.
Modulation of gene expression by extracellular signals can occur via activation of G protein-coupled membrane receptors leading to increased levels of intracellular messengers such as calcium and cAMP (reviewed in Refs. 1–4). Genes responsive to cAMP have been found to have a common DNA promoter sequence, the cAMP-regulatory element (CRE) (5). Elevation of intracellular cAMP concentration results in phosphorylation of specific proteins by protein kinase A (2). An important protein target of protein kinase A-mediated phosphorylation is the transcriptional trans-acting factor CRE-binding protein (CREB) (1). CREB and other proteins that bind to the CRE are members of the bZip superfamily, containing a basic leucine zipper. These regulatory proteins interact to form homo- or heterodimers to bind DNA and regulate transcription of responsive genes (2).
Transcriptional regulation leading to altered expression of critical genes is a fundamental aspect of the response to adverse external or internal signals, i.e. stress. A key mediator activating both the hormonal and behavioral responses to stress is the neuropeptide CRF. Like a number of genes that participate in the stress response, the CRF promoter contains a CRE consensus sequence, and its transcription is regulated by alterations in cAMP concentration and CRE binding (6, 7).
It has been documented that changes in CRE-binding activity and CREB phosphorylation can occur rapidly in the adult rat brain in response to diverse environmental stimuli, resulting in activation (or in some cases inhibition) of transcription (2). However, it is unclear whether the same transcriptional regulatory factors are activated in the central nervous system (CNS) of the developing rat. Differences between the mature and developing hormonal responses to stress signals have been established. The first 2 postnatal weeks are considered a stress hyporesponsive period with respect to hypothalamic-pituitary-adrenal activation (8–12). This developmental period has been characterized by attenuated hormonal responses and altered gene regulation in response to stress as compared with the adult. For example, expression of c-fos, an immediate early gene that is rapidly up-regulated by a variety of stress and activation signals in the mature brain, is not up-regulated in the immature rat brain even by the profound neuronal activation associated with kainic acid-induced seizures (13). Similarly, stress-induced up-regulation of CRF mRNA levels occurs only after the first postnatal week (14). Transcription of both the CRF and c-fos gene is believed to be regulated (at least in part) by binding of trans-acting regulatory proteins to the CRE sequence in the promoters of these genes (6, 7, 15, 16).
This study addressed two principal questions: First, do hypothalamic and cortical cells express CRE-binding activity during the first 2 postnatal weeks and is this CRE-binding capacity related to CREB? Second, is the developing organism capable of rapid, adaptive regulation of its gene repertoire by increasing the activity of transcriptional regulatory factors to alter expression of a group of genes such as those containing the CRE sequence?
RESULTS
CRE-Binding Activity and CREB in the Anterior Hypothalamus of the Developing Rat
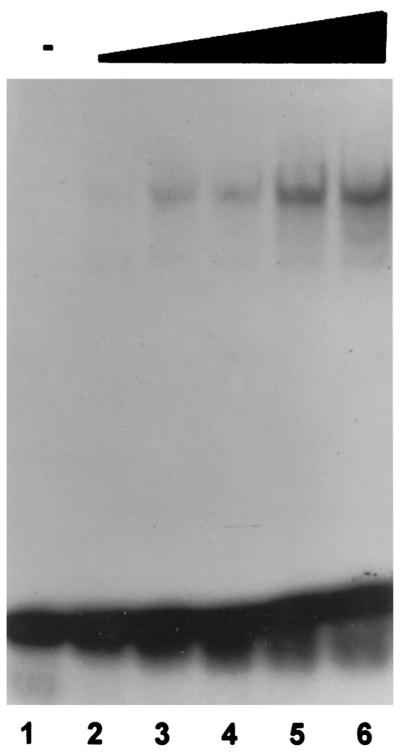
CRE-binding activity in protein extracts of 12- to 13-day-old rat brain was demonstrated using gel shift analysis. Protein extracts from both the anterior hypothalamus (Fig. 1) and the cerebral cortex (data not shown) from stress-free, control rats resulted in band retardation consistent with the presence of binding activity for the CRE consensus sequence in these extracts. Increasing concentrations of protein extract resulted in a corresponding increase in signal intensity of the retarded band (Fig. 1).
Fig. 1. The CRE-Binding Activity Is Present in Extracts from the Anterior Hypothalamus of the Developing Rat.
Gel shift analysis of a radioactively labeled oligonucleotide containing the CRE consensus sequence that was preincubated with increasing concentrations of protein extract (1.25, 2.5, 5, 10, and 20 μg) from the anterior hypothalamus of 12-to 13-day-old rats. No shift of the DNA fragment was detectable in the absence of brain extract (lane 1).
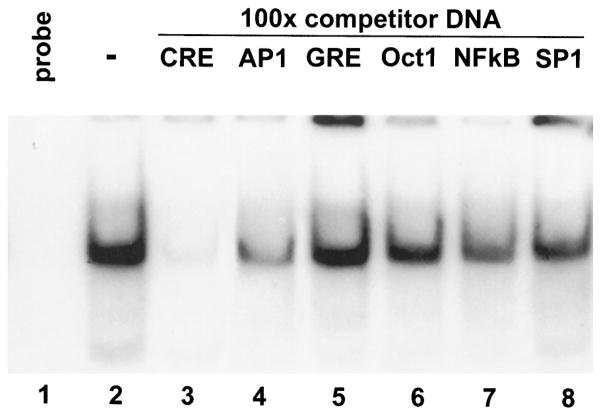
The specificity of the DNA-binding activity to the CRE consensus sequence was confirmed via competition experiments. CRE-binding activity was essentially eliminated by preincubation of the tissue extracts with 100-fold excess of unlabeled oligonucleotide containing the CRE consensus sequence (Fig. 2, compare lane 2 with lane 3). This DNA binding was not eliminated by 100-fold excess of unlabeled oligonucleotides containing other DNA consensus sequences including AP1, glucocorticoid response element (GRE), octomer binding protein 1 (Oct1), nuclear factor (NF)κB, and promoter–specific transcription factor (SP1) (Fig. 2).
Fig. 2. The CRE-Binding Activity in Hypothalamic Extracts Is Specific.
Gel shift analysis of a radioactively labeled oligonucleotide containing the CRE consensus sequence incubated with 10 μg protein extract from the anterior hypothalamus of 12- to 13-day-old rats (lanes 2–8). DNA-binding activity (lane 2) is eliminated by 100-fold excess unlabeled DNA fragment containing the CRE consensus sequence (lane 3) but not by 100-fold excess DNA fragments containing consensus sequences for AP1 (lane 4), GRE (lane 5), Oct1 (lane 6), NFκB (lane 7), or SP1 (lane 8).
The presence of the protein CREB was determined in extracts from the anterior hypothalamus and the cerebral cortex using Western immunoblot analysis (Fig. 3). Two different antibodies were used to identify CREB: the first was a polyclonal antiserum directed against CREB-1 (epitope corresponding to amino acids 295–321), and the other was a polyclonal antiserum specific for the phosphorylated form of CREB (epitope corresponding to amino acids 128–141) (17). Use of each antibody in Western immunoblot analysis of size-fractionated, membrane-bound extracts from both the anterior hypothalamus and the cerebral cortex resulted in a single predominant band with a molecular mass of approximately 43 kDa, consistent with the molecular mass of CREB (18).
Fig. 3. CREB and Phosphorylated CREB Are Present in Both Anterior Hypothalamic and Cortical Extracts of the Developing Rat.
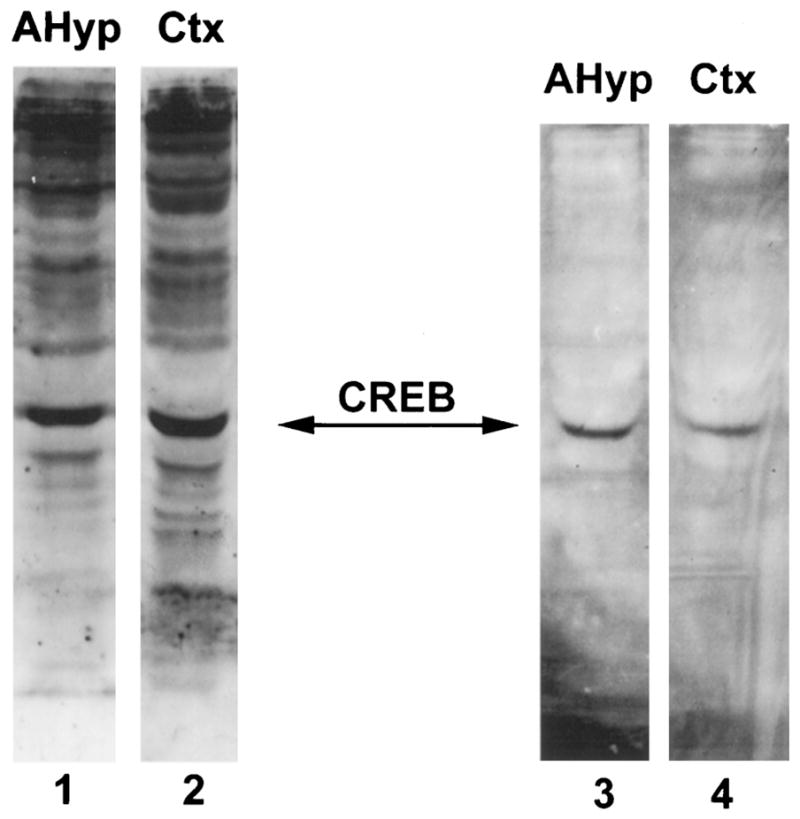
Western blot analysis of 10 μg protein extract from the anterior hypothalamus (lanes 1 and 3) and cerebral cortex (lanes 2 and 4) of 12- to 13-day-old rats using antisera directed against CREB-1 (lanes 1 and 2) and phosphorylated CREB (lanes 3 and 4). A predominant, 43-kDa immunolabeled band is evident in all lanes, consistent with CREB. CREB and the phosphorylated form of CREB are present in the anterior hypothalamus (lanes 1 and 3) and in the cerebral cortex (lanes 2 and 4).
Further confirmation that CREB was at least partially responsible for the CRE-binding activity of anterior hypothalamic extracts was obtained using gel shift analysis of this DNA-binding activity after incubation of the protein extract with the same anti-CREB antibodies used for the Western immunoblot analysis. The antiserum directed against phosphorylated CREB resulted in a supershifted band (Fig. 4, lane 3). The antiserum directed against CREB-1 blocked the CRE-binding activity of the extract, likely via steric hindrance (Fig. 4, lane 4).
Fig. 4. Functional and Antigenic Specificity of the CRE-Binding Activity of Rat Hypothalamic Extract.
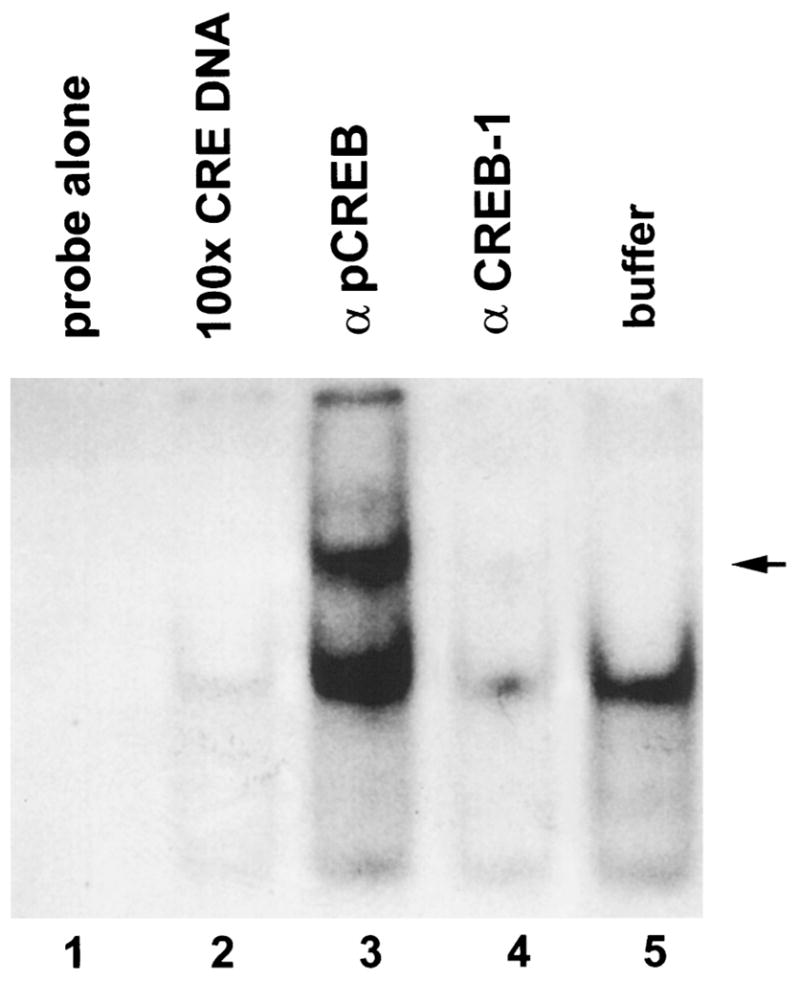
Gel shift analysis of a radioactively labeled oligonucleotide containing the CRE consensus sequence with 10 μg protein extract from the anterior hypothalamus of 12- to 13-day-old rats (lanes 2–5). Before incubation with the labeled oligonucleotide, samples were treated as follows. A, Incubation with 100-fold excess of unlabeled oligonucleotide containing the CRE consensus sequence resulted in elimination of the retarded band representing CRE-binding activity (lane 2); B, incubation with an antiserum directed against phosphorylated CREB (βpCREB) caused additional retardation with a supershifted band (lane 3, indicated by arrow in the margin); C, incubation with antiserum directed against CREB-1 (βCREB-1) resulted in nearly complete inhibition of DNA-binding activity (lane 4); D, incubation with buffer alone (lane 5).
Hypothermic Stress Results in an Increase of CRE-Binding Activity in the Anterior Hypothalamus but Not the Cerebral Cortex
Experiments were performed to determine whether there was an alteration in CRE-binding activity in the developing rat brain in response to stress. Hypothermic stress, which is a potent age-specific stressor for the immature rat (14), was employed. Animals were killed at several time points after the hypothermia, and plasma was analyzed for corticosterone levels as a measure of stress. Consistent with previous results (11, 14), hypothermic stress resulted in a significant increase in plasma corticosterone levels (Fig. 5).
Fig. 5. Plasma Corticosterone Levels after Acute Hypothermic Stress.
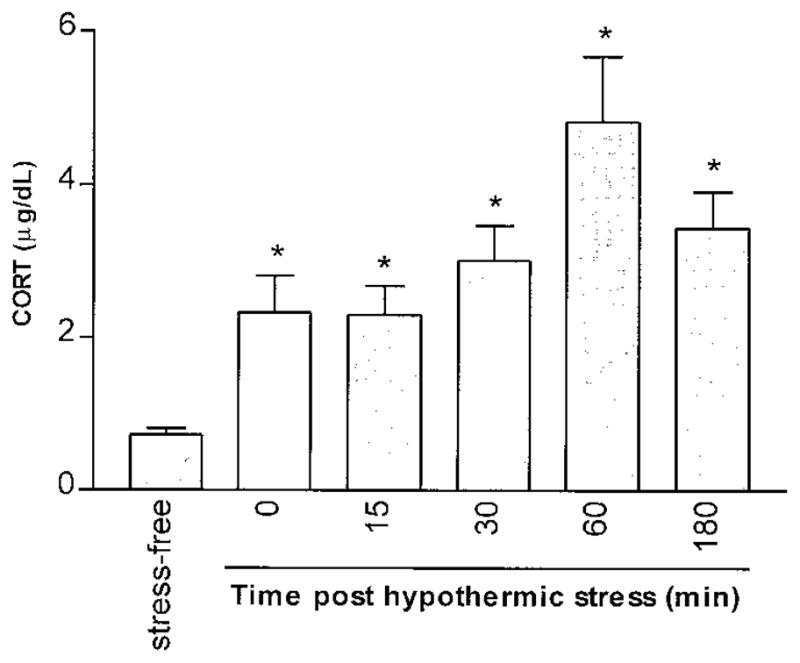
Plasma corticosterone (CORT) was measured in stress-free rats and at several time points after the termination of hypothermic stress. A robust stress-induced elevation of plasma CORT is evident by the end of the hypothermia period and peaks 60 min later. Each column represents the mean of at least four 12- to 13-day-old rats with bars indicating the SEM. Asterisks represent a significant difference from the stress-free group (P < 0.0001) determined by unpaired Student’s t test.
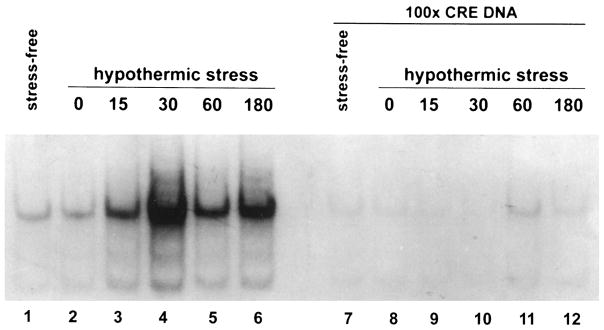
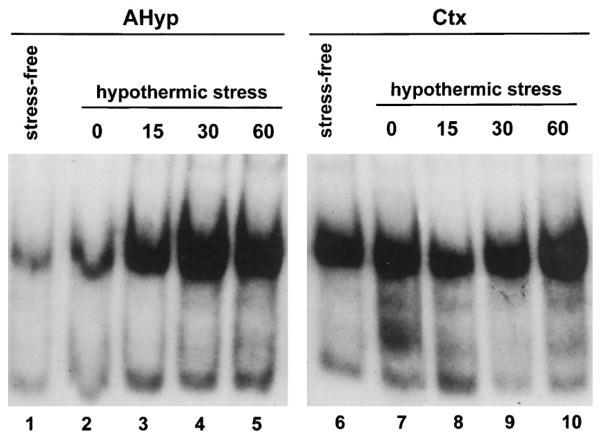
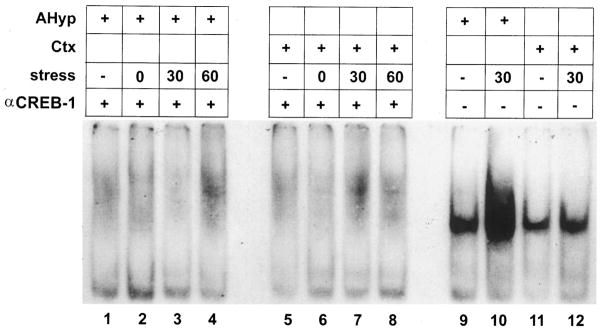
Extracts from the anterior hypothalamus and the cerebral cortex were analyzed for CRE-binding activity by gel shift analysis. Acute hypothermic stress resulted in an increased CRE-binding activity in hypothalamic extracts as indicated by enhanced signal intensity of the retarded band (Fig. 6). Maximal amounts of CRE-binding activity were detected 30 min after the termination of the hypothermic stress. Levels of CRE-binding activity at 180 min remained elevated relative to tissue derived from stress-free controls. The CRE-binding activity of these extracts was substantially reduced by competition with 100-fold excess unlabeled oligonucleotide containing the CRE consensus sequence (Fig. 6), suggesting that this DNA-binding activity is specific for the CRE. Unlike the stress-induced increase in CRE-binding activity in the anterior hypothalamus, there was no consistent change in the CRE-binding capacity of cortical extracts after hypothermic stress (Fig. 7). The CRE-binding activity in samples from both the anterior hypothalamus and the cerebral cortex of rats subjected to hypothermic stress was blocked by exposure of the extracts to the antiserum directed against CREB-1 (Fig. 8). In addition, Southwestern blot analysis of hypothalamic protein extracts indicated that a protein with molecular mass consistent with that of CREB was capable of binding an oligonucleotide containing the CRE consensus sequence (Fig. 9A). This CRE-binding capacity was increased in extracts obtained from animals subjected to hypothermic stress, although overall CREB immunoreactivity did not increase in response to stress (Fig. 9B).
Fig. 6. Increased CRE-Binding Activity in the Anterior Hypothalamus in Response to Hypothermic Stress.
Gel shift analysis of the DNA containing the CRE consensus sequence using rat anterior hypothalamic extracts at several time points after hypothermic stress. Each lane represents 10 μg of protein extract from 12- to 13-day-old rats. Samples in lanes 1 and 7 were derived from rats under stress-free conditions. For the other samples, rats were killed at 0 min (lanes 2 and 8), 15 min (lanes 3 and 9), 30 min (lanes 4 and 10), 60 min (lanes 5 and 11) or 180 min (lanes 6 and 12) after termination of hypothermic stress. A progressive increase in the CRE-binding capacity of the hypothalamic extracts is evident after stress, peaking at the 30-min time point (lane 4). Specificity of the CRE-binding activity was determined by competing with 100-fold excess of an unlabeled oligonucleotide containing the CRE consensus sequence. All of the stress-induced CRE-binding activity is eliminated by excess unlabeled CRE (lanes 7–12)
Fig. 7. Stress Increases the CRE-Binding Activity in the Anterior Hypothalamus but Not the Cerebral Cortex.
Gel shift analysis of DNA containing the CRE consensus sequence using 10 μg protein extracts from the anterior hypothalamus (lanes 1–5) and the cerebral cortex (lanes 6–10). Lanes represent protein extracts from 12- to 13-day-old rats under stress-free conditions (lanes 1 and 6) or 0 min (lanes 2 and 7), 15 min (lanes 3 and 8), 30 min (lanes 4 and 9), and 60 min (lanes 5 and 10) after maximal hypothermic stress. Increased intensity of the CRE-binding activity signal is evident in the hypothalamic extracts after stress (lanes 3–5) as compared with the stress-free condition (lane 1). Signal intensity of the CRE- binding activity of cortical extracts is not consistently influenced by exposure to stress (lanes 7–10 compared with lane 6).
Fig. 8. Stress-Induced CRE-Binding Activity Is Blocked with an Antiserum Directed against CREB-1.
Gel shift analysis of DNA containing the CRE consensus sequence using 10 μg protein extracts from the anterior hypothalamus (lanes 1–4 and 9 and 10) and the cerebral cortex (lanes 5–8 and 11 and 12) of 12- to 13-day-old rats. Samples were incubated with antiserum directed against CREB-1 (αCREB-1), which blocks the DNA-binding activity of CREB (lanes 1–8), or with buffer (lanes 9–12) before addition of the labeled oligonucleotide containing the CRE consensus sequence. Protein extracts were obtained from rats under stress-free conditions (lanes 1, 5, 9, and 11), or 0 min (lanes 2 and 6), 30 min (lanes 3, 7, 10, and 12), and 60 min (lanes 4 and 8) after maximal hypothermic stress. The stress-free levels of CRE-binding activity in the hypothalamus (lane 9) and cortex (lanes 11 and 12) and the stress-induced increase in CRE-binding activity in the hypothalamus (lane 10) are eliminated by preincubation of the extracts with the αCREB-1 antibody (lanes 1–8).
Fig. 9. Stress-Induced CRE-Binding Activity in the Hypothalamus Is Specific.
A, Southwestern blot analysis of protein extracts from the anterior hypothalamus. Protein-bound membranes were incubated with 32P-labeled double-stranded oligonucleotide containing the CRE consensus sequence. Protein extracts obtained from 12- to 13-day-old rats exposed to stress shows reveal increased signal intensity compared with stress-free control. Size of resultant bands are consistent with CREB (43 kDa). B, Western blot analysis of same membrane using an antiserum directed against CREB-1. No increase in CREB immunoreactivity in extracts from animals exposed to stress is apparent.
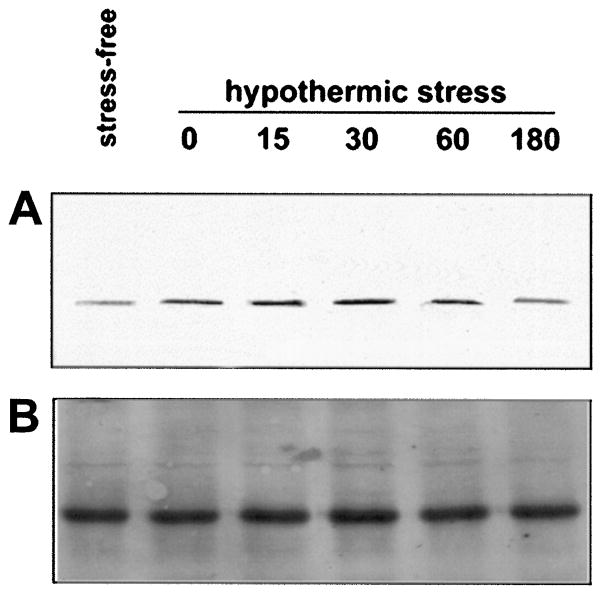
The augmented CRE-binding activity in the anterior hypothalamus after hypothermic stress was not associated with an overall increase of CREB immunoreactivity in these extracts (Fig. 10, top). However, immunoreactive phosphorylated CREB was increased with stress (Fig. 10, bottom) as indicated by Western blot analysis. This increase in phosphorylated CREB abundance was also specific to the anterior hypothalamus since it was not observed in cortical extracts.
Fig. 10. Stress Increases Phosphorylated CREB, but not CREB Immunoreactivity, in the Hypothalamus.
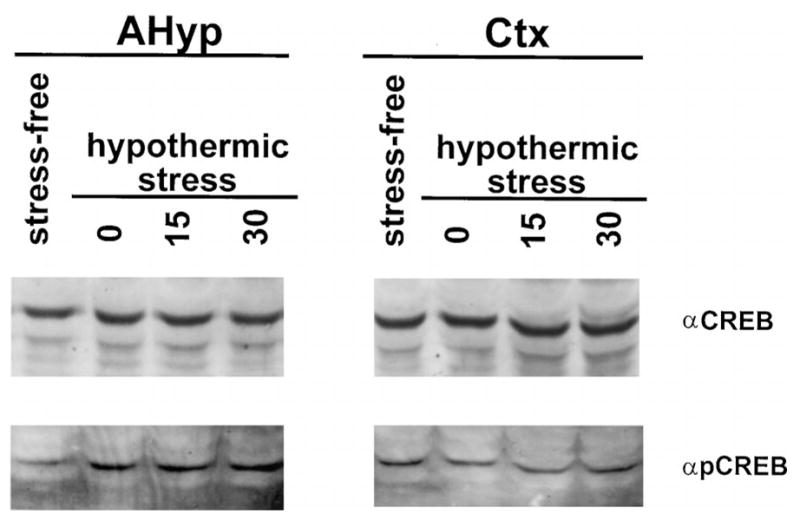
Western blot analysis of 20 μg protein extract from the anterior hypothalamus (left) or the cerebral cortex (right) of the 12- to 13-day-old rat under stress-free conditions, or 0 min, 15 min, and 30 min after the termination of hypothermic stress. Blots were bound with an antiserum directed against CREB (αCREB) and phosphorylated CREB (αpCREB). There is no apparent stress-induced change in the abundance of CREB immunoreactivity in either the hypothalamic or cortical extracts. However, there is an apparent increase in phosphorylated CREB immunoreactivity in hypothalamic (left bottom) but not cortical (right bottom) extracts after hypothermic stress as compared with extracts from the stress-free rats.
These results, shown here for 12- to 13-day-old rats, were replicated using 4- to 6-day-old rats; i.e. CRE-binding activity and the stress-induced enhancement of both CRE-binding and phosphorylated CREB in the anterior hypothalamus were demonstrated throughout the first 2 postnatal weeks.
DISCUSSION
The current study demonstrates increases in CRE-binding activity and phosphorylation of CREB that provide a mechanism for stress-induced up-regulation of the transcription of CRE-containing genes (such as CRF) in the developing hypothalamus. First, these results provide evidence that CRE-binding activity is present in the developing rat hypothalamus and cerebral cortex. Furthermore, CREB is likely to be the principal contributor to this CRE-binding activity since DNA binding is essentially blocked by an antiserum directed against CREB-1 (Fig. 4). Previous investigations have demonstrated DNA binding activity of CREB (19) and constitutive expression of CREB-mRNA (20) in the developing rat brain. This study documents that phosphorylated CREB is present and is involved in DNA binding in hypothalamic and cortical extracts of the developing rat, as suggested by the supershift (Fig. 4) and Western blot (Fig. 3) analyses using an antiserum specific for the phosphorylated form of CREB as well as a Southwestern blot (Fig. 9A) showing that a protein with molecular mass consistent with CREB binds to DNA containing the CRE consensus sequence.
The observed stress-induced increase in CRE binding was specific, as indicated by the ability of excess unlabeled oligonucleotide containing the CRE consensus sequence to compete for this binding (Fig. 6). CREB is likely to be a major constituent not only of the basal CRE-binding activity as shown above, but also of the stress-induced increase in CRE-binding activity. The evidence for this fact is provided by the elimination of the majority of CRE-binding capacity of the hypothalamic and cortical extracts by exposure to antiserum directed against CREB-1, which blocks the interaction of CREB with the CRE-DNA fragment (Fig. 8), and by the observed increase of CRE binding by a protein of a size consistent with CREB (43 kDa) by Southwestern blot analysis (Fig. 9A). In addition to enhanced CRE-binding capacity, hypothermic stress resulted in increased phosphorylated CREB in extracts from the anterior hypothalamus, but not the cerebral cortex (Fig. 10). These findings indicate that the developing hypothalamus exhibits plasticity in its complement of activated genes in response to environmental alterations.
The mechanism for the stress-induced enhancement of CREB-mediated DNA binding may be deduced from analysis of the data presented above. Stress did not lead to increased levels of total CREB in the hypothalamus or cortex (Fig. 10). In contrast, phosphyrylated CREB and CRE-binding activity were both enhanced in the hypothalami of cold-stressed rat pups. These findings are consistent with a posttranslational mechanism by which stress acts to activate existing CREB and increase CRE-binding capacity. CREB phosphorylation may be accompanied by activation of other components of the physiological CRE-binding protein complex.
CRE-binding activity and CREB play an important role in gene regulation. The promoters of numerous genes possess the CRE consensus sequence in a position suggesting a role for this element in transcriptional modulation. For example, the immediate early gene c-fos (15, 16), as well as the genes for arginine vasopressin (AVP) (21), proenkephalin (22, 23), and CRF (6, 7) are likely regulated by transcriptional factors that bind to the CRE sequence in their promoters. The regulation of these genes is of particular interest because their expression is enhanced in the adult rat hypothalamus after stress (15, 24–29). A recent comparative, quantitative time course analysis of the levels of mRNA of these genes in the adult rat hypothalamus has shown concurrent expression of phosphorylated CREB and CRF heteronuclear RNA, but delayed expression of c-fos and AVP heteronuclear RNA (29). This observation suggests that, in addition to phosphorylated CREB, other regulatory factors (such as AP1) may contribute to expression of certain genes in response to stress. In addition, the presence, phosphorylation, and dimerization of CREB is required but not always sufficient to activate a CRE-possessing promoter (1, 2).
In contrast to the significant information available regarding the regulation of stress-responsive, CRE-possessing genes in the mature CNS, relatively little is known about the mechanisms of transcriptional regulation by adverse external signals during development. Early studies suggested that the immature rat does not possess a robust hormonal response to stress. However, previous studies from our laboratory have revealed that age-appropriate, hypothermic stress increased steady-state CRF mRNA in the hypothalamus of rats starting in the second postnatal week (14). The current study demonstrates that phosphorylated CREB- and CRE-binding activity are present and inducible in the rat anterior hypothalamus during the first 2 postnatal weeks. Enhanced CREB phosphorylation and binding activity may thus provide the mechanism for augmentation of the transcription of stress-responsive genes in the developing hypothalamus. Specifically, CREB-mediated increased transcription of CRE-possessing genes, such as CRF, in response to hypothermic stress may provide a critical adaptive capacity to the neonatal rat: the stress neurohormone CRF activates both hormonal and behavioral responses that mediate survival in adverse situations.
As determined in the current study, hypothermic stress increased CRE-binding activity in the hypothalamus, but not in the cerebral cortex, of developing rats (Fig. 7). This finding is consistent with several hypotheses: First, the lack of alteration in CRE binding in the cerebral cortex may derive from the functional immaturity of this brain region in the rat during the first 2 weeks of life. Alternatively, the regulatory mechanisms of CREB-responsive genes in the hypothalamus may differ from those in the cerebral cortex. Finally, the stimulus of hypothermic stress, which activates relevant genes in the hypothalamus, may not be transmitted to the cortex in a context leading to gene activation.
The ability to adapt rapidly to stimuli is likely to be critical for the survival of the organism in a changing environment. This issue has been discussed in detail in a body of work addressing the stress response in the developing rat (8–12). Early work suggested that during the first 2 weeks of life there is little hormonal stress response with only small changes in plasma levels of the stress hormones ACTH and corticosterone. More recent studies have demonstrated that the developing hypothalamus does respond to external stimuli via secretion of CRF (14) and vasopressin (30, 31) which, in turn, leads to elevated plasma ACTH and corticosterone (11). The current study shows that transcriptional regulation of gene expression in the developing hypothalamus can be modulated by envirinmental changes (i.e. stress). Thus, these environmental stimuli are capable of inducing both immediate (hormonal) and long-term alterations in hypothalamic function by qualitatively and quantitatively modulating the repertoire of gene expression in this critical CNS region.
In conclusion, this study demonstrates that transcription of hypothalamic genes that are regulated by CRE binding is likely to be modulated by environmental stimuli during the first 2 postnatal weeks. This is consistent with physiological observations and confirms the critically important adaptability of the functions subserved by this brain region.
MATERIALS AND METHODS
Animals and Stress Paradigm
Timed pregnancy Sprague Dawley-derived rats (Zivic-Miller, Zelienople, PA) were housed under a 12-h light, 12-h dark cycle and given unlimited access to food and water. Delivery was verified at 12-h intervals and the day of birth was considered day 0; litters were culled within 24 h of delivery to 12 pups of both sexes. Each experiment included all of the experimental groups and was performed at least twice. Samples from each timepoint consisted of a pool of tissue from two to three pups without attention to sex. To avoid potential effects of litter size on brain maturation, members of each litter were used at a single age so that litter size was stable from birth to the time of death. Before each experiment, cages were undisturbed for 24 h, and all experiments were performed at 0800–1100 h. All experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care Committee.
Twelve or 13-day-old rats (four litters) were individually subjected to maximally tolerated hypothermic stress, defined by development of rigor, little response to tactile stimulus, and average core temperature of 9.8 C, as previously described in detail (14). This required cold exposure for 40–100 min for individual rats. These experiments were repeated on 4- to 6-day-old rats (three litters, data not shown). Maximal cold stress at this age required 30–60 min. After cold-separation stress, pups were placed on a euthermic pad where they regained normal core temperature of 33–34 C in 10–15 min. Rats were rapidly decapitated at 0, 15, 30, 60, or 180 min following the hypothermic stress. Stress-free controls were killed within 2 min of disturbance. Trunk blood was collected at time of death for analysis of plasma corticosterone levels by RIA (ICN, Irvine, CA) as previously described (32). Assay sensitivity was 0.5 μg/dl and interassay variability was less than 5%.
Preparations of Brain Extracts
Brains were dissected on ice for isolation of the anterior hypothalamus and the cerebral cortex. The anterior hypothalamus consisted of a tissue block including the paraventricular, suprachiasmatic, and supraoptic nuclei, as well as the anterior hypothalamic and medial preoptic areas. The cerebral cortex tissue block consisted of the occipital cortical area. At each timepoint, tissue from two pups was pooled, immersed into 1 ml cold PBS (pH 7.4), and homogenized (micro tissue grinder, Kontes, Vineland, NJ) on ice. The homogenate was centrifuged at 4,000 × g for 10 min at 4 C and the resulting pellet was frozen at −80 C. The pellet was resuspended in 5 volumes of extraction buffer [10 mM HEPES, pH 7.9, 400 mM NaCl, 100 μM EGTA, 500 μM dithiothreitol (DTT), 500 μM phenylmethylsulfonylfluoride, and 5% (vol/vol) glycerol] by trituration, vortex, and homogenization. Membranes and debris were separated by centrifugation at 10,000 × g for 15 min at 4 C. The resulting supernatant was aliquoted and frozen at −80 C. Extracts were analyzed for protein concentration by protein assay (Bio-Rad, Hercules, CA) using BSA as a standard.
Oligonucleotide Preparation
For gel shift analysis, 50 ng (3.5 pmol) of double-stranded DNA oligonucleotide (22 nucleotides in length) containing the CRE consensus sequence (Stratagene, La Jolla, CA) were labeled with [32P]ATP (New England Nuclear, Boston, MA) using 30 U of T4 polynucleotide kinase (Promega Corp., Madison, WI) according to the manufacturer’s protocol. The oligonucleotide was extracted first with phenol-chloroform-isoamyl alcohol (48:48:1) and then with chloroform-isoamyl alcohol (48:1) followed by column chromotography (NucTrap, Stratagene). Incorporation of 32P was determined as 35–75% with specific activity of 5 × 105–2 × 106 cpm/μg. For Southwestern blot analysis, 2 pmol CRE oligonucleotide were labeled with 20 pmol [32P]-α-deoxy-ATP (New England Nuclear) using terminal deoxynucleotidyl transferase (Promega Corp.) according to the manufacturer’s instructions. The labeled oligonucleotide was purified by column chromotography (NucTrap, Stratagene).
Gel Shift Analysis
Gel shift analysis was modified from published protocols (33, 34). Briefly, 10 μg protein extract were incubated for 1 h at room temperature with 100 pg labeled oligonucleotide containing the CRE consensus sequence. The final incubation volume was 20 μl containing 10 mM Tris pH, 7.5, 50 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 1 mM DTT, 1 μg poly (dI-dC) (Sigma, St. Louis, MO), and 0.1% (wt/vol) Triton X-100. After incubation, 2 μl of loading buffer (50% glycerol, 0.5% bromphenol blue, 0.5% xylenecyanole) was added, and the samples were electrophoresed on a 5% polyacrylamide gel (acrylamide-bisacrylamide, 37.5:1) in 0.5 × TBE buffer (44.5 mM Tris-borate, 44.5 mM boric acid, and 1 mM EDTA) at 160 V. Gels were dried and exposed to film (Reflection NEF, Dupont, Boston, MA) using a Reflection intensifying screen. Each assay was repeated at least once to verify results.
Competition experiments were performed by including 10 ng unlabeled double-stranded DNA in the reaction mixture. The oligonucleotides used for competition were each 22 nucleotides long and included the consensus sequence for CRE, AP1, GRE, Oct1, NFκB, or SP1 (Stratagene).
Supershift and antibody-blocking experiments were performed by incubating the protein extract with antisera (1:100) for 2 h at 4 C before the addition of radiolabeled oligonucleotide.
Western Immunoblot and Southwestern Blot Analysis
Samples were analyzed by Western immunoblot and Southwestern blot by size fractionation of 20 μg protein extract through denaturing 10% SDS-PAGE (35) under reducing conditions, followed by transfer to a nitrocellulose membrane (Schleicher & Schuell, Keene, NH) (36). Prestained molecular mass markers (Bio-Rad) were included on each gel. All incubations were performed at room temperature unless otherwise noted.
For Western immunoblots, membranes were stained with Ponceau S (Sigma) (37) to verify transfer. Membranes were then blocked for 1 h with buffer consisting of 0.5% nonfat dry milk (NFDM, Carnation) and 0.05% Tween-20 (Fisher Scientific, Pittsburgh, PA) in Tris-balanced saline (TBS, 50 mM Tris-HCl, pH 7.5, and 150 mM NaCl) followed by overnight incubation at 4 C with 1:1000 dilution of antisera in the same buffer. The membranes were washed three times in TBS, incubated for 2 h with 1:1000 dilution of horseradish peroxidase-conjugated secondary antibody (goat anti-rabbit IgG, Sigma), washed five times in TBS, and visualized using a chemiluminescent substrate (ECL, Amersham, Arlington Heights, IL).
Southwestern blots were performed based on published protocols (38, 39). First, membranes were rehydrated in HEPES buffer (25 mM HEPES, pH 7.9, 25 mM NaCl, 5 mM MgCl2, 0.5 mM DTT) for 30 min. Then, membranes were blocked for 1 h in 5% NFDM in HEPES buffer, followed by a rinse in 0.25% NFDM in HEPES buffer. Probe was bound by incubating membranes overnight with 2 pmol of 32P-labeled CRE oligonucleotide in 5 ml of HEPES buffer containing 0.25% NFDM and 5 μg poly(dI-dC) (Sigma). Membranes were rinsed three to five times in HEPES buffer containing 0.25% NFDM, air dried, and exposed to film.
Antibodies
Rabbit polyclonal antiserum 5322, specific for the phosphorylated form of CREB (17) (epitope corresponding to amino acids 128–141, with antibodies to the unphosphorylated polypeptide removed by adsorbtion), was a generous gift from Dr. M. Montminy. Rabbit polyclonal antiserum specific for CREB-1 (epitope corresponding to amino acids 295–321) and non-cross-reactive with other ATF/CREB transcription factors was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Acknowledgments
We thank Dr. M. Montminy for kindly providing the antiserum specific for phosphorylated CREB. The technical support of L. Schultz is greatly appreciated. We also thank Dr. M. Villalba, B. Tantayanubutr, and M. Eghbal-Ahmadi for comments on this manuscript.
This work was supported by NIH Grant NS-28912 (to T.Z.B.).
References
- 1.Brindle PK, Montminy MR. The CREB family of transcription activators. Curr Opin Genet Dev. 1992;2:199–204. doi: 10.1016/s0959-437x(05)80274-6. [DOI] [PubMed] [Google Scholar]
- 2.Lee KA, Masson N. Transcriptional regulation by CREB and its relatives. Biochim Biophys Acta. 1993;1174:221–233. doi: 10.1016/0167-4781(93)90191-f. [DOI] [PubMed] [Google Scholar]
- 3.Meyer TE, Habener JF. Cyclic adenosine 3′,5′-monophosphate response element binding protein (CREB) and related transcription-activating deoxyribonucleic acid-binding proteins. Endocr Rev. 1993;14:269–290. doi: 10.1210/edrv-14-3-269. [DOI] [PubMed] [Google Scholar]
- 4.Ginty DD. Calcium regulation of gene expression: isn’t that spatial? Neuron. 1997;18:183–186. doi: 10.1016/s0896-6273(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 5.Deutsch PJ, Hoeffler JP, Jameson JL, Habener JF. Cyclic AMP and phorbol ester-stimulated transcription mediated by similar DNA elements that bind distinct proteins. Proc Natl Acad Sci USA. 1988;85:7922–7926. doi: 10.1073/pnas.85.21.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seasholtz AF, Thompson RC, Douglass JO. Identification of a cyclic adenosine monophosphate-responsive element in the rat corticotropin-releasing hormone gene. Mol Endocrinol. 1988;2:1311–1319. doi: 10.1210/mend-2-12-1311. [DOI] [PubMed] [Google Scholar]
- 7.Itoi K, Horiba N, Tozawa F, Sakai Y, Sakai K, Abe K, Demura H, Suda T. Major role of 3′,5′-cyclic adenosine monophosphate-dependent protein kinase A pathway in corticotropin-releasing factor gene expression in the rat hypothalamus in vivo. Endocrinology. 1996;137:2389–2396. doi: 10.1210/endo.137.6.8641191. [DOI] [PubMed] [Google Scholar]
- 8.Levine S. The pituitary-adrenal system and the developing brain. Prog Brain Res. 1970;32:79–85. doi: 10.1016/S0079-6123(08)61521-6. [DOI] [PubMed] [Google Scholar]
- 9.Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:65–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- 10.Stanton ME, Gutierrez YR, Levine S. Maternal deprivation potentiates pituitary-adrenal stress responses in infant rats. Behav Neurosci. 1988;102:692–700. doi: 10.1037//0735-7044.102.5.692. [DOI] [PubMed] [Google Scholar]
- 11.Walker CD, Scribner KA, Cascio CS, Dallman MF. The pituitary-adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology. 1991;128:1385–1395. doi: 10.1210/endo-128-3-1385. [DOI] [PubMed] [Google Scholar]
- 12.Rosenfeld P, Suchecki D, Levine S. Multifactorial regulation of the hypothalamic-pituitary-adrenal axis during development. Neurosci Biobehav Rev. 1992;16:553–568. doi: 10.1016/s0149-7634(05)80196-4. [DOI] [PubMed] [Google Scholar]
- 13.Schreiber SS, Tocco G, Najm I, Finch CE, Johnson SA, Baudry M. Absence of c-fos induction in neonatal rat brain after seizures. Neurosci Lett. 1992;136:31–35. doi: 10.1016/0304-3940(92)90640-s. [DOI] [PubMed] [Google Scholar]
- 14.Yi SJ, Baram TZ. Corticotropin-releasing hormone mediates the response to cold stress in the neonatal rat without compensatory enhancement of the peptide’s gene expression. Endocrinology. 1994;135:2364–2368. doi: 10.1210/endo.135.6.7988418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sassone-Corsi P, Visvader J, Ferland L, Mellon PL, Verma IM. Induction of proto-oncogene fos transcription through the adenylate cyclase pathway: characterization of a cAMP-responsive element. Genes Dev. 1988;2:1529–1538. doi: 10.1101/gad.2.12a.1529. [DOI] [PubMed] [Google Scholar]
- 16.Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- 17.Hagiwara M, Brindle P, Harootunian A, Armstrong R, Rivier J, Vale W, Tsien R, Montminy MR. Coupling of hormonal stimulation and transcription via the cyclic AMP-responsive factor CREB is rate limited by nuclear entry of protein kinase A. Mol Cell Biol. 1993;13:4852–4859. doi: 10.1128/mcb.13.8.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montminy MR, Bilezikjian LM. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987;328:175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- 19.Pennypacker KR, Hudson PM, Hong JS, McMillian MK. DNA binding activity of CREB transcription factors during ontogeny of the central nervous system. Brain Res Dev Brain Res. 1995;86:242–249. doi: 10.1016/0165-3806(95)00033-a. [DOI] [PubMed] [Google Scholar]
- 20.Imaki J, Yoshida K, Yamashita K. A developmental study of cyclic AMP-response element binding protein (CREB) by in situ hybridization histochemistry and immunocytochemistry in the rat neocortex. Brain Res. 1994;651:269–274. doi: 10.1016/0006-8993(94)90706-4. [DOI] [PubMed] [Google Scholar]
- 21.Verbeeck MA, Adan RA, Burbach JP. Vasopressin gene expression is stimulated by cyclic AMP in homologous and heterologous expression systems. FEBS Lett. 1990;272:89–93. doi: 10.1016/0014-5793(90)80455-r. [DOI] [PubMed] [Google Scholar]
- 22.Comb M, Birnberg NC, Seasholtz A, Herbert E, Goodman HM. A cyclic AMP- and phorbol ester-inducible DNA element. Nature. 1986;323:353–356. doi: 10.1038/323353a0. [DOI] [PubMed] [Google Scholar]
- 23.Hyman SE, Comb M, Lin YS, Pearlberg J, Green MR, Goodman HM. A common trans-acting factor is involved in transcriptional regulation of neurotransmitter genes by cyclic AMP. Mol Cell Biol. 1988;8:4225–4233. doi: 10.1128/mcb.8.10.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovacs KJ, Mezey E. Dexamethasone inhibits corticotropin-releasing factor gene expression in the rat paraventricular nucleus. Neuroendocrinology. 1987;46:365–368. doi: 10.1159/000124846. [DOI] [PubMed] [Google Scholar]
- 25.Lightman SL, Young WS. Vasopressin, oxytocin, dynorphin, enkephalin and corticotrophin-releasing factor mRNA stimulation in the rat. J Physiol (Lond) 1987;394:23–39. doi: 10.1113/jphysiol.1987.sp016858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lightman SL, Young WS. Corticotrophin-releasing factor, vasopressin and pro-opiomelanocortin mRNA responses to stress and opiates in the rat. J Physiol (Lond) 1988;403:511–523. doi: 10.1113/jphysiol.1988.sp017261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown ER, Sawchenko PE. Hypophysiotropic CRF neurons display a sustained immediate-early gene response to chronic stress but not to adrenalectomy. J Neuroendocrinol. 1997;9:307–316. doi: 10.1046/j.1365-2826.1997.00586.x. [DOI] [PubMed] [Google Scholar]
- 28.Borsook D, Konradi C, Falkowski O, Comb M, Hyman SE. Molecular mechanisms of stress-induced proenkephalin gene regulation: CREB interacts with the proenkephalin gene in the mouse hypothalamus and is phosphorylated in response to hyperosmolar stress. Mol Endocrinol. 1994;8:240–248. doi: 10.1210/mend.8.2.8170480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovacs KJ, Sawchenko PE. Regulation of stress-induced transcriptional changes in the hypothalamic neurosecretory neurons. J Mol Neurosci. 1996;7:125–133. doi: 10.1007/BF02736792. [DOI] [PubMed] [Google Scholar]
- 30.Priou A, Oliver C, Grino M. In situ hybridization of arginine vasopressin (AVP) heteronuclear ribonucleic acid reveals increased AVP gene transcription in the rat hypothalamic paraventricular nucleus in response to emotional stress. Acta Endocrinol (Copenh) 1993;128:466–472. doi: 10.1530/acta.0.1280466. [DOI] [PubMed] [Google Scholar]
- 31.Paulmyer-Lacroix O, Anglade G, Grino M. Insulin-induced hypoglycaemia increases colocalization of corticotrophin-releasing factor and arginine vasopressin mRNAs in the rat hypothalamic paraventricular nucleus. J Mol Endocrinol. 1994;13:313–320. doi: 10.1677/jme.0.0130313. [DOI] [PubMed] [Google Scholar]
- 32.Baram TZ, Schultz L. Fetal and maternal levels of corticosterone and ACTH after pharmacological adrenalectomy. Life Sci. 1990;47:485–489. doi: 10.1016/0024-3205(90)90607-s. [DOI] [PubMed] [Google Scholar]
- 33.Taylor JD, Ackroyd AJ, Halford SE. The gel-shift assay for the analysis of DNA-protein interactions. In: Kneale GG, editor. Methods in Molecular Biology. Humana Press; Clifton, NJ: 1994. pp. 263–278. [DOI] [PubMed] [Google Scholar]
- 34.Verri A, Mazzarello P, Biamonti G, Spadari S, Focher F. The specific binding of nuclear protein(s) to the cAMP responsive element (CRE) sequence (TGACGTCA) is reduced by the misincorporation of U and increased by the deamination of C. Nucleic Acids Res. 1990;18:5775–5780. doi: 10.1093/nar/18.19.5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laemmli UK, Favre M. Maturation of the head of bacteriophage T4. J Mol Biol. 1973;80:575–581. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- 36.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salinovich O, Montelaro RC. Reversible staining and peptide mapping of proteins transferred to nitrocellulose after separation by sodium dodecylsulfate-polyacrylamide gel electrophoresis. Anal Biochem. 1986;156:341–347. doi: 10.1016/0003-2697(86)90263-0. [DOI] [PubMed] [Google Scholar]
- 38.Singh H, Clerc RG, LeBowitz JH. Molecular cloning of sequence-specific DNA binding proteins using recognition site probes. Biotechniques. 1989;7:252–261. [PubMed] [Google Scholar]
- 39.Dooley S, Welter C, Blin N. Nonradioactive Southwestern analysis using chemiluminescent detection. Biotechniques. 1992;13:540–543. [PubMed] [Google Scholar]