Abstract
Background aims
A hierarchy of endothelial colony forming cell (ECFC) with different levels of proliferative potential has been identified in human circulating blood and blood vessels. ECFC has recently become an attractive target for new vascular regenerative therapies; however, in vitro expansion of ECFC typically depends on the presence of fetal bovine serum or fetal calf serum (FBS or FCS) in the culture medium; which is not appropriate for its therapeutic application.
Methods
To identify optimal conditions for in vitro expansion of ECFC, the effects of human endothelial serum free medium (SFM) supplemented with six proangiogenic cytokines and human umbilical cord blood plasma (HCP) were investigated. The in vitro morphology, proliferation, surface antigen expression, and in vivo vessel-forming ability were utilized for examining the effects of medium on ECFC.
Results
This novel formulation of endothelial cell culture medium that, for the first time, allows us to efficiently isolate and expand human ECFC in vitro with a low concentration of HCP (1.5%) and without bovine serum additives. In this serum reduced medium (SRM), human ECFC colony yields remain quantitatively similar as those cultured in a high concentration (10%) of bovine serum supplemented medium. SRM cultured ECFC display robust clonal proliferative ability in vitro and human vessel-forming capacity in vivo.
Conclusions
Thus, the present study provides a novel method for the expansion of human ECFCs in vitro and will help to advance approaches for using the cells in human therapeutic trials.
Keywords: endothelial colony forming cells (ECFCs), vessel formation, human umbilical cord blood plasma (HCP), serum reduced medium (SRM)
INTRODUCTION
The progenitor cells for the endothelial lineage play critical roles in vascular homeostasis and regeneration in adult subjects [1–5]. High proliferative potential endothelial colony forming cells (HPP-ECFCs) have been identified as endothelial progenitor cells (EPCs) with robust proliferative potential in vitro and vessel-forming ability in vivo [6, 7]. Additionally, recent studies reveal that the concentration of ECFCs in circulation increases after vascular ischemia, which implies a possible contribution to vascular repair [8, 9]. Thus, ECFCs have become an attractive target for new vascular regenerative therapies. However, the therapeutic study of ECFCs is hampered by multiple challenges in culturing the cells.
Current protocols for in vitro expansion of ECFCs mostly depend on the presence of fetal bovine serum or fetal calf serum (FBS or FCS) in the culture medium. FBS/FCS may contain potentially harmful xenogenic compounds associated with risks of transmitting infectious agents and inducing immune reactions when used in a human transplantation setting [10–14]. Therefore, a well-defined, animal serum-free culture medium for in vitro isolation and expansion of ECFCs is greatly needed.
Human blood derivatives have been considered as alternatives to FBS. Human autologous or allogeneic serum/plasma, human umbilical cord serum, platelet enriched plasma, platelet lysate and platelet-released growth factors have been used as supplements in culture media to promote proliferation, migration and differentiation of mesenchymal stromal stem cells (MSCs) [15–20]. Similarly, human platelet lysate [21] or platelet-derived growth factors [17] are able to enhance proliferation and migration of endothelial cells (ECs) and retain their vessel-forming ability.
In this study, we describe a novel culture medium supplemented with six growth factors and 1.5% human umbilical cord blood plasma (HCP), to efficiently recover ECFCs from human cord blood mononuclear cells (MNCs). ECFCs can be propagated in this serum reduced medium (SRM), retaining their endothelial and progenitor cell phenotype and function in vitro and in vivo. Moreover, when cultured in SRM, ECFCs exhibit the complete hierarchy of proliferative potential distribution as observed in standard FCS/FBS supplemented medium.
MATERIALS AND METHODS
Media and supplements
Human Endothelial Serum Free Medium (SFM; Invitrogen, Grand Island, NY) was supplemented with 20 ng/ml human recombinant basic fibroblast growth factor (hrbFGF) (Invitrogen), 10 ng/ml human recombinant epidermal growth factor (hrEGF) (R&D, Minneapolis, MN), 10 ng/ml human recombinant vascular endothelial growth factor 165 (hrVEGF165) (R&D), 10 ng/ml human recombinant VEGF121 (hrVEGF121) (R&D), 10 ng/ml human recombinant stem cell factor (hrSCF) (R&D), 5 ng/ml stromal cell derived factor 1 alpha (SDF1 α) (R&D), 10 ng/ml human recombinant interleukin 6 (hrIL6) (R&D), 1.5% human umbilical cord blood plasma (HCP) and 1.0% penicillin/streptomyocin (Invitrogen), to create our serum reduced medium (SRM). As a control, human EGM-2 medium (Lonza, Walkersville, MD) was supplemented with 10% FBS (Hyclone, Logan, UT) and 1.0% penicillin/streptomyocin, and called complete EGM-2 medium (cEGM-2).
Preparation of pooled HCP
Human umbilical cord blood (UCB) samples (50–100 mL) were collected in heparin-coated syringes (20 to 30 USP units of heparin/mL of blood) from healthy newborns (38–40 weeks gestation). The Institutional Review Board at Indiana University School of Medicine reviewed and approved this study with exempt IRB status. UCB was diluted 1:1 with Dulbecco’s Phosphate Buffered Saline (DPBS) (Invitrogen) and overlaid onto Ficoll-Paque PLUS (GE Healthcare, Piscataway, NJ) according to the manufacturer’s instructions. Cells were centrifuged for 30 minutes at room temperature at 1500 rpm. After centrifugation, the MNCs were collected for culturing ECs, and the supernatant was collected for preparing HCP. Subsequently, the supernatant was aliquoted and frozen at −80°C. After thawing, aliquots with the same volume from at least 20 samples were pooled and sterilely filtered through a 0.2 μm filter. The pooled HCP was then added to SRM.
Isolation and culture of UCB-derived ECFCs
MNCs were isolated and washed with DPBS. For outgrowth of ECFC colonies, MNCs either were resuspended in SRM or cEGM-2 medium. MNCs (3 × 107/well) were seeded onto 6-well tissue culture plates precoated with Type I rat-tail collagen (BD Biosciences; Bedford, MA) and cultured as previously described [6]. Spindle-shaped ECFC colonies emerged sequentially from MNCs and the first day of ECFC colony emergence was recorded. The frequency of ECFC colonies was determined by measuring the total number of colonies in the primary culture on day 10 (as no ECFC ever emerged at a later time point). Subsequently, the ECFC-derived ECs were released from the primary culture dish by TrypLE™ Express (Gibco, Grand Island, NY) and replated onto 25 cm2 tissue culture flasks pre-coated with Type I rat-tail collagen for subsequent passage.
Immunophenotyping of ECFC derived ECs
Early passaged (1–2) ECFC-derived ECs (5 × 104) were incubated at 4°C for 30 minutes in 100 μl of medium with varying concentrations of the primary or isotype control antibody as outlined below, washed three times, and analyzed by fluorescence-activated cell sorting (FACS®) (Becton Dickinson, San Diego, CA). The primary antibodies we used included anti-human CD31 conjugated to phycoerythrin (PE) (BD Biosciences Pharmingen; Bedford, MA), anti-human CD34 conjugated to allophycocyanin (APC) (BD Biosciences Pharmigen), anti-human CD144 conjugated to PE (BD Biosciences Pharmingen), anti-human CD146 conjugated to PE (BD Biosciences Pharmingen), anti-human cKIT conjugated to APC (eBioscience; San Diego, CA), anti-human vascular endothelial growth factor receptor 1 (VEGFR1) conjugated to PE (BD Biosciences Pharmingen), anti-human VEGFR2 conjugated to fluorescein isothiocyanate (FITC) (BD Biosciences Pharmingen), anti-human VEGFR3 conjugated to APC (R&D), anti-human Neuropilin-1 (Nrp1) conjugated to PE (Miltenyi Biotec; Auburn, CA), anti-human Nrp2 (R&D) conjugated to Alexa Fluor 647 (Molecular Probes, Eugene, OR), anti-human CD14 conjugated to PE (BD Biosciences Pharmingen), anti-human CD45 conjugated to FITC (BD Biosciences Pharmingen), anti-human AC-133 conjugated to APC (Miltenyi Biotec) and anti-human CXCR4 conjugated to FITC (BD Biosciences Pharmingen). For negative controls, we used directly conjugated mouse IgG isotypes (BD Biosciences Pharmingen).
Single cell clonogenic assays
Early passaged (1–2) ECFC-derived ECs were plated at one cell per well into 96 well plates pre-coated with Type I rat-tail collagen in 200 μl of cEGM-2 medium. Cells were cultured at 37°C in a humidified incubator with 5% CO2. Media were changed every five days. After 14 days of culturing, cells were fixed with 4% paraformaldehyde (Sigma; St. Louis, MO) in phosphate-buffered saline for 30 minutes at room temperature, then washed twice, stained with 1.5 μg/ml DAPI, and examined for the growth of ECs. Those wells containing two or more cells were identified as positive for proliferation under a fluorescent microscope at 10x magnification. Wells containing fewer than 50 cells were counted by visual inspection with a fluorescent microscope at 40x magnification. For those wells with more than 50 cells, colonies were imaged and cell number quantified using an Image J1.36v program (Wayne Rasband, NIH).
In vivo matrix implantation assays
Early passaged (3–5) ECFC-derived ECs (2 × 106 cells/mL) were suspended in a 1.5 mg/mL collagen-fibronectin matrix as previously described [7, 22]. Aliquotes (250μl) were pipetted into wells of 48 well plates, allowed to polymerize at 37°C for 30 minutes, and covered with 500μl of culture medium for overnight incubation at 37°C, in 5% CO2. After 18 hours of ex vivo culture, cellularized matrices were implanted into the flanks of 6–8 weeks old NOD/SCID mice as previously described [7, 22]. After 14 days, mice were euthanized and the grafts were harvested, fixed in formalin-free zinc fixative (BD Biosciences), paraffin embedded, bisected, and sectioned (6 μm) for analysis by histology and immunohistochemistry (n=6).
Histology and Immunohistochemistry
Sections were stained as previously described [7, 22]. Paraffin-embedded tissue sections were deparaffinized and then either directly stained with hematoxylin and eosin (H&E) or immersed in retrieval solution (Dako, Carpenteria, CA) for 20 minutes at 90–99°C. Slides were incubated at room temperature for 30 minutes with anti-human CD31 antibody (clone JC70/A, Abcam, Cambridge, MA), followed by a 10-minute incubation with LASB2 link-biotin and streptavidin-HRP (Vector Laboratories, Burlingame, CA), then developed with DAB (Vector Laboratories) solution for 5 minutes.
Statistical Analysis
Results are shown as the mean ± the standard error of the mean (SEM). Comparison of the time and the frequency of ECFC colony emerged from MNCs in two culture media were assessed by Student’s paired t-test. The colony number and size distribution, the vessel number and size distribution were evaluated by Student’s unpaired t test. The significant differences were set at P < 0.05. All analyses were performed using GraphPad InStat software (GraphPad Software Inc, La Jolla, CA).
RESULTS
Isolation and expansion of human UCB ECFCs
We and others have successfully isolated human ECFC-derived EC colonies from low-density MNCs in UCB by utilizing cEGM-2 medium [6, 23, 24]. To evaluate whether SRM is able to promote ECFC outgrowth and proliferation, we compared these two culture media directly. MNCs from the same donor were divided into portions with half of them cultured in SRM and the rest in cEGM-2. The first ECFC colonies were detected after 4.35 ± 0.25 days in SRM, compared to 6.10 ± 0.38 days in cEGM-2 (p < 0.001, Figure 1A). No difference was observed in the frequency of ECFCs recovered on day 10 under these two conditions (Figure 1B). Colonies in SRM displayed a cobblestone appearance with variations in colony size, which indicated their heterogeneous proliferative abilities (Figure 1C) as previously reported [6].
Figure 1. Isolation of human cord blood ECFC-derived EC colonies from UCB MNCs by using SRM.
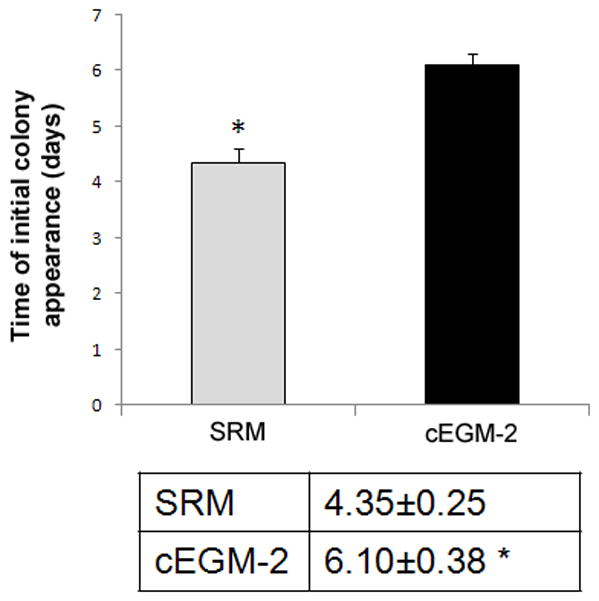
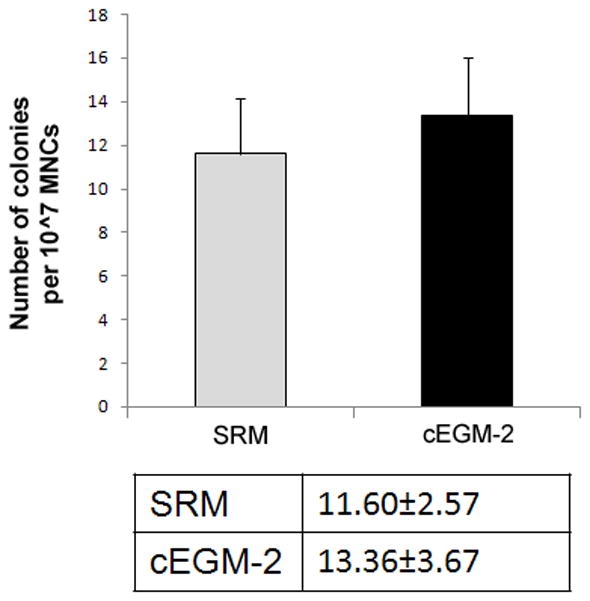
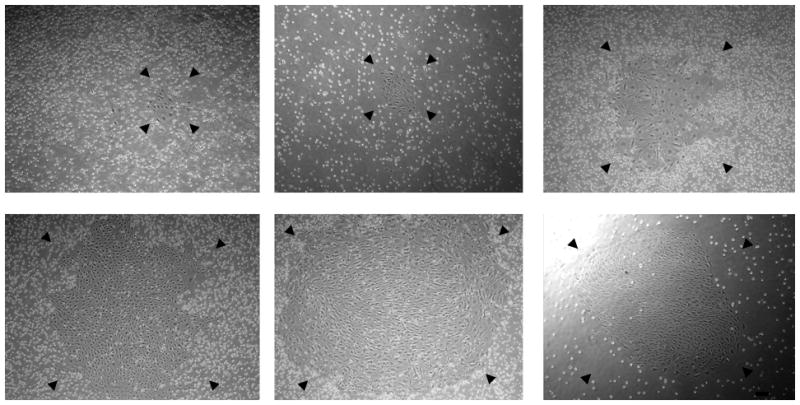
(A) Time of initial ECFC derived EC colonies emerged from MNCs after culture initiation in SRM and cEGM-2. Results represent the mean number of days before initial EC appearance ± SEM (Blood sample number N = 23, *P < 0.001). (B) Number of ECFC-derived EC colonies outgrown per 107 MNCs after 10 days of culture initiation in SRM and cEGM-2. Results represent the average number of EC colonies ± SEM (Blood sample number N = 23). (C) Representative photomicrographs of individual human ECFC-derived EC colonies from UCB in SRM. Scale bar represents 100 μm.
Phenotypic characterization of human UCB ECFCs
The ECFC colonies expanded and formed an endothelial monolayer in both types of culture media conditions. Immunophenotyping of the endothelial monolayer (Figure 2) revealed that ECs cultured in SRM expressed endothelial cell surface antigens CD31, CD34, CD144, CD146, VEGFR1, VEGFR2 and VEGFR3, which was similar with those cultured in cEGM-2 medium. However, the expression of cKIT (the receptor of SCF) and CXCR4 (the receptor of SDF1α) was higher in ECs in SRM than in cEGM-2 (40.57±4.50% vs. 19.81±3.40% and 60.31±5.42% vs. 2.41±0.70%, p<0.05, respectively). This observation can be the result of the addition of hrSCF and hrSDF1α in the endothelial culture medium [25, 26]. Most importantly, the EC colonies cultured in SRM did not express the hematopoietic cell surface antigens CD11b, CD14, CD45 or AC133, which indicates that the HCP-supplemented culture environment was devoid of hematopoietic cell contamination.
Figure 2. Phenotypic analysis of human cord blood ECFC-derived ECs cultured in SRM.
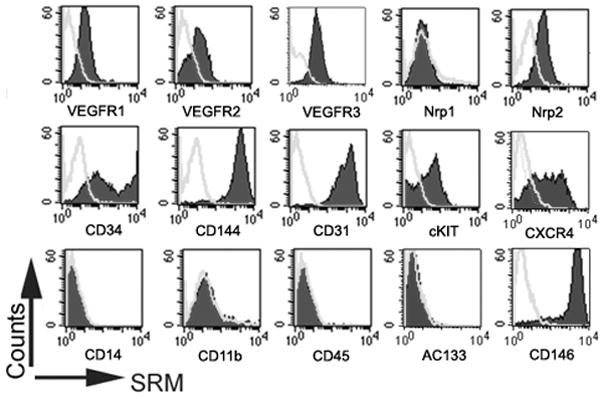
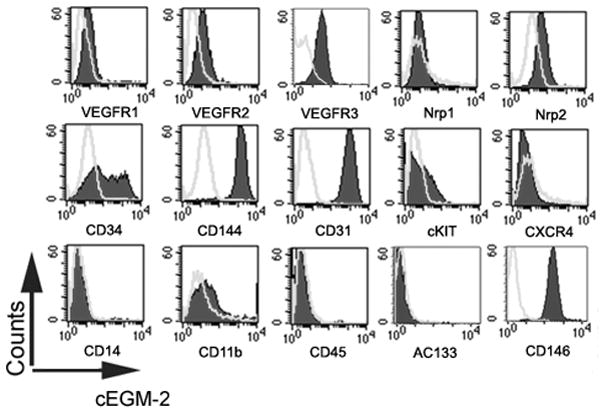
Immunophenotyping of EC from the cultured monolayer derived from human cord blood ECFC in SRM (A) and cEGM-2 (B) by fluorescence cytometry. Similar to cells grown in cEGM-2, the ECs cultured in SRM expressed CD31, CD34, CD144, CD146, VEGFR1, VEGFR2, VEGFR3, and Nrp2 but not CD45, CD14, CD11b or AC133. Moreover, the expression of cKIT and CXCR4 was detectable in ECs grown in SRM, but not in cEGM-2.
Clonogenic ability maintained in human UCB ECFCs
A complete hierarchy of ECFC in human peripheral blood and UCB derived ECs, based on proliferative and clonogenic abilities, has been previously identified [6]. To determine whether such a proliferative hierarchy is also present in the ECs cultured in SRM, a single-cell clonogenic assay was performed. After single cells were plated in culture, some cells didn’t divide, while other cells divided and formed colonies of different sizes comprised of varying cell numbers. The frequency of single cells undergoing division was similar between samples cultured in SRM and those in cEGM-2 (28.10 ± 21.04 vs 34.30 ± 20.89, respectively). Moreover, the entire hierarchy of ECFCs, composed of high proliferative (HPP)-, low proliferative (LPP)-ECFC, endothelial-cluster and non-dividing mature ECs, was exhibited in ECs cultured in SRM (Figure 3).
Figure 3. Quantitation of the clonogenic and proliferative potential of single ECs derived from human cord blood cultured in SRM.
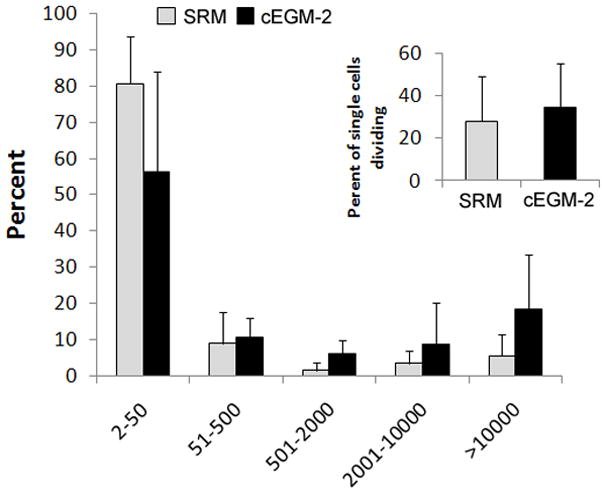
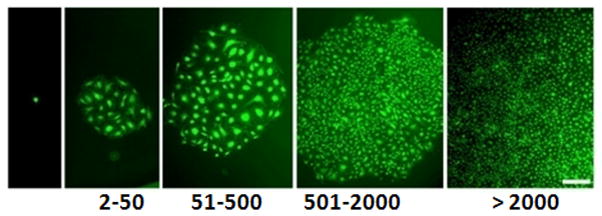
(A) The distribution of colony sizes, where colonies were derived from single ECs grown in individual wells after 14 days of culture. The complete hierarchy of ECFCs was present in ECs cultured in SRM; that is similar to those grown in cEGM-2. Inset chart is the percentage of single ECs dividing at least once after growing 14 days in culture. No statistical difference in this frequency was observed between cells grown in SRM and those in cEGM-2. (N = 5) (B) Representive photomicrographs of EC colonies with varied sizes derived from single ECs. Scale bar represents 100μm.
In vivo formation of chimera blood vessels
We [7, 22] and others have demonstrated that human UCB-derived ECFCs possess the potential to form de novo blood vessels when suspended in a collagen-fibronectin matrix or Matrigel and implanted subcutaneously into immunodeficient mice [23, 24]. To test the in vivo vessel-forming ability of UCB ECFCs cultured in SRM, the same methods were employed. After 14 days of carrying implants containing ECFC cultured in SRM or cEGM2, the mice were euthanized, the grafts were harvested, and analyzed for human or murine blood vessel formation.
H&E staining revealed the formation of human microvessels perfused with murine red blood cells in the graft, indicating human vessel anastomoses with the surrounding murine vasculature (Figure 4A). To further verify the human origin of these vessels, an immunohistochemistry study with a specific anti-human CD31 antibody was conducted and the results are shown on Figure 4A. Thus, ECFC progeny cultured in SRM can also form functional human-murine chimeric vessels in a short-term xenograft model of blood vessel formation similar to cEGM2 medium cultured cells. Quantification of human microvessels that carry murine erythrocytes (Figure 4B) showed that there was no statistical difference between the implanted cells cultured in these two culture media (SRM vs cEGM-2 is 28.48 ± 14.86 vs. 14.73 ± 6.69 vessels/mm2). Furthermore, the size distribution of these functional microvessels formed by ECFC cultured in SRM was similar to those cultured in cEGM-2 (Figure 4C).
Figure 4. Human cord blood ECFC-derived ECs cultured in SRM demonstrate the potential to form functional microvessels in immunodeficient mice.
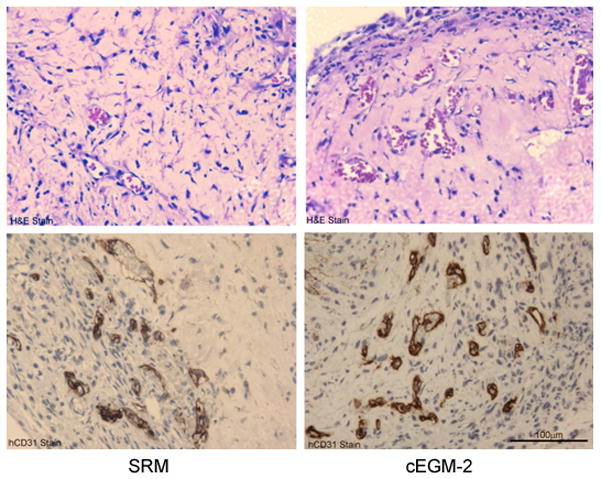
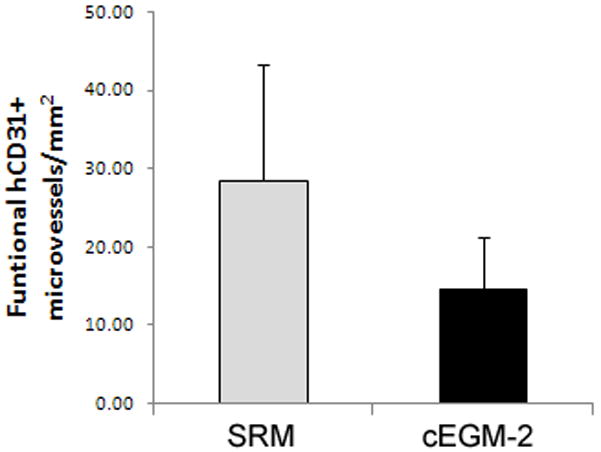
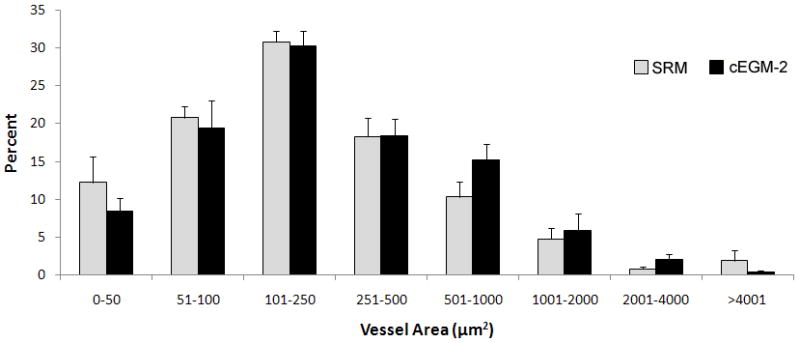
(A) H&E staining indicates microvessel formation in collagen-fibronectin gel after 14 days of implantation in NOD/SCID mice. Anti-human CD31 staining further confirms the human origin of these vessels. Scale bar represents 100 μm. (B) The number of vessels formed by human cord blood derived ECFCs (from a pool of 5 different blood samples) and perfused with murine red blood cells per mm2 in the gel after 14 days of implantation. (n=6) (C) The size distribution of the microvessels formed by human cord blood ECFCs. These data indicate that there is no difference in the vessel-forming abilities of ECs cultured in SRM versus those in cEGM-2.
DISCUSSION
We have demonstrated a novel method that, for the first time, allows us to efficiently isolate and expand human ECFCs in vitro with a low concentration of HCP (1.5%) with no bovine serum additives. In SRM, human ECFC colony yields remained quantitatively similar as those in standard cEGM-2. The SRM cultured ECFC displayed robust proliferative ability in vitro and vessel forming capacity in vivo indicative of maintenance of proliferative potential during culture. Thus, our novel SRM formulation permits optimal culture of human ECFC.
A recent study illustrated that human platelet lysate (HPL) can replace FBS for large-scale propagation of human ECFCs [21]. This study supported the idea that human blood products can maintain EC growth in vitro similar to the support provided to MSC [15, 20, 27, 28]. Human endothelial serum-free medium (SFM), the basal medium in this culture system, is commercially available and when supplemented with 20 ng/ml hrbFGF and10 ng/ml hrEGF is reported by the manufacturer to support the isolation and long-term culture of human umbilical cord artery and vein endothelial cells (HUAECs and HUVECs) and human dermal microvascular endothelial cells (HMVECs) (Invitrogen manuals). However, that culture medium formulation did not support human cord blood ECFC outgrowth in our pilot assay. Consequently, we had to consider addition of other factors.
An increasing number of cytokines and chemokines have been demonstrated as displaying proangiogenic properties. Among them, FGF, EGF, VEGF, SCF, SDF1α and IL6 have been extensively studied for their effects on angiogenesis [29–37]. Thus, we added these cytokines to the basal media separately or in combination (data not shown) to examine whether they could promote ECFC emergence from human low-density MNCs. We found a mixture of growth factors with added 1.5% HCP to maximally promote ECFCs to emerge from MNCs and propagate with maintenance of proliferative potential.
While 1.5% HCP substituted for 10% FBS in our studies, we were unable to effectively isolate and expand the ECFC in the absence of HCP. This indicates that some yet-unidentified factors reside in HCP that are critical for EC survival and proliferation. Therefore, it is very important to attempt to define such components, as that will ultimately allow the establishment of a completely defined, serum-depleted culture system. Additionally, the molecular mechanisms underlying the interaction of these factors supporting ECs growth need to be further investigated, to help us fully understand the biology of ECs.
ECFCs cultivated in SRM emerged from MNCs earlier (Figure 1A) but propagated slower compared to those in cEGM-2 medium (doubling time SRM vs. cEGM-2 is 2.53 ± 0.34 days vs. 1.52 ± 0.26 days). SRM cultured ECFCs exhibited genomic stability when examined with Fluorescence in situ hybridization (FISH) experiments targeting chromosomes 17 and X in the early passage (supplemental Figure 1). Actually, we found that there was no significant difference between ECFCs grown in SRM and those in cEGM-2 in the incidence of abnormal polyploid and aneuploid formation during 30 days of tissue culture (supplemental Figure 1). Emerging research debates whether human serum is better for maintaining genomic stability in human cells than FBS. Some studies suggest that autologous serum may favor chromosomal stability as compared to FBS [28, 38], while other reports indicate that chromosomal stability is independent of the serum source [39–41]. Our current observations agreed with the latter opinion. However, the mechanism underlying the maintenance of genomic stability is complicated and remains poorly understood [42] and thus further studies aimed to reveal the mechanisms involved in maintenance of chromosome stability are required.
In summary, we report the development of a novel formulation of endothelial cell culture medium that is free of FBS and is supplemented with 1.5% HCP for expansion of human cord blood ECFCs in vitro. We have demonstrated that ECFCs grown in this medium retain their phenotype and function, as well as their proliferative and in vivo vasculogenic properties. This work contributes to the development of a complete serum-free culture system for ECFCs that we and others [21] believe will be required for future use of ECFC as a human cell therapeutics.
Supplementary Material
(A) Fluorescence in situ hybridization (FISH) analysis of ECs using centromere probes specific for the X chromosome (red) and chromosome 17 (cyan). Nuclei are stained with DAPI (blue). Normal (diploid) female cells display two X chromosomes (red spots) and two chromosome 17s (cyan spots). Tetraploid female cells display four X chromosomes (4 red spots) and four chromosome 17s (4 cyan spots). (B) FISH analysis of > 200 ECs after 30 days of culture initiation revealed a similar frequency of diploid content in cells grown in SRM and in cEGM-2. Chromosomally aberrant cells (tetraploid and aneuploid) were detectable at a low incidence in both culture conditions. (N=4)
Acknowledgments
The authors would like to thank Coleen P. Mallett for her help in collection of human umbilical cord blood samples.
This work was supported by grant AHA- 0810062Z (L.H and M.C.Y) and the Riley Children’s Foundation.
Footnotes
DISCLOSURE OF INTEREST
Dr. Mervin C. Yoder is a co-founder and receives consulting fees from EndGenitor Technologies, Inc.
References
- 1.Asahara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9(6):702–12. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 3.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28(9):1584–95. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khakoo AY, Finkel T. Endothelial progenitor cells. Annu Rev Med. 2005;56:79–101. doi: 10.1146/annurev.med.56.090203.104149. [DOI] [PubMed] [Google Scholar]
- 5.Kovacic JC, et al. Endothelial progenitor cells, angioblasts, and angiogenesis--old terms reconsidered from a current perspective. Trends Cardiovasc Med. 2008;18(2):45–51. doi: 10.1016/j.tcm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Ingram DA, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104(9):2752–60. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 7.Yoder MC, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109(5):1801–9. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guven H, et al. The number of endothelial progenitor cell colonies in the blood is increased in patients with angiographically significant coronary arterydisease. J Am Coll Cardiol. 2006;48(8):1579–87. doi: 10.1016/j.jacc.2006.04.101. [DOI] [PubMed] [Google Scholar]
- 9.Huang L, et al. Acute myocardial infarction in swine rapidly and selectively releases highly proliferative endothelial colony forming cells (ECFCs) into circulation. Cell Transplant. 2007;16(9):887–97. doi: 10.3727/096368907783338181. [DOI] [PubMed] [Google Scholar]
- 10.Medicinal and other products and human and animal transmissible spongiform encephalopathies: memorandum from a WHO meeting. Bull World Health Organ. 1997;75(6):505–13. [PMC free article] [PubMed] [Google Scholar]
- 11.Doerr HW, et al. Prions and orthopedic surgery. Infection. 2003;31(3):163–71. doi: 10.1007/s15010-003-3108-3. [DOI] [PubMed] [Google Scholar]
- 12.Halme DG, Kessler DA. FDA regulation of stem-cell-based therapies. N Engl J Med. 2006;355(16):1730–5. doi: 10.1056/NEJMhpr063086. [DOI] [PubMed] [Google Scholar]
- 13.Mannello F, Tonti GA. Concise review: no breakthroughs for human mesenchymal and embryonic stem cell culture: conditioned medium, feeder layer, or feeder-free; medium with fetal calf serum, human serum, or enriched plasma; serum-free, serum replacement nonconditioned medium, or ad hoc formula? All that glitters is not gold! Stem Cells. 2007;25(7):1603–9. doi: 10.1634/stemcells.2007-0127. [DOI] [PubMed] [Google Scholar]
- 14.Spees JL, et al. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther. 2004;9(5):747–56. doi: 10.1016/j.ymthe.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Bieback K, et al. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells. 2009;27(9):2331–41. doi: 10.1002/stem.139. [DOI] [PubMed] [Google Scholar]
- 16.Doucet C, et al. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005;205(2):228–36. doi: 10.1002/jcp.20391. [DOI] [PubMed] [Google Scholar]
- 17.Kilian O, et al. Effects of platelet growth factors on human mesenchymal stem cells and human endothelial cells in vitro. Eur J Med Res. 2004;9(7):337–44. [PubMed] [Google Scholar]
- 18.Muller I, et al. Animal serum-free culture conditions for isolation and expansion of multipotent mesenchymal stromal cells from human BM. Cytotherapy. 2006;8(5):437–44. doi: 10.1080/14653240600920782. [DOI] [PubMed] [Google Scholar]
- 19.Lindroos B, et al. Serum-free, xeno-free culture media maintain the proliferation rate and multipotentiality of adipose stem cells in vitro. Cytotherapy. 2009;11(7):958–72. doi: 10.3109/14653240903233081. [DOI] [PubMed] [Google Scholar]
- 20.Horn P, et al. Impact of individual platelet lysates on isolation and growth of human mesenchymal stromal cells. Cytotherapy. 2010 doi: 10.3109/14653249.2010.501788. [DOI] [PubMed] [Google Scholar]
- 21.Reinisch A, et al. Humanized large-scale expanded endothelial colony-forming cells function in vitro and in vivo. Blood. 2009;113(26):6716–25. doi: 10.1182/blood-2008-09-181362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Critser PJ, et al. Collagen matrix physical properties modulate endothelial colony forming cell-derived vessels in vivo. Microvasc Res. 2010;80(1):23–30. doi: 10.1016/j.mvr.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Au P, et al. Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. Blood. 2008;111(3):1302–5. doi: 10.1182/blood-2007-06-094318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melero-Martin JM, et al. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109(11):4761–8. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 25.Broudy VC, et al. Human umbilical vein endothelial cells display high-affinity c-kit receptors and produce a soluble form of the c-kit receptor. Blood. 1994;83(8):2145–52. [PubMed] [Google Scholar]
- 26.Volin MV, et al. Chemokine receptor CXCR4 expression in endothelium. Biochem Biophys Res Commun. 1998;242(1):46–53. doi: 10.1006/bbrc.1997.7890. [DOI] [PubMed] [Google Scholar]
- 27.Schallmoser K, et al. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion. 2007;47(8):1436–46. doi: 10.1111/j.1537-2995.2007.01220.x. [DOI] [PubMed] [Google Scholar]
- 28.Schallmoser K, et al. Rapid large-scale expansion of functional mesenchymal stem cells from unmanipulated bone marrow without animal serum. Tissue Eng Part C Methods. 2008;14(3):185–96. doi: 10.1089/ten.tec.2008.0060. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed Z, Bicknell R. Angiogenic signalling pathways. Methods Mol Biol. 2009;467:3–24. doi: 10.1007/978-1-59745-241-0_1. [DOI] [PubMed] [Google Scholar]
- 30.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438(7070):937–45. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 31.Fan Y, et al. Interleukin-6 stimulates circulating blood-derived endothelial progenitor cell angiogenesis in vitro. J Cereb Blood Flow Metab. 2008;28(1):90–8. doi: 10.1038/sj.jcbfm.9600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerritsen ME, et al. Using gene expression profiling to identify the molecular basis of the synergistic actions of hepatocyte growth factor and vascular endothelial growth factor in human endothelial cells. Br J Pharmacol. 2003;140(4):595–610. doi: 10.1038/sj.bjp.0705494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heidemann J, et al. Mucosal angiogenesis regulation by CXCR4 and its ligand CXCL12 expressed by human intestinal microvascular endothelial cells. Am J Physiol Gastrointest Liver Physiol. 2004;286(6):G1059–68. doi: 10.1152/ajpgi.00417.2003. [DOI] [PubMed] [Google Scholar]
- 34.Murakami M, et al. The FGF system has a key role in regulating vascular integrity. J Clin Invest. 2008;118(10):3355–66. doi: 10.1172/JCI35298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piao CS, et al. The role of stem cell factor and granulocyte-colony stimulating factor in brain repair during chronic stroke. J Cereb Blood Flow Metab. 2009;29(4):759–70. doi: 10.1038/jcbfm.2008.168. [DOI] [PubMed] [Google Scholar]
- 36.Vertesaljai M, et al. Drugs, gene transfer, signaling factors: a bench to bedside approach to myocardial stem cell therapy. Heart Fail Rev. 2008;13(2):227–44. doi: 10.1007/s10741-007-9047-9. [DOI] [PubMed] [Google Scholar]
- 37.Yao JS, et al. Interleukin-6 upregulates expression of KDR and stimulates proliferationof human cerebrovascular smooth muscle cells. J Cereb Blood Flow Metab. 2007;27(3):510–20. doi: 10.1038/sj.jcbfm.9600365. [DOI] [PubMed] [Google Scholar]
- 38.Shahdadfar A, et al. In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells. 2005;23(9):1357–66. doi: 10.1634/stemcells.2005-0094. [DOI] [PubMed] [Google Scholar]
- 39.Dahl JA, et al. Genetic and epigenetic instability of human bone marrow mesenchymal stem cells expanded in autologous serum or fetal bovine serum. Int J Dev Biol. 2008;52(8):1033–42. doi: 10.1387/ijdb.082663jd. [DOI] [PubMed] [Google Scholar]
- 40.Meza-Zepeda LA, et al. High-resolution analysis of genetic stability of human adipose tissue stem cells cultured to senescence. J Cell Mol Med. 2008;12(2):553–63. doi: 10.1111/j.1582-4934.2007.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tarte K, et al. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood. 2010;115(8):1549–53. doi: 10.1182/blood-2009-05-219907. [DOI] [PubMed] [Google Scholar]
- 42.Lingle WL, Lukasiewicz K, Salisbury JL. Deregulation of the centrosome cycle and the origin of chromosomal instability in cancer. Adv Exp Med Biol. 2005;570:393–421. doi: 10.1007/1-4020-3764-3_14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Fluorescence in situ hybridization (FISH) analysis of ECs using centromere probes specific for the X chromosome (red) and chromosome 17 (cyan). Nuclei are stained with DAPI (blue). Normal (diploid) female cells display two X chromosomes (red spots) and two chromosome 17s (cyan spots). Tetraploid female cells display four X chromosomes (4 red spots) and four chromosome 17s (4 cyan spots). (B) FISH analysis of > 200 ECs after 30 days of culture initiation revealed a similar frequency of diploid content in cells grown in SRM and in cEGM-2. Chromosomally aberrant cells (tetraploid and aneuploid) were detectable at a low incidence in both culture conditions. (N=4)


