Abstract
Corticotropin releasing factor (CRF) activates two known receptor types, CRF1 and CRF2. In the adult rat brain, CRF2 has a distinct distribution pattern, suggesting that it may mediate functions exclusive of CRF1. The goal of this study was to determine the age-dependent distribution of CRF2-messenger RNA (CRF2-mRNA) in the rat brain. Brains from rats sacrificed under stress-free conditions on fetal days (F) 15, 16, 17 and 19, and postnatal days 1, 3, 5, 7, 9, 12, 15, 25, 49 and 90 (adult) were analyzed using semiquantitative in situ hybridization histochemistry. The onset and distribution of CRF2-mRNA in the developing rat brain revealed important differences from the adult expression pattern: earliest expression of CRF2-mRNA was observed in the ventromedial hypothalamus (VMH) on F16. High levels of CRF2-mRNA were present in the fronto-parietal cortex in the fetal and early postnatal brain but not later. Conversely, no CRF2-mRNA was detectable in the ventroposterior (lateral and medial) thalamic nuclei prior to postnatal day 7. Distinct developmental profiles of CRF2-mRNA were also observed in the lateral septum, medial, basal and cortical amygdala nuclei, and in several hippocampal fields. In conclusion, CRF2 is expressed in the hypothalamus on F16, prior to the detection of CRF itself in the paraventricular nucleus. The differential levels and distributions of CRF2-mRNA in hypothalamic and limbic brain regions indicate a precise regulation of this receptor's expression during development, as shown for CRF1. Regulation of the levels of CRF2 may modulate the effects of CRF (and related ligands) on target neurons, consistent with differential maturation of the functions mediated by this receptor.
Keywords: Corticotropin releasing factor, Receptor, Ventromedial hypothalamus, Ontogeny, Neuroendocrine, Rat
1. Introduction
Corticotropin releasing factor (CRF) is the key mediator of the neuroendocrine response to stress [43]. Release of hypothalamic CRF from the paraventricular nucleus (PVN) leads to elevations of plasma ACTH and glucocorticoids [38,43]. Outside the hypothalamus, CRF has been shown to act as a neurotransmitter in specific central nervous system (CNS) regions [44,51]. The peptide has been implicated in the central mediation of the response to stress, as well as in anxiety, anorexia and neuroexcitation [16,28,33,42]. Certain actions of CRF on limbic neurons are age-dependent, such as the peptide's excitatory effects on neurons in the amygdala [4] and the hippocampus [20,39].
CRF activates G-protein coupled, membrane-bound receptors [11,14,17,21]. Two major types of CRF receptors have been characterized and named CRF1 and CRF2 [10,23,30]. The CRF2 receptor exists in at least two isoforms [22]. The distribution and expression of the two CRF receptors have been documented in the adult rat brain [10,31,47]. The differential anatomical distributions of the two receptors indicate that they may serve distinct functional roles.
The onset of expression and the distribution of the CRF1 receptor in the developing rat brain has been determined using both receptor-binding techniques and in situ hybridization histochemistry for receptor messenger ribonucleic acid (mRNA) [2,21]. Unique developmental expression patterns of CRF1-mRNA were found in the amygdala, hippocampus, and cortex. CRF1 has recently been demonstrated to mediate the proconvulsant actions of CRF [3], and the observed high levels of CRF1-mRNA in limbic structures may provide the mechanism for the dramatic excitatory effects of CRF in these regions during development [4,6,7]. Based on these facts, the current study focused on the developmental profile of CRF2-mRNA in hypothalamic, limbic and cortical regions of the rat brain to gain insight of the potential functions of this receptor in the developing brain.
2. Materials and methods
2.1. Animals and tissue preparation
Timed pregnancy Sprague–Dawley rats were purchased from Zivic-Miller (Zelienople, PA). In this strain, gestation typically lasts for 21 days. Rats were maintained in NIH-approved animal facilities and kept on a 12-h light/dark cycle with access to unlimited lab chow and water. Cages were monitored for the presence of pups at 12-h intervals, and the date of birth was considered day 0 [48,49]. Fetal rats were delivered by cesarian-section on fetal days 15, 16, 17, and 19, and decapitated heads were rapidly immersed in powdered dry ice. Postnatal rats aged 1, 3, 5, 7, 9, 12, 15, 25, 49, and 90 days were sacrificed within 45 s of disturbance. Brains were rapidly dissected onto powdered dry ice and stored at −80°C. Sections were cut coronally at 20 μm using a cryostat, mounted on gelatin-coated slides and stored at −80°C. All experimental procedures were approved by the Institutional Animal Care Committee and conformed to National Institutes of Health guidelines.
2.2. Synthesis and preparation of the riboprobe
A plasmid containing the 461 base-pair fragment of CRF2 cDNA was linearized with HindIII [23]. Radioactive antisense cRNA was synthesized by incubating T3 RNA polymerase (30 U, Promega, Madison, WI) with 1 μg linearized plasmid in 2.5 mM ATP/GTP/UTP, 6 μM [α-35S]-CTP (New England Nuclear, Boston, MA), 10 mM DTT, 40 mM Tris–HCl (pH 7.5), 6 mM MgCl2, 2 mM spermidine, 10 mM NaCl and 40 U RNase inhibitor (RNasin, Promega). After 2 h at 37°C, 3 U of RNase-free DNase (RQ1-DNase, Promega) was added for 15 min at 37°C. Integrity of each transcript was determined by acrylamide gel electrophoresis. The probe was subjected to alkaline hydrolysis and purified by column chromatography (Select-D[RF], 5′–3′, Boulder, CO). The specific activity of the probe was 1–3×106 cpm/μg. Further details of characterization and specificity of the probe to CRF2-mRNA (and sense-strand controls) are provided in Ref. [10]. This CRF2-riboprobe hybridizes with both CRF2a and CRF2b isoforms of the CRF2-mRNA. However, in the adult rat brain, the CRF2b form is found only in the choroid plexus and arterioles [22].
2.3. In situ hybridization histochemistry (ISH)
ISH method was modified from published protocols [23,48–50]. Sections were brought to room temperature, air dried and fixed in fresh 4% buffered paraformaldehyde for 20 min, followed by dehydration and rehydration through graded ethanols. Subsequently, sections were exposed to 0.25% acetic anhydride in 0.1 M triethanolamine (pH 8) for 8 min and were dehydrated through graded ethanols and air dried. Following a 1-h prehybridization, using hybridization buffer devoid of probe at 55°C in a humidity chamber, sections were hybridized at the same temperature for approximately 20 h with the 35S-labeled ribonucleotide probe in hybridization buffer containing 50% formamide, 5× SET, 0.2% SDS, 5× Denhardt's solution, 0.5 mg/ml sheared salmon sperm DNA, 25 μg/μl yeast tRNA, 100 mM dithiothreitol, and 10% dextran sulfate. Posthybridization, sections were washed in 4× and 2× SSC (1× SSC denotes 0.15 M NaCl, 15 mM trisodium citrate buffer, pH 7) for 5 min at room temperature, and were digested using RNase-A (200 μg/ml for 30 min at 37°C) (Calbiochem, La Jolla, CA). The sections underwent successive washes at 55°C in 2× SSC and 1× SSC for 5 min, 0.25× SSC for 30 min, and 0.1× and 0.03× SSC for 1 h each. Finally, sections were dehydrated through graded ethanols and were apposed to film (Hyperfilm B-Max, Amersham, IL) for 6–10 days. Specificity controls for the hybridization reaction consisted of sections which were subjected to RNase digestion prior to the ISH. Representative sections were also dipped in NTB2 nuclear emulsion (Eastman Kodak, Rochester, NY) and exposed for 2–3 weeks. Slides were developed (D-19 developer, KODAK) for 2.5 min at 16°C, and counter-stained using hematoxylin.
2.4. Acquisition and quantitative analysis of CRF2-mRNA ISH signal
Semiquantitative analysis of CRF2-mRNA was accomplished using the ImageTool software program (University of Texas Health Science Center, San Antonio). Films were scanned using a StudioStar scanner (AGFA, resolution 1200×1200 dots per inch), equipped with a transparency bed and the Fotolook 32 software. Digitized images were acquired onto a Dell Computer and analyzed. For each age group, 2–5 brains were utilized and a minimum of four sections were analyzed for each structure. Optical densities were determined over the lateral septum (LS), hippocampal CA1 and CA3 fields, the dentate gyrus (DG), medial, basal, and cortical amygdaloid nuclei, ventroposterior lateral and medial thalamic nuclei, paraventricular thalamic nucleus, ventromedial hypothalamic nucleus, frontal and piriform cortices, and the choroid plexus. Densities were calibrated using 14C standards, as well as brain-paste [48–50], and are expressed in arbitrary units. The significance (p<0.05) of observed quantitative differences among different age groups was evaluated using ANOVA or Student's t-test, as appropriate, with Bonferroni multiple comparison post-hoc test (Prism GraphPad software, San Diego, CA).
3. Results
A distinct developmental profile of CRF2-gene expression was observed in many of the brain areas previously found to contain CRF2-mRNA in the adult. In addition, several regions were found to express CRF2-mRNA only during certain stages of development.
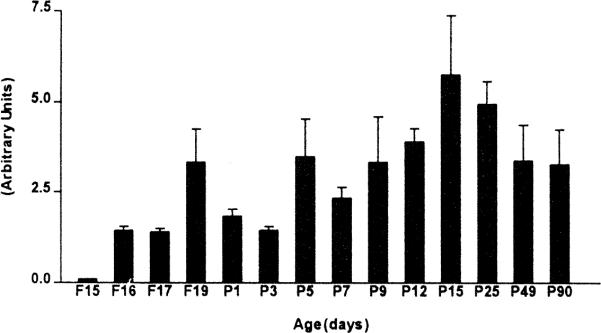
Earliest expression of CRF2-mRNA was observed in the ventromedial hypothalamus (VMH) occurring as early as the 16th fetal day (F16; Fig. 1). There was a significant trend for increased CRF2-mRNA expression in the VMH with age, reaching adult levels on postnatal day 15 (p<0.05, one way ANOVA with Bonferroni post-hoc test, Fig. 2).
Fig. 1.
Coronal brain hemi-sections at the level of the diencephalon, illustrating CRF2-mRNA presence in the ventromedial hypothalamus (VMH) of developing rats at the indicated ages. Sections were subjected to in situ hybridization using a riboprobe for the CRF2-mRNA (see Section 2). CRF2 -mRNA expression is evident in the VMH (arrowhead) as early as the 16th fetal day (F16). In the basal amygdala (BasA) nucleus, CRF2-mRNA is detectable as early as F19, and the signal is robust by P1 (arrow). CRF2-mRNA signal is also visible over the medial amygdala (MeA) and cortical amygdala (CoA) nuclei. Note that no hybridization signal is present over the thalamus in sections derived from fetal or neonatal rats, while signal in the hippocampal CA1 and CA3 is relatively unchanged with age. VPL and VPM are the ventroposterior lateral and medial thalamic nuclei, respectively. ChP denotes CRF2-mRNA expression in the choroid plexus. Section magnification varies.
Fig. 2.
Quantitative analysis of CRF2-mRNA levels in the ventromedial hypothalamus. Each bar represents means with standard errors of the means of data from a minimum of four sections from three rats. Subsequent to in situ hybridization for CRF2-mRNA, sections were analyzed as described in Section 2. CRF2 expression increases with age to reach adult levels by postnatal day 15 (p<0.05, one way ANOVA with Bonferroni post-hoc test).
Detectable levels of CRF2-mRNA were present in the lateral septum starting on the 19th fetal day (Fig. 3). CRF2-mRNA hybridization signal was evident primarily in the intermediate region of the lateral septum, with significant involvement of the ventral subdivision (Fig. 3). The adult pattern of distribution, showing a cluster of highly CRF2-mRNA expressing cells at the basal portion of the lateral septum, was evident by postnatal day 7 (P7 in Fig. 3). At higher magnifications, discrete groups of CRF2-expressing cells confined to the lateral septum were visible (Fig. 4). Visual inspection suggested a trend towards increased CRF2-mRNA levels in the septum with age (Fig. 5), which was not confirmed by statistical analysis.
Fig. 3.
Coronal forebrain hemi-sections at the level of septum, derived from developing rats at the indicated ages. Sections were subjected to in situ hybridization histochemistry using a riboprobe for the CRF2-mRNA (see Section 2). Hybridization signal is evident over the lateral septum as early as the 19th fetal day (F19). CRF2-mRNA can be detected over the piriform cortex starting on the first postnatal day (P1). The RNase Control section, from a 9-day-old pup was treated with RNase prior to the in situ hybridization histochemistry (see Section 2), demonstrating the specificity of the CRF2-mRNA riboprobe. Section magnification varies.
Fig. 4.
Brightfield (A) and darkfield (B) photomicrographs of the lateral septum region from a 25-day-old rat. (A) Shows anatomic landmarks; open arrow denotes choroid plexus, closed arrow points to the lateral ventricle. SPLd, SPLi, and SPLv denote the dorsal, intermediate, and ventral septal nuclei, respectively. Scale bar=300 μm. (B) Darkfield photomicrographs of the area delimited by the broken line in (A). Scale bar=120 μm. Expression of CRF2-mRNA in the SPLi and SPLv is evident. Virtually no silver grains are found in the striatum (arrow).
Fig. 5.
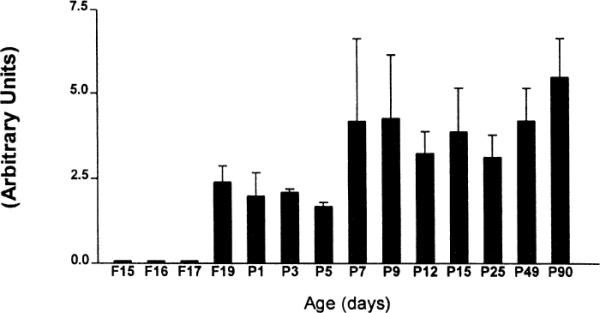
Quantitative analysis of CRF2-mRNA levels in the lateral septum. Each bar represents means and standard errors of data from a minimum of four sections from three rats. Expression of CRF2-mRNA is observed in the lateral septum starting on fetal day 19 (F19). No significant correlation of CRF2-mRNA expression with age is apparent (p=0.58; one way ANOVA).
In the amygdala, discrete CRF2-mRNA signal was evident over the medial (MeA) and basal (BasA) nuclei as early as the 17th fetal day (Figs. 1 and 6A,B). In the cortical amygdaloid nucleus, however, onset of CRF2-expression occurred only on the third postnatal day. Unlike other nuclei of the amygdala where CRF2-mRNA levels remained fairly stable to adulthood (Fig. 6A,B), an early postnatal increase of CRF2- receptor mRNA production was evident in the cortical nucleus (p<0.01, one way ANOVA; Fig. 6C). In the hippocampal formation, CRF2-mRNA was initially observed on the first postnatal day. In both CA1 and CA3 fields of Ammon's horn, as well as in the dentate gyrus, levels of CRF2-mRNA were relatively constant throughout development (Fig. 7A–C).
Fig. 6.
Comparison of CRF2-mRNA levels in three nuclei of the amygdaloid complex. In the medial nucleus (A) and in the basal group (medial and lateral basal nuclei) (B) CRF2-mRNA is first detectable on fetal day 17 (F17). Receptor expression remains fairly stable until adulthood (P90). Conversely, in the cortical amygdala (C), CRF2-mRNA is not observed until P3, and message levels increase with age (p<0.01; one way ANOVA with Bonferroni post-hoc test, P3 to P90). Values for each bar are the mean from a minimum of three animals per age group with bars representing the standard error of the means.
Fig. 7.
Quantitative analysis of the developmental expression of CRF2-mRNA in the hippocampal formation. Each bar represents data derived from a minimum of three animals per age group. (A) and (B) depict CRF2-mRNA levels in the CA1 and CA3 regions of Ammon's horn, respectively. In both regions, levels do not change with development. (C) is a graphic representation of CRF2-mRNA levels over the granule cell layer of the dentate gyrus. No statistically significant correlation of CRF2 expression with age can be discerned (p=0.09; one way ANOVA).
CRF2-gene expression was evident in the frontal cortex by the 17th fetal day. Expression of this transcript was transient as no CRF2-mRNA signal was detectable in this region subsequent to the 12th postnatal day (Fig. 8A). A very different expression pattern was observed in the piriform cortex, where a moderate CRF2-mRNA signal was first detectable on the first postnatal day (Fig. 3) and remained rather stable to adulthood (Fig. 8B).
Fig. 8.
Quantitative analysis of CRF2-mRNA levels in two cortical regions, illustrating the differential temporal patterns of receptor mRNA expression in these two areas. In the frontal cortex (A) CRF2-mRNA is expressed by the 17th fetal day (F17). In contrast, in the piriform cortex (B), CRF2-mRNA is observed starting on the first postnatal day (P1, see also Fig. 3), and robust expression of the receptor persists to adulthood. The mean from a minimum of three animals per age is shown with error bars representing the standard error of the means.
The current study revealed the presence of CRF2-mRNA in specific thalamic nuclei (P90 in Fig. 1), regions which have not been previously shown to express this receptor in the adult [10,23]. A distinct developmental pattern of CRF2-mRNA was demonstrated in the thalamus: we observed CRF2-mRNA-specific signal in the paraventricular thalamic nucleus starting on postnatal day 25 (Fig. 9A). In addition, the onset of detectable CRF2-mRNA signal over the ventroposterior lateral and medial thalamic nuclei (VPM and VPL) occurred on the seventh postnatal day (Fig. 9B) and persisted to adulthood. Other thalamic nuclei did not express appreciable levels of CRF2-mRNA. In addition, CRF2-mRNA was expressed in the choroid plexus as early as the 17th fetal day and steady-state mRNA levels remained stable through postnatal day 90 (data not shown). In the habenula, CRF2-mRNA expression was present starting on the 17th fetal day (data not shown) and remained at constant levels through adulthood (Fig. 9A).
Fig. 9.
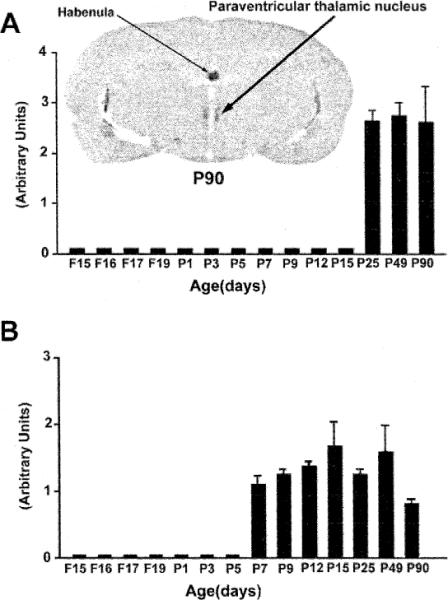
Quantitative analysis of CRF2-mRNA levels in two thalamic nuclei, illustrating the distinct spatiotemporal expression patterns of the receptor in these thalamic regions. Each bar represents means of data derived from a minimum of three animals, with the standard error of the means. The paraventricular thalamic nucleus (A) expresses significant levels of CRF2-mRNA only starting on the fourth postnatal week, while in the ventroposterior lateral and medial thalamic nuclei (VPL and VPM) (B) CRF2-mRNA is observed starting on postnatal day 7 (P7). A strong hybridization signal is observed over the habenula.
4. Discussion
This study demonstrates that CRF2-gene expression exhibits region- and age-specific patterns during development. These distinct spatial and temporal distribution patterns may provide important information regarding the regulation and function of this receptor in the developing brain.
CRF2-mRNA expression was evident in the ventromedial hypothalamus (VMH) by the 16th fetal day. Neurons of the VMH are born on the 13th–14th fetal day in the rat [1], so that initial expression of CRF2 occurs in post-mitotic cells. However, CRF2-mRNA expression in the VMH precedes detectable mRNA signal for CRF itself in the hypothalamic paraventricular nucleus (PVN) [5,18]. The parvocellular neurons of the PVN are born around F16 [1], and start expressing CRF within 24 h, i.e., on F17 [5,18]. The onset of CRF2 receptor expression prior to the availability of its putative ligand, CRF, suggests that the CRF2 receptor may serve functions that are independent from activation by CRF during the early developmental period. Such functions may include cellular growth and differentiation. Alternatively, the CRF2 receptor may be activated by a non-CRF ligand [40,45]. The endogenous neuropeptide urocortin has been suggested as a likely candidate ligand for CRF2 receptors in the adult, due to the relatively high affinity of its binding to CRF2 [45] and its appetite suppressing properties [40]. To our knowledge, the ontogeny of urocortin in the VMH has not been described. In the adult brain, modest signal corresponding to urocortin-mRNA has been observed over the VMH [46].
On microscopic visualization, VMH neurons appear undifferentiated during the first postnatal week, with few organelles [32]. These neurons achieve adult characteristics and a full array of synapses by the 20th postnatal day. The observation that CRF2-mRNA expression was detected as early as the 16th fetal day, with a gradual increase in signal intensity peaking on the 15th–25th postnatal days, suggests that full functional maturation of VMH neurons may not be necessary for activation of CRF2 production.
What are the potential role(s) of the CRF2 receptor in the VMH of the developing rat? Available data is consistent with a number of important functions. Activation of the VMH depresses feeding, while VMH lesions lead to obesity [33]. CRF has anorectic actions when given systemically or into the hypothalamus [19,33]. CRF also prevents the excessive body weight gain of genetically obese rats [36]. Thus, CRF may interact with CRF2 receptors in the VMH to control feeding [36]. In support of this possibility, CRF2-mRNA levels have been demonstrated to be lower in obese Zucker rats [34]. As food intake and its regulation is most critical to the survival of the neonatal rat, an early and well developed complement of CRF2 receptors in the VMH should be expected, as found in this study.
The VMH is also a key structure in the complex coordination of diurnal variation in the hypothalamic-pituitary–adrenal (HPA) axis tone [41] VMH lesions disrupt a number of functions including the circadian rhythms of food intake, insulin secretion and adrenocortical function [13]. A key mechanism by which the VMH mediates signals inhibiting the HPA axis is via occupancy of mineralocorticoid receptors on VMH neurons [41]. However, a role for activation of CRF2 receptors in modulating some aspects of the diurnal cycle of the HPA axis tone cannot be excluded [12]. CRF1-mRNA levels in the hypothalamus and pituitary are clearly regulated by a variety of stresses [24,25,27,35], by glucocorticoids and other steroid hormones [24,25,27], and by CRF itself [26]. Recently, down-regulation of CRF2-mRNA levels in the VMH of 9-day-old rats after a 24-h maternal separation period, which involves both stress and food deprivation, has been documented [15]. Whether stress, feeding state, diurnal phase, diet constituents or body weight regulate CRF2 gene expression during development remains to be determined.
The VMH projects directly to the paraventricular nucleus of the thalamus [9], a structure found in this study to express CRF2 in the adult rat. Previous investigations have not reported on the presence of CRF2-mRNA in this nucleus [10,23]. This fact is probably due to the small size, closeness to the midline and unusual anatomy of this nucleus. The thalamic paraventricular nucleus has been implicated in a number of autonomic and behavioral responses to stress [8], and receives input from the CRH expressing neurons in the central nucleus of the amygdala [29]. The presynaptic terminals of these neurons are likely the source of the endogenous CRF impinging on the thalamic paraventricular CRF2 receptors demonstrated in the current study.
CRF2-mRNA was found exclusively in three of the amygdala nuclei. Furthermore, the onset and time-course of CRF2-gene expression in these nuclei displayed two discrete profiles. In the medial (including the corticomedial region) and basal amygdaloid nuclei, CRF2-mRNA was evident starting on the 17th fetal day. Interestingly, a direct projection of CRF-containing neurons from the medial amygdala to the VMH has been demonstrated [37]. In the cortical nucleus, onset of CRF2-expression was postnatal (Fig. 6C) and levels of CRF2-mRNA increased with age, suggesting that CRF2 is probably not involved in trophic or developmental regulatory functions in this nucleus. Little CRF2-mRNA signal was detected in the central nucleus of the amygdala. This nucleus plays a major role in the transduction of stress signals and contains a large cluster of CRF-expressing neurons, as well as modest amounts of CRF1-mRNA [2,10,14,31,38]. Current knowledge of the distribution of the two members of the CRF receptor family in the amygdala is insufficient to determine the relative roles of these receptors in mediating the effects of CRF on the hormonal and behavioral responses to stress.
The distribution of CRF2-mRNA in hypothalamic and limbic brain regions, and the transcript levels during development are distinct from those established for CRF1 (Fig. 10) [2]. For CRF1, messenger RNA levels in the hippocampus peak at the end of the first postnatal week, while levels in the amygdala are maximal during the second postnatal week. This developmental profile of CRF1-mRNA may provide the mechanism for the enhanced potency of CRF for neuronal excitation, which is mediated by activation of the CRF1 receptor [3,4]. In contrast, the developmental profile of CRF2 reveals early-onset expression in the hypothalamus and in circuits involved in autonomic and neuroendocrine functions which are critical throughout the organism's life-span. These functions of CRF2 would require fairly constant levels of receptor synthesis (and CRF2-mRNA levels) throughout development, as demonstrated in this study in a majority of brain regions. Both receptor-mRNAs, however, are more abundant in the frontal cortex during the first postnatal week than at later periods (Fig. 8A) [2]. CRF1-mRNA is found in the fronto-parietal cortex throughout adult life, while little CRF2-mRNA signal is evident over the mature frontal cortex [10]. The role of both receptors in the developing cortex is unclear, but they may mediate trophic actions of CRF on developing cortical neurons during the time of the formation of neuronal connections.
Fig. 10.
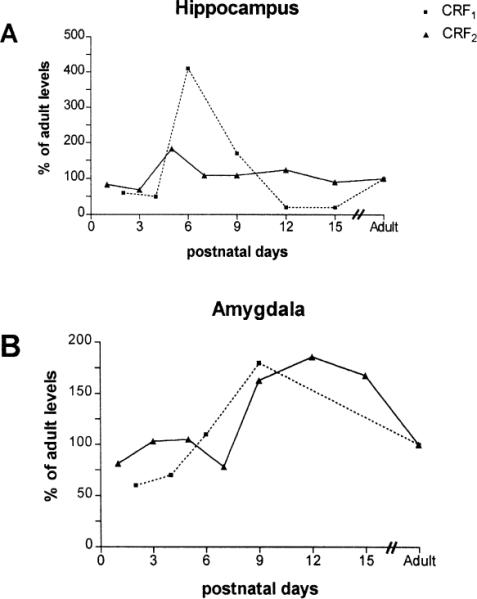
Comparative developmental profiles of CRF1-mRNA and CRF2-mRNA in the hippocampus (A) and amygdala (B). Values have been normalized to adult levels. In the hippocampus (CA1 and CA3), CRF1-mRNA expression peaks at 400% of adult levels on postnatal day 6. This is not evident for CRF2-mRNA. In the amygdala, CRF1-mRNA is expressed primarily in the central and lateral nuclei [2], while CRF2-mRNA hybridization signal is concentrated over the medial, basal, and cortical nuclei. A developmental `hyperexpression' for both receptors is evident during the second postnatal week, in comparison with the adult levels. Values for the amygdala are a composite of signal over all of the nuclei which express each receptor, as described above. The data for CRF1-mRNA are modified from [2], and printed with permission.
In conclusion, the spatiotemporal profile of CRF2-mRNA indicates a precise regulation of the expression of this receptor during development in distinct brain regions. Furthermore, modulation of CRF2 receptor levels may provide an important means for regulating certain effects of CRF itself during the developmental period.
Acknowledgements
The technical assistance of L. Schultz is appreciated. Supported by NIH NS 28912 (TZB).
References
- [1].Altman J, Bayer SA. The development of the rat hypothalamus. Adv. Anat. Embryol. Cell Biol. 1986;100:1–178. [PubMed] [Google Scholar]
- [2].Avishai-Eliner S, Yi SJ, Baram TZ. Developmental profile of messenger RNA for the corticotropin-releasing hormone receptor in the rat limbic system. Dev. Brain Res. 1996;91:159–163. doi: 10.1016/0165-3806(95)00158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Baram TZ, Chalmers DT, Chen C, Kotsoukos Y, De Souza EB. The CRF1 receptor mediates the excitatory actions of corticotropin releasing factor in the developing rat brain: in vivo evidence using novel, selective, non-peptide CRF receptor antagonist. Brain Res. 1997;770:89–95. doi: 10.1016/s0006-8993(97)00759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Baram TZ, Hirsch E, Snead OC, Schultz L. Corticotropin-releasing hormone-induced seizures in infant rats originate in the amygdala. Ann. Neurol. 1992;31:488–494. doi: 10.1002/ana.410310505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Baram TZ, Lerner SP. Ontogeny of corticotropin-releasing hormone gene expression in rat hypothalamus—comparison with somatostatin. Int. J. Dev. Neurosci. 1991;9:473–478. doi: 10.1016/0736-5748(91)90033-i. [DOI] [PubMed] [Google Scholar]
- [6].Baram TZ, Ribak CE. Peptide-induced infant status epilepticus causes neuronal death and synaptic reorganization. NeuroReport. 1995;6:277–280. doi: 10.1097/00001756-199501000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Baram TZ, Schultz L. Corticotropin-releasing hormone is a rapid and potent convulsant in the infant rat. Dev. Brain Res. 1991;61:97–101. doi: 10.1016/0165-3806(91)90118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Beck CH, Fibiger HC. Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J. Neurosci. 1995;15:709–720. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus νulgaris leucoagglutinin study in the rat. J. Comp. Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- [10].Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J. Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chang CP, Pearse RV, O'Connell S, Rosenfeld MG. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron. 1993;11:1187–1195. doi: 10.1016/0896-6273(93)90230-o. [DOI] [PubMed] [Google Scholar]
- [12].Choi S, Horsley C, Aguila S, Dallman MF. The hypothalamic ventromedial nuclei couple activity in the hypothalamo-pituitary–adrenal axis to the morning fed or fasted state. J. Neurosci. 1996;16:8170–8180. doi: 10.1523/JNEUROSCI.16-24-08170.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dallman MF. Viewing the ventromedial hypothalamus from the adrenal gland. Am. J. Physiol. 1984;246:R1–R12. doi: 10.1152/ajpregu.1984.246.1.R1. [DOI] [PubMed] [Google Scholar]
- [14].De Souza EB, Insel TR, Perrin MH, Rivier J, Vale WW, Kuhar MJ. Corticotropin-releasing factor receptors are widely distributed within the rat CNS: an autoradiographic study. J. Neurosci. 1985;5:3189–3203. doi: 10.1523/JNEUROSCI.05-12-03189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Eghbal-Ahmadi M, Hatalski CG, Avishai-Eliner S, Baram TZ. Corticotropin-releasing factor receptor type II (CRF2) messenger ribonucleic acid levels in the hypothalamic ventromedial nucleus of the infant rat are reduced by maternal deprivation. Endocrinology. 1997;138:5048–5051. doi: 10.1210/endo.138.11.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ehlers CL, Henriksen SJ, Wang M, Rivier J, Vale W, Bloom FE. Corticotropin-releasing factor produces increases in brain excitability and convulsive seizures in rats. Brain Res. 1983;278:332–336. doi: 10.1016/0006-8993(83)90266-4. [DOI] [PubMed] [Google Scholar]
- [17].Grigoriadis DE, Heroux JA, De Souza EB. Characterization and regulation of corticotropin-releasing factor receptors in the central nervous, endocrine and immune systems. Ciba. Found. Symp. 1993;172:85–101. doi: 10.1002/9780470514368.ch5. [DOI] [PubMed] [Google Scholar]
- [18].Grino M, Young III WS, Burgunder JM. Ontogeny of expression of the corticotropin-releasing factor gene in the hypothalamic paraventricular nucleus and of the proopiomelanocortin gene in rat pituitary. Endocrinology. 1989;124:60–68. doi: 10.1210/endo-124-1-60. [DOI] [PubMed] [Google Scholar]
- [19].Heinrichs SC, Menzaghi F, Merlo-Pich E, Hauger RL, Koob GF. Corticotropin-releasing factor in the paraventricular nucleus modulates feeding induced by neuropeptide Y. Brain Res. 1993;611:18–24. doi: 10.1016/0006-8993(93)91771-j. [DOI] [PubMed] [Google Scholar]
- [20].Hollrigel GS, Chen K, Baram TZ, Soltesz I. The pro-convulsant actions of corticotropin-releasing hormone in the hippocampus of infant rats. Neurosci. 1998 doi: 10.1016/s0306-4522(97)00499-5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Insel TR, Battaglia G, Fairbanks DW, De Souza EB. The ontogeny of brain receptors for corticotropin-releasing factor and the development of their functional association with adenylate cyclase. J. Neurosci. 1988;8:4151–4158. doi: 10.1523/JNEUROSCI.08-11-04151.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lovenberg TW, Chalmers DT, Liu C, De Souza EB. CRF2 alpha and CRF2 beta receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology. 1995;136:4139–4142. doi: 10.1210/endo.136.9.7544278. [DOI] [PubMed] [Google Scholar]
- [23].Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc. Natl. Acad. Sci. U.S.A. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Luo X, Kiss A, Makara G, Lolait SJ, Aguilera G. Stress-specific regulation of corticotropin-releasing hormone receptor expression in the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J. Neuroendocrinol. 1994;6:689–696. doi: 10.1111/j.1365-2826.1994.tb00636.x. [DOI] [PubMed] [Google Scholar]
- [25].Makino S, Schulkin J, Smith MA, Pacak K, Palkovits M, Gold PW. Regulation of corticotropin-releasing hormone receptor messenger ribonucleic acid in the rat brain and pituitary by glucocorticoids and stress. Endocrinology. 1995;136:4517–4525. doi: 10.1210/endo.136.10.7664672. [DOI] [PubMed] [Google Scholar]
- [26].Mansi JA, Rivest S, Drolet G. Regulation of corticotropin-releasing factor type 1 (CRF1) receptor messenger ribonucleic acid in the paraventricular nucleus of rat hypothalamus by exogenous CRF. Endocrinology. 1996;137:4619–4629. doi: 10.1210/endo.137.11.8895325. [DOI] [PubMed] [Google Scholar]
- [27].Nappi RE, Rivest S. Ovulatory cycle influences the stimulatory effect of stress on the expression of corticotropin-releasing factor receptor messenger ribonucleic acid in the paraventricular nucleus of the female rat hypothalamus. Endocrinology. 1995;136:4073–4083. doi: 10.1210/endo.136.9.7649116. [DOI] [PubMed] [Google Scholar]
- [28].Nemeroff CB. New vistas in neuropeptide research in neuropsychiatry: focus on corticotropin-releasing factor. Neuropsychopharmacology. 1992;6:69–75. [PubMed] [Google Scholar]
- [29].Otake K, Nakamura Y. Sites of origin of corticotropin-releasing factor-like immunoreactive projection fibers to the paraventricular thalamic nucleus in the rat. Neurosci. Lett. 1995;201:84–86. doi: 10.1016/0304-3940(95)12148-w. [DOI] [PubMed] [Google Scholar]
- [30].Perrin MH, Donaldson CJ, Chen R, Lewis KA, Vale WW. Cloning and functional expression of a rat brain corticotropin-releasing factor (CRF) receptor. Endocrinology. 1993;133:3058–3061. doi: 10.1210/endo.133.6.8243338. [DOI] [PubMed] [Google Scholar]
- [31].Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko PE, Vale W. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pozzo Miller LD, Aoki A. Postnatal development of the hypothalamic ventromedial nucleus: neurons and synapses. Cell Mol. Neurobiol. 1992;12:121–129. doi: 10.1007/BF00713366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Richard D. Involvement of corticotropin-releasing factor in the control of food intake and energy expenditure. Ann. New York Acad. Sci. 1993;697:155–172. doi: 10.1111/j.1749-6632.1993.tb49930.x. [DOI] [PubMed] [Google Scholar]
- [34].Richard D, Rivest R, Naimi N, Timofeeva E, Rivest S. Expression of corticotropin-releasing factor and its receptors in the brain of lean and obese Zucker rats. Endocrinology. 1996;137:4786–4795. doi: 10.1210/endo.137.11.8895348. [DOI] [PubMed] [Google Scholar]
- [35].Rivest S, Laflamme N, Nappi RE. Immune challenge and immobilization stress induce transcription of the gene encoding the CRF receptor in selective nuclei of the rat hypothalamus. J. Neurosci. 1995;15:2680–2695. doi: 10.1523/JNEUROSCI.15-04-02680.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rohner-Jeanrenaud F, Walker CD, Greco-Perotto R, Jeanrenaud B. Central corticotropin-releasing factor administration prevents the excessive body weight gain of genetically obese (fa/fa) rats. Endocrinology. 1989;124:733–739. doi: 10.1210/endo-124-2-733. [DOI] [PubMed] [Google Scholar]
- [37].Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 1986;382:213–238. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- [38].Sawchenko PE, Imaki T, Potter E, Kovacs K, Imaki J, Vale W. The functional neuroanatomy of corticotropin-releasing factor. Ciba. Found. Symp. 1993;172:5–21. doi: 10.1002/9780470514368.ch2. [DOI] [PubMed] [Google Scholar]
- [39].Smith BN, Dudek FE. Age-related epileptogenic effects of corticotropin-releasing hormone in the isolated CA1 region of rat hippocampal slices. J. Neurophysiol. 1994;72:2328–2333. doi: 10.1152/jn.1994.72.5.2328. [DOI] [PubMed] [Google Scholar]
- [40].Spina M, Merlo-Pich E, Chan RK, Basso AM, Rivier J, Vale W, Koob GF. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science. 1996;273:1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- [41].Suemaru S, Darlington DN, Akana SF, Cascio CS, Dallman MF. Ventromedial hypothalamic lesions inhibit corticosteroid feedback regulation of basal ACTH during the trough of the circardian rhythm. Neuroendocrinology. 1995;61:453–463. doi: 10.1159/000126868. [DOI] [PubMed] [Google Scholar]
- [42].Tsigos C, Chrousos GP. Physiology of the hypothalamic pituitary adrenal axis in health and dysregulation in psychiatric and autoimmune disorders. Endocrinol. Metab. Clin. North Am. 1994;23:451–466. [PubMed] [Google Scholar]
- [43].Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- [44].Valentino RJ, Page ME, Curtis AL. Activation of noradrenergic locus ceruleus neurons by hemodynamic stress is due to local release of corticotropin-releasing factor. Brain Res. 1991;555:25–34. doi: 10.1016/0006-8993(91)90855-p. [DOI] [PubMed] [Google Scholar]
- [45].Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, Rivier J, Sawchenko PE, Vale W. Urocortin; a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- [46].Wong ML, Al-Shekhlee A, Bongiorno PB, Esposito A, Khatri P, Sternberg EM, Gold PW, Licinio J. Localization of urocortin messenger RNA in rat brain and pituitary. Mol. Psychiatry. 1996;1:307–312. [PubMed] [Google Scholar]
- [47].Wong ML, Licinio J, Pasternak KI, Gold PW. Localization of corticotropin-releasing hormone (CRH) receptor mRNA in adult rat brain by in situ hybridization histochemistry. Endocrinology. 1994;135:2275–2278. doi: 10.1210/endo.135.5.7956950. [DOI] [PubMed] [Google Scholar]
- [48].Yi SJ, Baram TZ. Corticotropin-releasing hormone mediates the response to cold stress in the neonatal rat without compensatory enhancement of the peptide's gene expression. Endocrinology. 1994;135:2364–2368. doi: 10.1210/endo.135.6.7988418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yi SJ, Masters JN, Baram TZ. Effects of a specific glucocorticoid receptor antagonist on corticotropin-releasing hormone gene expression in the paraventricular nucleus of the neonatal rat. Dev. Brain Res. 1993;73:253–259. doi: 10.1016/0165-3806(93)90145-z. [DOI] [PubMed] [Google Scholar]
- [50].Yi SJ, Masters JN, Baram TZ. Glucocorticoid receptor mRNA ontogeny in the fetal and postnatal rat forebrain. Mol. Cell Neurosci. 1994;5:385–393. doi: 10.1006/mcne.1994.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Young III WS, Walker LC, Powers RE, De Souza EB, Price DL. Corticotropin-releasing factor mRNA is expressed in the inferior olives of rodents and primates. Brain Res. 1986;387:189–192. doi: 10.1016/0169-328x(86)90010-0. [DOI] [PubMed] [Google Scholar]