Abstract
Corticotropin-releasing hormone (CRH) excites hippocampal neurons and induces death of selected CA3 pyramidal cells in immature rats. These actions of CRH require activation of specific receptors that are abundant in CA3 during early postnatal development. Given the dramatic effects of CRH on hippocampal neurons and the absence of CRH-containing afferents to this region, we hypothesized that a significant population of CRHergic neurons exists in developing rat hippocampus. This study defined and characterized hippocampal CRH-containing cells by using immunocytochemistry, ultrastructural examination, and colocalization with gamma-aminobutyric acid (GABA)-synthesizing enzyme and calcium-binding proteins. Numerous, large CRH-immunoreactive (ir) neurons were demonstrated in CA3 strata pyramidale and oriens, fewer were observed in the corresponding layers of CA1, and smaller CRH-ir cells were found in stratum lacunosum-moleculare of Ammon's horn. In the dentate gyrus, CRH-ir somata resided in the granule cell layer and hilus. Ultrastructurally, CRH-ir neurons had aspiny dendrites and were postsynaptic to both asymmetric and symmetric synapses. CRH-ir axon terminals formed axosomatic and axodendritic symmetric synapses with pyramidal and granule cells. Other CRH-ir terminals synapsed on axon initial segments of principal neurons. Most CRH-ir neurons were coimmunolabeled for glutamate decarboxylase (GAD)-65 and GAD-67 and the majority also contained parvalbumin, but none were labeled for calbindin. These results confirm the identity of hippocampal CRH-ir cells as GABAergic interneurons. Further, a subpopulation of neurons immunoreactive for both CRH and parvalbumin and located within and adjacent to the principal cell layers consists of basket and chandelier cells. Thus, axon terminals of CRH-ir interneurons are strategically positioned to influence the excitability of the principal hippocampal neurons via release of both CRH and GABA.
Keywords: hippocampus, interneurons, neuropeptides, parvalbumin, development
INTRODUCTION
Corticotropin-releasing hormone (CRH) is a neuropeptide with both neuroendocrine and neurotransmitter properties (Vale et al., 1981; Young, 1992). The neuroendocrine effects of CRH, the key mediator of the stress response, originate from clusters of peptidergic cells in the hypothalamic paraventricular nucleus (Lightman and Harbuz, 1993; Herman and Cullinan, 1997). CRH is also a neuromodulator in a number of limbic and autonomic brain circuits (Fox and Gruol, 1993; Curtis et al., 1995). CRH-producing neurons are widely but specifically distributed in the brain (Sawchenko et al., 1993), including the central nucleus of the amygdala, which is considered a major source for nonendocrine CRH-mediated neurotransmission (Herman and Cullinan, 1997). Target neurons for CRH, expressing specific receptors, are found in brain regions including the hippocampus (Chalmers et al., 1995; Avishai-Eliner et al., 1996). Physiological effects of CRH on hippocampal neurons include facilitating memory retention and increasing protein phosphorylation (Lee et al., 1992; Behan et al., 1995). Abnormalities of CRH-mediated neurotransmission may contribute to neurological disorders such as depression (Nemeroff, 1992) or Alzheimer's disease (Behan et al., 1995).
In general, CRH is an excitatory neurotransmitter (Young, 1992; Curtis et al., 1995), and CRH-induced excitation has been demonstrated in the mature rat amygdala (Ehlers et al., 1983; Rainnie et al., 1992; Weiss et al., 1993). In the adult rat, in vivo administration of CRH into the cerebral ventricles results in epileptiform discharges in the amygdala and the hippocampus, which progress to seizures 3–7 hours later (Ehlers et al., 1983). Indirect evidence suggests that CRH may also be involved in excitotoxic neuronal death in the mature brain: increased CRH levels have been observed in brain regions undergoing injury after kainic-acid-induced status epilepticus (Piekut et al., 1996), and administration of CRH receptor blockers has been reported to decrease ischemic neuronal injury (Lyons et al., 1991; Maecker et al., 1997).
In the immature rat (10–13 postnatal days of age), synthetic CRH produces rapid-onset and prolonged limbic seizures involving the amygdala and hippocampus (Baram et al., 1992; Baram and Hatalski, 1998). Furthermore, repeated administration of synthetic CRH in vivo results in death of some hippocampal pyramidal neurons in CA3 (Baram and Ribak, 1995; Ribak and Baram, 1996). The mechanisms by which CRH activates immature hippocampal neurons have been investigated by using in vitro hippocampal slice preparations. Smith and Dudek (1994) found an increase in the amplitude of population spikes in the CA1 region. Using single-cell patch-clamp recording, Hollrigel et al. (1998) demonstrated that CRH dramatically increases the frequency of excitatory synaptic events in CA3 pyramidal neurons.
Hippocampal CA3 and CA1 pyramidal neurons possess receptors for CRH. Messenger-RNA levels of the type I CRH receptor (CRF1) are highest during development in the rat, particularly in the second postnatal week (Avishai-Eliner et al., 1996), an age during which the excitatory effects of CRH on hippocampal neurons are maximal. The role of CRF1 receptor activation in mediating the excitant effects of CRH is supported by the complete elimination of CRH-induced seizures after blocking the CRF1 receptor (Baram et al., 1997). The source of endogenous CRH, the physiological ligand activating the CRF1 receptors on hippocampal neurons, is unclear. Although CRH has been demonstrated in regions providing afferents to the hippocampal formation, such as the entorhinal cortex, CRH-containing neuronal pathways reaching Ammon's horn have not been described.
Previous studies regarding CRH-immunoreactive (ir) neurons in the hippocampal formation have described variable numbers and distribution of such cells. Using antisera directed against ovine CRH, Olschowka et al. (1982) concluded that CRH-ir neurons or fibers were not detectable in the adult rat hippocampus, even after colchicine treatment, while Swanson et al. (1983) and Merchenthaler (1984) described scattered “interneuron-like” cells in this region. More recent studies using antisera against the rat/human CRH have described relatively small numbers of CRH-ir cell bodies scattered in Ammon's horn and in the hilus of the dentate gyrus (Sakanaka et al., 1987). Although CRH-ir hippocampal cells were considered to be interneurons, they have not been subjected to detailed morphological, ultrastructural, and neurochemical characterization.
The goal of the present study was to test the hypothesis that a significant population of interneurons in the hippocampus contain CRH. We aimed to define the neuroanatomical features of these neurons by using both light and electron microscopic (EM) methods and to study the colocalization of CRH with glutamate decarboxylase (GAD) and calcium-binding proteins.
MATERIALS AND METHODS
Animals and Tissue Preparation
Rats (n = 24) were offspring of timed-pregnancy Sprague-Dawley-derived dams (Zivic-Miller, Zelienople, PA). Dams were caged individually in NIH-approved animal facilities and kept on a 12-hour light/dark cycle with access to unlimited lab chow and water. Delivery was verified at 12-hour intervals, and the date of birth was considered to be day 0 (Yi and Baram, 1994). For analysis of the distribution and structure of CRH-containing neurons in the hippocampal formation of the unstressed rat, 10–13-day-old pups were perfused. Because CRH is a neuropeptide known to be regulated and secreted with stress, pups were perfused under stress-free conditions. These conditions consisted of at least 24 hours without any disturbance, after which the pups were deeply anesthetized with pentobarbital (100 mg/kg, intraperitoneally) within 45 sec of initial handling. Pups were then removed to the laboratory for perfusion. Eighteen of these rats, used for light microscopic analysis, were perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) after a short vascular rinse with saline. The remaining six rats, used for EM preparations, were perfused with the above fixative with added 0.5% glutaraldehyde (four rats) or 0.1% glutaraldehyde and 15% picric acid (two rats).
Brains were left in situ overnight at 4°C and dissected from the skull. Brains were postfixed in the perfusion solution for 4 hours, transferred to 0.1 M phosphate buffered saline (PBS), pH 7.4, and immersed in 25% cold sucrose for cryoprotection. The cerebrum was cut coronally at 20 or 40 μm in a cryostat. Twenty-micrometer-thick sections were directly mounted on gelatin-coated slides and the 40-μm-thick sections were collected in tissue-culture wells in cold PBS. Additional sections were prepared for Nissl staining with cresyl violet. Brains for EM analysis were removed immediately after vascular perfusion, cut with a Vibratome at 30 μm, and placed in tissue-culture wells.
Immunocytochemistry for CRH
Immunostaining for CRH was performed on slide-mounted or free-floating sections by using standard avidin-biotin complex (ABC) methods. After several washes with PBS, sections were treated with 0.05% hydrogen peroxide for quenching endogenous peroxidase activity and then preincubated in PBS containing 0.4% normal goat serum and 1.5% bovine serum albumin for blocking nonspecific reactivity (PBS+). Sections were then incubated in a solution of rabbit-CRH antibody (final dilution 1:9,000) in PBS+ containing 0.03% Triton X-100 overnight at room temperature. After several rinses with PBS, sections were transferred to a solution containing 1% biotinylated goat anti-rabbit IgG with Triton X-100 for 1 hour at room temperature, followed by a 1-hour incubation in the 1% ABC solution (Vectastain, Vector Laboratories, Burlingame CA). The immunoreaction product was visualized using 0.005% hydrogen peroxide and 0.05% 3,3 diaminobenzidine (DAB) with or without NiCl (0.5%). The rabbit antiserum directed against rat/human CRH, a generous gift from Dr. W.W. Vale (Salk Institute, La Jolla, CA), does not cross-react with other neuropeptides such as somatostatin, neuropeptide Y, endorphin, enkephalin, or vasopressin (Vale et al., 1983).
EM Methods
Preimbedding immunocytochemistry was used to characterize the ultrastructure of CRH-ir neurons in the hippocampal formation of nonstressed infant rats by using modifications of previously published methods (Ribak et al., 1996). Briefly, 30-μm-thick sections were immunostained for CRH as described above, except that Triton X-100 was omitted in the incubation solution to preserve tissue ultrastructure. Sections were postfixed with osmium tetroxide, dehydrated with ethanol and acetone, and flat-embedded in Epon. Semithin (2 μm) sections for light microscopy were cut from these Epon blocks to identify CRH-ir neurons for orienting the EM sections. Thin sections from all regions of the hippocampal formation containing CRH-ir neurons were cut on a microtome, stained with uranyl acetate and lead citrate, and then examined with a Philips CM10 transmission EM.
Double-Labeling Procedures
Free-floating sections (40 μm thick) were processed for concurrent immunolabeling of CRH and other markers by using a modification of a previously described dual-chromogen procedure (Levey et al., 1986; Kordower et al., 1994). Briefly, sections were first immunostained for CRH as described above without nickel intensification, yielding a homogeneous brown reaction product within the cytoplasm. Following this reaction for localization of CRH, sections were washed with PBS on a shaker, preincubated in buffer with 5% normal horse serum (NHS) for 1 hour, after which the sections were incubated overnight, with agitation, at 4°C in a PBS solution containing 5% NHS and one of the following primary antibodies. A monoclonal mouse antibody against GAD-65 (Boehringer Mannheim, Indianapolis, IN) was diluted at 1:2,000, and a polyclonal rabbit anti-GAD-67 serum (Chemicon Inc., Temecula, CA) was diluted at 1:5,000. Monoclonal mouse antisera directed against parvalbumin and calbindin were purchased from Sigma Chemical Co. (St. Louis, MO), and both were diluted at 1:8,000. After the incubation with the primary antibodies, sections were incubated with 1% universal IgG (for GAD-67) or horse anti-mouse IgG (for the other markers) for 1 hour, followed by 1% ABC solution for another hour. After this step, the sections were processed through several changes of 0.02 M phosphate buffer, pH 6.5, to lower the pH and ionic strength. This process permitted visualization of immunoreaction products of the second group of primary antibodies by using benzidine dihydrochloride, leading to the formation of granular blue-black deposits.
Morphometric Analysis of CRH-ir Cells in the Hippocampal Formation
To obtain a semiquantitative distribution map of CRH-ir neurons, low-magnification camera lucida drawings were prepared of the immunolabeled neurons in 12 representative sections of hippocampal sections obtained from four stress-free rats. The laminar boundaries of Ammon's horn and the dentate gyrus were obtained from adjacent Nissl sections and were transferred to these maps.
For a measure of the extent of colocalization of CRH with GAD isoforms or calcium binding proteins in hippocampal neurons, cell counts were performed on four double-labeled sections from each of two animals by using a light microscope equipped with a reticule grid. CRH-ir neurons were examined for their staining features (single or double label) and their laminar location in Ammon's horn or the dentate gyrus. The fraction of CRH-ir neurons colabeled for each marker in each laminar region was determined, without attempting to obtain an absolute count of single or double-labeled neurons. The long diameter of each CRH-ir cell in sections double labeled for CRH and parvalbumin was measured by using a higher magnification.
RESULTS
Distribution and Morphology of CRH-ir Neurons
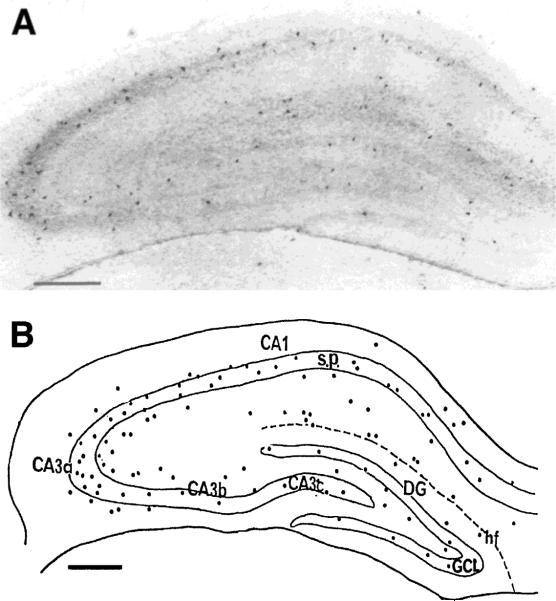
CRH-ir neurons were present in all regions and layers of the hippocampal formation (Fig. 1). In Ammon's horn, labeled cell bodies were most abundant in strata pyramidale and oriens, less frequent in stratum lacunosum-moleculare, and only occasional in stratum radiatum. The abundance of CRH-ir cells was highest in CA3, especially the CA3a subfield, where stratum pyramidale curves ventrodorsally (Fig. 1A,B). Fewer somata were located in CA1 and the CA3b and CA3c subfields of CA3 (Figs. 1, 2D,E). In the dentate gyrus itself, CRH-ir cell bodies were mainly found in the granule cell layer and the hilus (Fig. 1). Many CRH-ir cells were located at the upper and lower borders of the granule cell layer, and only a few labeled somata were detected in the molecular layer (Fig 1B).
FIGURE 1.
Distribution of corticotropin-releasing hormone-immunoreactive (CRH-ir) neurons in the developing rat hippocampus. Low magnification photomicrograph (A) and camera lucida drawing (B) of neurons from representative (20 μm) coronal sections through the hippocampal formation of a stress-free 13-day-old rat. CRH-ir cell bodies are found both in the hippocampus (CA1, CA3) and dentate gyrus (DG). In Ammon's horn, these cells are predominantly clustered in stratum pyramidale (s.p.) and are concentrated in the CA3a subfield. In the dentate gyrus, CRH-ir somata are distributed mainly within the granule cell layer (GCL) and hilus. hf, hippocampal fissure. Scale bars = 200 μm.
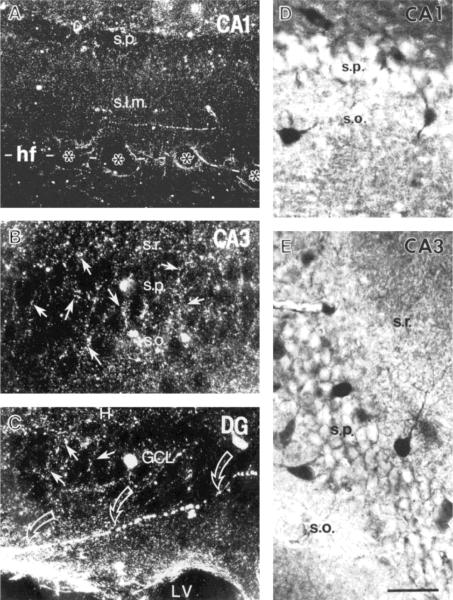
FIGURE 2.
Distribution and morphology of CRH-ir cell bodies and processes in the hippocampal formation of a 10-day-old rat. Darkfield photomicrographs (A–C) demonstrate CRH-ir terminals (arrows in B and C) mainly in the cellular layers of the hippocampus and dentate gyrus (DG). In both CA1 and CA3 (A and B, respectively), CRH-ir terminals outline unlabeled cell bodies in stratum pyramidale (s.p.). In A, axons are found mainly in stratum lacunosummoleculare (s.l.m.), and some labeled processes are associated with blood vessels (asterisks) in the hippocampal fissure (hf). C shows the distribution of CRH-ir terminals (arrows) in the granule cell layer (GCL) of the DG, which “outlines” GC somata. Beaded axons (open arrows) course toward the lateral ventricle (LV). Brightfield photomicrographs (D–E) show the detailed morphology of several CRH-ir neurons in CA1 (D) and CA3a (E). Cell bodies are medium to large and are located within the upper and lower borders of stratum pyramidale. Two or more dendrites are observed, with a branching pattern characteristic for interneurons. s.r., stratum radiatum; s.o., stratum oriens. Scale bar = 100 μm for A, 50 μm for B–E.
Although CRH-ir cell bodies did not possess the morphological features of pyramidal neurons, numerous unlabeled cells in both the pyramidal and granule cell layers were “outlined” by CRH-ir terminals (Fig. 2B,C). Similar CRH-labeled fine processes formed a loose plexus within the stratum lacunosum-moleculare of CA1 (Fig. 2A) and occasionally of CA3 and extended to the adjacent molecular layer of the dentate gyrus. In addition, CRH-ir processes were associated with blood vessels, particularly in the stratum lacunosum-moleculare of Ammon's horn and the adjacent molecular layer of the dentate gyrus (Fig. 2A). Long thin axonal fibers were also found to extend toward the surface of the lateral ventricles (Fig. 2C).
CRH-ir neurons in the hippocampal formation possessed the morphological characteristics of interneurons. Labeled cells in strata pyramidale and oriens of Ammon's horn had large or medium-sized cell bodies (>15 μm), the long axis of which was perpendicular to that of the pyramidal cell layer (Fig. 2D,E). These CRH-ir cells had more than two dendrites originating from their soma and extending to strata radiatum and oriens, respectively (Figs. 2D,E,3A). Small (>10 μm) CRH-ir cells in the same layers were essentially bipolar in shape (Fig. 2D), and in stratum lacunosum-moleculare CRH-ir neurons were small and multipolar (Fig 2A). In the dentate gyrus, CRH-ir somata in the granule cell layer and hilus were mostly medium to large multipolar or bipolar neurons and small multipolar CRH-ir neurons were occasionally observed in the molecular layer (see below).
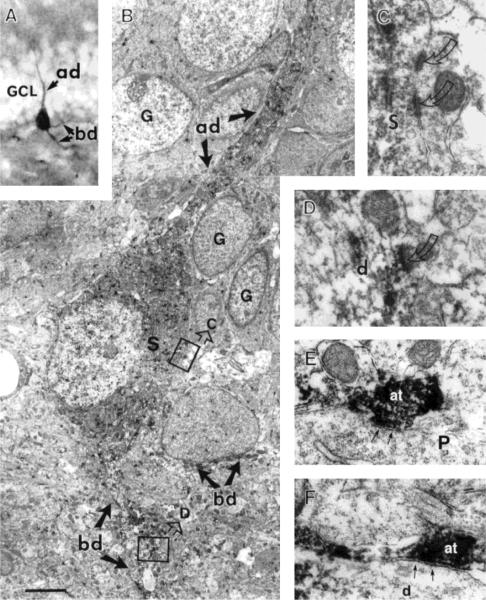
FIGURE 3.
Light and electron micrographs of CRH-ir hippocampal neurons from 13-day-old rats. A: A CRH-ir basket-like cell located in the lower border of the granule cell layer (GCL) of the dentate gyrus. This CRH-ir neuron possesses an apical dendrite (ad) oriented toward the molecular layer and two basal dendrites (bd) in the hilus. B: EM of the same cell. Both the soma (S) and dendrites (ad, bd) are devoid of spines. Several unlabeled granule cells (G) adjacent to the apical dendrite appear in different developmental stages. The two boxed regions in B are enlarged to show examples of asymmetric synapses (curved arrows) on the soma (S in C) and dendrite (d in D) of this cell. The presynaptic terminals of both synapses are not labeled for CRH. E, F: CRH-ir axon terminals (at) in stratum pyramidale of CA3, forming symmetric synapses (arrows) with an unlabeled pyramidal soma (P) and a dendrite (d), respectively. Scale bar = 50 μm for A, 2 μm for B, 0.5 μm for C,D, 0.3 μm for E,F.
Ultrastructure of CRH-ir Neurons
As described above, one of the most prevalent types of CRH-ir cells in the hippocampal formation of the immature rat was a multipolar neuron located within or adjacent to the principal cell layers, i.e., the pyramidal and granule cell layers (Figs. 2D,E, 3A). Ultrastructural analysis reported in the present study focused on these CRH-ir neurons because their laminar localization may provide a morphological base for the robust effects of CRH on hippocampal principal cells. For example, the ultrastructure of a representative CRH-ir neuron located within the granule cell layer of the dentate gyrus is shown in Figure 3. This CRH-ir neuron possessed an apical dendrite directed to and arborizing within the molecular layer (Fig. 3A, ad). Basal dendrites of the same cell can be observed, oriented toward the hilus and branching in this layer (Fig. 3A, bd). CRH-ir neurons in the granule cell layer and in CA3 and CA1 pyramidal cell layers did not possess spines on either their soma or their dendrites (Fig. 3B). These neurons were postsynaptic to asymmetric synapses on both their soma and dendrites as is shown for the dentate gyrus (Fig. 3C,D) and for CA1 (Fig. 4A). Symmetric synapses from unlabeled terminals on CRH-ir soma were encountered, as is shown for CA1 neurons in Figure 4B.
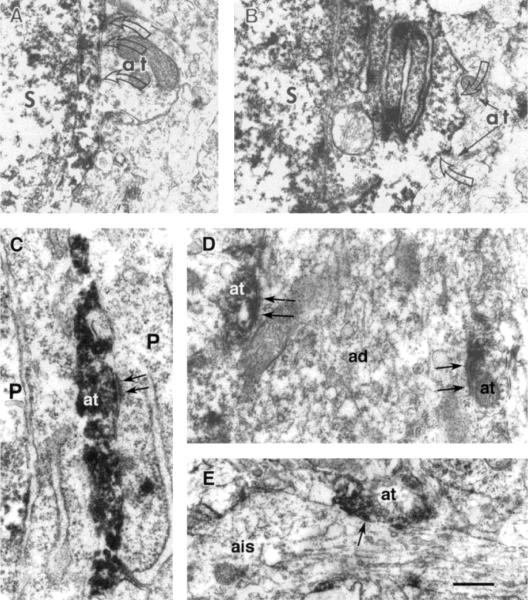
FIGURE 4.
Ultrastructural characteristics of the synaptic relationship of CRH-ir neurons in CA1 of the hippocampus of a 13-day-old rat. Portions of two CRH-ir somata (S) located within stratum pyramidale are illustrated, showing asymmetric (A) and symmetric (B) axosomatic synapses (open arrows) from unlabeled axon terminals (at). C–E show CRH-ir axon terminals forming symmetric synapses (arrows) with pyramidal cells (P). In C, a CRH-ir axon terminal synapses on the soma; in D, two CRH-ir axon terminals form synapses with an apical dendrite (ad). E shows an immunolabeled axon terminal (at) that synapses (arrow) with an axon initial segment (ais) of a pyramidal cell. Scale bar = 0.3 μm for A,D, 0.5 μm for B, 0.25 μm for C, 0.4 μm for E.
As predicted from the darkfield results, CRH-ir axon terminals were observed within the principal cell layers of the hippocampal formation. In the pyramidal cell layer of CA1 and CA3, CRH-ir axon terminals were commonly observed to form symmetric synapses on unlabeled somata (Figs. 3E, 4C) and dendrites (Figs. 3F, 4D) of pyramidal cells. Thus, among more than 30 synapses with CRH-labeled presynaptic terminals, no asymmetric synapses were found. CRH-containing axon terminals were also shown to form synapses on axon initial segments of principal hippocampal cells (Fig. 4E).
Colocalization of CRH With GAD and Calcium-Binding Proteins
Localization of CRH with GAD or one of the calcium-binding proteins within an individual neuron was easily recognized based on the simultaneous presence of the brown homogeneous DAB reaction-product for CRH and the blue granular deposits for other markers (Fig. 5). The vast majority of CRH-ir somata in the hippocampal formation were also labeled for GAD-65 (210 of 221 counted cells in Ammon's horn; 53 of 65 CRH-ir cells in the dentate gyrus). Similar results were found for the colocalization of CRH and GAD-67 (230 of 233 counted cells in Ammon's horn and 44 of 47 in the dentate gyrus). Colocalization of CRH and GAD isoforms is illustrated for strata pyramidale (Fig. 5A), oriens (Fig. 5B), and lacunosum-moleculare (Fig. 5D) of Ammon's horn, and the hilus of the dentate gyrus (Fig. 5C) and was evident in CRH-ir neurons of diverse sizes and shapes. Immunolabeling for GAD-65 was usually present in both soma and dendrites and overlapped with CRH immunoreactivity (Fig. 5B), while that for GAD-67 was most prominent in the soma of CRH-ir neurons (Fig. 5C,D). CRH-containing cells were only a subpopulation of GAD-containing hippocampal interneurons, as evident from the large number of cells labeled only for GAD-65 or GAD-67 but not for CRH (Fig. 5A).
FIGURE 5.
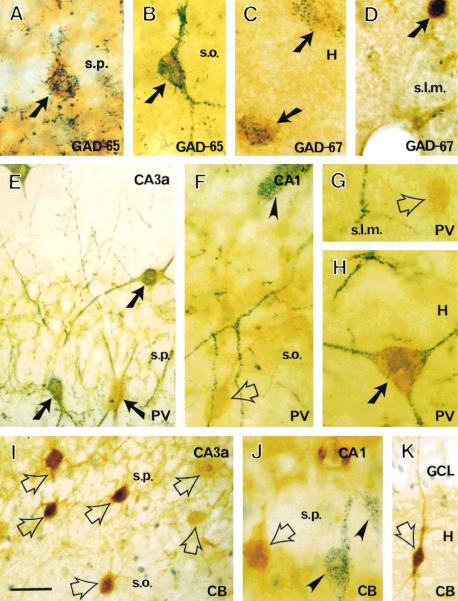
Colocalization of CRH with other interneuronal markers in the hippocampus of a 13-day-old rat. CRH-ir in this figure appears yellow; CRH-ir for GAD-65 (A,B), GAD-67 (C–D), parvalbumin (PV; E–H), and calbindin (CB; I–K) appears as blue, granular deposits. CRH-ir neurons (arrows) in stratum pyramidale (s.p.), oriens (s.o.), and lacunosum-moleculare (s.l.m.) of Ammon's horn and in the dentate gyrus are universally stained for GAD-65 (A,B) or GAD-67 (C,D). Large CRH-ir neurons in all hippocampal regions are immunolabeled for PV (E,H). In contrast, small CRH-ir cells (open arrows) are not PV-ir (F,G). CRH and calbindin do not coexist in hippocampal neurons from Ammon's horn (I,J) or the dentate gyrus (K). Double-labeled neurons are indicated with solid arrows. Single-labeled CRH-ir cells are indicated with open arrows. PV- or CB-labeled neurons that do not contain CRH are indicated with arrowheads. Scale bar = 50 μm for E,I,K, 20 μm for the other panels.
Colocalization analysis of CRH and parvalbumin demonstrated a different pattern from that of CRH and the GABA synthesizing enzyme (Fig. 5E–H). Most CRH-ir neurons in the hippocampus and dentate gyrus, particularly those with mediumsized and large cell bodies (long somal diameter > 15 μm), were also parvalbumin immunoreactive (Fig. 5E). Thus, 289 of 321 counted neurons in strata pyramidale, oriens, and radiatum were double labeled for CRH and parvalbumin. In the stratum lacunosum-moleculare, where the long somal diameter was typically less than 10 μm, none of the 45 CRH-ir counted neurons contained parvalbumin. Parvalbumin immunoreaction products occurred both in the soma and in neuronal processes (Fig. 5E,H). These data indicate that 79% of the CRH-ir neurons in Ammon's horn contain parvalbumin. In the same preparations, we found that 79.6% of the parvalbumin-ir neurons in Ammon's horn were immunostained for CRH. In the granule cell layer and hilus of the dentate gyrus, most CRH-ir neurons were large and also contained parvalbumin (75 of 88 counted cells). The number of parvalbumin-ir neurons that contained CRH was similar (75 of 87 cells). CRH–parvalbumin double-labeled neurons were not found in the molecular layer, which contained rare, small CRH-ir neurons (Fig. 5F,G).
In sections subjected to double labeling for CRH and calbindin, calbindin-ir cells with the morphological features of interneurons were demonstrated throughout the hippocampal formation (Fig. 5I,J), and calbindin was also observed in granule cells (Fig. 5K). However, of 307 CRH-ir cells counted in Ammon's horn in these sections, none contained calbindin. This was also true for the 69 CRH-ir cells sampled in the dentate gyrus.
DISCUSSION
The major findings of this study are (a) CRH-containing neurons are abundant in the hippocampal formation of the unstressed developing rat, (b) CRH-ir cells are GABAergic interneurons, and (c) the location and the morphological, neurochemical, and ultrastructural characteristics of these neurons suggest that they form a heterogenous population, with diverse interactions with the hippocampal principal cells.
CRHergic Cells Possess the Morphological Features of Interneurons
In this study, CRH-containing neurons were immunolabeled not only in their cell bodies but throughout a significant portion of their dendritic arbors. Primary, secondary, and tertiary dendrites were often labeled for several hundred microns, permitting the identification of morphological characteristics that distinguish the CRH-containing cells as nonprincipal hippocampal neurons. Thus, CRH-containing neurons located within stratum pyramidale lacked the distinctive apical dendrite and two basal dendrites typical of pyramidal cells. Furthermore, dendrites of CRH-containing neurons did not possess spines. This observation is significant because most hippocampal interneurons have aspiny dendrites, whereas the dendrites of pyramidal and granule cells are very spiny (Freund and Buzsáki, 1996).
CRH-ir Cells Possess the Neurochemical Markers of GABAergic Interneurons
Double-label immunocytochemistry showed that the overwhelming majority of CRH-ir neurons contained one of the GABA synthesizing enzyme isoforms, GAD-65 and GAD-67. This fact and the morphological features discussed above suggest that the CRH-containing neurons of the hippocampal formation represent a subpopulation of GABAergic interneurons. Colocalization of CRH and GAD in individual cells has been reported in neuronal populations elsewhere in the brain, such as the dorsome-dial portion of the parvocellular paraventricular nucleus of the hypothalamus (Meister, 1993). In the hippocampal formation, the additional double-label studies indicated that the population of CRH-ir GABAergic interneurons is not homogeneous. Thus, large and medium-sized CRH-ir neurons, located primarily adjacent to or within the pyramidal and granule cell layers typically contained parvalbumin. This colocalization of CRH and parvalbumin was rare among small CRH-ir neurons in both Ammon's horn and the dentate gyrus. It needs to be emphasized that the percentage of parvalbumin neurons with CRH-ir was similar to that for the CRH-containing neurons with parvalbuminir. Therefore, the hippocampal CRH and parvalbumin neurons represent two closely overlapping subpopulations of GABAergic neurons. In contrast, practically no CRH-ir neurons were immunolabeled with an antiserum directed against calbindin, indicating that they are two separate subpopulations.
What Type of Interneurons do CRH-ir Cells Represent?
Several interneuron populations have been defined based on cell morphology and location, dendritic arbor orientation and connectivity, and postsynaptic targets (Gulyas et al., 1993; Halasy and Somogyi, 1993; Freund and Buzsáki, 1996). As is evident from Figures 2 and 5 and from the discussion above, CRH-containing interneurons do not represent a uniform population vis-á-vis the cardinal defining characteristics of interneuronal types. Thus, CRH-ir cells are found both within and adjacent to the pyramidal and granule cell layers but also in strata lacunosummoleculare and oriens in Ammon's horn and in the molecular layer and hilus of the dentate gyrus. Furthermore, the dendritic arbors of these neurons are variable in orientation throughout the hippocampal formation. Finally, the size, shape, and neurochemical profile of these neurons are not homogeneous. These facts notwithstanding, subpopulations of the CRH-containing neurons of the hippocampal formation can be further defined.
For example, GABAergic basket cells form synapses with the somata and proximal dendrites of principal neurons (pyramidal and granule cells), and GABAergic chandelier cells form synapses with axon initial segments of principal neurons (Ribak and Seress, 1983; Katsumaru et al., 1988; Soriano et al., 1990; Gulyas et al., 1993; Halasy and Somogyi, 1993). Both types of interneurons are characteristically labeled for parvalbumin (Nitsch et al., 1990; Ribak et al., 1990). In the current study, darkfield illumination showed CRH-containing axon terminals in the principal cell layers, and EM demonstrated many CRH-ir axon terminals forming symmetric synapses with dendrites and somata of principal neurons. Thus, a subpopulation of large (parvalbumin-immunolabeled) CRH-ir neurons with axon terminals synapsing on somata and dendrites of principal cells is identified as basket cells. The axon terminals of an additional subset of CRH-ir neurons were observed to form synapses on axon initial segments of unlabeled pyramidal cells in stratum oriens, thereby supporting their identification as chandelier cells. This finding is consistent with that of a previous report of CRH-ir chandelier cells in the monkey neocortex, particularly in the posterior cingulate and association cortices (Lewis et al., 1989). Rounded, vertically oriented CRH-ir somata in layer IV possessed thick clusters of densely labeled terminal boutons that encased axon initial segments of unlabeled cortical pyramidal neurons. Although Lewis et al. (1989) did not include EM analysis, they considered the size, morphology, and relationship to pyramidal neurons sufficient evidence for the identification of the cortical CRH-ir interneurons as chandelier cells.
CRH-ir Neurons Are Abundant in the Hippocampal Formation of the Nonstressed, Immature Rat
This study demonstrated numerous CRH-ir interneurons in the hippocampal formation, including well-described cell types such as basket and chandelier cells. Several causes may account for the apparent discrepancy between the current study and previous reports regarding the prevalence and distribution of CRH-ir neurons. First, the CRH antiserum used in this study has been shown to be exceptionally potent and specific for CRH (Vale et al., 1983), enhancing signal–noise ratio and increasing sensitivity. In addition, the hippocampi analyzed in the current study were derived from rats that were anesthetized for perfusion within 45 sec of their initial disturbance (see Materials and Methods). CRH is a stress neurohormone, and secretion of CRH from CRH-ir neurons in the hypothalamus may occur within minutes after the onset of a stressful stimulus in both adult (Rivier et al., 1983) and immature (Yi and Baram, 1994) rats. A similar rapid, stress-induced transport of CRH from somata to axons may occur within the hippocampal formation, diminishing the intensity of CRH-ir signal over cell bodies (pilot unpublished observations). For blocking axonal transport, Merchenthaler (1984) administered colchicine to some of the animals he studied and reported no increase in the intensity or numbers of CRH-ir neurons in the treated animals. However, this author did not mention whether any precautions were taken to eliminate the stress associated with handling the animals. Rapid release of CRH after handling injection stress has been demonstrated (Yi and Baram, 1994). Thus, using stress-free rats may account for the relatively high abundance and strong signal of CRH-ir cells in this study compared with previous reports because CRH can be expected to be released with even the mild stress of handling and preparation of the animals for perfusion.
In addition, the current study focused on developing rats during the second postnatal week. The rationale for this choice was based on the established robust effects of CRH on hippocampal pyramidal cells at this age (Smith and Dudek, 1994; Baram and Ribak, 1995; Hollrigel et al., 1998) and the demonstrated high abundance of CRH receptors in the CA3 pyramidal cell layer during this developmental period (Avishai-Eliner et al., 1996). We hypothesized that such well-established electrophysiological and biochemical postsynaptic effects of CRH required a significant array of presynaptic CRH-ir neurons. Since a systematic developmental analysis of hippocampal CRH-ir neurons was not a focus of the current study, a direct comparison of these results with data from adult rats, to determine whether a larger population of CRHergic neurons exists in the immature hippocampus, is not possible at this time.
Apparent Structural Immaturity of the CRH-ir Neurons
Certain features of the CRH-ir neurons described in this study, observed by both light microscopy and EM, are consistent with the immaturity of the CRH-ir neurons in the hippocampal formation. First, the axonal plexus of neurons labeled for both CRH and parvalbumin around pyramidal and granule cell bodies was not as extensive as that demonstrated for parvalbumin-containing axon terminals in the adult hippocampus (Kosaka et al., 1987; Sloviter, 1989; Nitsch et al., 1990; Ribak et al., 1990). Second, many of the CRH-ir axon terminals, observed with EM, were small, explaining their poor visualization in light microscopic preparations. Furthermore, as seen in Figure 3, some CRH-ir cells with otherwise characteristic features of basket cells lacked nuclear infoldings and intranuclear rods, which are typical of mature basket cells (Seress and Ribak, 1990).
In contrast to the apparent immaturity of CRH-ir cells in the developing hippocampus, several lines of evidence have demonstrated a robust functional response of hippocampal neurons of the infant rat to synthetic CRH (Baram et al., 1992). CRH receptors, particularly CRF1, have been localized to the CA1 and CA3 pyramidal layers as early as the first postnatal week (Insel et al., 1988). Moreover, in these regions, CRF1-mRNA abundance is maximal at the end of the first postnatal week (Avishai-Eliner et al., 1996). The presence of functional second messenger cascade downstream from activation of the CRH receptor has also been documented in the infant rat (Insel et al., 1988; Pihoker et al., 1992). More directly, in vivo studies have shown that activation of CRH receptors, specifically CRF1, leads to overt and prolonged seizures during the first two postnatal weeks in the rat. In the in vitro hippocampal slice preparation, application of CRH increases the excitability of hippocampal CA1 and CA3 neurons and also diminishes inhibition (Smith and Dudek, 1994; Hollrigel et al., 1998).
The current study is consistent with these established robust physiological effects of CRH in the rat hippocampus during the second postnatal week. Mature synapses formed by CRH-ir axon terminals on pyramidal and granule cells indicate that CRH-mediated neurotransmission occurs and may contribute to the enhanced excitability in hippocampal circuitry at this age (Swann et al., 1993). Furthermore, the location of the synapses between CRH-ir neurons and principal hippocampal cells suggests that a subpopulation of CRH-containing neurons is strategically positioned to influence directly the excitability of hippocampal principal cells. This novel population of CRH-ir neurons may thus provide the neuroanatomical basis for the dramatic effects of CRH on neuronal excitability in the developing hippocampus.
Acknowledgments
We thank Dr. Ivan Soltesz for his critical reading of the manuscript. The technical assistance of A. Gerth and M. Shiba-Noz is appreciated. This study was supported in part by NIH grants NS 28912 (T.Z.B.) and NS 15669 (C.E.R.).
Grant sponsor: NIH; Grant numbers: NS 28912 and NS 15669.
REFERENCES
- Avishai-Eliner S, Yi SJ, Baram TZ. Developmental profile of messenger RNA for the corticotropin-releasing hormone receptor in the rat limbic system. Dev Brain Res. 1996;91:159–163. doi: 10.1016/0165-3806(95)00158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Hatalski CG. Mechanisms of non-genetic, provoked seizures in neonatal and infant brain. In: Moshe SL, Wasterlain C, Nehlig A, editors. Developmental epilepsies. 1998. In press. [Google Scholar]
- Baram TZ, Ribak CE. Peptide-induced infant status epilepticus causes neuronal death and synaptic reorganization. NeuroReport. 1995;6:277–280. doi: 10.1097/00001756-199501000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Hirsch E, Snead OC, Schultz L. Corticotropin-releasing hormone-induced seizures in infant rats originate in the amygdala. Ann Neurol. 1992;31:488–494. doi: 10.1002/ana.410310505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Chalmers DT, Chen C, Koutsoukos EB, De Souza EB. The CRF1 receptor mediates the excitatory actions of corticotropin releasing factor (CRF) in the developing rat brain: in vivo evidence using a novel, selective, non-peptide CRF receptor antagonist. Brain Res. 1997;770:89–95. doi: 10.1016/s0006-8993(97)00759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan DP, Heinrichs SC, Troncoso JC, Liu XJ, Kawas CH, Ling N, De Souza EB. Displacement of corticotropin releasing factor from its binding protein as a possible treatment for Alzheimer's disease. Nature. 1995;378:284–287. doi: 10.1038/378284a0. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AL, Pavcovich LA, Grigoriadis DE, Valentino RJ. Previous stress alters corticotropin-releasing factor neurotransmission in the locus coeruleus. Neuroscience. 1995;65:541–550. doi: 10.1016/0306-4522(94)00496-r. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Henriksen SJ, Wang M, Rivier J, Vale W, Bloom FE. Corticotropin releasing factor produces increases in brain excitability and convulsive seizures in rats. Brain Res. 1983;278:332–336. doi: 10.1016/0006-8993(83)90266-4. [DOI] [PubMed] [Google Scholar]
- Fox EA, Gruol DL. Corticotropin-releasing factor suppresses the afterhyperpolarization in cerebellar Purkinje neurons. Neurosci Lett. 1993;149:103–107. doi: 10.1016/0304-3940(93)90358-r. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Miles R, Hajos N, Freund TF. Precision and variability in postsynaptic target selection of inhibitory cells in the hippocampal CA3 region. Eur J Neurosci. 1993;5:1729–1751. doi: 10.1111/j.1460-9568.1993.tb00240.x. [DOI] [PubMed] [Google Scholar]
- Halasy K, Somogyi P. Subdivisions in the multiple GABAergic innervation of granule cells in the dentate gyrus of the rat hippocampus. Eur J Neurosci. 1993;5:411–429. doi: 10.1111/j.1460-9568.1993.tb00508.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Hollrigel G, Baram TZ, Soltesz I. Neuroscience. 1998. Corticotropin releasing hormone increases excitatory synaptic transmission in the hippocampus of infant rats. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Battaglia G, Fairbanks DW, De Souza EB. The ontogeny of brain receptors for corticotropin-releasing factor and the development of their functional association with adenylate cyclase. J Neurosci. 1988;8:4151–4158. doi: 10.1523/JNEUROSCI.08-11-04151.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumaru H, Kosaka T, Heizmann CW, Hama K. Immunocytochemical study of GABAergic neurons containing the calcium-binding protein parvalbumin in the rat hippocampus. Exp Brain Res. 1988;72:347–352. doi: 10.1007/BF00250256. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chen EY, Sladek JR, Mufson EJ. TRK-immunoreactivity in the monkey central nervous system: forebrain. J Comp Neurol. 1994;349:20–35. doi: 10.1002/cne.903490103. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Katsumaru H, Hama K, Wu JY, Heizmann CW. GABAergic neurons containing the Ca+2-binding protein parvalbumin in the rat hippocampus and dentate gyrus. Brain Res. 1987;419:119–130. doi: 10.1016/0006-8993(87)90575-0. [DOI] [PubMed] [Google Scholar]
- Lee EH, Hung HC, Lu KT, Chen WH, Chen HY. Protein synthesis in the hippocampus associated with memory facilitation by corticotropin-releasing factor in rats. Peptides. 1992;13:927–937. doi: 10.1016/0196-9781(92)90051-4. [DOI] [PubMed] [Google Scholar]
- Levey AI, Bolam JP, Rye DB, Hallanger AE, Meduth RM, Mesulam MM, Wainer BH. A light and electron microscopic procedure for sequential double antigen localization using diaminobenzidine and benzidine dihydrochloride. J Histochem Cytochem. 1986;34:1449–1457. doi: 10.1177/34.11.2430010. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Foote SL, Cha CI. Corticotropin-releasing factor immunoreactivity in monkey neocortex: an immunohistochemical study. J Comp Neurol. 1989;290:599–613. doi: 10.1002/cne.902900412. [DOI] [PubMed] [Google Scholar]
- Lightman SL, Harbuz MS. Expression of corticotropin-releasing factor mRNA in response to stress. Ciba Found Symp. 1993;172:173–187. doi: 10.1002/9780470514368.ch9. [DOI] [PubMed] [Google Scholar]
- Lyons MK, Anderson RE, Meyer FB. Corticotropin releasing factor antagonist reduces ischemic hippocampal neuronal injury. Brain Res. 1991;545:339–342. doi: 10.1016/0006-8993(91)91310-w. [DOI] [PubMed] [Google Scholar]
- Maecker H, Desai A, Dash R, Rivier J, Vale W, Sapolsky R. Astressin, a novel and potent CRF antagonist, is neuroprotective in the hippocampus when administered after a seizure. Brain Res. 1997;744:166–170. doi: 10.1016/s0006-8993(96)01207-3. [DOI] [PubMed] [Google Scholar]
- Meister B. Gene expression of chemical diversity in hypothalamic neurosecretory neurons. Mol Neurobiol. 1993;7:87–110. doi: 10.1007/BF02935638. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I. Corticotropin releasing factor-like immunoreactivity in the rat central nervous system. Extrahypothalamic distribution. Peptides. 1984;5:53–69. doi: 10.1016/0196-9781(84)90265-1. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Stankovics J, Gallyas F. A highly sensitive one-step method for silver intensification of the nickel diaminobenzidine end product of peroxidase reaction. J Histochem Cytochem. 1989;37:1563–1565. doi: 10.1177/37.10.2674275. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. New vistas in neuropeptide research in neuropsychiatry: focus on corticotropin-releasing factor. Neuropsychopharmacology. 1992;6:69–75. [PubMed] [Google Scholar]
- Nitsch R, Soriano E, Frotscher M. The parvalbumin-containing nonpyramidal neurons in the rat hippocampus. Anat Embryol. 1990;181:413–425. doi: 10.1007/BF02433788. [DOI] [PubMed] [Google Scholar]
- Olschowka JA, O'Donohue TJ, Mueller GP, Jacobowitz DM. Hypothalamic and extra-hypothalamic distribution of CRF-like immunoreactive neurons in the rat brain. Neuroendocrinology. 1982;35:305–308. doi: 10.1159/000123398. [DOI] [PubMed] [Google Scholar]
- Piekut D, Phipps B, Pretel S, Applegate C. Effects of generalized convulsive seizures on corticotropin-releasing factor neuronal systems. Brain Res. 1996;743:63–69. doi: 10.1016/s0006-8993(96)00970-5. [DOI] [PubMed] [Google Scholar]
- Pihoker C, Cain ST, Nemeroff CB. Postnatal development of regional binding of CRF and adenylate cyclase activity in the rat brain. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16:581–586. doi: 10.1016/0278-5846(92)90063-k. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Fernhout BJ, Shinnick-Gallagher P. Differential actions of corticotropin releasing factor on basolateral and central amygdaloid neurones, in vitro. J Phamacol Exp Ther. 1992;263:846–858. [PubMed] [Google Scholar]
- Ribak CE, Baram TZ. Selective death of hippocampal CA3 pyramidal cells with mossy fiber afferents after CRH-induced status epilepticus in infant rats. Dev Brain Res. 1996;91:245–251. doi: 10.1016/0165-3806(95)00183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribak CE, Seress L. Five types of basket cell in the hippocampal dentate gyrus: a combined Golgi and electron microscopic study. J Neurocytol. 1983;12:577–597. doi: 10.1007/BF01181525. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Nitsch R, Seress L. Proportion of parvalbumin-positive basket cells in the GABAergic innervation of pyramidal and granule cells of the rat hippocampal formation. J Comp Neurol. 1990;300:449–461. doi: 10.1002/cne.903000402. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Tong WMY, Brecha NC. GABA plasma membrane transporters, GAT-1 and GAT-3, display different distributions in the rat hippocampus. J Comp Neurol. 1996;367:595–606. doi: 10.1002/(SICI)1096-9861(19960415)367:4<595::AID-CNE9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Rivier J, Spiess J, Vale W. Characterization of rat hypothalamic corticotropin-releasing factor. Proc Natl Acad Sci USA. 1983;80:4851–4855. doi: 10.1073/pnas.80.15.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Corticotropin releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt-glucose oxidase-diaminobenzidine. J Comp Neurol. 1987;260:256–298. doi: 10.1002/cne.902600209. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Imaki T, Potter E, Kovacs K, Imaki J, Vale W. The functional neuroanatomy of corticotropin-releasing factor. Ciba Found Symp. 1993;172:5–21. doi: 10.1002/9780470514368.ch2. [DOI] [PubMed] [Google Scholar]
- Seress L, Ribak CE. Postnatal development of the light and electron microscopic features of basket cells in the hippocampal dentate gyrus of the rat. Anat Embryol. 1990;181:547–565. doi: 10.1007/BF00174627. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Calcium-binding protein (calbindin-D28k) and parvalbumin immunocytochemistry: localization in the hippocampus with specific reference to the selective vulnerability of hippocampal neurons to seizure activity. J Comp Neurol. 1989;280:183–196. doi: 10.1002/cne.902800203. [DOI] [PubMed] [Google Scholar]
- Smith BN, Dudek FE. Age-related epileptogenic effects of corticotropin-releasing hormone in the isolated CA1 region of rat hippocampal slices. J Neurophysiol. 1994;72:2328–2333. doi: 10.1152/jn.1994.72.5.2328. [DOI] [PubMed] [Google Scholar]
- Soriano E, Nitsch R, Frotscher M. Axo-axonic chandelier cells in the rat fascia dentata: Golgi electron microscopy and immunocytochemical studies. J Comp Neurol. 1990;293:1–25. doi: 10.1002/cne.902930102. [DOI] [PubMed] [Google Scholar]
- Swann JW, Smith KL, Brady RJ. Localized excitatory synaptic interactions mediate the sustained depolarization of electrographic seizures in developing hippocampus. J Neurosci. 1993;13:4680–4689. doi: 10.1523/JNEUROSCI.13-11-04680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Vale W, Vaughan J, Yamamoto G, Bruhn T, Douglas C, Dalton D, Rivier C, Rivier J. Assay of corticotropin releasing factor. Methods Enzymol. 1983;103:565–577. doi: 10.1016/s0076-6879(83)03040-2. [DOI] [PubMed] [Google Scholar]
- Weiss GK, Castillo N, Fernandez M. Amygdala kindling rate is altered in rats with a deficit in the responsiveness of the hypothalamo-pituitary-adrenal axis. Neurosci Lett. 1993;157:91–94. doi: 10.1016/0304-3940(93)90650-a. [DOI] [PubMed] [Google Scholar]
- Yi SJ, Baram TZ. Corticotropin-releasing hormone mediates the response to cold stress in the neonatal rat without compensatory enhancement of the peptide's gene expression. Endocrinology. 1994;135:2364–2368. doi: 10.1210/endo.135.6.7988418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WS. Regulation of gene expression in the hypothalamus: hybridization histochemical studies. Ciba Found Symp. 1992;168:127–138. [PubMed] [Google Scholar]