Abstract
Corticotropin releasing factor (CRF) is the key coordinator of the neuroendocrine and behavioral responses to stress. In the central nervous system, CRF excites select neuronal populations, and infusion of CRF into the cerebral ventricles of infant rats produces severe age-dependent limbic seizures. These seizures, like other CRF effects, result from activation of specific receptors. Both of the characterized members of the CRF receptor family (CRF1 and CRF2), are found in the amygdala, site of origin of CRF-induced seizures, and may therefore mediate these seizures. To determine which receptor is responsible for the excitatory effects of CRF on limbic neurons, a selective, non-peptide CRF1 antagonist was tested for its ability to abolish the seizures, in comparison to non-selective inhibitory analogues of CRF. Pretreatment with the selective CRF1 blocker (NBI 27914) increased the latency and decreased the duration of CRF-induced seizures in a dose-dependent manner. The higher doses of NBI 27914 blocked the behavioral seizures and prevented epileptic discharges in concurrent electroencephalograms recorded from the amygdala. The selective CRF1 blocker was poorly effective when given systemically, consistent with limited blood-brain barrier penetration. Urocortin, a novel peptide activating both types of CRF receptors in vitro, but with preferential affinity for CRF2 receptors in vivo, produced seizures with a lower potency than CRF. These limbic seizures, indistinguishable from those induced by CRF, were abolished by pretreatment with NBI 27914, consistent with their dependence on CRF1 activation. In summary, CRF induces limbic seizures in the immature rat, which are abolished by selective blocking of the CRF1 receptor. CRF1-messenger RNA levels are maximal in sites of seizure origin and propagation during the age when CRF is most potent as a convulsant. Taken together, these facts strongly support the role of the developmentally regulated CRF1 receptor in mediating the convulsant effects of CRF in the developing brain.
Keywords: Corticotropin releasing factor, Corticotropin releasing factor receptor, Seizure model, Selective antagonist, Electroencephalogram, Limbic epilepsy
1. Introduction
Corticotropin releasing factor (CRF) is a 41 amino acid neuropeptide which activates target pituitary cells and neurons via specific membrane receptors [16,38]. CRF release during the hormonal response to stress induces the secretion of corticotropin (ACTH) from the pituitary, an action blocked by analogues which compete for the receptors [33,39] In the central nervous system (CNS), CRF mediates ‘central’ components of the stress response, activating neurons in the amygdala as well as locus ceruleus, hippocampus and cerebellum [1,20,21,40]. In the immature rat, synthetic CRF excites hippocampal neurons [35] and produces prolonged limbic seizures [3,7]. Electroencephalographic (EEG) mapping has been used to localize the origin of these seizures to the amygdala [4], and propagation of the seizures to the hippocampus has been demonstrated. The postsynaptic mechanisms of the limbic seizures induced by CRF, and the reason for the increased convulsant potency of the peptide during development have not been established.
CRF activates postsynaptic receptors on target neurons [16,22]. Two members of the CRF receptor family are currently known, and consist of membrane-spanning G-protein coupled molecules [14,27,30]. The first, CRF1, is found in the CNS and the pituitary [32,44], and is the candidate mediator for many of the endocrine effects of CRF. The second receptor, CRF2, is found in at least two splice forms in the rat (CRF2α and CRF2β ; [27,28]). One of these subtypes, CRF2α, is found primarily in the CNS. Although the messenger ribonucleic acid (mRNA) distribution patterns for CRF1 and CRF2 in the adult rat brain are quite distinct, both genes are expressed in limbic neurons of the amygdala and the hippocampus [13]. Therefore, both types of CRF receptor are candidates for mediating the convulsant effects of CRF. In the developing rat brain, CRF receptors have been demonstrated in the amygdala and hippocampus via binding assays [24,31]. Recently, CRF1-mRNA has been localized to the amygdala and hippocampus of the developing rat [2].
The goal of this study was to determine the receptor type that is responsible for mediating the excitatory effects of CRF on limbic neurons. The study compared the effects of a novel, selective CRF1 blocker and of established non-selective CRF1/CRF2 receptor antagonists on the behavioral and electrographic seizures induced by CRF. In addition, the convulsive effects of a second endogenous ligand for both types of CRF receptor, urocortin, were eliminated by selective blocking of CRF1.
2. Materials and methods
2.1. Animals
Infant rats (n = 185) were offspring of time-pregnant, Sprague–Dawley rats. They were born in the University of California, Irvine (UCI) federally approved animal facility, kept on a 12 h lightrdark cycle (lights on at 07.00). and given access to unlimited food and water. The time of birth of pups was determined every 12 h, and the day of birth was considered day 0. Litters were culled to 12 pups and mixed among experimental groups. Thus, for each experiment, controls were littermates of, or precisely age-matched, to the experimental groups. Cages were maintained in a quiet, uncrowded room. Pups were implanted with stainless steel cannulae directed to the right cerebral ventricle 24 h prior to experiments. Cannulation was carried out under halothane anesthesia, using a stereotaxic apparatus, as described in detail elsewhere [3,4,7,8,10] and cannula position was verified in all cases [4]. Peptide infusion was carried out on postnatal days 9–13, but each experiment consisted of a comparison of experimental and control pups on the same day of life. Each pup was subjected to CRF or urocortin administration into the cerebral ventricle (i.c.v.), with or without a receptor antagonist, once only [4].
2.2. Materials
CRF, α-helical CRF9–41 and [D-Phe12,Nle21,38,C-MeLeu37]CRF12 – 41 (i.e., D-Phe-CRF12 – 41), were purchased from Bachem (Torrance, CA). NBI 27914 was synthesized at Neurocrine Biosciences as described elsewhere [15]. Urocortin was a generous gift from Dr. Nicholas Ling (Neurocrine Biosciences).
Peptides were dissolved in distilled water containing a dye marker (Fast Green, Sigma, St. Louis, MO). Final concentrations were 0.15 nmolrml for CRF and 0.5 nmol/μl for the peptide antagonists. Urocortin was diluted to 2 μg/μl or 1 μg/μl. NBI 27914 was dissolved in dimethyl sulfoxide (DMSO) at a final concentration of 10 μg/μl for i.c.v. administration, and 20 μg/μl for systemic administration. Control vehicles consisted of the dye-water solution for CRF, α-helical CRF and urocortin, and dye-DMSO for NBI 27914.
2.3. Experimental design
2.3.1. Experiment 1. Does i.c.v. administration of a specific CRF1 blocker eliminate CRF-induced seizures? How does NBI 27914 compare with a peptide CRF1/CRF2 receptor antagonist?
Experimental groups (n = 5–12) received NBI 27914 or a non-selective CRF1/CRF2 antagonist 15–20 min prior to CRF infusion, while controls received vehicle. Drugs were administered i.c.v. via the indwelling cannula using a micro-infusion pump, while the pups were freely moving on a warming pad (34°C) [3,4,7,8]. The CRF dose (0.15 nmol in 1 μl) was chosen to result in significant (2–3 h long) seizures with a short latency. Subsequent to the infusion of CRF, seizure latency and duration were monitored for a minimum of 180 min. Animals were scored at 5 min intervals for the behavioral limbic seizures induced by CRF using the previously established methodology [3,4,8].
2.3.2. Experiment 2. Does NBI 27914 block CRF-induced seizures when given systemically?
2.3.2.1. Intraperitoneal (i.p.) administration
The experimental group (n = 12) received the selective CRF1 receptor blocker i.p., using a 1 ml tuberculin syringe. Based on pilot experiments and on previous studies of CRF antagonists [10], the injections were carried out either 15–20 min or 30–40 min prior to CRF infusion. The dose tested was 10 mg/kg. Control rats received an equal volume of DMSO.
2.3.2.2. Oral administration
Experimental animals received NBI 27914 (20 mg/kg) by gavage, using a 1 ml tuberculin syringe attached to a pre-calibrated polyethylene tube. In pilot experiments, the administration of a DMSO-dye solution via the tube resulted in obvious staining of stomach contents. Because of the persistence of dye in the gastric cavity for at least 2 h, the compound was administered 0.5, 1 or 2 h prior to the CRF infusion.
2.3.3. Experiment 3. Does a CRF1 receptor blocker elimi-nate the epileptic EEG correlates of seizures induced by CRF?
For EEG recordings, a separate group of rats (n = 6) was implanted with bipolar electrodes directed to the amygdala and dorsal hippocampus, using previously established methods [4,5]. Briefly, pups were subjected to halothane anesthesia and placed in an infant rat stereotactic apparatus (Kopf Instruments, Tujunga, CA). Electrodes were implanted using age-appropriate coordinates [4], and were aimed at the basal amygdala and/or dorsal hippocampus. Each animal carried amygdala – or amygdala and hippocampus – electrode arrays in addition to the i.c.v. cannulae. The placement of the electrode tips was verified for each animal at the end of the experiment as described elsewhere [4]. EEGs were recorded using a GRASS 78E polygraph, connected via long, flexible wires to freely moving animals on a warming pad, as described elsewhere [3–5]. All infusions were given i.c.v. and carried out at 08.00–10.00 h, to avoid the effects of circadian variability in endogenous CRF [43].
2.3.4. Experiment 4. Can limbic seizures be generated by activation of CRF receptors by the novel agonist, urocortin? Do the seizures persist upon blocking CRF1 by NBI 27914?
Four experimental groups (on postnatal days 10 and 11) were evaluated for the presence, latency, duration and phenomenology of limbic seizures. Twelve pups received 0.15 nmol of urocortin i.c.v., and the other twelve were infused i.c.v. with 0.75 nmol of urocortin. Half of the animals in each group were pretreated with NBI 27914 (1 mg/kg. 20 min prior to urocortin administration.
Statistical analysis was performed using non-parametric tests (Mann–Whitney unpaired two-tailed comparison, INSTAT software) without assumptions regarding the distribution of values.
3. Results
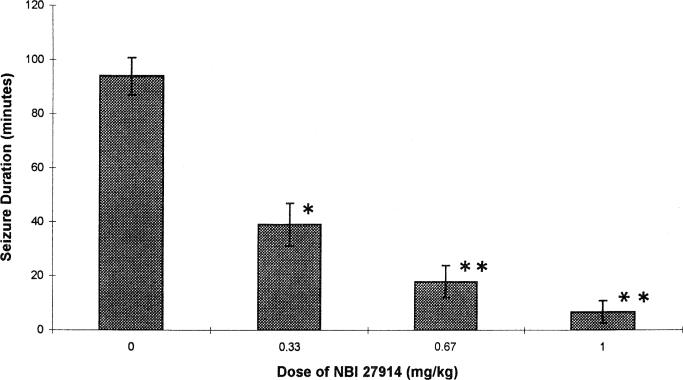
NBI 27914 given i.c.v. increased the latency to the onset of CRF-induced seizures and decreased their duration in a dose-dependent manner (Table 1, Fig. 1). As is evident from the table, increasing doses of NBI 27914 (0.33, 0.67 and 1.0 mg/kg) abolished the seizures entirely in 40, 62.5 and 67% of rats, respectively. In comparison, the i.c.v. pre-administration of the non-selective CRF receptor blocker α-helical CRF9 – 41 eliminated CRF-induced seizures in 33% of the animals. In rats implanted with depth electrodes, pre-administration of NBI 27914 prevented the development of epileptic discharges in EEG recordings from the amygdala (Fig. 2).
Table 1.
Effect of NBI 27914 and non-selective CRF receptor antagonists on the latency of CRF-induced seizures
| Receptor blocker | Dose (mg/kg) | Group size (n) | Latency of seizures (min) |
|---|---|---|---|
| None | 23 | 9.96 ± 1.10 | |
| NBI 27914 | 0.33 | 7 | 2 = no seizures |
| 5 = 29.40 ± 3.53 * | |||
| 0.67 | 8 | 5 = no seizures | |
| 3 = 30.67 ± 3.33 * | |||
| 1.0 | 3 | 2 = no seizures | |
| 1 = 59 min latency | |||
| α-Helical CRF9–41 | 0.5 | 12 | 4 = no seizures |
| 8= 21.4 ± 4.4 * | |||
| d-Phe-CRF12–41 | 0.25 | 11 | 1 = no seizures |
| 10= 41.3 ± 11.8 * |
Animals received the indicated CRF antagonist or vehicle 15–20 min prior to the infusion of 0.15 nmol of CRF, via an i.c.v. cannula. Latency and duration of seizures were scored as described in the text.
Statistically different from control (P < 0.001, two-tailed Mann–Whitney U- test).
Fig. 1.
Dose effect of pretreatment with NBI 27914 on the duration of seizures evoked by CRF. Rat pups received the selective CRF1 inhibitor at the doses indicated on the x-axis (see Section 2.3.1). Values are means ± S.E.M. *Significantly different from control values, P-0.05; ** P-0.01 (Mann–Whitney U-test).
Fig. 2.
Effects of pretreatment with NBI 27914 on EEG features of seizures induced by CRF. Six immature rats were implanted with bipolar electrodes directed at the left amygdala on the 10th postnatal day (see text). Depth EEGs were recorded on the following morning. Panel A shows low-voltage, non-rhythmic background activity in all six tracings (1–6). Occasional brief movement artifacts are noted by the arrows. Panel B was obtained 30 min after the administration of 1 mg/kg of NBI 27914 to rats Nos. 2, 3 and 6, and 13–15 min after the infusion of 0.15 nmol CRF to all six pups. The EEG features of animals Nos. 2, 3 and 6 reveal only increased overall frequency of movement artifacts. High-voltage spike-wave and polyspike epileptic discharges are evident in rats subjected to CRF without pretreatment with NBI 27914 (curved arrows). Behavioral limbic seizures lasted for 150, 180 and 110 min in rats Nos. 1, 4 and 5, respectively. No behavioral seizures were observed in rats Nos. 3 and 6. Rat No. 2 had one episode of wet dog shakes, and a seizure lasting 25 min. Horizontal marker indicates 1 s; vertical marker corresponds to 50 μV.
The effect of intraperitoneally (i.p.) administered NBI 27914 on CRF-induced seizures was highly dependent on the time of administration relative to that of CRF infusion. As is evident from Table 2, an interval of 15–20 min did not alter any parameter of CRF-induced seizures significantly. Seizure latency (10.0 ± 1.0 min) and duration (129.0 ± 27.4 min) did not differ from control values (8.5 ± 1.3 and 138.8 ± 9.9 min). However, an interval of 30–40 min prior to CRF infusion permitted NBI 27914 given i.p. to markedly increase the latency (23.5 ± 3.6 min; P = 0.0008) and decrease the duration (104.8 ± 5.8; P = 0.018) of seizures induced by CRF. In comparison, i.p. administration of two doses of α-helical CRF9 – 41 did not diminish the duration or increase the latency of CRF-induced seizures (Table 2). The higher dose tested, 4.0 mg/kg, was administered either 20 or 40 min prior to CRF. The time to the onset of the seizures (12.8 ± 3.5 and 8.0 ± 1.6 min) and their duration did not differ between the 20 min and 40 min groups (P = 0.31, Mann–Whitney U-test). Therefore, the data for these groups were combined in Table 2.
Table 2.
Effect of intraperitoneal NB1 27914 or α-helical CRF9–41 on latency and duration of CRF induced seizures
| Group | Dose (mg/kg) | Interval to CRF (min) | n | Seizure latency (min) | Seizure duration (min) |
|---|---|---|---|---|---|
| Control | Not applicable | 12 | 8.5 ± 1.3 | 138.0 ±9.9 | |
| NBI 27914 | 10–20 | 15–20 | 6 | 10.0 ± 1.1 | 129.0 ± 27.4 |
| 30–40 | 6 | 23.5 ± 3.6 * | 104.8 ± 5.8 ** | ||
| Control | Not applicable | 11 | 7.5 ± 1.1 | 161.0 ± 8.8 | |
| α-Helical CRF | 1.0 | 20–30 | 6 | 7.2 ± 0.5 | 169.2 ± 7.1 |
| 4.0 | 20 and 40 | 10 | 10.4 ± 2.0 | 161.0 ± 9.6 |
CRF receptor antagonists or vehicle were injected into the peritoneal cavity at the indicated time prior to infusion of a convulsant dose (0.15 nmol) of CRF into the lateral cerebral ventricle. Seizure latency and duration determination are described in the text. Values are means ± S.E.M.
Significantly different from control (P = 0.0008, Mann–Whitney U-test).
P = 0.018, significantly different from control values.
Oral administration of NBI 27914 to suckling rats resulted in a highly variable rate and extent of absorption and penetration into the CNS. Thirty minutes did not suffice to allow the compound to reach CRF receptors (Table 3). Elimination of the seizures or a significant decrease in their duration were noted upon administration of NBI 27914 1 h prior to CRF infusion. When the CRF1 blocker was given 120 min prior to the convulsant challenge, a marked inter-animal variability was observed. Three pups had no seizures, but the other three had no evident effect of the antagonist, with a short latency (Table 3) and prolonged duration (180 min) of the seizures.
Table 3.
Effect of oral NBI 27914 on CRF-induced seizures
| Group | Interval to CRF (min) | n | Seizure latency (min) | Seizure duration (min) |
|---|---|---|---|---|
| Control | Not-applicable | 17 | 9.6 ± 1.8 | 125.3 ± 12.8 |
| NBI 27914 | 30 | 3 | 9.0 ± 1.0 | 123.3 ± 6.0 |
| 60 | 6 | 2 = no seizures | 53.3 ± 27.9 * | |
| 4= 20.2 ± 8.6 | ||||
| 120 | 6 | 3 = no seizures | 77.5 ± 35.4 | |
| 3= 8 ± 0.67 |
The selective CRF1 receptor antagonist or a DMSO-dye vehicle were administered by gavage to infant rats at the indicated time prior to infusion of CRF into the cerebral ventricles. The latency and duration of CRF-induced seizures were determined.
Significantly different from controls (P = 0.03, Mann–Whitney U-test).
Urocortin administration to immature rats resulted in the development of behavioral limbic seizures, but with a longer latency and significantly shorter duration than those produced by equivalent doses of CRF (Table 4). Pre-administration of NBI 27914 practically eliminated the effects of the lower dose of urocortin, while seizures induced by the higher dose were significantly attenuated (P = 0.02 vs. the group not receiving NBI 27914, Table 4).
Table 4.
Effects of NBI 27914 on urocortin-induced behavioral phenomena in immature rats
| Urocortin dose (nmol) | NBI 27914 | n | Latency of seizures (min) | Seizure duration (min) |
|---|---|---|---|---|
| 0.75 | – | 6 | 10.3 ± 2.3 | 98.2 ± 24.0 |
| + | 6 | 2 = no seizures | 18.3 ± 8.7 * | |
| 4 = 29.8 ± 13.0 | ||||
| 0.15 | – | 5 | 1=no seizures | 53.0 ± 13.5 |
| 4= 11.0 ± 3.6 | ||||
| + | 6 | 5=no seizures | 0 | |
| 1 = 38 | 10 | |||
| CRF0.15 | – | 17 | 9.6 ± 1.8 | 125.3 ± 12.8 |
The CRF-related peptide, urocortin, which activates both types of CRF receptors, was given via an i.c.v. cannula, at the doses shown. The CRF1 receptor blocker abolished the convulsant effects of urocortin, indicating their mediation via CRF1.
Significantly different from the same dose of urocortin without NBI 27914 (P < 0.05).
4. Discussion
The current study demonstrates that a selective competitive antagonist of the CRF1 receptor type abolishes the neuronal excitation effect of CRF in developing rats. The behavioral and electroencephalographic seizures induced by CRF in neonatal (first postnatal week) and infant (second postnatal week) rats have been described and characterized previously [3,4,7,8]. CRF leads to seizures that originate in the amygdala and spread to other limbic structures [4]. The doses required for seizure production (picomolar range) are insufficient to activate the pituitary, and do not increase plasma glucocorticoids [3]. Elevation of plasma corticosterone is only evident after the onset of the seizures which are clearly a major activator of the stress response. CRF-induced seizures are therefore a paradigm of the central effects of the peptide on its receptor(s). The seizures can be eliminated by non-selective CRF1/CRF2 receptor antagonists, but not by, for example, glutamate receptor antagonists [3,9,10].
Administration of the selective CRF1 receptor blocker, NBI 27914, into the lateral cerebral ventricle of infant rats led to a dose-dependent attenuation of CRF-induced seizures. The higher doses tested were at least as potent as non-selective peptide CRF1/CRF2 antagonists. These data are strongly consistent with the assignment of the CRF1 as the receptor mediating the excitatory, convulsant effects of CRF.
Several additional lines of evidence further support CRF1 activation as a prerequisite for neuronal excitation by CRF. First, CRF-induced seizures involve the amygdala and hippocampus [4], areas expressing high levels of CRF1-mRNA [13]. Moreover, CRF has been demonstrated to have a highly age-dependent convulsant potency, which peaks during the second postnatal week [4,7]. The developmental profile of the CRF1-mRNA in the hippocampus and amygdala mirrors the pattern of the peptide's potency. Maximal levels of steady-state CRF1-mRNA are found during the second postnatal week, potentially accounting for the enhanced excitatory effects of the peptide during this period [2].
An intriguing question is whether activation of CRF1 is involved in, or is required for, other limbic seizures generated in the developing rat during the second postnatal week. In our hands, seizures induced during this period by i.p. administration of kainic acid are not modified by pretreatment with peptide, non-selective blockers of both CRF receptors (Baram and Schultz, unpublished observations). However, using a model for febrile seizures [11], the administration of non-selective CRF receptor blockers prior to exposure to hyperthermia results in elevation of the threshold temperature at which seizures occur [6]. This is consistent with a role for CRF receptor activation in the mechanism of generating febrile seizures which are highly age-specific developmental seizures [6].
The selective CRF1 inhibitor blocked CRF-induced seizures reliably when administered into the lateral cerebral ventricles. Systemic administration suggested either an erratic absorption from both the peritoneal and gastric cavities, or poor penetration of the blood-brain barrier. Prolonging the interval between intraperitoneal administration and the CRF challenge resulted in attenuation of CRF-induced seizures at doses approximately 30-fold higher than those yielding a comparable effect when given i.c.v. This suggests that some of the systemically given NBI 27914 did penetrate the blood-brain barrier of the immature rat. Conversely, peptide inhibitory analogues of CRF given systemically did not alter any parameter of the CRF-induced seizures. This finding is consistent with either a rapid degradation of the peptides, or their inability to penetrate the blood-brain barrier.
CRF binds the CRF1 receptor with a higher affinity than the CRF2 receptor type [27]. Recently, a CRF-related peptide, urocortin, has been characterized [41], which demonstrates comparable affinities to both types of CRF receptors in vitro [18]. Because of its relatively high affinity for CRF2, it has been suggested that urocortin might be the ‘native ligand’ of this receptor [27]. Indeed, in vivo, urocortin is a far more potent anorexia-inducing agent than CRF: the median effective dose was 0.19 μg for urocortin and 6.82 μg for CRF. Suppression of food intake is generally considered to depend on activation of CRF2 receptors in the hypothalamic ventromedial nucleus [36]. Conversely, urocortin was approximately 20-fold less potent than CRF in producing ‘anxiety’-type behaviors [36]. Using the in vivo paradigm described in the present study, urocortin initiated behaviors that were indistinguishable from those produced by CRF. However, based on the duration of seizures induced by 0.15 and 0.75 nmol of urocortin (Table 4) the potency of this peptide in neuronal excitation was only about 20% of that of CRF. In addition, inhibiting the activation of the CRF1 receptor by urocortin using NBI 27914 essentially eliminated the effects of urocortin in this paradigm, which is highly suggestive of the mediation of these excitatory actions by the CRF1 receptor type.
CRF is emerging as an important neuromodulator in several CNS regions [34] which may have significant clinically relevant effects [17,29,37]. For example, the peptide induces protein phosphorylation in the hippocampus [23] and may enhance learning and memory [12,26]. CRF, probably via effects at amygdala receptors, is also highly anxiogenic [19,25,42], and leads to appetite suppression. In the developing brain, CRF is a potent convulsant, probably via actions on receptors in the amygdala and hippocampus [2,4]. The presence of at least two types of CRF receptors raises the possibility of selective enhancement of desired effects of CRF and abrogation of its detrimental actions. The assignment of the neuroexcitant actions of CRF to CRF1 thus offers an opportunity for the development of potentially clinically useful drugs which directly and selectively modulate the activation of this receptor.
Acknowledgements
The technical assistance of Linda Schultz is appreciated. These studies were partially supported by NIH NS 28912 (T.Z.B.).
References
- 1.Aldenhoff JB, Gruol DL, Rivier J, Vale W, Siggins GR. Corticotropin-releasing factor decreases postburst hyperpolarization and excites hippocampal neurons. Science. 1983;221:875–877. doi: 10.1126/science.6603658. [DOI] [PubMed] [Google Scholar]
- 2.Avishai-Eliner S, Yi S-J, Baram TZ. Developmental profile of CRH-receptor messenger RNA in the rat limbic system. Dev. Brain Res. 1996;91:159–163. doi: 10.1016/0165-3806(95)00158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baram TZ, Schultz L. CRH is a rapid and potent convulsant in the infant rat. Dev. Brain Res. 1991;61:97–101. doi: 10.1016/0165-3806(91)90118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baram TZ, Hirsch E, Snead OC, III, Schultz L. CRH induced seizures in the infant brain originate in the amygdala. Ann. Neurol. 1992;31:488–494. doi: 10.1002/ana.410310505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baram TZ, Hirsch E, Schultz L. Short-interval amygdala kindling in neonatal rats. Dev. Brain Res. 1993;73:79–83. doi: 10.1016/0165-3806(93)90048-f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baram TZ, Schultz L. Corticotropin releasing hormone receptor antagonist is effective for febrile seizures in the infant rat. Ann. Neurol. 1994;36:487. [Google Scholar]
- 7.Baram TZ, Ribak CE. Peptide-induced infant status epilepticus causes neuronal death and synaptic reorganization. NeuroReport. 1995;6:277–280. doi: 10.1097/00001756-199501000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baram TZ, Schultz L. ACTH does not control neonatal seizures induced by the administration of exogenous corticotropin releasing hormone. Epilepsia. 1995;36:174–178. doi: 10.1111/j.1528-1157.1995.tb00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baram TZ, Avishai-Eliner S, Schultz L. Seizure threshold to kainic acid in infant rats is markedly decreased by corticotropin releasing hormone. Epilepsia. 1995;36(Suppl.) Abstr. B-05. [Google Scholar]
- 10.Baram TZ, Koutsoukos Y, Schultz L, Rivier J. The effect of ‘Astressin’, a novel antagonist of corticotropin releasing hormone (CRH), on CRH-induced seizures in the infant rat: comparison with two other antagonists. Mol. Psychiatry. 1996;1:223–226. [PMC free article] [PubMed] [Google Scholar]
- 11.Baram TZ, Gerth A, Schultz L. Febrile seizures: an appropriate aged model suitable for long-term studies. Dev. Brain Res. 1997;98:170–265. doi: 10.1016/s0165-3806(96)00190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behan DP, Heinrichs SC, Troncoso JC, Liu XJ, Kawas CH, Ling N, De Souza EB. Displacement of corticotropin releasing factor from its binding protein as a possible treatment for Alzheimer's disease. Nature. 1995;378:284–287. doi: 10.1038/378284a0. [DOI] [PubMed] [Google Scholar]
- 13.Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang CP, Pearse RV, O'Connell S, Rosenfeld M. Identification of a 7 transmembrane helix receptor for CRF and sauvagine in mammalian brain. Neuron. 1993;11:1187–1195. doi: 10.1016/0896-6273(93)90230-o. [DOI] [PubMed] [Google Scholar]
- 15.Chen C, Dagnino R, De Souza EB, Grigoriadis DE, Huang CQ, Kim K-I, Liu Z, Moran T, Webb TR, Witten JP, Xie YF, McCarthy JR. Design and synthesis of a series of non-peptide high affinity human corticotropin releasing factor-1 receptor antagonists. J. Med. Chem. 1996;39:4358–4360. doi: 10.1021/jm960149e. [DOI] [PubMed] [Google Scholar]
- 16.De Souza EB, Insel TR, Perrin MH, Rivier J, Vale WW, Kuhar MJ. Corticotropin-releasing factor receptors are widely distributed within the rat CNS: an autoradiographic study. J. Neurosci. 1985;5:3189–3203. doi: 10.1523/JNEUROSCI.05-12-03189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Souza EB, Whitehouse PJ, Kuhar MJ, Price DL, Vale WW. Reciprocal changes in corticotropin releasing factor (CRF)-like immunoreactivity and CRF receptors in cerebral cortex of Alzheimer disease. Nature. 1986;319:593–595. doi: 10.1038/319593a0. [DOI] [PubMed] [Google Scholar]
- 18.Donaldson CJ, Sutton SW, Perrin MH, Corrigan AZ, Lewis KA, Rivier JE, Vaughan J, Vale WW. Cloning and characterization of human urocortin. Endocrinology. 1996;137:2167–2170. doi: 10.1210/endo.137.5.8612563. [DOI] [PubMed] [Google Scholar]
- 19.Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin- releasing factor administration: is CRF a mediator of anxiety or stress response? Brain Res. Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- 20.Ehlers CL, Henriksen SJ, Wang M, Rivier J, Vale WW, Kuhar MJ. Corticotropin releasing factor produces increases in brain excitability and convulsive seizures in rats. Brain Res. 1983;278:332–336. doi: 10.1016/0006-8993(83)90266-4. [DOI] [PubMed] [Google Scholar]
- 21.Fox EA, Gruol DL. CRF suppresses the afterhyperpolarization in cerebellar Purkinje neurons. Neurosci. Lett. 1993;149:103–107. doi: 10.1016/0304-3940(93)90358-r. [DOI] [PubMed] [Google Scholar]
- 22.Grigoriadis DE, Heroux JA, De Souza EB. CRF: CIBA Found. Symp. Vol. 172. Wiley, NY: 1993. Characterization and regulation of corticotropin-releasing factor receptors in the central nervous, endocrine and immune systems; pp. 85–107. [DOI] [PubMed] [Google Scholar]
- 23.Hung HC, Chou C-KK, Chiu TH, Lee EHY. CRF increases protein phosphorylation and enhances retention performance in rats. NeuroReport. 1992;3:181–184. doi: 10.1097/00001756-199202000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Insel TR, Battaglia G, Fairbanks DW, De Souza EB. The ontogeny of brain receptors for corticotropin-releasing factor and the development of their functional association with adenylate cyclase. J. Neurosci. 1988;8:4151–4158. doi: 10.1523/JNEUROSCI.08-11-04151.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koob GF, Bloom FE. Corticotropin releasing factor and behavior. Fed. Proc. 1985;44:259–263. [PubMed] [Google Scholar]
- 26.Lee EHY, Hung HC, Lu KT, Chen WH, Chen HY. Protein synthesis in the hippocampus associated with memory facilitation by CRF in rats. Peptides. 1992;13:927–927. doi: 10.1016/0196-9781(92)90051-4. [DOI] [PubMed] [Google Scholar]
- 27.Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. Cloning and characterization of functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc. Natl. Acad. Sci. USA. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lovenberg TW, Chalmers DT, Liu C, De Souza EB. CRF2 alpha and CRF2 beta receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology. 1995;136:4139–4142. doi: 10.1210/endo.136.9.7544278. [DOI] [PubMed] [Google Scholar]
- 29.Nemeroff CB. New vistas in neuropeptide research in neuropsychiatry: focus on corticotropin releasing factor. Neuropsychopharmacology. 1992;6:69–75. [PubMed] [Google Scholar]
- 30.Perrin MH, Donaldson CJ, Chen R, Lewis KA, Vale WW. Cloning and functional expression of a rat brain corticotropin releasing factor receptor. Endocrinology. 1993;133:3058–3061. doi: 10.1210/endo.133.6.8243338. [DOI] [PubMed] [Google Scholar]
- 31.Pihoker C, Cain ST, Nemeroff CB. Postnatal development of regional binding of CRF and adenylate cyclase activity in the rat brain. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 1992;16:581–586. doi: 10.1016/0278-5846(92)90063-k. [DOI] [PubMed] [Google Scholar]
- 32.Potter E, Sutton S, Donaldson C, Chen R, Perrin MH, Lewis K, Sawchenko PE, Vale WW. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc. Natl. Acad. Sci. USA. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivier J, Rivier C, Vale W. Synthetic competitive antagonists of corticotropin releasing factor: effect on ACTH secretion in the rat. Science. 1984;224:889–891. doi: 10.1126/science.6326264. [DOI] [PubMed] [Google Scholar]
- 34.Sawchenko PE, Swanson LW. Organization of CRF immunoreactive cells and fibers in the rat brain: immunohistochemical studies. In: De Souza EB, Nemeroff CB, editors. Corticotropin-releasing Factor: Basic and Clinical Studies of a Neuropeptide. CRC; Boca Raton, FL: 1990. pp. 29–46. [Google Scholar]
- 35.Smith BN, Dudek FE. Age-related epileptogenic effects of corticotropin releasing hormone in the isolated CA1 region of rat hippocampal slices. J. Neurophysiol. 1994;72:2328–2333. doi: 10.1152/jn.1994.72.5.2328. [DOI] [PubMed] [Google Scholar]
- 36.Spina M, Merlo-Pich E, Chan RKW, Basso AM, Rivier J, Vale W, Koob GF. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science. 1996;273:1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- 37.Tsigos C, Chrousos GP. Physiology of the hypothalamic pituitary adrenal axis in health and dysregulation in psychiatric and autoimmune disorders. Endocrinol. Metab. Clin. North Am. 1994;23:451–466. [PubMed] [Google Scholar]
- 38.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 39.Vale W, Rivier C, Brown MR, Spiess J, Koob G, Swanson L, Bilezikjian L, Bloom F, Rivier J. Chemical and biological characterization of corticotropin releasing factor. Recent Prog. Hormone Res. 1983;39:339–375. doi: 10.1016/b978-0-12-571139-5.50010-0. [DOI] [PubMed] [Google Scholar]
- 40.Valentino RJ, Page ME, Curtis AL. Activation of noradrenergic locus ceruleus neurons by hemodynamic stress is due to local release of CRF. Brain Res. 1991;555:25–34. doi: 10.1016/0006-8993(91)90855-p. [DOI] [PubMed] [Google Scholar]
- 41.Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, Rivier J, Sawchenko PE, Vale W. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- 42.Veldhuis D, de Wied D. Differential behavioral action of corticotropin releasing factor. Pharmacol. Biochem. Behav. 1984;21:707–713. doi: 10.1016/s0091-3057(84)80007-6. [DOI] [PubMed] [Google Scholar]
- 43.Watts AG, Swanson LW. Diurnal variation in the content of CRH-mRNA in the hypothalamic PVN of rats of both sexes as measured by ISH. Endocrinology. 1989;125:1734–1738. doi: 10.1210/endo-125-3-1734. [DOI] [PubMed] [Google Scholar]
- 44.Wong ML, Licinio J, Gold PW. Localization of CRH receptor mRNA in adult rat by ISH. Endocrinology. 1994;135:2275–2278. doi: 10.1210/endo.135.5.7956950. [DOI] [PubMed] [Google Scholar]