Summary
Thromboembolic events was most important adverse event for coil embolization for intracerebral aneurysm.The present study investigated possible risk factors for thromboembolic events during coil embolization using diffusion-weighted imaging (DWI), comparing unruptured and ruptured lesions.
Key words: cerebral aneurysm, thromboembolic, DWI
Introduction
Endovascular coil embolization can prevent rupture in cerebral aneurysm. Coil placement in ruptured aneurysms as an alternative to surgical clipping will presumably increase with the development of devices and interventional skills. However, unlike with surgical clipping, the risk of thromboembolic events should be considered for such procedures.
Thromboembolic complications represent a major adverse event in endovascular surgery. Reported rates for periprocedure ischemic stroke following endovascular treatment of aneurysms using Guglielmi detachable coils (GDCs) range from 2.3% to 10.4%1-7. If coil embolization is to become the golden standard for the treatment of intracranial cerebral aneurysm, efforts must be made to decrease the frequency of this adverse event. Previous investigators have suggested adjunctive technique such as a balloon-assisted or stent-assisted techniques would increase the risk of silent thromboembolic events8,9. In endovascular surgery for ruptured aneurysms, special management is warranted to avoid thromboembolic complications. The present study investigated possible risk factors for thromboembolic events during coil embolization using diffusion-weighted imaging (DWI), comparing unruptured and ruptured lesions.
Methods
Patient Populations
Between March 1, 2003, and October 31, 2004, a total of 81 consecutive patients with 82 intracranial saccular aneurysms were treated using GDC placement. Aneurysms were ruptured in 30 patients (31 aneurysms) and unruptured in 51 patients (51 aneurysms). All except seven patients underwent DWI ≤ two days after GDC embolization. DWI data were not available due to unstable conditions after SAH or motion artifacts. As a result, a total of 45 unruptured aneurysm (Group U) and 29 ruptured aneurysms (Group R) were included in this study. Aneurysm size was classified as: s, small (<4 mm); m/s, medium (5-9 mm) with small neck (<4 mm); m/w, medium (5-9 mm) with wide neck (>4 mm); large (>10 mm); or giant (>25 mm). Locations and sizes of aneurysms are shown in table 1.
Table 1.
Locations and sizes of aneurysms.
| unruptured aneurysms | ruptured aneurysms | ||
| n (%) | n (%) | ||
| total | 45 | 29 | |
| size | s | 7 (15.6) | 15 (51.7) |
| m/s | 10 (22.2) | 5 (17.2) | |
| m/w | 15 (33.3) | 5 (17.2) | |
| large | 13 (28.9) | 4 (13.8) | |
| locations | ACA | 4 (8.9) | 5 (17.2) |
| ICA | 34 (75.6) | 12 (41.3) | |
| MCA | 3 (6.7) | 5 (17.2) | |
| VA-BA | 4 (8.8) | 7 (24~1) | |
| anti-platelet drugs | 40 (88.9) | 0 | |
| Balloon assist tehnique | 22 (48.9) | 0 | |
Endovascular Procedures
All patients were treated under general anesthesia and activated clotting time (ACT) was kept >250 s during procedures. For unruptured aneurysms, patients were administered heparin 5000 IU before the procedure and heparin 1000 IU/h during the procedure. ACTs were not tested during procedure. Sheaths and guiding catheters were continuously flushed with an infusion of saline with heparin. In all cases, systemic heparinization was not reversed, and patients were transferred to the intensive care unit under systemic heparinization. If no thromboembolic events were detected and if the aneurysm was completely occluded, heparinization was stopped. If a risk of thromboembolic events was suspected, systemic heparinization was stopped 24 h after the procedure, and anticoagulants were given for 23 days afterwards. For unruptured aneurysms, 40 of the 45 patients (88.9%) were administered anti-platelet agents (Bayasprin® 100 mg; Bayer Yakuhin, Tokyo, Japan) before the procedure. A 6-F guide catheter was navigated into the parent artery using a system of continuous heparinized saline flushing, and a microcatheter was advanced into the aneurysm. In cases of wide-necked aneurysms, balloon-assisted technique (BAT) was employed in 22 of the 45 unruptured aneurysms (48.9%). BAT was not used in ruptured aneurysms.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) was scheduled for ≤ two days after procedures. Imaging was performed using a Magnetom Vision 1.5-T system (Simens, Erlangen, Germany) with a multisection, single-shot, spin-echo, echo planar imaging sequence.
Diffusion gradients were applied in each of the x, y and z axes with two values of b (0 and 1000 s/mm2). Imaging parameter were as follows: TR/TE, 2600/82; matrix, 256; field of view, 23 x 23 cm; section thickness, 5 mm; and intersection gap, 2.5 mm.
Evaluation
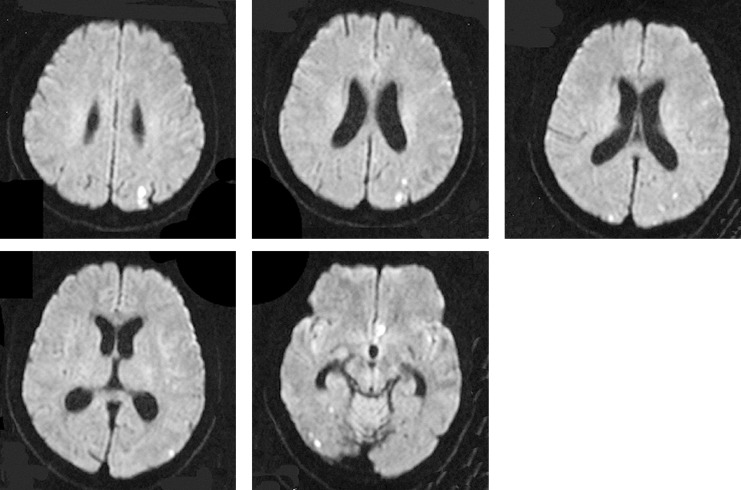
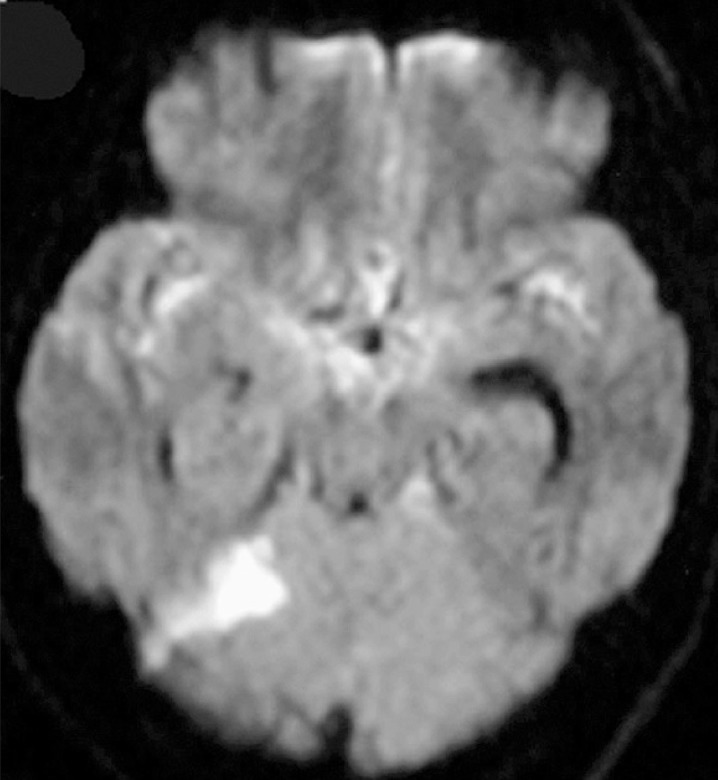
Signal hyperintensity on DWI was counted by a radiologist and evaluated by both the radiologist and neuroendovascular surgeons. Patterns of signal hyperintensity on DWI comprised small single or multiple spots (figure 1), and aggregations of high signal intensity and large high intensity areas. In this study, two groups were employed for evaluation of the occurrence of the signal hyperintensity. The group with few hyperintensities (few-HIs) displayed 0-4 signal hyperintensities (<4 mm), while the group with multiple hyperintensities (multi-HIs) displayed ≥ 5 hyperintensities, aggregation or large areas of hyperintensity (>4 mm) on DWI (figure 2).
Figure 1.
Multiple hyperintensities (multi-HIs) displayed on DWI after coil embolization for ruptured left MCA aneurysm.
Figure 2.
Large areas of hyperintensity were shown in left cerebellar haemisphere after coil embolization for ruptured right ICA aneurysm. There was no neurological deficit related to this lesion.
Statistical Analysis
The association between hyperintense lesions and ruptured or unruptured status was tested using chi-square for independence test. Values of P<0.05 were considered indicative of a significant association.
Results
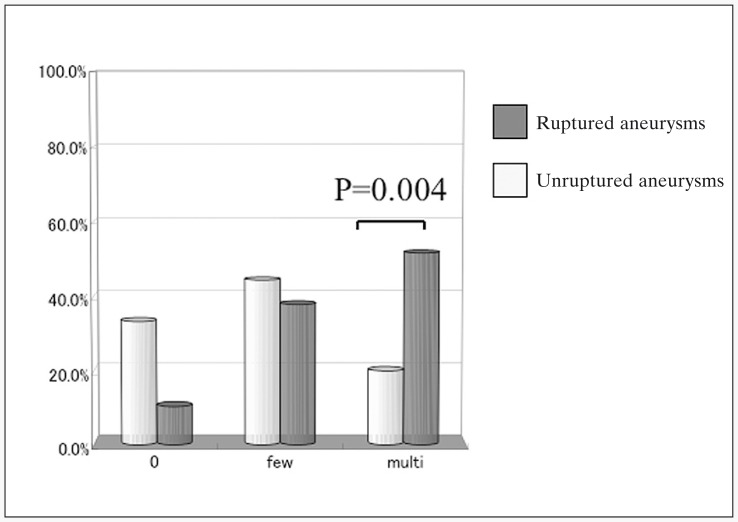
Frequency of signal hyperintensity on DWI after the endovascular procedure was 66.7% in Group U and 89.7% in Group R. In Group R, 48% were few-HIs and 52% were multi-HIs. Only 3 procedures showed 0 HIs in Group R (10.3%).
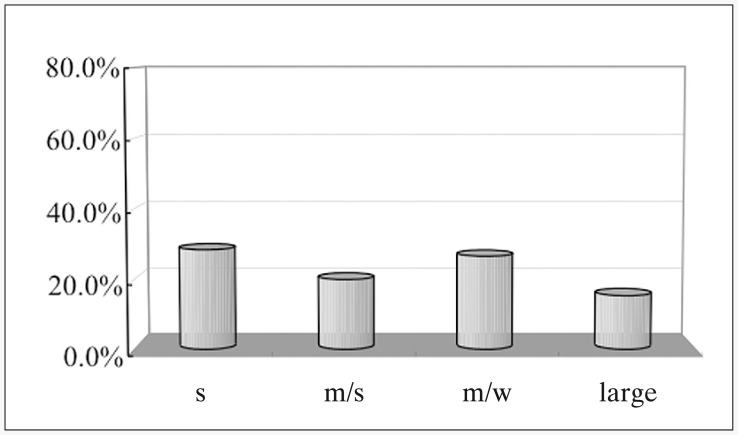
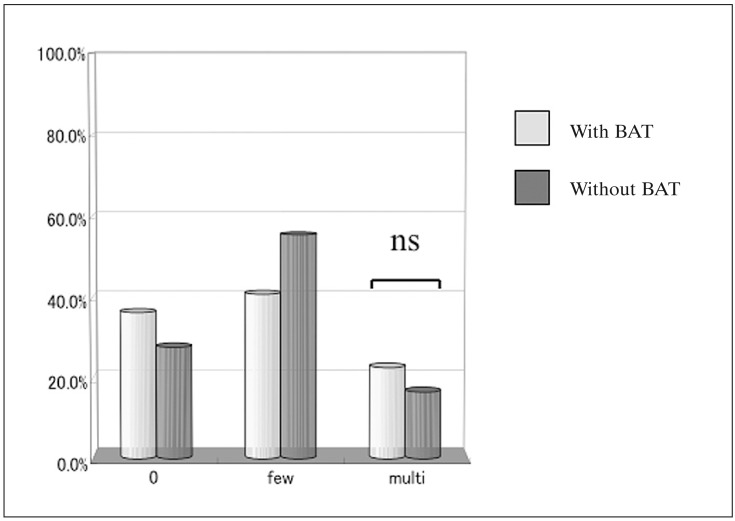
In contrast, Group U displayed 78% few-HIs and 22% multi-HIs, and 33.3% of Group U showed 0 HIs. Frequency of multi-HIs was significantly higher in Group R than in Group U (P=0.004; figure 3). Frequency of 0 HIs was significantly higher in Group U than in Group R. Sizes and locations did not influence the frequency of signal hyperintensity after GDC embolization in this study (figure 4). BAT also did not influence the frequency of signal hyper-intensity after GDC embolization among patients who had taken anti-platelet drugs before the procedures (figure 5). In Group U, 16% of HIs displayed no relation to the side of the aneurysms. In contrast, 46% of HIs in Group R displayed no relation to the side of the aneurysms. All except one patient with signal hyper-intensities on DWI after the endovascular procedure showed no symptomatic neurological deficits.
Figure 3.
Comparison with the incidence of hyperintense lesion on DWI between unruptured aneurysms and ruptured aneurysm. Incidence of multiple hyperintensities on DWI was significantly higher for coil embolization for ruptured aneurysms than that of unruptured aneurysms.
Figure 4.
Incidence of hyperintense lesions according to the size of the unruptured aneurysms. There was no significant difference between each size of the aneurysm.
Figure 5.
Comparison of the incidence of hyperintense lesion on DWI between with or without balloon-assisted technique. Balloon-assisted technique did not influence the incidence of the multiple hyperintensities lesion.
Discussion
Thromboembolic complications represent one of the most common and serious complications of endovascular coil embolization. Soeda et Al.6 noted that thromboembolic events resulting from GDC embolization procedures may be even more common than previously reported10. In their experience, thromboembolic events on DWI associated with GDC embolization in unruptured aneurysms occur at a frequency of 61%, with persisting deficits affecting approximately 40% of patients. The present result suggests that care must be taken during coil embolization for ruptured aneurysms. Furthermore, adjunctive techniques such as BAT do not appear to influence the frequency of thromboembolic events. This report provides new information on endovascular coils.
The reasons underlying the higher frequency of HIs on DWI in coil embolization for ruptured aneurysms than for unruptured aneurysms remain unclear. Different methods of heparin administration may influence the incidence of HIs in ruptured aneurysms compared with unruptured aneurysms. Various methods may be employed for haeparin use during endovascular coil embolization for ruptured aneurysms. In our cases, systemic heparinization was delayed until after successful placement of one or more coils.
The importance of antiplatelet therapy during endovascular procedures has gained increasing recognition. We routinely use antiplatelet drugs before embolization for unruptured aneurysms, to avoid thromboembolic complications. However, we could not use antiplatelet drugs before embolization for ruptured aneurysms. This may be one reason for the high frequency of HIs on DWI after embolization for ruptured anurysms.
The frequency of thromboembolic complication is assumed to increase with the use of adjunctive technique. Some authors have suggested that BATs carry a greater risk than conventional GDC procedures11. Albayram8 suggested that the frequency of new ischemic lesions detected on DWI was strongly influenced by the number of times the microcatheter or coil is repositioned or removed. Our results differed from previous reports, and no difference was found in the frequency of HIs on DWI after BAT. The effect of antiplatelet drugs may again represent one reason for these results. Antiplatelet therapy may restrain thrombus formation following damage to the blood vessel wall. In addition, newly developed intracranial assist balloons may contribute to the decrease in thromboembolic events for coil embolization with BAT. The need for adjunctive techniques such as BAT or stents seems likely to increase in the near future, and attention should be paid to periprocedural thromboembolic events.
In conclusion, great care must be taken in procedures for ruptured aneurysms. Moreover, the risks of thromboembolism with adjunctive techniques such as BAT may be decreased by administering anti-platelet drugs. Periprocedural management for thromboembolic events is crucial in endovascular surgery.
References
- 1.Derdeyn CP, Cross DT, et al. Postprocedure ischemic events after treatment of intracranial aneurysms with Guglielmi detachable coils. J Neurosurg. 2002;96(5):837–843. doi: 10.3171/jns.2002.96.5.0837. [DOI] [PubMed] [Google Scholar]
- 2.Friedman JA, Nichols DA, et al. Guglielmi detachable coil treatment of ruptured saccular cerebral aneurysms: retrospective review of a 10-year single-center experience. Am J Neuroradiol. 2003;24(3):526–533. [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez N, Murayama Y, et al. Treatment of unruptured aneurysms with GDCs: clinical experience with 247 aneurysms. Am J Neuroradiol. 2003;25(5):577–583. [PMC free article] [PubMed] [Google Scholar]
- 4.Murayama Y, Nien YL, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years' experience. J Neurosurg. 2003;98(5):959–966. doi: 10.3171/jns.2003.98.5.0959. [DOI] [PubMed] [Google Scholar]
- 5.Park HK, Horowitz M, et al. Periprocedural morbidity and mortality associated with endovascular treatment of intracranial aneurysms. Am J Neuroradiol. 2005;26(3):506–514. [PMC free article] [PubMed] [Google Scholar]
- 6.Pelz DM, Lownie SP, et al. Thromboembolic events associated with the treatment of cerebral aneurysms with Guglielmi detachable coils. Am J Neuroradiol. 1998;19(8):1541–1547. [PMC free article] [PubMed] [Google Scholar]
- 7.Wanke I, Doerfler A, et al. Endovascular treatment of unruptured intracranial aneurysms. Am J Neuroradiol. 2002;23(5):756–761. [PMC free article] [PubMed] [Google Scholar]
- 8.Albayram S, Selcuk H, et al. Thromboembolic events associated with balloon-assisted coil embolization: evaluation with diffusion-weighted MR imaging. V. 2004;25(10):1768–1777. [PMC free article] [PubMed] [Google Scholar]
- 9.Soeda A, Sakai N, et al. Thromboembolic events associated with Guglielmi detachable coil embolization with use of diffusion-weighted MR imaging. Part II. Detection of the microemboli proximal to cerebral aneurysm. Am J Neuroradiol. 2003;24(10):2035–2038. [PMC free article] [PubMed] [Google Scholar]
- 10.Rordorf G, Bellon RJ, et al. Silent thromboembolic events associated with the treatment of unruptured cerebral aneurysms by use of Guglielmi detachable coils: prospective study applying diffusion-weighted imaging. Am J Neuroradiol. 2001;22(1):5–10. [PMC free article] [PubMed] [Google Scholar]
- 11.Qureshi AI, Luft AR, et al. Prevention and treatment of thromboembolic and ischemic complications associated with endovascular procedures: Part II--Clinical aspects and recommendations. Neurosurgery. 2000;46(6):1360–1375. doi: 10.1097/00006123-200006000-00014. [DOI] [PubMed] [Google Scholar]