Summary
A 40-year-old man was transferred to our hospital due to sudden headache while swimming in the pool. CT revealed cerebellar haematoma within vermis associated with subarachnoid haemorrhage (SAH). Digital subtraction angiography (DSA) showed dural arteriovenous fistulas (DAVFs) with venous pouch on the surface of cerebellar vermis. Fistulas were on the meningeal surface near the sinus confluence. Draining veins formed venous pouch invaginating into cerebellar vermis.
Transarterial embolization (TAE) was performed under the concept that main feeder should be embolized last to occlude the DAVFs completely. Post-embolization 3D-CT showed the cast of N-butyl -cyanoacrylate (NBCA) in the fistulas as well as in the drainer. The good order of occlusion made the embolization complete.
Key words: dural arteriovenous fistulas, transarterial embolization, the order of embolization
Introduction
Endovascular treatment is usually the choice for DAVFs in most cases. Transarterial approach is chosen when there is no sinus or draining veins suitable for coil packing. Before occluding nidus as well as drainer completely, special care should be taken not to occlude dangerous anastomoses. We repot a case of DAVFs presenting with intracranial haemorrhage and SAH, which was cured with successful TAE. Crucial factors for complete obliteration of DAVFs are discussed.
Case report
A previously healthy 40-year-old man was transferred to our hospital due to sudden headache with nausea and vomiting during swimming. CT revealed cerebellar hematoma within the vermis associated with SAH. Consecutive post-contrast CT showed abnormal vasculature around haematoma (figure 1). DAVFs with venous pouch on the surface of cerebellar vermis became apparent in DSA (figure 2). Fistulas, on the meningeal surface near the sinus confluence, consist of bilateral occipital arteries and posterior meningeal arteries. Draining veins formed a venous pouch invaginating into cerebellar vermis. It drained into right transverse sinus. No direct drainage from fistulas into dural sinuses was noted. Main feeder was a meningeal branch of the right occipital artery. There was no reflux into cerebellar cortical veins. DSA also showed a hypoplastic left transverse sinus and the absence of inferior vermian veins.
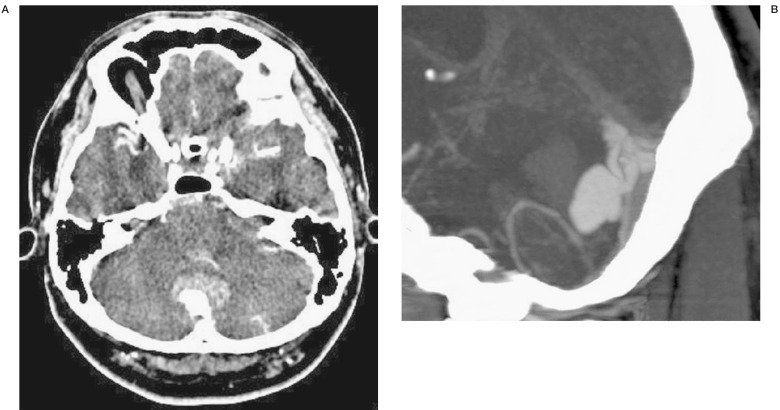
Figure 1.
Post-contrast CT at admission. A) CT revealed hematoma within the cerebellar vermis. A large enhancing lesion behind the hematoma was noted. B) Sagittal MPR showed dilated veins with a pouch near the sinus confluence.
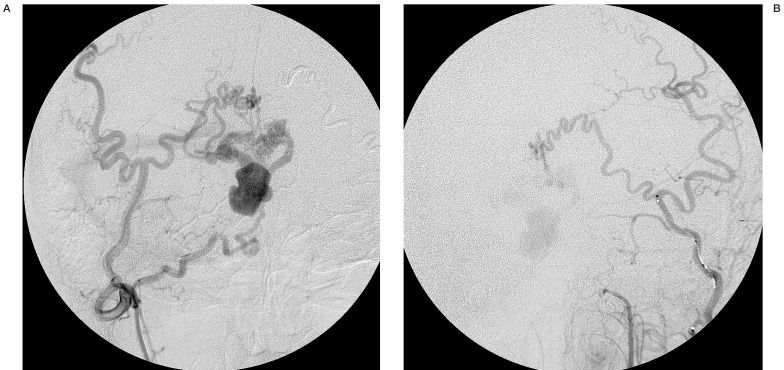
Figure 2.
DSA shows DAVFs with venous pouch on the surface of cerebellar vermis. A) Right occipital artery angiogram. AP view. The meningeal branch of the occipital artery mainly feeds the fistulas. Indirect drainage into the transverse sinus via the pouch is shown. B) Left external carotid angiogram. AP view.
Endovascular treatment
TAE was performed a week after admission. The order of occlusion was planned as follows: 1) left posterior meningeal artery, 2) left occipital artery, 3) right posterior meningeal artery, 4) right occipital artery, 5) meningeal branch of right occipital artery (main feeder). First the left posterior meningeal artery was occluded with PVA particles of 250 micrometer. Next the medial branch of the left occipital artery was occluded with 20% NBCA.
Then the right posterior meningeal artery was occluded with PVA particles and 20% NBCA. The medial branch of the right occipital artery was embolized with 33% NBCA. Last the meningeal branch of the right occipital artery, the main feeder, was catheterized with Magic catheter.
The tip of the catheter was placed near the fistulas or nidus (figure 3). Under flow control by manual compression of the right carotid artery, the main feeder was embolized with 50% NBCA. On the DSA view, the first cluster of NBCA run through the nidus and slewed around in the pouch and stopped in the draining vein just before the junction to the sinus. The drainer as well as fistulas was embolized effectually in consequence.
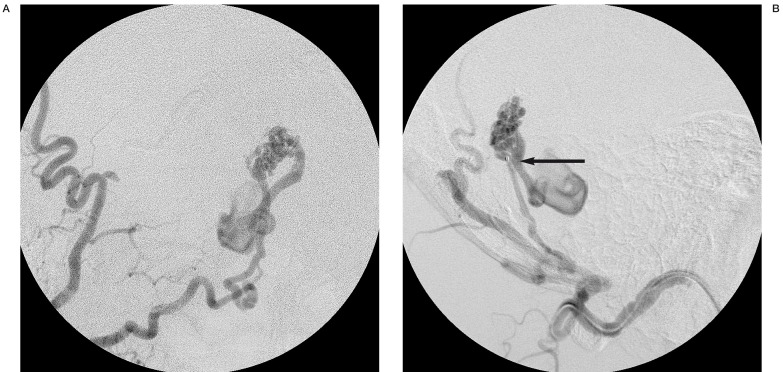
Figure 3.
Right occipital angiogram during embolization. A) AP view. After embolization of the medial branch of the occipital artery, the meningeal branch (main feeder) was left. B) Lateral view. The tip of the catheter was placed near the fistulas (arrow).
Results
Post-treatment 3D-CT showed the cast of NBCA in the fistulas as well as in the drainer. Although no cast was seen in the venous pouch, the complete obliteration of the DAVFs was accomplished (figure 4).
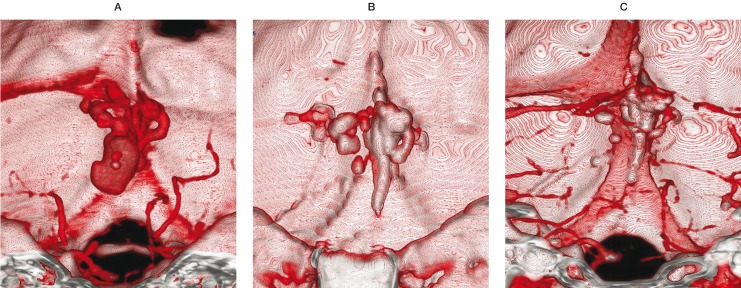
Figure 4.
3D-CT, AP view. A) Pretreatment contrast 3D-CT shows the DAVFs with venous pouch. B) Post-treatment noncontrast 3D-CT shows the cast of NBCA in the fistulas as well as in the drainer. C) Post-treatment contrast 3D-CT. Although no cast was seen in the venous pouch, the DAVFs are completely occluded.
Discussion
Our case reported here is classified into the type IV DAVFs according to Cognard et Al.1,2,3. The type IV DAVFs has a high risk of bleeding because of direct cortical drainage with venous dilatation 4,5. Endovascular treatment is currently a standard therapy for DAVFs. It includes transarterial and/or transvenous embolization and sometimes with surgical intervention6.
As for this case, TAE is the choice of treatment because there is no venous drainage to be packed with coils. It is not indispensable to occlude the venous pouch inavaginating in the vermis, which is responsible for bleeding.
Among several crucial factors in successful transarterial embolization, the order of feeder occlusion is thought to be of importance to achieve complete obliteration of the DAVFs. It is essential to identify main feeder(s), which should be embolized last. Then smaller feeders should be embolized first. In our case, the left posterior meningeal artery, the smallest feeder, was embolized first. Then the other remaining feeders were embolized one by one with slightly denser NBCA. Finally only the main feeder was left. It passes through the skull into dura and forms fistulas on the surface of the dura. To occlude the DAVFs completely, the catheter tip needs to be placed close to the nidus as much as possible. A flow-guided Magic catheter served the purpose
It goes without saying that before treatment we should understand the angio-architecture of individual DAVFs and dangerous anastomoses7. In practice, however, the precise structure of DAVFs sometimes become clearer during treatment procedure. Moreover the appearance of the DAVFs could change dramatically as the embolization proceeds.
3D-CT is a good tool for the evaluation of the distribution of the NBCA cast. By comparing with the pretreatment 3D-CT, the distribution of NBCA could easily be identified.
Conclusions
We reported a case of DAVFs that has successfully treated with TAE. The key components of success in TAE are to know the precise anatomy of individual lesion and dangerous anastomoses. Also the awareness of the possible alteration in appearance of the DAVFs during treatment is essential. In practice it is crucial to make a decision of the order of embolization before procedure. More specifically we have to decide in advance which feeder should be embolized last. 3D-CT is a good tool for the post treatment assessment of NBCA distribution in the DAVFs.
References
- 1.Cognard C, Gobin YP, et al. Cerebral dural arteriovenous fistulas: Clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671–680. doi: 10.1148/radiology.194.3.7862961. [DOI] [PubMed] [Google Scholar]
- 2.Lasjaunias P, Chiu M, et al. Neurological manifestations of intracranial dural arteriovenous malformations. J Neurosurg. 1986;64:724–730. doi: 10.3171/jns.1986.64.5.0724. [DOI] [PubMed] [Google Scholar]
- 3.Borden JA, Wu JK, et al. A proposed classification for spinal and cranial dural arteriovenous fistulas malformations and implications for treatment. J Neurosurg. 1995;82:166–179. doi: 10.3171/jns.1995.82.2.0166. [DOI] [PubMed] [Google Scholar]
- 4.Willinsky R, Goyal M, et al. Tortuous, engorged pial veins in intracranial dural arteriovenous fistulas: Correlations with presentation, location, and MR findings in 122 patients. Am J Neuroradiol. 1999;20:1031–1036. [PMC free article] [PubMed] [Google Scholar]
- 5.Kuwayama N, Kubo M, et al. The treatment of intracranial (dural) arteriovenous fistulas in unusual locations. Interventional Neuroradiology. 1999;5(Suppl 1):115–120. doi: 10.1177/15910199990050S121. [DOI] [PubMed] [Google Scholar]
- 6.Goto K, Sidipratomo P, et al. Combining endovascular and neurosurgical treatment of high-risk dural arteriovenous fistulas in the lateral sinus and the confluence of the sinuses. J Neurosurg. 1999;90:289–299. doi: 10.3171/jns.1999.90.2.0289. [DOI] [PubMed] [Google Scholar]
- 7.Hacein-Bey L, Daniels DL, et al. The ascending pharyngeal artery] branches, anastomoses, and clinical significance. Am J Neuroradiol. 2002;23:1246–1256. [PMC free article] [PubMed] [Google Scholar]