Summary
Intracranial artery angioplasty utilizing coronary stent is now widely tried as an effective alternative for treating intracranial artery stenosis, and several successful result of stent-assisted angioplasty for intracranial artery were reported.
Authors experienced a case of the basilar artery stenosis, in which re-stenosis progressed rapidly after simple balloon angioplasty and resulted in vessel rupture during stent-assisted angioplasty. Pathological result achieved by autopsy showed vessel wall disruption at the stent and multiple interruptions and defect of elastic laminar.
Key words: basilar artery, stenosis, stent
Introduction
Recent technical progress of the balloons and stents for coronary arteries enabled their insertion into torturous arteries, and several successful result of their application for intracranial artery has been reported1-11. The stent-assisted angioplasty for intracranial vessels, especially for the lesions at vertebral artery (VA) or the basilar artery (BA) will be one of effective treatment options at present.
In this paper, we report the pathological findings of a case of BA stenosis that showed rapid progress of re-stenosis after initial balloon angioplasty and ruptured during re-treatment with coronary stent.
Case Report
A 75-year-old man with the histories of diabetes mellitus and hyperlipidemia received a medical check up and abnormal finding of the intracranial arteries were not pointed out on magnetic resonance angiogram (MRA). Four month after the initial MRA, the pateint presented dysarthria and ataxia, and visited a local hospital. A magnetic resonance image (MRI) showed small pontine infarction and MRA and angiogram showed severe stenosis at the middle portion of the BA (figure 1,2). The symptoms were improved with conservative treatments; however, double vision and ataxia relapsed after alteration of treatments to oral administration of anti-platelets (aspirin 200 mg, ticropidine 200 mg). The patient was referred to us for angioplasty of the refractory to the medical therapy lesion
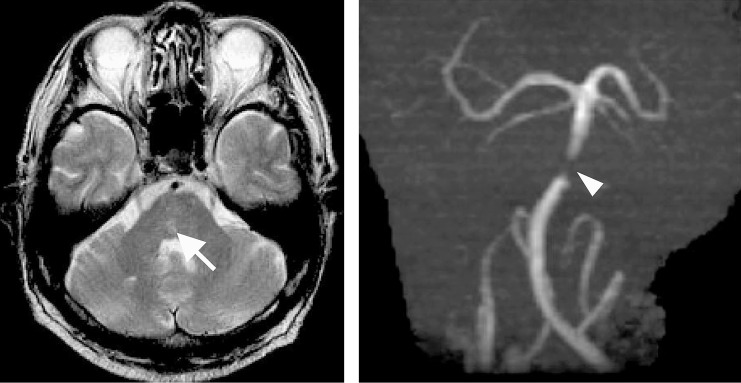
Figure 1.
MRI (Left) and MRA (Right) obtained the day of the initial episode. MRI shows small brainstem infarction (arrow) and MRA suggests severe basilar artery trunk stenosis. (arrowhead).
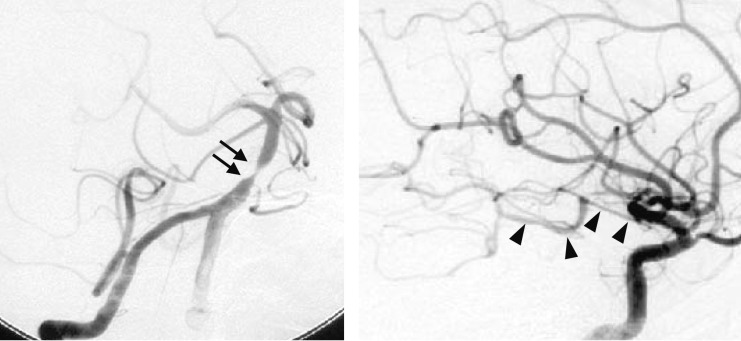
Figure 2.
Angiogram obtained two weeks after the initial episode. Right vertebral angiogram (left) showing severe stenosis at the basilar artery (arrows). Right carotid angiogram (right) shows poor collateral circulation to PCA via posterior communicating artery (arrowheads).
One month after initial episode, angioplasty with 3.0 mm balloon (Gateway; Boston Scientific, Fremont, USA) was made under local anesthesia and the stenosis was improved with small dissection (figure 3). After the angioplasty, his symptoms disappeared and the patient was free from symptoms for two months with oral anti-platelets administration (aspirin 200 mg, ticropidine 200 mg). Two months after the initial treatment, the patient noticed ataxia again and the follow-up MRA showed severe re-stenosis.
Figure 3.
Left vertebral angiogram after angioplasty shows that the stenotic basilar artery is successfully dilated
The stent-assisted angioplasty was made three months after initial episode. The lesion was pre-dilated with 2.5 mm balloon (Gateway; Boston Scientific, Fremont, USA) and then dilated with 3.5- x 12-mm stent (S670; AVE, Minneapolis, USA) up to 3.5 mm with nominal pressure (8 atm.). The angiogram just after the stent placement showed massive extravasation (figure 4).
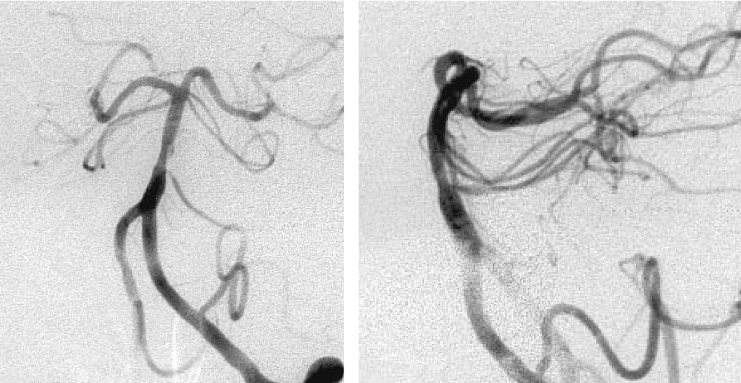
Figure 4.
Angiograms obtained after stent placement. Angiogram just after angioplasty shows massive extravasation form lower part of the lesion (left) and left vertebral angiogram after haemostasis shows good dilation of the lesion (middle, right).
Pathological findings at autopsy disclosed that the muscular layer of the arterial wall was completely disconnected and covered only with fibrin at the margin of the plaque; the elastic lamina had multiple small disruptions and wasdiscontinued widely at the plaque (figure 5). The arterial wall out of the lesion, the elastic lamina was hypertrophic and partially duplicated (figure 6).
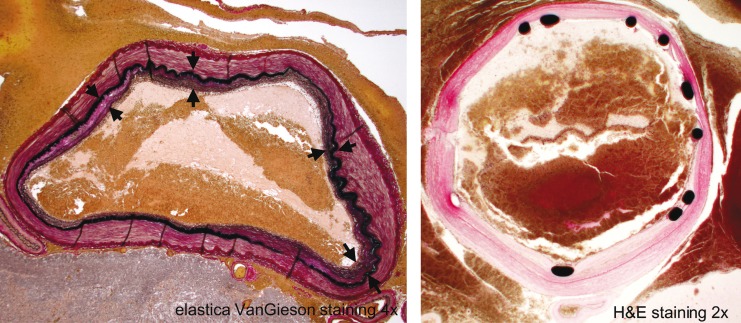
Figure 5.
Cross sectional specimen of the ruputured part shows complete disconnection of the muscular layer (arrows) and disrupted part is covered only with fibrin. Multiple disruptions (arrowheads) of the elastic lamina are observed especially at the margin of the intimal hypertrophy.
Figure 6.
Cross sectional image of basilar artery proximally to the stent (left) and distal end of the stent (right) obtained from autopsied specimen shows hypertrophic elastic lamina with duplication (arrows).
Thicken arterial wall was considered as the fibrous hyperplasia due to hypertension but not as the atheromatous plaque.
Discussion
Since the outcome of the symptomatic basilar artery stenosis is poor, angioplasty of the basilar artery stenosis has been introduced since early 1990's12-15. The initial results of balloon angioplasty were not satisfactory for the high risk of intraplaque dissection, plaque dislodgement and high rate of re-stenosis16-19.
Recent technical evolution for coronary stents improved the rigidity and trackability of the devices, and made them available to navigate into intracranial arteries. Since late 1990's, clinical applications of coronary stent are started for treating intracranial arterial stenosis. In the previously reported 56 cases, morbidity due to brainstem infarction was 7% (four cases) and mortality was 9% (five cases)1-11.
In our case, thicken arterial wall was fibrous but not the atheromatous plaque and the elastic laminar was multiply disrupted. The arterial wall ruptured at the margin of the plaque where the elastic lamina was absent. This pathological result suggests that the arterial wall had multiple fragile parts due to disruption of elastic laminar. In the simple balloon angioplasty for intracranial arteries, 50% residual stenosis after angioplasty was considered as a safety result15.
In the stent-assisted angioplasty, placing the stent to fit the surrounding arterial wall is necessary and has the risk of arterial wall disruption at the lesion. Discreet decision is important to indicate the stent-assisted angioplasty for intracranial arteries.
Conclusions
Stent-assisted angioplasty is a fascinating alternative for treating intracranial artery. But the detailed structure of the lesion cannot be investigated with the examinations at present. The arterial wall with sclerosis has multiple disruption of the elastic lamina and is fragile. Discreet indication is necessary for the stent-assisted angioplasty for intracranial arteries.
References
- 1.Lanzino G, Fessler RD, et al. Angioplasty and stenting of basilar artery stenosis: Technical case report. Neurosurgery. 1999;45:404–407. doi: 10.1097/00006123-199908000-00047. [DOI] [PubMed] [Google Scholar]
- 2.Horowitz MB, Pride GL, et al. Percutaneous transluminal angioplasty and stenting of midbasilar stenosis: Three technical case reports and literature review. Neurosurgery. 1999;45:925–930. doi: 10.1097/00006123-199910000-00043. [DOI] [PubMed] [Google Scholar]
- 3.Gometz CR, Misra VK, et al. Elective stenting of symptomatic basilar artery stenosis. Stroke. 2000;31:95–99. doi: 10.1161/01.str.31.1.95. [DOI] [PubMed] [Google Scholar]
- 4.Joseph GJ, Goldstein J, et al. Endovascular stenting of aeteriosclerotic stenosis in a basilar artery after unsuccessful angioplasty. Am J Radiol. 1999;174:383–385. doi: 10.2214/ajr.174.2.1740383. [DOI] [PubMed] [Google Scholar]
- 5.Phatouros CC, Lefler JE, et al. Primary stenting for high-grade basilar artery stenosis. Am J Neuroradiol. 2000;21:1744–1749. [PMC free article] [PubMed] [Google Scholar]
- 6.Piotin M, Blank R, et al. Primary basilar artery stenting: Immediate and long-term results in one patient. Am J Radiol. 2000;175:1367–1369. doi: 10.2214/ajr.175.5.1751367. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen PA, Perl II J, et al. Stent-assisted angioplasty of intracranial vertebrobasilar atherosclerosis: An initial experience. J Neurosurg. 2000;92:771–778. doi: 10.3171/jns.2000.92.5.0771. [DOI] [PubMed] [Google Scholar]
- 8.Levy EI, Horowitz MB, et al. Transluminal stent-assisted angioplasty of the Intracranial vertebrobasilar system for medically refractory, posterior circulation ischemia: Early results. Neurosurgery. 2001;48:1215–1223. doi: 10.1097/00006123-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Barakate MS, Snook KL, et al. Angioplasty and stenting in the posterior cerebral circulation. J Endovasc Ther. 2001;8:558–565. doi: 10.1177/152660280100800604. [DOI] [PubMed] [Google Scholar]
- 10.Gondim FAA, Cruz-Flores, et al. Angioplasty and stenting for symptomatic basilar artery stenosis. J Neuroimaging. 2002;12:55–58. doi: 10.1111/j.1552-6569.2002.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 11.Levy EI, Hanel RA, et al. Comparison of periprocedure complications resulting from direct stent placement compared with those due to conventional and staged stent placement in the basilar artery. J Neurosurg. 2003;99:653–660. doi: 10.3171/jns.2003.99.4.0653. [DOI] [PubMed] [Google Scholar]
- 12.Ausman JI, Shrontz CE, et al. Vertebrobasilar insufficiency: A review. Arch Neurol. 1985;42:803–808. doi: 10.1001/archneur.1985.04210090071021. [DOI] [PubMed] [Google Scholar]
- 13.Ahuja A, Guterman LR, et al. Angioplasty for basilar artery atherosclerosis. J Neurosurg. 1992;77:941–944. doi: 10.3171/jns.1992.77.6.0941. [DOI] [PubMed] [Google Scholar]
- 14.Higashida RT, Tsai FY, et al. Transluminal angioplasty for atherosclerotic disease of the vertebral and basilar arteries. J Neurosurg. 1993;78:192–198. doi: 10.3171/jns.1993.78.2.0192. [DOI] [PubMed] [Google Scholar]
- 15.Nakatsuka H, Veda T, et al. Successful percutaneous transluminal angioplasty for basilar artery stenosis: Technical case report. Neurosurgery. 1996;39:161–164. doi: 10.1097/00006123-199607000-00034. [DOI] [PubMed] [Google Scholar]
- 16.Clark WM, Barnwell SL, et al. Safety and efficacy of percutaneous transluminal angioplasty for intracranial atherosclerotic stenosis. Stroke. 1995;26:1200–1204. doi: 10.1161/01.str.26.7.1200. [DOI] [PubMed] [Google Scholar]
- 17.Higashida RT, Tsai FY, et al. Cerebral percutaneous transluminal angioplasty. Hart Dis Stroke. 1993;2:497–502. [PubMed] [Google Scholar]
- 18.Kellogg JX, Nesbit GM, et al. The role of angioplasty in the treatment of cerebrovascular disase. Neurosurgery. 1998;43:549–556. doi: 10.1097/00006123-199809000-00077. [DOI] [PubMed] [Google Scholar]
- 19.Yokote H, Terada T, et al. Percutaneous transluminal angioplasty for intracranial arteriosclerotic lesions. Neuroradiology. 1998;40:590–596. doi: 10.1007/s002340050651. [DOI] [PubMed] [Google Scholar]