Abstract
Inhalation of diesel exhaust particles (DEPs) is associated with pulmonary and cardiovascular disease. One contributor to pathogenesis is inhaled particles reaching and injuring the lung capillary endothelial cells, and possibly gaining access to the blood stream. Using in vitro capillary tubes as a simplified vascular model system for this process, it was previously shown that DEPs induce the redistribution of vascular endothelial cell-cadherin (VE-Cad) away from the plasma membrane to intracellular locations. This allowed DEPs into the cell cytoplasm and tube lumen, suggesting the tubes may have become permeable (Chao et al., 2011). Here some of the mechanisms responsible for endothelial tube changes after DEP exposure were examined. The results demonstrate that endothelial tube cells mounted an oxidative stress response to DEP exposure. Hydrogen peroxide and oxidized proteins were detected after 24 hr of exposure to DEPs. Particles induced relocalization of Nrf2 from the cytoplasm to the nucleus, upregulating the expression of the enzyme heme oxygenase-1 (HO-1). Surprisingly, vascular endothelial cell growth factor-A (VEGF-A), initially termed “vascular permeability factor” (VPF), was found to be up-regulated in response to the HO-1 expression induced by DEPs. Similar to DEPs, applied VEGF-A induced relocalization of VE-Cadherin from the cell membrane surface to an intracellular location, and relocalization of VE-cadherin was associated with permeability. These data suggest that the DEPs may induce or contribute to the permeability of capillary-like endothelial tube cells via induction of HO-1 and VEGF-A.
Keywords: diesel exhaust particles, endothelial tubes, toxicity, ROS, VEGF-A, VEGFR2, VE-cadherin, endothelial permeability
1. INTRODUCTION
Diesel exhaust particles (DEPs) are a major component of atmospheric particulate matter (PM) air pollution, which is associated with adverse pulmonary and cardiovascular health effects (Brook et a., 2008; ). In the atmosphere, DEPs contributes to fine PM (particles with diameters of 2.5 µm or smaller, or PM2.5) and ultrafine PM (diameters of 0.1 µm, i.e., 100 nm, or smaller, or PM0.1), both of which are able to reach alveolar regions of the lung. Several mechanisms have been proposed to explain how inhalation of DEPs and other PM can cause cardiovascular health effects. Inducing production of harmful ROS (Bai et al., 2001; Becker et al., 2005), initiating inflammatory responses (Salvi et al., 1999); alteration of autonomic balance (Pope et al., 1999), and disruption of vascular function (Ikeda et al., 1998) are a few. All of these mechanisms may contribute to the final pathology. Our interest is in endothelial dysfunction induced by DEPs. Vasoconstriction has been demonstrated in humans who inhaled ambient PM2.5 and ozone for 2 hr (Brook et al., 2002; Brook, 2008), and impairment of vasodilatation was observed 24 hr after a 1 hr diesel exhaust inhalation study (Tornqvist et al., 2007). Importantly, 24 hr after intratracheal installation of DEPs or DEP extracts, the water content of mouse lungs increased (Inoue et al., 2006), indicating an increase in pulmonary endothelial permeability.
DEPs likely injure alveolar epithelia, and possibly also lung capillaries (Bai et al., 2001; Brook et al., 2002; Salvi et al., 1999). If the effect on epithelial and endothelial cells is indirectly induced by DEPs, the inflammatory mediators released by neutrophils and macrophages in response to DEPs may explain the permeability observed in vivo. In addition, DEPs may have direct effects on capillary endothelia. Inhaled nanoparticles have been shown to reach the circulation (Geiser et al., 2005; Kreyling et al., 2002; Kreyling et al., 2009; Nemmar et al., 2001; Nemmar et al., 2002; Nemmar et al., 2004; Nemmar et al., 2005; Oberdorster et al., 2004). While these data are largely based on TiO2 and iridium particle inhalation studies (Geiser et al., 2005; Kreyling et al., 2002; Nemmar et al., 2001; Nemmar et al., 2002; Nemmar et al., 2004; Oberdorster et al., 2002), a more recent report used carbon particles fitting the designation of PM0.1. These particles consisted of labeled 80 nm and 20 nm nanoparticles, and, 24 hr after inhalation, they were found in many organs, including blood (Kreyling et al., 2009). This suggests that very small particles are able to reach the alveolar spaces, have a direct effect on the endothelium, and traverse the alveolar membrane to enter the bloodstream. DEPs may distribute similarly after inhalation.
Our recent data with confocal microscopy used in vitro-assembled endothelial tubes as a model system to visualize the effect of PM2.5 DEPs on the capillary-like structures. By particle number, about 14% of these DEPs were between 20 and 100 nm (i.e., PM0.1) and about 85% were less than 2.5 µm. The results of our previously reported in vitro endothelial tube culture experiments demonstrated that the adherens junction component, VE-cadherin, moved from cell-cell junctions to intracellular locations after exposure (Chao et al., 2011). Such rearrangement of cell-cell junctions is associated with permeability (for review see Dejana et al., 2008), and VE-cadherin is an important player in this property. A hypothesis was developed from our results, incorporating the following observations from the literature: (1) Adherens junctions are organizers of other cell-cell junctions, and interruption of them causes the endothelial cells to become leaky (for reviews see Dejana et al., 1995; Dejana et al., 2008); (2) In non-DEP studies, monolayers of bovine pulmonary artery endothelial cells treated with hydrogen peroxide (H2O2) caused junctional components such as VE-cadherin to become disorganized, resulting in gaps between the cells (Kevil et al., 2001), This indicated that the generation of H2O2 may be induced by DEP, thereby contributing to endothelial permeability; (3) In non-DEP studies on vascular smooth muscle cells, ROS-induced hemeoxygenase-1 (HO-1) resulted in expression of vascular endothelial cell growth factor-A (VEGF-A) (Dulak et al., 2002), which is an inducer of vascular permeability. In fact, VEGF-A was first discovered functionally, and was initially named vascular permeability factor (VPF) (Connolly et al., 1989; Ferrara and Henzel, 1989; Keck et al., 1989; Senger et al., 1983; Senger et al., 1990). (4) The basis for VEGF’s affect on VE-cadherin has been shown to be associated with its interaction with the VEGF receptor 2 (VEGFR2) at the endothelial plasma membrane (Weis et al., 2004). VEGF stimulation transiently dissociates the complex, inducing gaps between the endothelial cells (Weis et al., 2004). With these issues in mind, we hypothesized that the human and murine pulmonary permeability that is observed after inhalation of, or exposure to, DEPs, may in part be due to DEP-induced generation of ROS, leading to HO-1 expression, which in turn stimulates secretion of VEGF-A. This would thereby facilitate permeability by disrupting the endothelial cell-cell junctions.
The goal of the experiments described below was to explore this potential connection between DEP exposure, redistribution of VE-cadherin, VEGF expression, and the VEGFR2 interaction with VE-cadherin. Because the known association between VE-cadherin and VEGFR2 in the cell membrane (Weis et al., 2004), we asked whether DEPs would induce VEGF-A/VPF, since this factor has potential to be a mechanistic cause of internalization of VE-cadherin and permeability. Our results support this idea in an a 3 dimensional culture model system, demonstrating that in in vitro capillary-like endothelial tubes, free of the confounding effects of inflammatory cells, DEP induce ROS such as H2O2, and that the endothelial cells junctions become disrupted. In addition, the induced upregulation of HO-1 stimulates VEGF-A secretion, thereby suggesting a possible pathway that might explain one aspect of how vascular permeability is initiated by inhalation of DEP in vivo.
2. MATERIALS AND METHODS
2.1. Diesel exhaust particles and cell culture
Japanese automobile diesel exhaust particles (DEPs) were collected by Dr. Masaru Sagai, who donated them to researchers at UCLA. Particles were a gift from Dr. David Diaz-Sanchez, formerly of UCLA. The effects of these particles have been studied in a number of in vitro and in vivo models (Bai et al., 2001; Inoue et al., 2006; Ito et al., 2000; Kumagai et al., 1997; Sagai et al., 1993; Singh et al., 2004). A 10 mg/ml DEP stock solution was made by suspending 0.1 g of DEP in 10 ml in PBS, 0.05% Tween 80. Particles were vortexed for 3 minutes, then sonicated at 60 Hz for 5 minutes, then assessed six times (120 sec/run) by dynamic light scattering using a Zetasizer Nano ZS90. This yielded particles of ~30 nm to ~1050 µm (see Chao et al., 2010), fitting into the commonly used category of PM2.5 (2.5µm diameter and smaller). After vortexing and sonicating, the stock suspension was immediately diluted to 1, 10 or 100 µg/ml in medium, ending up with a final concentration of 1X PBS, 0.05% Tween 80 due to the particle suspension. (1X PBS is 137 mM NaCl, 2.7 mM KCl, 10 mM Sodium Phosphate dibasic, 2 mM Potassium Phosphate monobasic, pH 7.4.) The dilutions were immediately applied to the endothelial tube cultures via pipet.
Human umbilical vein endothelial cells (HUVECs) were cultured in endothelial cell growth medium EBM-2 Bulletkit (Lonza) as previously described (Chao et al., 2011). Medium was supplemented with phosphate buffered saline and Tween-80 to make a final concentration of 1X PBS, 0.05% Tween-80. The PBS/Tween-80 was always included to minimize differences between non-DEP-exposed controls and DEP-treated samples, since DEPs are suspended in 1X PBS, 0.05% Tween-80. (This Tween-medium with dispersed DEPs was prepared in exactly the same manner as that used in the Zetasizer Nano ZS90 determination of particle size, ensuring the particles were consistent in all experiments.) HUVECs at passage 5 to 15 were used to assemble tubes on 10 mg/ml LDEV-free Matrigel (BD Biosciences), that was solidified onto 2-well chamber slides (120 µl Matrigel/well) or 6-well (150 µl Matrigel/well) plastic tissue culture plates. The Matrigel was solidified for at least 30 min before adding cells. HUVECs were plated at 156 cells/mm2 on both the 2-well chamber slides (6 X 104 cells/well), and on the 6-well plastic dishes (1.5 X 105 cells/well), to ensure that parameterns were equivalent. Endothelial tubes were allowed to form for 12 hr prior to adding Tween/medium containing dispersed DEPs. Medium was changed daily. For monolayer cultures, endothelial cells were treated exactly the same, but were plated at the same density directly on plastic wells without Matrigel. Once confluent, cells were allowed to incubate for 12 hr, the same time allotted for the cells forming tubes on Matrigel. Well characterized DEPs (Bai et al., 2001; Singh et al., 2004) were prepared as previously described to create a suspension resulting in sizes of PM2.5 (Chao et al., 2011). A volume of medium proportional to the well size was added, delivering either medium (medium always with PBS-Tween alone (i.e., 0 µg DEPs/ml), or 1, 10, or 100 µg DEPs/ml to each well in a volume to make exposures always equivalent per cell between the two well sizes. Unless otherwise indicated, analysis was performed after a 24 hr incubation at 37°C. Since Matrigel is a liquid at 4°C, endothelial tubes could always be separated from the Matrigel by putting the culture dishes in the refrigerator, then pipetting out the Matrigel. If samples were at room temperature, cells could be lysed in the culture dishes, then separated from the substratum and cell debris by scraping the entire contents into a centrifuge tube, and spinning it at 10K × g.
2.2. Reagents
N-Acetyl Cysteine was obtained from Sigma-Aldrich (St. Louis, MO). Tin protoporphyrin IX (SnPP) was bought from Frontier Scientific, Inc. (Logan, UT). Other reagents employed are incorporated into the appropriate method sections below.
2.3. Analysis of intracellular ROS accumulation
The ROS detection studies were performed using an Image-iT™ Live Green Reactive Oxygen Species Detection Kit (Molecular Probes/Invitrogen). The method involved analyzing the intracellular accumulation of ROS due to H2O2 generation, based on the conversion of the non-fluorescent probe carboxy-H2DCFDA (2’, 7’-dichlorohihydrofluorescein diacetate) to green-fluorescent carboxy-DCF. Endothelial tubes were treated with 0, 1, 10 and 100 µg DEPs/ml for 24 hr. DEPs were then washed away with 37°C 1X PBS, and the samples were incubated for 25 min at 37°C with fresh medium containing 25 µM carboxy-H2DCFDA, which penetrates cells. After washing again with 37°C 1 X PBS, ROS detection was visualized with epifluorescence microscopy at 200X magnification (emission at 495–529 nm, Olympus IX71 Inverted Microscope), examining the carboxy-DCF which could not be transported out of the cells.
2.4. Measurement of H2O2 production
H2O2 production was assessed with 10-acetyl-3,7-dihydroxyphenoxazine using a commercially available Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit (Molecular Probes/ Invitrogen), with some protocol modifications. First a standard curve was prepared using serial dilutions of H2O2. Endothelial tubes were treated with various concentrations of DEPs (0, 1, 10, and 100 µg/ml) for 24 hr, then were scraped from wells and collected in pH adjusted SDS-PAGE buffer (25 mM Tris, 192 mM glycine, pH 7.4, 0.1% SDS) to which 1/1000 volume of Sigma’s protease inhibitor cocktail (cat. # P2714, containing AEBSF, Aprotinin, bestatin hydrochloride, E-64, EDTA and Leupeptin) was added. The buffer contained no azide, so as not to interfere with the Amplex Red. The protein in the lysate was quantitated, and made 4000 µg/ml. A 2.5 µl aliquot of lysate was added to 47.5 µl 1X PBS, then 50 µl of Amplex Red reagent/0.2 U/ml horse radish peroxidase, 0.5 mM NADPH solution were added, and the mixture was incubated at room temperature for 30 min. The absorbance of samples was read on an HTS 7000 Plus Bio Assay Reader (540 nm excitation, 590 nm emission-Perkin Elmer Life Sciences). The amount of H2O2 was determined by comparison to the standard curve.
2.5. Assessment of oxidative modifications of proteins
ROS-induced oxidative alterations in proteins (i.e., amino acid side chains modified with carbonyl groups by ROS) was detected using an OxyBlot Protein Oxidation Detection kit (Chemicon). Briefly, the HUVEC tubes were scraped from wells, sonicated for 1 min with pH adjusted SDS-PAGE buffer (as above) containing a final concentration of 0.1% Sigma’s P2714 protease inhibitor cocktail, then Matrigel and cell debris were removed by centrifugation at 10,000 × g for 10 min. Protein concentrations were determined using the bicinchoninic acid method (BCA Protein Assay, Pierce) at absorbance 540 nm. Lysate proteins (5 µg/sample) were derivatized with 1,3-dinitro-phenyl-hydrazine (DNP) following the manufacturer’s protocol, and subjected to SDS-PAGE on 12% gels. Proteins were electrotransferred to 0.22 µm nitrocellulose membrane blots (Bio-Rad). Blots were incubated for 1 hr at room temperature in blocking buffer (3% BSA with 0.02% NaN3 in TBST [25 mM Trizma base, pH 7.3, 3.0 mM KCl, 140 mM NaCl and 0.05% Tween-20,]), then incubated with the kit’s polyclonal DNP antibody (diluted 1:150) for 1 hr at room temperature. This was followed by incubation with goat anti-rabbit HRP-conjugated IgG secondary antibody (Bio-Rad cat. #170-6515), diluted 1-5000, for 1 hr at room temperature. The nitrocellulose membrane was treated with Super Signal West Pico chemiluminescence reagent (Pierce) for visualizing immunoreactive proteins on X-ray film. The band intensities of the many carbonyl-modified proteins in DEP-exposed sample lanes were visually compared with that of the negative control (i.e., endothelial tubes not exposed to DEPs) to make a qualitative evaluation, but differences were not quantitated.
2.6. Protein Preparation, Immunoprecipitation and Western Blot analysis
For Western blots of cell lysates, HUVEC tubes were collected and lysed by sonication for 1 min in pH adjusted SDS-PAGE buffer supplemented with 0.1% Sigma’s protease inhibitor cocktail, then Matrigel and cell debris were separated out by centrifugation at 10,000 × g for 10 min. The concentration of protein in each sample was measured using the bicinchoninic acid method (BCA Protein Assay, Pierce). Cell lysate proteins were loaded (30 µg per well) onto SDS polyacrylamide gels for electrophoresis.
Nuclear protein extracts were prepared from the endothelial tube cells by adapting a one hour minipreparation technique (Deryckere and Gannon, 1994). Briefly, cells were collected and sonicated 1 min in a relatively low salt lysis buffer (0.6 % Nonidet P-40 [NP-40], 150 mM NaCl, 10 mM HEPES pH 7.9, 1 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride [PMSF]), then centrifuged for 30 sec at 2500 rpm. The supernatant, which contained the nuclei, was next incubated for 5 min on ice, then centrifuged for 5 min at 5000 rpm. The pelleted nuclei were resuspended in a higher salt lysis solution (25% glycerol, 20 mM HEPES pH 7.9, 420 mM NaCl, 1.2 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 0.5 mM PMSF, 2 mM benzamidine, and 5 µg/ml of aprotinin), and incubated on ice for 20 min. Insoluble nuclear debris was pelleted by a 30 sec centrifugation. The protein concentration of the whole cell extract and the supernatant containing the nuclear extract was determined using a BCA Assay (Pierce), and the samples were frozen in liquid nitrogen and stored at −80°C until used for SDS-PAGE, when 20 µg protein per lane was applied to the gels.
For immunoprecipitation, 1.5 mg/50 µl Dynabeads (Immunoprecipitation kit-Dynabeads Protein A, Invitrogen) were incubated with primary rabbit monoclonal anti-human VE-Cad antibody (1:80 dilution, Abcam) at 4°C, overnight. The conjugated Dynabeads-antibody were placed on a magnet, the supernatant was removed, then cell extracts were added. The protein in the extract was quantitated, and made 4 mg/ml, and a 250 µl aliquot was added to the beads (= 1 mg) for a 10 min incubation with rotation at room temperature. The Dynabeads-antibody-extracts were then washed and the immunotargeted protein was eluted from the Dynabead sample following the manufacturer’s instructions. The eluates were denatured by heating at 95°C for 5 min and loaded onto 7.5% SDS polyacryamide gels for electrophoresis. Dynabeads conjugated with rabbit IgG (cat # 011-000-003, Jackson ImmunoResearch) were incubated with cell lysates as negative controls.
Because endothelial tubes are always plated at the same density and incubated in the same relative volume of medium, for secreted products, 40 µl of medium was used directly for SDS-PAGE. When this was done, samples were applied in order on the gels twice. After electrophoresis, the gel was cut in half. One half was used for the Western blot and the other half was stained with Coomassie blue to evaluate the equivalence of lane loading.
For all of the protein preparations mentioned (i.e., whole cell, nuclear, immunoprecipitated and medium proteins), after electrophoresis the proteins were electrophoretically transferred to 0.22 µm nitrocellulose membrane. A 1 hr incubation at room temperature was performed in blocking buffer (3% BSA with 0.02% NaN3 in TBST) to reduce non-specific reactivity of antibodies. All primary antibodies were diluted 1 to 1000 for use unless indicated. These were: rabbit anti-rat HO-1 antibody (SPA-895, Stressgen); rabbit anti-human anti-Nrf2 peptide antibody (ab31163, Abcam); rabbit anti-human polyclonal antibody against VEGF-A, VEGF-B, VEGF-C, VEGF-D and VEGFR2 from Abcam; rabbit anti-mouse GAPDH antibody (G9547, Sigma). Antibodies against VEGF-B and C were diluted 1to 1000, but VEGF-A required a 1 to 500 dilution, VEGF-D was 1–250 and VEGFR2 1 to 200. The respective companies all indicated that the non-human antibodies cross react with the corresponding human proteins, and this was indeed the case. Incubations were at 4°C, overnight. The secondary antibody for each was goat anti-rabbit IgG (H+L) conjugated to horseradish peroxidase (HRP) (Bio-Rad cat # 170-6515), diluted 1 to 5000, incubating blots for 1 hr at room temperature. Blots were enhanced with Super Signal West Pico chemiluminescence reagent (Pierce), and exposed to X-ray film.
2.7. Immunofluorescence Microscopy
HUVECs were plated as monolayers or were seeded onto Matrigel-coated 2-well chamber slides for tube formation. After DEP treatment for 24 hr, the HUVECs were fixed with 4% paraformaldehyde for 10 min. When antibody was required to reach intracellular locations, the fixed endothelial cells were permeabilized with 0.05% Triton X-100 for 10 min at room temperature. Nonspecific reactivity was blocked by incubation with 2% normal goat serum plus 0.02% NaN3 in PBS for 1 hr at room temperature. Endothelial tubes were incubated with primary polyclonal antibody against Nrf2 (Abcam ab31163) at a 1:100 dilution for 1 hr at room temperature, followed by a 1 hr room temperature incubation with goat anti-rabbit secondary antibody labeled with Alexa 488 (Molecular Probes/ Invitrogen cat # A11008), diluted 1 to 200. The VE-Cad antibody was used on monolayer and endothelial tube cells as in Chao et al. (2011), and the VEGFR2 antibody (Abcam) was diluted 1 to 200 prior to use.
Prolong Gold anti-fade mounting media including DAPI (Invitrogen) was added to sections, and slides were covered for an overnight incubation at 4°C. Monolayer and comparative endothelial tube images were observed at 100X and 400X magnifications on a wide field (epifluorescence) microscope (Olympus IX71 Inverted Microscope). Other endothelial tube images were observed at 630X magnification on a Leica TCS SP5 Spectral Confocal Microscope.
2.8. Permeability Assays
For monolayer cultures of HUVECs, cells were seeded at 1.5 X 105 cells per well and grown to confluence on fibronectin (7 µg/ml)-coated filters of transwell units (6.5 mm, 0.4 µm pore size, Corning Costar). A non-confluent monolayer culture (1.5 X 104 cells per well, 15.6 cells/mm2) served as a control for 100% permeability. Culture medium volume in each upper chamber was 100 µl and in each lower chambers was 600 µl. When the cultures reached confluence (3 to 5 days), the cells in the upper wells were exposed to various concentrations of DEP (0, 1, 10, 100 µg/ml), incubating them for 24 hours at 37°C. Then, FITC-conjugated dextran (molecular mass 70 kDa, Sigma) was added and allowed time to penetrate to the lower chamber. In initial experiments, unexposed confluent monolayers were used to see how long it took for any FITC-dextran to reach the medium in the lower chamber. Aliquots (100 µl/well) were collected in 3 hours intervals (0–3, 3–6, 6–9, and 9–12 hours time frames) while continuously incubating the cultures at 37°C. Small quantities of fluorescent dextran, assessed at 490 nm, penetrated the confluent monolayers and gained access to the lower chamber between 1–6 hrs. Since no dextran was obtained in the 7–9 hour period after adding the agent (data not shown), and since >98% of this dextran entered the lower chamber in the first 4 of the 6 hr preliminary test time, 4 hr after FITC-dextran application was chosen as the time to analyze the monolayers for permeability. Samples, including non-confluent control samples, were also evaluated by phase contrast microscopy (200X, Zeiss-Axiovert 40 Inverted Microscope).
Since no method exists for evalulating the permeability of in vitro endothelial tubes, it was reasoned that if DEP caused leakiness of the capillary-like structures, the dextran would be able to enter leaky cells of the capillary-like structures due to the lack of blood flow. First endothelial tubes were made, seeding 1.5 X 105 HUVECs per well onto Matrigel-coated 6 well plates. Tubes were allowed to form over 12 hr. As controls, some wells were coated with Matrigel only (no cells were plated), and some wells were coated with Matrigel, then plated with cells to form tubes, but were never exposed to DEP (0 µg/ml DEP). Test sample wells were coated with Matrigel, then cells were added and allowed to form tubes for 12 hr before exposure to 1, 10, or 100 µg/ml DEPs for 24 hours at 37°C. After incubation, free DEPs were washed away with PBS, and medium (1 ml per well) containing FITC conjugated-dextran at a final concentration of 1 mg/ml was added into the culture dishes and incubated for 4 hours. At the end of this time period, the medium/dextran was removed and discarded. The endothelial tubes were washed twice with PBS and collected by scraping them from the wells into eppendorfs containing 25 mM Tris, 192 mM glycine, pH 7.4, 0.1% SDS to which 1/1000 volume of Sigma’s protease inhibitor cocktail (cat. # P2714, containing AEBSF, Aprotinin, bestatin hydrochloride, E-64, EDTA and Leupeptin) had been added. The sample was gently pipetted up and down to make an extract of the cells. After BCA quantitation, samples were all adjusted to 4 mg protein per ml. To ascertain if any FITC label had been able to access the tube structures, an aliquot of 200 µl of each lysate was loaded into 96-well plates for determination of the relative fluorescence units (RFU) of each sample. Reading was done on an HTS 7000 Plus Bio Assay Reader at 490 nm-Perkin Elmer Life Sciences. The “Matrigel only” samples were treated identically as the test samples, although there were no cells to extract. The FITC-dextran adhering to Matrigel samples was averaged and plotted, and is indicated as the blank in Figure 4D. The RFU value of the second control, dishes of endothelial tubes receiving no DEPs, represented the baseline adherence of dextran to unexposed tubes, and is indicated as 0 µg/ml DEP in Figure 4D. These values are indicated on the graph in Figure 4. RFU values greater than this baseline value are considered to be representative of the passage of FITC-dextran into the cells of permeable capillary-like structures, thereby indicating a level of endothelial tube permeability after DEP exposure.
2.9. Statistics
For statistical analysis, each experiment was performed at least 2 times, and each time, samples were run in triplicate. The results were expressed as means ± SD and were analyzed using Student t-tests and one way parametric ANOVA. p < 0.05 and p < 0.01 indicated by * and ** respectively, were considered statistically significant.
3. RESULTS
3.1. DEPs induce generation of ROS in capillary-like endothelial tubes
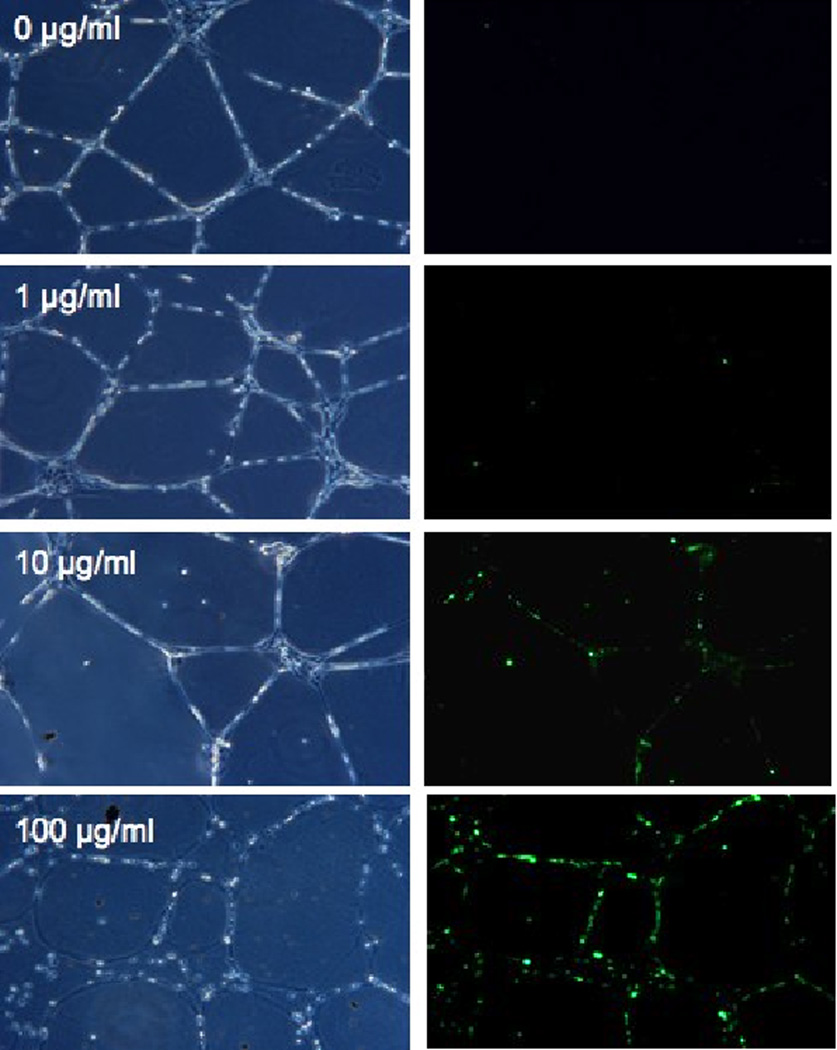
Previous work (Chao et al., 2011) demonstrated that a 24 hr exposure of DEP is cytotoxic to human umbilical vein endothelial cell (HUVEC) pre-assembled into capillary-like tubes. To evaluate whether cytotoxicity was a consequence of oxidative stress, as had been shown for monolayer HUVEC cultures (Bai et al., 2001; Furuyama et al., 2006), endothelial tubes were exposed to DEP (0, 1, 10, or 100 µg/ml) and assessed. Oxidative stress in response to DEPs was evaluated by labeling reactive oxygen species with carboxy-2’,7’-dichlorodihydrofluorescein diacetate and visualizing the fluorescence by microscopy (Fig. 1A). This method also allowed evaluation of changes in structural integrity of the capillary-like tubes. As seen in the left panels of Fig. 1A, HUVEC tube integrity is minimally affected by DEPs at 1 µg/ml, and mildly affected by 10 µg/ml. At 100 µg/ml the tube cells remain in the same position on the plate, but round up and lose cell-cell contacts. The right panels of Fig 1A show DEP-induced ROS as fluorescent green puncta (via fluorescent carboxy-DCF). When 1 µg DEPs/ml was used, ROS production was minimal, with only 5–10 small green punctate areas indicating ROS generation. Samples treated with 10 µg/ml DEPs showed more fluorescence. In the samples treated with 100 µg DEPs/ml for 24 hr, ROS generation was very abundant, with nearly all the cells fluorescing.
Fig. 1. DEPs cause generation of ROS in endothelial tubes.
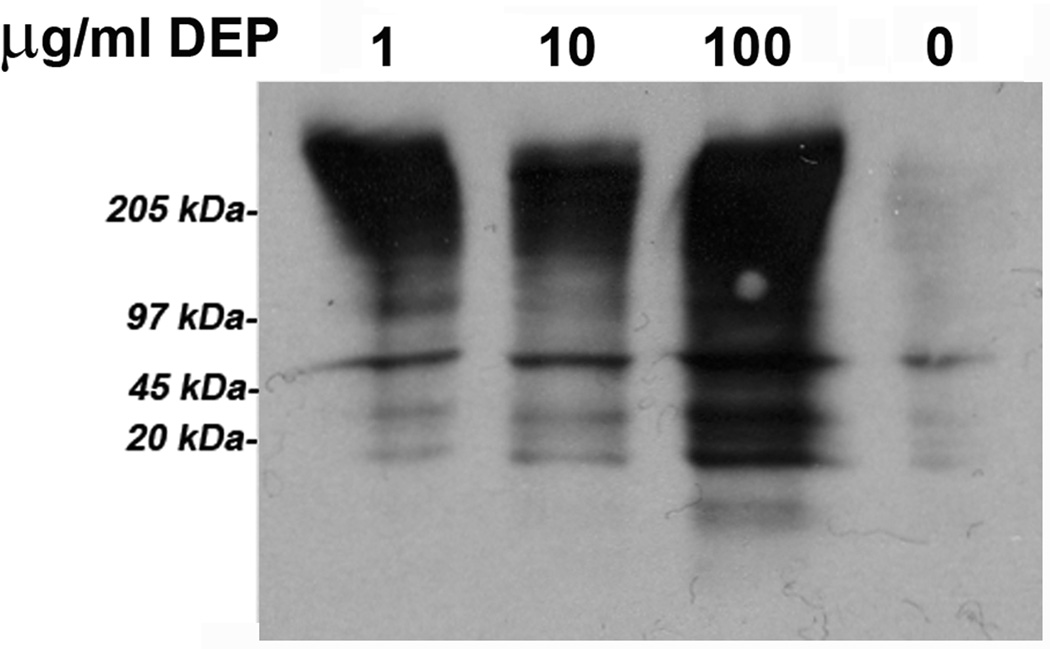
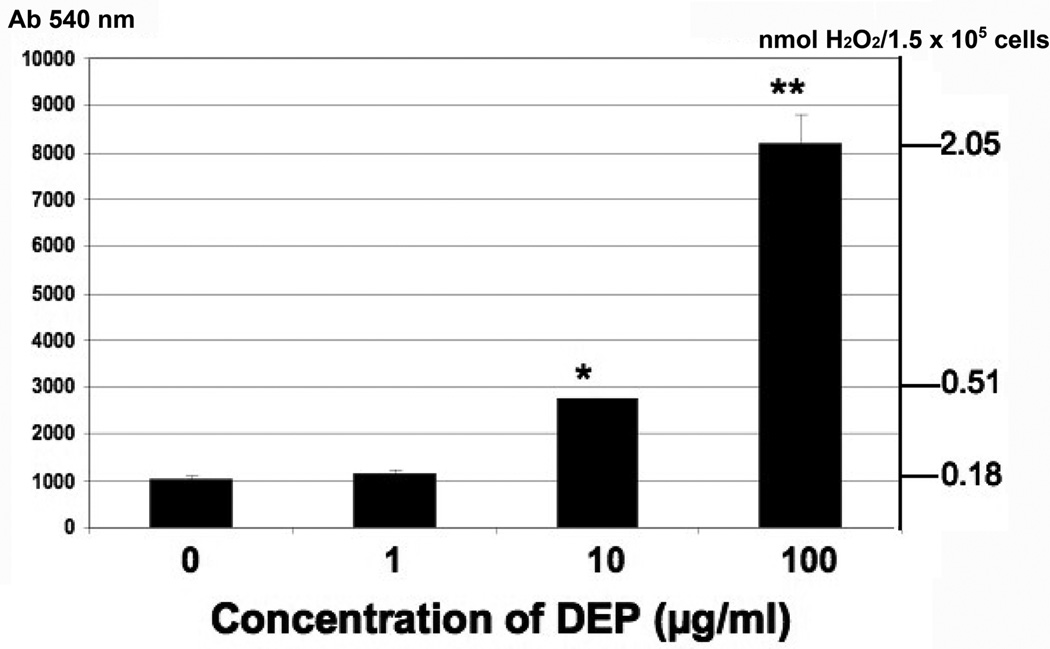
(A) HUVEC tubes were treated with no DEPs, or 1, 10, or 100 µg DEPs/ml for 24 hr. ROS detection was accomplished with the Image-iT™ Live Green Reactive Oxygen Species Detection Kit. Tubes were visualized at 200X magnifications on an Olympus IX71 Inverted Microscope. Phase contrast images are shown on the left, and the epifluorescence is shown on the right. The green punctuate fluorescence represents conversion of non-fluorescent DCFH-DA (2’, 7’-dichlorohihydrofluorescein diacetate) to fluorescent DCF by ROS; (B) ROS were also detected by an increase in carbonyl groups, indicating oxidization of proteins in endothelial tubes treated with increasing concentrations of DEPs. Protein lysates were made from HUVEC tubes after exposure to DEPs for 24 hr. Lysates were quantitated, and equal amounts of protein from each treatment group were used to derivatize carbonyl groups with dinitro-phenyl hydrazine (DNP). Samples were then electrophoresed and blotted for reaction with a DNP-specific antibody; (C) H2O2 production generated in endothelial tube cells in response to 0, 1, 10 and 100 µg DEPs/ml was assessed by Amplex Red assays, graphed as relative light units (Y axis, left side). The nmol amount of H2O2 was experimentally determined by comparison to a standard curve (Y axis, right side), calculated from a standard curve where assays were run with known nmol amounts of H2O2 from serial dilutions (not shown). Error bars indicate ± SD (n = 3). A parametric ANOVA statistical analysis was performed to determine significance (* indicates p < 0.05; ** indicates p < 0.01).
Endothelial tube extracts were made after DEP exposure to measure oxidative modifications to proteins. Equal amounts of protein were loaded in each well of SDS-polyacrylamide gels for Western blots probed with an antibody against derivatized carbonyls (Fig. 1B). DEPs caused increased protein oxidation as assessed by the intensity of the immunoreactivity as compared to endothelial tubes not exposed to particles. The two lower DEP exposures (1 and 10 µg/ml) were not significantly different from each other, however, the 100 µg DEPs/ml exposure greatly increased the immunoreactivity, indicating increased levels of oxidized carbonyl groups in endothelial tube cell proteins.
To identify if H2O2 is among the ROS generated in response to particles, endothelial tube cells were exposed to 0, 1, 10 or 100 µg DEPs/ml for 24 hr, then subjected to Amplex Red analysis. As shown in Fig 1C, cells exposed to 1 µg/ml DEPs generated only 1.1 times more H2O2 (0.18 nmol) than untreated cells (0.16 nmol). At the 10 µg/ml and 100 µµg/ml concentrations of DEPs, production of H2O2 in the tube cells was 2.8 times higher (0.51 nmol, p < 0.05) and 12.8 times higher (2.05 nmol), respectively, than controls.
3.2. Generation of ROS causes an Nrf2-dependent detoxification response
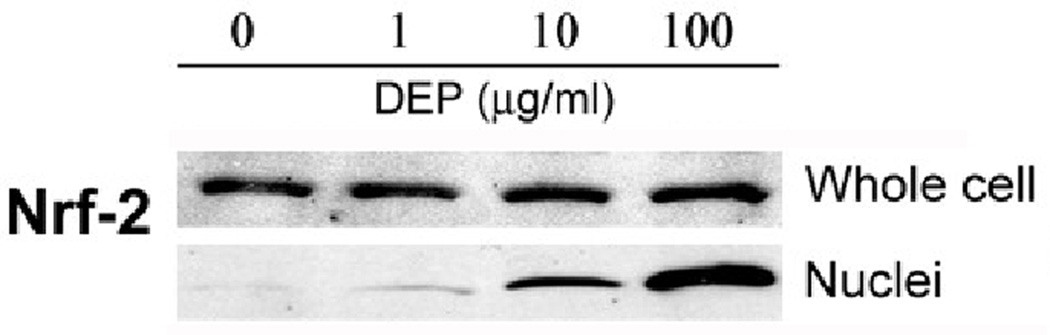
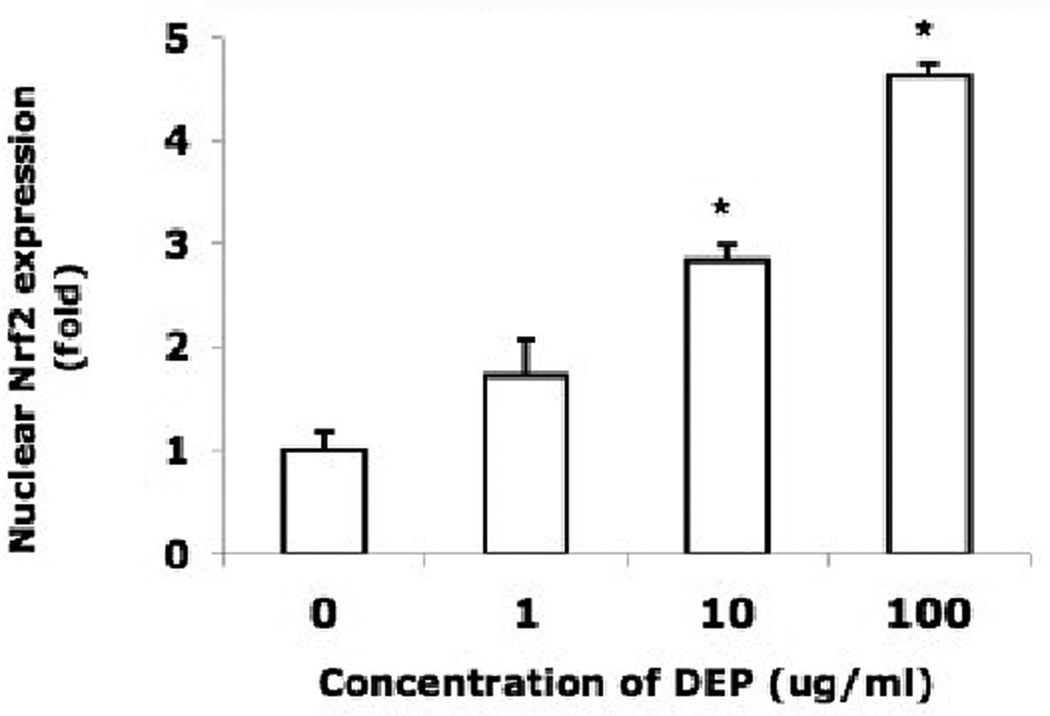
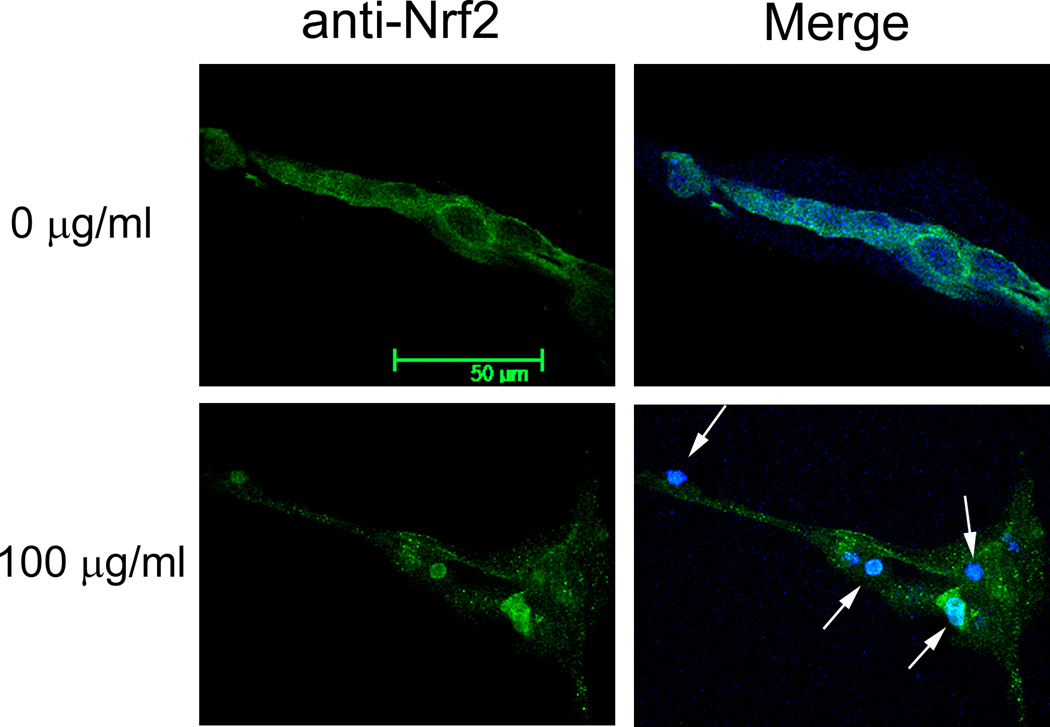
Detection of H2O2 and oxidative modifications of proteins suggests that endothelial tube cells would likely mount a detoxification response to enhance their chance of survival. Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor known to regulate detoxification enzymes. This factor responds to oxidative stress by translocating from the cytoplasm to the nucleus, where it binds to gene promoter regions, enhancing transcription of oxidative stress genes. To evaluate whether this occurs in the 3 dimensional capillary-like model, endothelial tubes were assessed for their expression of Nrf2 in response to DEPs. Immunoblots of Nrf2 derived from two types of protein extracts were performed. One was a whole cell extract of DEP-treated tubes; this blot demonstrated that the overall level of Nrf2 does not change in response to DEP exposure (Fig 2A, upper blot). A separate set of DEP-exposed endothelial tubes was used to isolate and prepare a nuclear protein extract. The levels of Nrf2 in the nuclear extract increased with increasing DEP concentration (Fig 2A, lower blot). At 1 and 10 µg/ml DEPs, the amount of nuclear Nrf2 was respectively 1.6 and 3.7 times higher than that of the non-exposed control (Fig. 2B). At the highest concentration of DEPs, it was 4.6 times greater. The shuttling of the transcription factor from the cytoplasm to the nucleus is shown in situ in Fig. 2C, where the Nrf2 immunofluorescence pattern of untreated endothelial tubes is compared to those treated with 100 µg DEPs/ml. In unexposed capillary-like tubes, Nrf2 (green) was present predominantly in the cytoplasm after 24 hr in culture (top panels). Following a 24 hr exposure to 100 µg DEPs/ml, the transport of Nrf2 to the nucleus was dramatic (lower panels). Merging the Nrf2 and DAPI images clearly shows much of the green Nrf2 colocalizing with the blue nuclei in the DEP-exposed endothelial tubes. These data place Nrf2 in the correct location after DEP exposure to facilitate the expression of detoxification response enzymes.
Fig. 2. DEPs induce translocation of Nrf2 from the cytoplasm to the nucleus.
(A) Capillary-like endothelial tubes were incubated with no DEPs, or 1, 10 or 100 µg DEPs/ml for 24 hr. Two western blots are shown, one of Nrf2 from whole cell protein isolates (top) and one of Nrf2 in extracts of isolated nuclei (bottom). The blots indicate that increasing amounts of DEPs increase the amount of Nrf2 that is translocated to the nucleus. (B) The nuclear blot was scanned and the value for the no DEP exposure sample was set to 1. The other nuclear band densities were normalized to this sample (* indicates p < 0.05); (C) Confocal microscopy of Nrf2 plus or minus DEPs: With no DEPs, Nrf2 immunofluorescence (green) is seen mostly in the cytoplasm with only minor overlapping of the blue fluorescence. With exposure to 100 µg DEPs/ml for 24 h, Nrf2 is found to be translocated to the nucleus. The magnification (630X) in all panels is same. Scale bar = 50 µm. Nuclei are stained blue with DAPI.
3.3. Expression of antioxidant protein HO-1
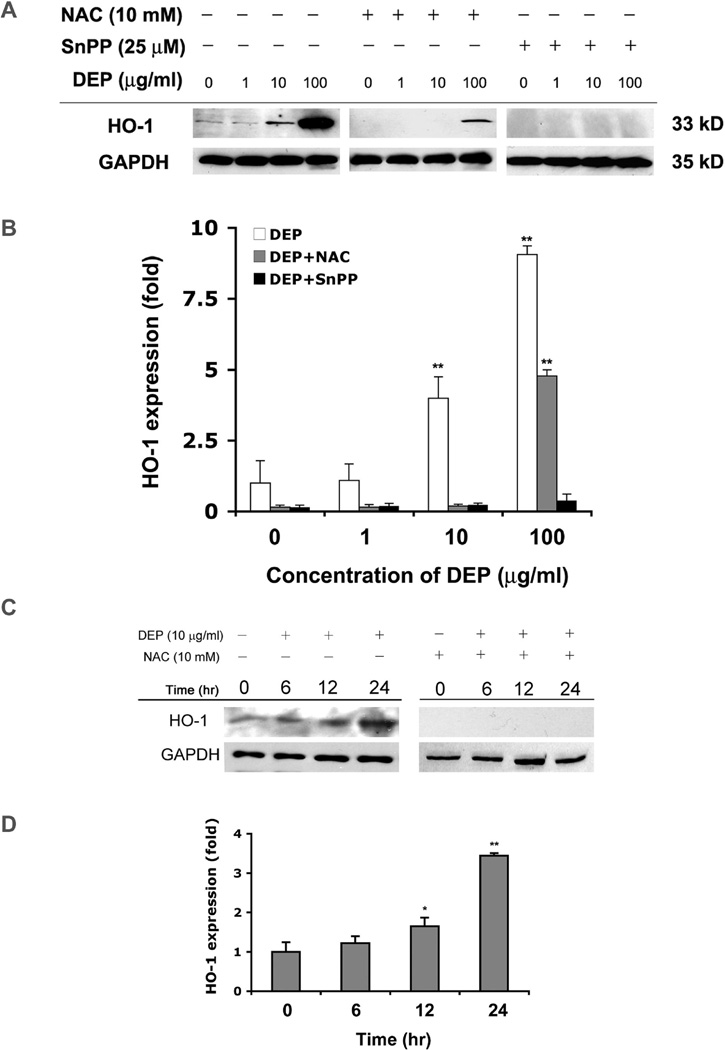
Heme oxygenase-1 (HO-1) is a detoxification enzyme whose expression is enhanced by Nrf2 (Buckley et al., 2003; Hsieh et al., 2009). Therefore, levels of HO-1 were evaluated as a representative of ROS-minimizing enzymes. After a 24 hr exposure to DEPs, HUVEC tube proteins were analyzed for HO-1 levels by Western analysis (Fig 3A). A strong increase was observed at the 10 µg/ml exposure, and an extreme response was observed at 100 µg/ml (Fig. 3A, left lanes). N-acetyl cysteine (NAC) was added to medium of one set of samples because of its ability to increase endogenous glutathione levels and reduce the effects of oxidative stress and cytotoxicity (Furuyama et al., 2006; Olivieri et al., 2001; Pocernich et al., 2000). NAC added to the cultures attenuated, but did not totally block, the induction of HO-1 expression (Fig 3A, middle lanes). The more specific inhibitor of HO-1, tin protoporphyrin (SnPP), totally inhibited HO-1 expression (Fig 3A, right 4 lanes). Fig 3B shows a quantitation of the blots, and accentuates that HO-1 is approximately 3.8 times higher after exposure to 10µg/ml DEPs, and 9 times higher after exposure to 100 µg/ml DEPs. NAC and SnPP significantly reduced the HO-1 response, with the specific HO-1 inhibitor having more effect at the 100 µg/ml DEPs concentration than NAC.
Fig. 3. DEPs induce endothelial capillary-like tube expression of HO-1.
(A) After a 24 hr exposure to no DEPs or 1, 10 or 100 µg/ml, endothelial tubes were lysed and proteins extracted for Western analysis. The same was done with unexposed and exposed samples plus or minus 10 mM NAC or 25 µM SnPP. The first 4 lanes of the immunoblot demonstrate that HO-1 is induced by DEPs in a dose dependent manner. ROS is an intermediary for HO-1 induction, since HO-1 levels are reduced when NAC is added (4 middle lanes). Furthermore, SnPP totally inhibits the HO-1 expression induced by DEPs (last 4 lanes). Equal protein loading was confirmed with GAPDH immunoreactivity. (B) Histograms show a quantitation of HO-1 levels after 24 hr exposure to DEPs. Bars are the relative fold change compared to the control with no added DEPs. Asterisks indicate statistical significance (* indicates p < 0.05 and ** indicates p < 0.01). (C) Western blotting was performed to determine the timing of HO-1 expression after addition of 10 µg DEPs/ml to tubes, plus and minus NAC. Equal protein loading was confirmed by GAPDH immunoreactivity. (D) Quantitation of HO-1 expression at 0, 6, 12, and 24 hr, derived from the Western blots. The HO-1 band intensity for the 0 time point was defined as one, and all other time points are shown as the fold-change relative to this point. Values are means ± SD, n = 3. Statistical analysis was by parametric ANOVA and asterisks indicate significance (p < 0.05).
To examine the timing for the increase in HO-1 expression, HUVEC tubes were treated with 10 µg/ml DEPs for 6, 12 and 24 hr, and HO-1 was evaluated by Western analysis. To attenuate ROS, 10 mM NAC was added to sister cultures at the same time the 10 µg/ml DEPs treatment was started. DEPs caused an increase in HO-1 by 6 hr, which grew stronger at 12 and at 24 hr (Fig 3C). Again, NAC totally inhibited the induction of HO-1 in tubes exposed to 10 µg/ml DEPs. Quantitation of the blots in histogram form (Fig 3D) shows HO-1 was increased over controls by 1.2 fold at 6 hr, by 1.6 fold at 12 hr, and by ~3.5 fold at 24 hr. This is in good agreement with the previous experiment, where the 24 hr response value was 3.8 fold higher than controls. These results suggest that exposure to 10 µg/ml DEPs causes HO-1 induction to begin slowly, taking between 6 and 12 hr to get a 60% increase over controls, but that the response intensifies by 24 hr, being 350% higher than controls.
3.4. DEPs cause endothelial permeability
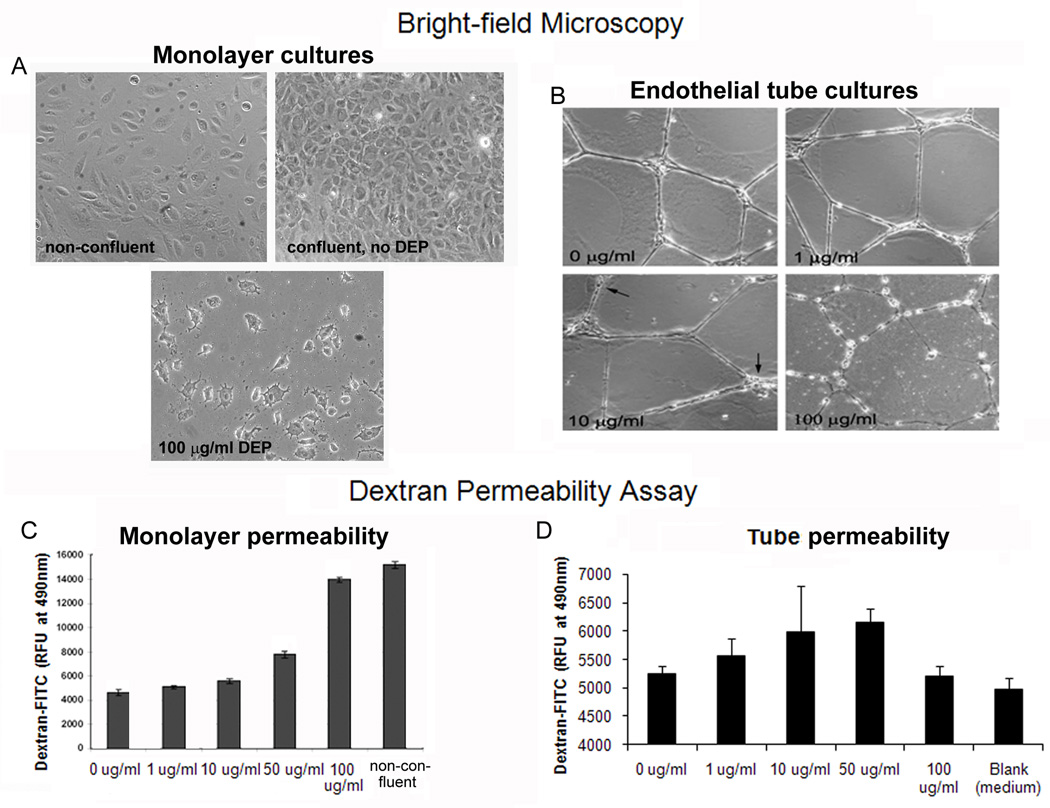
Since endothelial tube structures do not totally cover the culture dish, experiments to evaluate whether DEPs induce endothelial permeability were first performed using confluent monolayers of cells to assess whether permeability resulted after a 24 hr DEP exposure. This was done in a standard 2 chamber FITC-labeled dextran assay. The positive controls were non-confluent plates (Fig. 4A, left), where cell contact was infrequent and dextran would have free passage. The negative controls were fully confluent plates of cells (Fig. 4A, right) that had not been exposed to DEP, and thus represented the minimum permeability possible. As seen in Fig 4C, with increasing concentrations of DEPs, the levels of dextran able to reach the lower chamber increased. Cultures of endothelial tubes were set up and exposed to DEP (Fig. 4B). Lacking a standard method to assess the permeability of capillary-like tubes, we evaluated whether dextran was taken up by the endothelial tubes exposed for 24 hr to medium or DEP. After the 24 hr incubation, fluorescent dextran was added to the washed cultures and remained there for 4 hr. As seen in Fig. 4B, endothelial tube structures are altered with increasing concentration of DEPs. At 100 ug/ml, cells have rounded up, pulling and thinning the cytoplasm between them. Increasing concentrations of DEP correlated with small increments of dextran uptake, with the exception of the 100 µg/ml DEP sample (Fig. 4E). This is likely because this exposure causes ~50% of the cells to die by 24 hr (Chao et al., 2011). While the design of the endothelial tube experiment cannot be made to yield data as clear as monolayer cultures, there was a correlative trend between DEP exposure of endothelial tubes and how much fluorescence they were able to retain, suggesting permeability.
Fig. 4. DEPs induces endothelial permeability in monolayer and tube cultures.
(A) Bright field microscopy showing monolayers of confluent endothelia 0 µg/ml (no DEP), non-confluent cells, and cells treated with 100 µµg/ml DEPs. (B) Bright field microscopy showing endothelial tubes either not treated with DEPs or exposed to 1, 10 or 100 µg/ml DEPs. With increasing levels of DEPs, endothelial tube cells are increasingly pulling away from each other and rounding up. (C) Cell-cell junctions of monolayer cultures are disrupted by DEP exposure as determined by dextran gaining access to the lower chamber (i.e., the culture is permeabilized). (D) FITC-dextran was added to endothelial tubes for 4 hr post a 24 hr exposure to different concentrations of DEP. The RFU of FITC-dextran that was not removed by washing the endothelial tubes suggests the fluorescent dextran has entered inside the tube structures. At 100 µg/ml about 50% of the cells are dead, and the remaining cells look unhealty. It is assumed this is why they do not take up the dextran.
3.5 DEPs stimulate secretion of VEGF-A into the culture medium
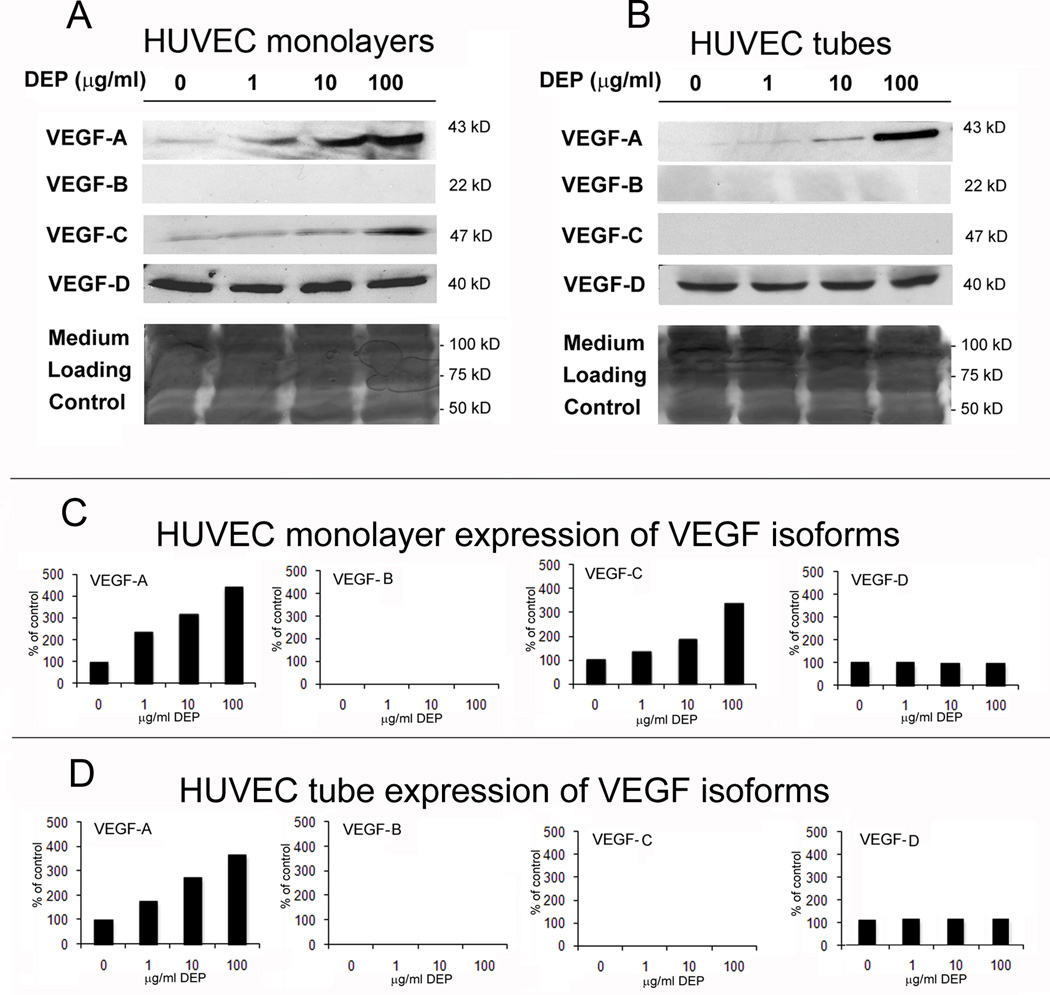
In experiments with monolayer cultures of HUVECs not involving DEP exposure, HO-1 expression had been shown to induce secretion of vascular endothelial cell growth factor-A (VEGF-A) (Dulak et al., 2002; Lin et al., 2008). Therefore, it seemed reasonable to expect that the HO-1 induced by DEPs might ultimately induce secretion of endothelial VEGF-A. Since the indications relating HO-1 and VEGF came from data using HUVECs in monolayer culture, and differences between the behaviors of monolayer and tube cultures had been observed (Chao et al., 2011), the expression of VEGF isoforms in HUVEC monolayers and capillary-like tubes was compared. Endothelial tubes and monolayers were treated for 24 hr with 0, 1, 10 or 100 µg/ml concentrations of DEP, then medium was isolated for Western analysis (Fig. 5A and B). While both capillary-like tubes and monolayer cultures secreted VEGF-A and VEGF-D into the medium, monolayer cultures also secreted VEGF-C. In both types of culture VEGF-D was constant, but the secretion of VEGF-A increased with increasing DEP concentration. This data verified that the key VEGF isoform known to be involved in endothelial permeability, VEGF-A, was induced in a dose dependent manner in DEP-treated HUVECs plated as monolayers and as capillary-like tubes. Comparative densities from the Western bands are graphed in Fig. 5C and D.
Fig. 5. Increasing DEP concentrations induce increasing secretion of VEGF-A by endothelial tube cells.
(A) Endothelial tubes and monolayers of endothelial cell cultures were exposed to no DEPs or 1, 10 or 100 µg/ml for 24 hr. Medium was collected from each exposure sample, and 40 µl of each were applied to the wells of an SDS polyacrylamide gel. After electrophoresis, each gel was cut in half. One half was used for Western analysis with the VEGF-A and the VEGF-B antibodies. This half was then stripped and reprobed with the VEGF C and VEGF D antibodies. The other gel halves were stained with Coomassie blue, to ascertain equal loading of samples by comparing the intensity of the medium proteins across the gel. (B) Densitometric scans were made of the blots, and the data was used to compare the relative levels of each VEGF isoform to the respective DEP concentrations.
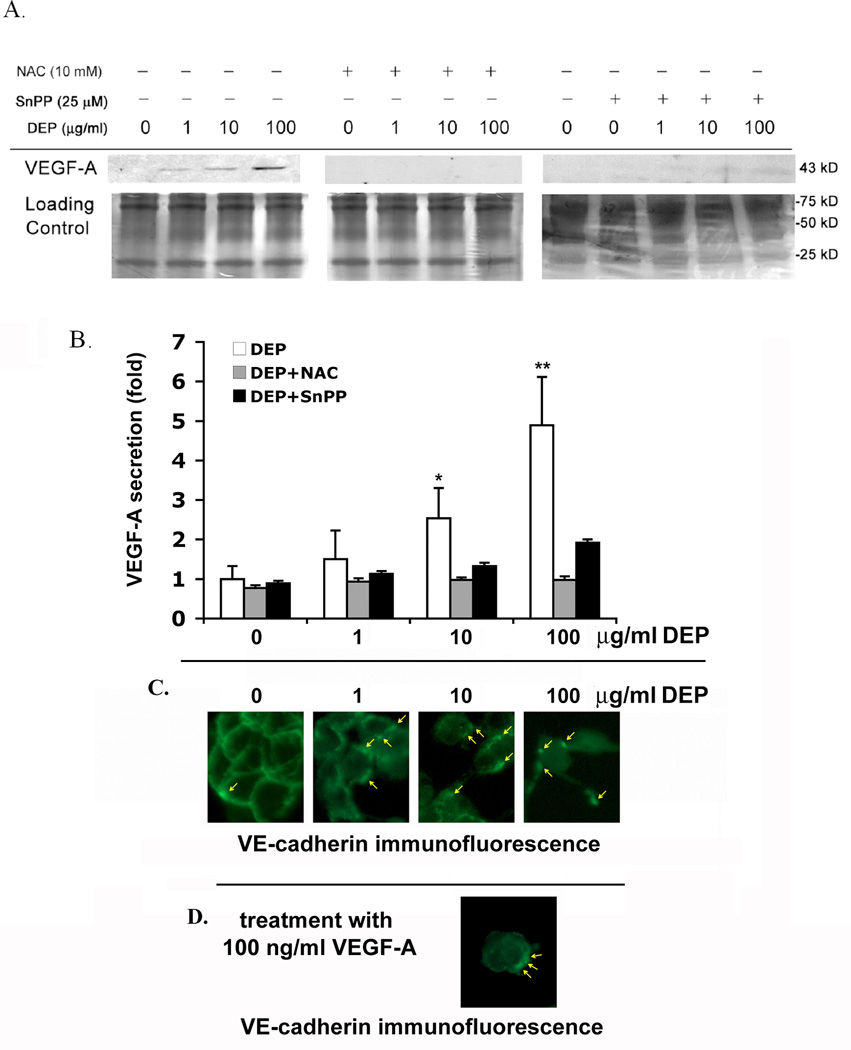
To evaluate whether DEP-induced ROS are responsible for the VEGF-A secretion, endothelial tubes were exposed to 0, 1, 10 or 100 µg/ml concentrations of DEP for 24 hr with or without 10 mM NAC. The Western blot in Fig 6A indicates that, although VEGF-A increases with increasing DEP concentrations, the addition of NAC totally inhibited its secretion. To investigate whether HO-1 is specifically responsible for VEGF-A secretion, the HO-1 inhibitor SnPP was employed. VEGF-A secretion was attenuated, but not totally diminished by SnPP treatment. This strongly suggests that the effect of DEPs is mediated by ROS, and that HO-1 expression is one of the major effectors of DEP-induced VEGF-A secretion, although not likely the only one. Relative expression of the VEGF-A levels from these experiments is shown graphically in Fig. 6B. VE-cadherin is a major player in preventing permeability of cells. The effect of DEP on VE-cadherin in endothelial monolayers is shown in Fig. 6C. Yellow arrows point out VE-cadherin internalization, and indicate that increasing amounts of DEP cause increasing levels of VE-cadherin to be pulled from the cell surface into globules under the cell membrane (Fig 6C.). To evaluate whether VEGF-A had the ability to rearrange the VE-cadherin pattern of monolayers, cells that were not exposed to DEP were treated for 24 hr with 100 ng/ml VEGF-A (Fig. 6D). Most of the cells had separated from each other, and the globules of submembrane VE-cadherin resembled the pattern of cells exposed to 10 µg/ml DEP. This suggests the possibility that VEGF-A contributes to the permeability of the cell junctions, as does DEP exposure.
Fig. 6. DEPs induce VEGF-A secretion, and both VEGF-A and DEPs disrupt membrane VE-cadherin.
(A) Secretion of VEGF-A from capillary endothelial tubes is related to DEP-induced HO-1 expression. HUVEC tubes were exposed to no DEPs or 1, 10 or 100 µg DEPs/ml plus or minus NAC (10 mM) or plus or minus SnPP for 24 hr. Medium was collected and run on gels for immunoblotting. Without NAC and SnPP, VEGF-A secretion increased in a dose dependent manner. NAC totally blocked VEGF-A secretion, and SnPP mostly blocked it, indicating that factors other than HO-1 may contribute to the induction of VEGF-A secretion. To assure that equal amounts of protein were loaded in each lane, half of each gel was used for the blot and the other half, loaded in the same order with the same amount of protein, was stained with Coomassie blue (loading control). (B) Densitimetric quantitation of VEGF-A secretion compared to each DEP exposure concentration. The relative intensity of VEGF-A bands from the Western was graphed, with the value of the 0 µg/ml DEP treatment sample set as 1. Values are mean ± SD, n = 3. Statistical analysis was by parametric ANOVA (* indicates p < 0.05 and ** indicates p < 0.01) compared with the control, and were considered significant. (C) The membrane localization of VE-cadherin is disrupted by DEP exposure as assessed by epifluorescence microscopy. Confluent endothelial monolayers were treated for 24 hr with and without DEP. With no DEP exposure, cells remain in a monolayer with VE-cadherin appearing continuously around the cells’ membrane. Increasing concentrations of DEPs result in cell-cell separations and in globules of VE-cadherin accumulating intracellularly near to, but no longer at, the cell membrane (yellow arrows). These globules have a more interior localization. (D) A confluent monolayer culture of endothelial cells was treated for 24 hr with 100 ng/ml VEGF-A, then reacted with VE-cadherin antibody. VEGF-A treatment caused dissociation of cells from each other and resulted in VE-cadherin disruption. Arrows point to areas where VE-cadherin has moved from the cell membrane to more interior, sub-membrane intracellular locations. The resulting VE-cadherin pattern most resembles that of endothelial cells treated with 10 µg/ml DEP.
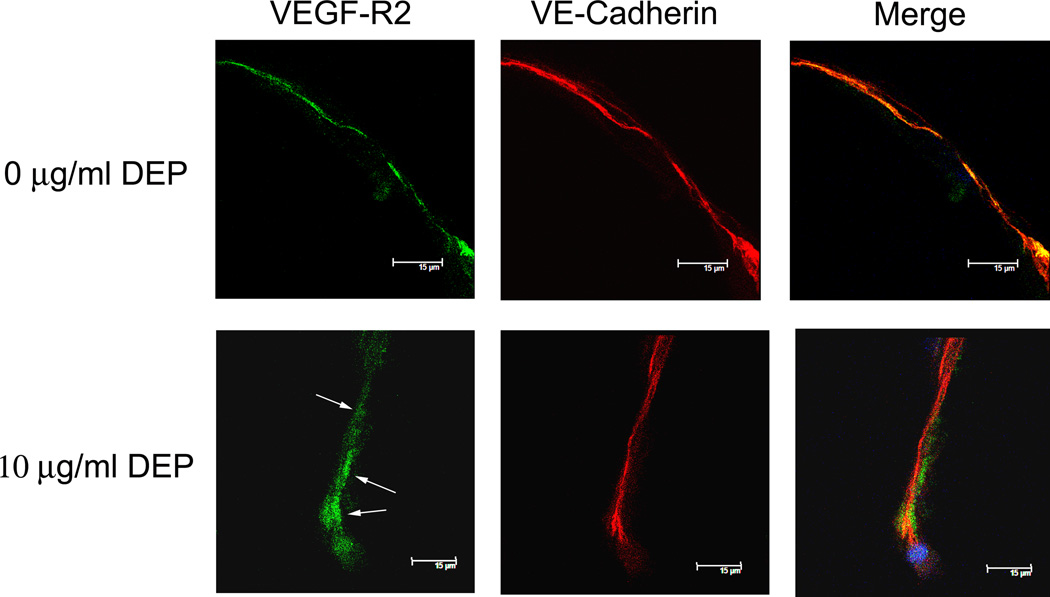
Lastly, DEP exposure and its impact on VEGFR2, the major VEGF-A receptor, was explored with respect to the changes in VE-cadherin previously reported (Chao et al., 2011). This was examined because VE-cadherin has been shown to associate and interact with VEGFR2 (Carmeliet et al., 1999; Lampugnani et al., 2003). Binding of VEGF-A to VEGFR2 has been implicated in interrupting the VE-cadherin-VEGFR2 interaction, internalizing VE-cadherin away from the cell surface and allowing for disruption of cell-cell junctions and permeability (Carmeliet et al., 1999). For these experiments, only the 0 µg/ml, 1 µg/ml and 10 µg/ml DEP exposures were employed in cell lysate pull down assays. The 100 µg/ml DEP concentration disrupted VE-cadherin too extremely and killed ~50% of the cells, so was not used. Cell lysates were prepared after the 24 hr DEP exposures. Western analysis was performed using antibodies against VE-cadherin and VEGFR2 (Fig. 7A, left). This showed both molecules were present. The avidity of the antibodies is unknown, so the bands cannot truly connote a quantitative ratio between the amount of VE-cadherin and VEGFR2 in the cell membrane. In Fig. 7A, right, a pull down assay is shown. Here, VE-cadherin had first been immunoprecipitated from the unexposed and exposed cell lysates. The immunoprecipitates were run in duplicate on an SDS polyacrylamide gel, then blotted. The blot was cut in half for reaction against each of the antibodies. The VE-cadherin levels at these DEP concentrations was mostly the same since it was the initial pull down epitope. However, the amount of VEGFR2 pulled down with VE-cadherin decreased dramatically with increasing concentrations of DEP. Less VEGFR2 was associated with VE-cadherin as the DEP concentration increased. Immunofluorescence analysis (Fig. 7B) demonstrated that, in unexposed endothelial tubes, VE-cadherin (red) and VEGFR2 (green) co-localize in areas of the membrane, indicated by the merged orange-yellow color (i.e., all green overlaps with red) for the 0 µg/ml DEP image. In the merged image of the 10 µg/ml DEP exposure, it is clear that the two molecules are largely disassociated by the distinct green and red visible in the merged image panel. The colocalization of VE-cadherin with VEGFR2 is greatly reduced. The immunofluorescence analysis supports the pull down assays, showing that, in the absence of DEP, nearly all the VEGFR2 and VE-cadherin are associated, but after DEP exposure, the interaction between the two molecules is attenuated.
Fig. 7. DEP interrupt interactions between VE-cadherin and VEGFR2.
A. Left, Western analysis showed VE-cadherin and VEGFR2 in endothelial tube cell lysates. Right, pull down assays were performed by first using VE-cadherin antibody to immunoprecipitate the adherens junction protein from the cell lysates of samples exposed to 0, 1, or 10 µg/ml DEPs. The immunoprecipitated product was applied to SDS polyacrylamide gels, then Western analysis was performed. The VE-cadherin immunoreactivity indicates that the protein was cleanly isolated by the antibody. The VEGFR2 immunoreactivity indicates that VEGFR2 was associated with VE-cadherin, allowing it to be pulled down with the molecule by the VE-cadherin antibody. With increasing DEP concentrations, less VEGFR2 was immunoprecipitated with VE-cadherin, suggesting that with increasing concentrations of DEPs, there is a loss of association of VE-cadherin and VEGFR2; (B) Confocal immunofluorescence microscopy with VEGFR2, detected by green fluorescence, and VE-cadherin, detected by red fluorescence: The data show that, in the absence of DEP exposure, VEGFR2 is co-localized with VE-cadherin (see orange-yellow merged color). However, 24 hr after 10 µg/ml DEP exposure, little orange-yellow is seen in the merged images. Instead, the red color localizing VE-cadherin, and the green localizing VEGFR2 are mostly distinct, indicating disassociation of the molecules.
4. DISCUSSION
To summarize our work, DEPs induced oxidative stress in endothelial cells. Hydrogen peroxide was generated, and, as expected, cells responded by transporting Nrf2 to the nucleus to facilitate transcription of genes to defend against ROS. Heme-oxygenase-1 was a defense enzyme induced by Nrf2. In response to DEP exposure, endothelial cells expressed VEGF-A and became permeable. There are several reports in the literature indicating that VEGF-A is a factor causing endothelial permeability (Esser et al., 1998; Kevil et al., 1998; Nagy et al., 2006; Roberts and Palade, 1995; Senger et al., 1983; Strugar et al., 1994; Weis and Cheresh, 2005). However, while our data is consistent with this interpretation, it cannot rule out the possibility that other aspects of oxidative stress cause the endothelial permeability.
The work was begun to gain an understanding of the effects DEPs might have on lung capillaries. HUVECs were chosen for the experiments for three reasons: This endothelial cell type has been used previously by others to study the effects of particulate exposures (Garcia et al., 1989; Sumanasekera et al., 2007; Yamawaki and Iwai, 2006); The cells have been extensively characterized, having been used in more than three hundreds publications studying angiogenesis; also in vitro endothelial capillary tubes share many similarities with in vivo capillary structures (Donovan et al., 2001; Grant et al., 1991; Zimrin et al., 1995). First, the in vitro morphology of the tubes approximates that of in vivo capillaries; second, cells in HUVEC tubes are proliferation prohibited (Chao et al., 2011), as are endothelia in in vivo capillaries (Hadley et al., 1985); Third, HUVEC tube adherens junctions, key regulators of permeability, have been demonstrated to have the same junctional components and structure as in in vivo capillaries by electron microscopic analysis (Schmelz and Franke, 1993). The drawback of the in vitro model system is that it cannot target any particular area of the lung, and it is lacking other cell types adversely influenced by DEPs, such as neutrophils and macrophages, which subsequently release components injurious to the endothelium. However, we considered it an advantage that the confounding effects of inflammatory cells, known to induce vascular permeability, were absent. This allowed the observation of direct effects of DEP on endothelial cells. Another limitation of the model system is that it can only evaluate what may occur if inhaled DEPs reach the alveolar or other vascular endothelia. It also does not differentiate what might be caused by the particles themselves versus the materials that may be solubilized from them. However, there is significant evidence in the literature indicating that particles in size ranges that overlap with the size range of the DEPs employed here, do indeed reach the capillaries (Geiser et al., 2005; Kreyling et al., 2002; Kreyling et al., 2009; Nemmar et al., 2001; Nemmar et al., 2002; Nemmar et al., 2004; Oberdorster et al., 2002). Thus, we suggest that HUVEC tubes can be effectively employed to model potential effects of DEPs on the vasculature, especially since the DEPs used to expose the endothelial tubes are already well characterized (Bai et al., 2001; Inoue et al., 2006; Ito et al., 2000; Kumagai et al., 1997; Sagai et al., 1993; Singh et al., 2004) and were dispersed to biologically relevant sizes. The majority of our dispersed particles ranged from 0.3 to 1.05 µm, with a bimodal size distribution that included a distribution in the range of 30 to 120 nm with a mode of 80 nm. Particles of this size would have the ability to penetrate deeper into the respiratory tract (Kreyling et al., 2002; Kreyling et al., 2009; Nemmar et al., 2006; Oberdorster et al., 1994), where capillaries may be covered by only the thin membrane of an alveolar type I cells. Thus, deep in the respiratory tract, particles may exert a direct effect on capillary endothelia.
Comparisons between biological effects of exposure to DEP our in vitro system and ‘real-world’ inhalation exposure to DEP are limited. In these in vitro experiments, the lowest DEP concentration at which observed significant increases in production of hydrogen peroxide and expression of HO-1 and Nrf2 was 10 µg/ml in the culture media. To date, in vivo concentrations of inhaled DEP, and/or soluble components, reaching endothelial surfaces have not been directly measured or reliably estimated. Comparative dosimetry would require assumptions about pulmonary ventilation rate, the site and rate of DEP deposition, the distribution of particle sizes reaching the lung periphery, efficiency and rate of pulmonary clearance mechanisms, and rates of transmigration of the particles and/or soluble components through the alveolar membranes to the pulmonary capillary endothelial cells. Furthermore, real-world exposure to DEP occurs with co-exposure to other gas-phase components of diesel engine emissions that may modify the toxicological effects of DEP. Thus, the data must be considered for what it is: simply a model for investigation what may occur in in vivo.
The results demonstrating that DEPs induce ROS agree with those using monolayer cultures of endothelia, as well as with animal models (Bai et al., 2001; Sagai et al., 1993), by. Protein oxidation was increased by DEP, there was a DEP-induced generation of H2O2, and the observation that N-acetyl cysteine attenuated DEP cytotoxicity have all been previously shown in different systems. The importance of H2O2 generation cannot be underestimated because it has been shown to induce VE-cadherin internalization in endothelial cell monolayer cultures, causing cell-cell gaps that make the monolayers measurably permeable (Kevil et al., 1998). Thus, the DEP-induced reorganization of VE-cadherin demonstrated in endothelial tubes (Chao et al., 2011) is, in part, likely due to the generation of H2O2 .
VEGF-A is also known to cause weakening of cell-cell junctions, increasing permeability (Connolly et al., 1989; Ferrara and Henzel, 1989; Keck et al., 1989; Senger et al., 1983). VEGF-A inhibitors are used to decrease edema accompanying certain cancers (Gerstner et al., 2009; Strugar et al., 1994). VEGF-A has also been shown to be induced in endothelia by HO-1 in studies unrelated to, and not employing DEPs (Cisowski et al., 2005; Jozkowicz et al., 2003); (for review see Dulak et al., 2008). Thus, a mechanism warranting consideration is the possibility that the permeability observed after DEP exposure is, in part, due to the DEP-induction of VEGF-A. It was our goal to determine whether DEP induced HO-1, and ultimately VEGF-A, and this turned out to be true. Nrf2 is a factor controlling expression of HO-1 (Buckley et al., 2003; Hsieh et al., 2009), and endothelial tubes exposed to DEPs transported Nrf2 from the cytoplasm to the nucleus. In addition, HO-1 expression was induced within 6–12 hr of initial exposure. The link between HO-1 and VEGF-A secretion was investigated by exposing endothelial tubes to DEP in the presence and absence of NAC and the HO-1 inhibitor SnPP, followed by measuring the amount of VEGF-A in the culture medium after 24 hr. VEGF-A secretion was induced by DEP, and the levels were attenuated by the addition of NAC and SnPP, indicating that DEP-induced HO-1 is a factor contributing to endothelial VEGF-A secretion. The experiments performed indicated that VEGF-A produced by the endothelial tubes in response to DEPs does not induce angiogenesis, since there was no sprouting of tubes after DEP addition. Nor did the VEGF-A induce endothelial cell proliferation when assessed by MTS assays (not shown). The other major function of VEGF-A is induction of permeability, and in our system as well as in vivo this may be how DEPs induce permeability.
The association of VEGFR2 and VE-cadherin in the endothelial cell membranes has been demonstrated, although it is not fully characterized and understood (Carmeliet and Collen, 2000; Lampugnani et al., 2003; Wallez and Huber, 2008). VEGF-A has been shown to modulate vascular permeability by inducing the phosphorylation of VE-cadherin, thereby loosening cell-cell contacts (Esser et al., 1998). While we did not examine VE-cadherin phosphorylation, our results are consistent with observations from the literature showing VE-cadherin internalization. DEPs induce VEGF-A, and it is likely that its binding to VEGFR2 causes dissociation from VE-cadherin. Since VE-cadherin is internalized in a dose dependent manner by DEP exposure (Chao et al., 2011), our data suggest that the vascular permeability found after DEP exposure may, in part, be due to this VE-cadherin internalization. The endothelial tube model represents a simplistic view of endothelial DEP exposure, since there are no gaseous components of diesel exhaust being considered, nor is there any decent correlation with actual in vivo exposure levels. Still, despite these pitfalls, the data that can be obtained, such as in this work, may illuminate different ideas and approaches for investigations of humans or animals exposed to diesel exhaust. There is a wealth of information on endothelial cell biology that should be used to uncover potential mechanisms of DEP toxicity.
Research Highlights.
Endothelial tube cells mount an oxidative stress response to DEP exposure
DEPs induce capillary-like endothelial tube cells to secrete VEGF-A
Colocalization of VE-cadherin and VEGFR2 is compromised by DEP
ACKNOWLEDGMENTS
We thank Linda Everett and Rita Hahn for their help with this manuscript, and Dr. Masaru Sagai for providing the DEP. The work was supported by the National Institute of Environmental Health Sciences (NIEHS) [P30ES005022] award to the UMDNJ/Rutgers Center for Environmental Exposures and Disease and K08 ES013520 to RL; by the National Eye Institute (NEI) award EY009056 to MKG; and by the National Institute of Arthritis and Musculoskeletal and Skin Disease (NIAMS) [U54AR055073] award to the UMDNJ/Rutgers CounterACT Center of Excellence.
Abbreviations
- DEPs
diesel exhaust particles
- HUVEC
human umbilical vein endothelial cells
- PM2.5
particulate matter with diameters equal or less than 2.5 µm
- VE-Cad
vascular endothelial cell cadherin
- ROS
reactive oxygen species
- VEGF-A
vascular endothelial cell growth factor-A
- VEGFR2
vascular endothelial cell growth factor receptor-2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bai Y, Suzuki AK, Sagai M. The cytotoxic effects of diesel exhaust particles on human pulmonary artery endothelial cells in vitro: role of active oxygen species. Free Radic. Biol. Med. 2001;30:555–562. doi: 10.1016/s0891-5849(00)00499-8. [DOI] [PubMed] [Google Scholar]
- Becker S, Dailey LA, Soukup JM, Grambow SC, Devlin RB, Huang YC. Seasonal variations in air pollution particle-induced inflammatory mediator release and oxidative stress. Environ. Health Perspect. 2005;113:1032–1038. doi: 10.1289/ehp.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105:1534–1536. doi: 10.1161/01.cir.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]
- Brook RD. Cardiovascular effects of air pollution. Clin. Sci. (Lond.) 2008;115:175–187. doi: 10.1042/CS20070444. [DOI] [PubMed] [Google Scholar]
- Buckley BJ, Marshall ZM, Whorton AR. Nitric oxide stimulates Nrf2 nuclear translocation in vascular endothelium. Biochem. Biophys. Res. Commun. 2003;307:973–979. doi: 10.1016/s0006-291x(03)01308-1. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Collen D. Molecular basis of angiogenesis. Role of VEGF and VE-cadherin. Ann. N.Y. Acad. Sci. 2000;902:249–262. doi: 10.1111/j.1749-6632.2000.tb06320.x. discussion 262-244. [DOI] [PubMed] [Google Scholar]
- Chao MW, Kozlosky J, Po IP, Strickland PO, Svoboda KK, Cooper K, Laumbach RJ, Gordon MK. Diesel exhaust particle exposure causes redistribution of endothelial tube VE-cadherin. Toxicology. 2011;279:73–84. doi: 10.1016/j.tox.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisowski J, Loboda A, Jozkowicz A, Chen S, Agarwal A, Dulak J. Role of heme oxygenase-1 in hydrogen peroxide-induced VEGF synthesis: effect of HO-1 knockout. Biochem. Biophys. Res. Commun. 2005;326:670–676. doi: 10.1016/j.bbrc.2004.11.083. [DOI] [PubMed] [Google Scholar]
- Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J. Clin. Invest. 1989;84:1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E, Corada M, Lampugnani MG. Endothelial cell-to-cell junctions. FASEB J. 1995;9:910–918. [PubMed] [Google Scholar]
- Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- Deryckere F, Gannon F. A one-hour minipreparation technique for extraction of DNA-binding proteins from animal tissues. Bio Techniques. 1994;16:405. [PubMed] [Google Scholar]
- Donovan D, Brown NJ, Bishop ET, Lewis CE. Comparison of three in vitro human 'angiogenesis' assays with capillaries formed in vivo. Angiogenesis. 2001;4:113–121. doi: 10.1023/a:1012218401036. [DOI] [PubMed] [Google Scholar]
- Dulak J, Jozkowicz A, Foresti R, Kasza A, Frick M, Huk I, Green CJ, Pachinger O, Weidinger F, Motterlini R. Heme oxygenase activity modulates vascular endothelial growth factor synthesis in vascular smooth muscle cells. Antioxid. Redox. Signal. 2002;4:229–240. doi: 10.1089/152308602753666280. [DOI] [PubMed] [Google Scholar]
- Dulak J, Deshane J, Jozkowicz A, Agarwal A. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis. Circulation. 2008;117:231–241. doi: 10.1161/CIRCULATIONAHA.107.698316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser S, Lampugnani MG, Corada M, Dejana E, Risau W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J. Cell Sci. 1998;111(Pt 13):1853–1865. doi: 10.1242/jcs.111.13.1853. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem. Biophys. Res. Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- Furuyama A, Hirano S, Koike E, Kobayashi T. Induction of oxidative stress and inhibition of plasminogen activator inhibitor-1 production in endothelial cells following exposure to organic extracts of diesel exhaust particles and urban fine particles. Arch. Toxicol. 2006;80:154–162. doi: 10.1007/s00204-005-0020-x. [DOI] [PubMed] [Google Scholar]
- Garcia JG, Dodson RF, Callahan KS. Effect of environmental particulates on cultured human and bovine endothelium. Cellular injury via an oxidant-dependent pathway. Lab. Invest. 1989;61:53–61. [PubMed] [Google Scholar]
- Geiser M, Rothen-Rutishauser B, Kapp N, Schurch S, Kreyling W, Schulz H, Semmler M, Im Hof V, Heyder J, Gehr P. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ. Health Perspect. 2005;113:1555–1560. doi: 10.1289/ehp.8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner ER, Duda DG, di Tomaso E, Ryg PA, Loeffler JS, Sorensen AG, Ivy P, Jain RK, Batchelor TT. VEGF inhibitors in the treatment of cerebral edema in patients with brain cancer. Nat. Rev. Clin. Oncol. 2009;6:229–236. doi: 10.1038/nrclinonc.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant DS, Lelkes PI, Fukuda K, Kleinman HK. Intracellular mechanisms involved in basement membrane induced blood vessel differentiation in vitro. In Vitro Cell Dev. Biol. 1991;27A:327–336. doi: 10.1007/BF02630910. [DOI] [PubMed] [Google Scholar]
- Hadley MA, Byers SW, Suarez-Quian CA, Kleinman HK, Dym M. Extracellular matrix regulates Sertoli cell differentiation, testicular cord formation, and germ cell development in vitro. J. Cell Biol. 1985;101:1511–1522. doi: 10.1083/jcb.101.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CY, Hsiao HY, Wu WY, Liu CA, Tsai YC, Chao YJ, Wang DL, Hsieh HJ. Regulation of shear-induced nuclear translocation of the Nrf2 transcription factor in endothelial cells. J. Biomed. Sci. 2009;16:12. doi: 10.1186/1423-0127-16-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda U, Takahashi M, Shimada K. Monocyte-endothelial cell interaction in atherogenesis and thrombosis. Clin. Cardiol. 1998;21:11–14. doi: 10.1002/clc.4960210103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Takano H, Sakurai M, Oda T, Tamura H, Yanagisawa R, Shimada A, Yoshikawa T. Pulmonary exposure to diesel exhaust particles enhances coagulatory disturbance with endothelial damage and systemic inflammation related to lung inflammation. Exp. Biol. Med. 2006;231:1626–1632. doi: 10.1177/153537020623101007. [DOI] [PubMed] [Google Scholar]
- Ito T, Ikeda M, Yamasaki H, Sagai M, Tomita T. Peroxynitrite formation by diesel exhaust particles in alveolar cells: Links to pulmonary inflammation. Environ Toxicol Pharmacol. 2000;9:1–8. doi: 10.1016/s1382-6689(00)00053-3. [DOI] [PubMed] [Google Scholar]
- Jozkowicz A, Huk I, Nigisch A, Weigel G, Dietrich W, Motterlini R, Dulak J. Heme oxygenase and angiogenic activity of endothelial cells: stimulation by carbon monoxide and inhibition by tin protoporphyrin-IX. Antioxid. Redox. Signal. 2003;5:155–162. doi: 10.1089/152308603764816514. [DOI] [PubMed] [Google Scholar]
- Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- Kevil CG, Payne DK, Mire E, Alexander JS. Vascular permeability factor/vascular endothelial cell growth factor-mediated permeability occurs through disorganization of endothelial junctional proteins. J. Biol. Chem. 1998;273:15099–15103. doi: 10.1074/jbc.273.24.15099. [DOI] [PubMed] [Google Scholar]
- Kevil CG, Okayama N, Alexander JS. H(2)O(2)-mediated permeability II: importance of tyrosine phosphatase and kinase activity. Am. J. Physiol. Cell. Physiol. 2001;281:C1940–C1947. doi: 10.1152/ajpcell.2001.281.6.C1940. [DOI] [PubMed] [Google Scholar]
- Kreyling WG, Semmler M, Erbe F, Mayer P, Takenaka S, Schulz H, Oberdorster G, Ziesenis A. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J. Toxicol. Environ. Health. 2002;A 65:1513–1530. doi: 10.1080/00984100290071649. [DOI] [PubMed] [Google Scholar]
- Kreyling WG, Semmler-Behnke M, Seitz J, Scymczak W, Wenk A, Mayer P, Takenaka S, Oberdorster G. Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhal. Toxicol. 2009;21(Suppl 1):55–60. doi: 10.1080/08958370902942517. [DOI] [PubMed] [Google Scholar]
- Kumagai Y, Arimoto T, Shinyashiki M, Shimojo N, Nakai Y, Yoshikawa T, Sagai M. Generation of reactive oxygen species during interaction of diesel exhaust particle components with NADPH-cytochrome P450 reductase and involvement of the bioactivation in the DNA damage. Free Radic. Biol. Med. 1997;22:479–487. doi: 10.1016/s0891-5849(96)00341-3. [DOI] [PubMed] [Google Scholar]
- Lampugnani MG, Zanetti A, Corada M, Takahashi T, Balconi G, Breviario F, Orsenigo F, Cattelino A, Kemler R, Daniel TO, Dejana E. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J. Cell Biol. 2003;161:793–804. doi: 10.1083/jcb.200209019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HH, Chen YH, Chang PF, Lee YT, Yet SF, Chau LY. Heme oxygenase-1 promotes neovascularization in ischemic heart by coinduction of VEGF and SDF-1. J. Mol. Cell. Cardiol. 2008;45:44–55. doi: 10.1016/j.yjmcc.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Nagy JA, Feng D, Vasile E, Wong WH, Shih SC, Dvorak AM, Dvorak HF. Permeability properties of tumor surrogate blood vessels induced by VEGF-A. Lab. Invest. 2006;86:767–780. doi: 10.1038/labinvest.3700436. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Vanbilloen H, Hoylaerts MF, Hoet PH, Verbruggen A, Nemery B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am. J. Respir. Crit. Care Med. 2001;164:1665–1668. doi: 10.1164/ajrccm.164.9.2101036. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, Vanbilloen H, Mortelmans L, Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105:411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoylaerts MF, Hoet PH, Nemery B. Possible mechanisms of the cardiovascular effects of inhaled particles: systemic translocation and prothrombotic effects. Toxicol. Lett. 2004;149:243–253. doi: 10.1016/j.toxlet.2003.12.061. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hamoir J, Nemery B, Gustin P. Evaluation of particle translocation across the alveolo-capillary barrier in isolated perfused rabbit lung model. Toxicology. 2005;208:105–113. doi: 10.1016/j.tox.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoylaerts MF, Nemery B. Effects of particulate air pollution on hemostasis. Clin. Occup. Environ. Med. 2006;5:865–881. doi: 10.1016/j.coem.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Ferin J, Lehnert BE. Correlation between particle size, in vivo particle persistence, and lung injury. Environ. Health Perspect. 1994;102(Suppl 5):173–179. doi: 10.1289/ehp.102-1567252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, Kreyling W, Cox C. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J. Toxicol. Environ. Health. 2002;A 65:1531–1543. doi: 10.1080/00984100290071658. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- Olivieri G, Baysang G, Meier F, Muller-Spahn F, Stahelin HB, Brockhaus M, Brack C. N-acetyl-L-cysteine protects SHSY5Y neuroblastoma cells from oxidative stress and cell cytotoxicity: effects on beta-amyloid secretion and tau phosphorylation. J. Neurochem. 2001;76:224–233. doi: 10.1046/j.1471-4159.2001.00090.x. [DOI] [PubMed] [Google Scholar]
- Pocernich CB, La Fontaine M, Butterfield DA. In-vivo glutathione elevation protects against hydroxyl free radical-induced protein oxidation in rat brain. Neurochem. Int. 2000;36:185–191. doi: 10.1016/s0197-0186(99)00126-6. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Verrier RL, Lovett EG, Larson AC, Raizenne ME, Kanner RE, Schwartz J, Villegas GM, Gold DR, Dockery DW. Heart rate variability associated with particulate air pollution. Am. Heart. J. 1999;138:890–899. doi: 10.1016/s0002-8703(99)70014-1. [DOI] [PubMed] [Google Scholar]
- Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J. Cell Sci. 1995;108:2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- Sagai M, Saito H, Ichinose T, Kodama M, Mori Y. Biological effects of diesel exhaust particles. I. In vitro production of superoxide and in vivo toxicity in mouse. Free Radic. Biol. Med. 1993;14:37–47. doi: 10.1016/0891-5849(93)90507-q. [DOI] [PubMed] [Google Scholar]
- Salvi S, Blomberg A, Rudell B, Kelly F, Sandstrom T, Holgate ST, Frew A. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am. J. Respir. Crit. Care Med. 1999;159:702–709. doi: 10.1164/ajrccm.159.3.9709083. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Franke WW. Complexus adhaerentes, a new group of desmoplakin-containing junctions in endothelial cells: the syndesmos connecting retothelial cells of lymph nodes. Eur.J. Cell Biol. 1993;61:274–289. [PubMed] [Google Scholar]
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Senger DR, Connolly DT, Van de Water L, Feder J, Dvorak HF. Purification and NH2-terminal amino acid sequence of guinea pig tumor-secreted vascular permeability factor. Cancer Res. 1990;50:1774–1778. [PubMed] [Google Scholar]
- Singh P, DeMarini DM, Dick CA, Tabor DG, Ryan JV, Linak WP, Kobayashi T, Gilmour MI. Sample characterization of automobile and forklift diesel exhaust particles and comparative pulmonary toxicity in mice. Environ. Health Perspect. 2004;112:820–825. doi: 10.1289/ehp.6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strugar J, Rothbart D, Harrington W, Criscuolo GR. Vascular permeability factor in brain metastases: correlation with vasogenic brain edema and tumor angiogenesis. J. Neurosurg. 1994;81:560–566. doi: 10.3171/jns.1994.81.4.0560. [DOI] [PubMed] [Google Scholar]
- Sumanasekera WK, Ivanova MM, Johnston BJ, Dougherty SM, Sumanasekera GU, Myers SR, Ali Y, Kizu R, Klinge CM. Rapid effects of diesel exhaust particulate extracts on intracellular signaling in human endothelial cells. Toxicol. Lett. 2007;174:61–73. doi: 10.1016/j.toxlet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Tornqvist H, Mills NL, Gonzalez M, Miller MR, Robinson SD, Megson IL, Macnee W, Donaldson K, Soderberg S, Newby DE, Sandstrom T, Blomberg A. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am. J. Respir. Crit. Care Med. 2007;176:395–400. doi: 10.1164/rccm.200606-872OC. [DOI] [PubMed] [Google Scholar]
- Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim. Biophys. Acta. 2008;1778:794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Weis SM, Cui J, Barnes L, Cheresh DA. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J. Cell Biol. 2004;167:223–229. doi: 10.1083/jcb.200408130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437:497–504. doi: 10.1038/nature03987. [DOI] [PubMed] [Google Scholar]
- Yamawaki H, Iwai N. Mechanisms underlying nano-sized air-pollution-mediated progression of atherosclerosis: carbon black causes cytotoxic injury/inflammation and inhibits cell growth in vascular endothelial cells. Circ J. 2006;70:129–140. doi: 10.1253/circj.70.129. [DOI] [PubMed] [Google Scholar]
- Zimrin AB, Villeponteau B, Maciag T. Models of in vitro angiogenesis: endothelial cell differentiation on fibrin but not matrigel is transcriptionally dependent. Biochem. Biophys. Res. Commun. 1995;213:630–638. doi: 10.1006/bbrc.1995.2178. [DOI] [PubMed] [Google Scholar]