Abstract
Many important experiments in cancer research are initiated with cell line data analysis due to the ease of accessibility and utilization. Recently, the ability to capture and characterize circulating tumor cells (CTCs) has become more prevalent in the research setting. This ability to detect, isolate, and analyze CTCs allows us to directly compare specific protein expression levels found in patient CTCs to cell lines. In this study, we use immunocytochemistry to compare the protein expression levels of total cytokeratin (CK) and androgen receptor (AR) in CTCs and cell lines from patients with prostate cancer to determine what translational insights might be gained through the use of cell line data. A non-enrichment CTC detection assay enables us to compare cytometric features and relative expression levels of CK and AR by indirect immunofluorescence from prostate cancer patients against the prostate cancer cell line LNCaP. We measured physical characteristics of these two groups and observed significant differences in cell size, fluorescence intensity, and nuclear to cytoplasmic (N/C) ratio. We hope that these experiments will initiate a foundation to allow cell line data to be compared against characteristics of primary cells from patients.
Introduction
The majority of all cancer deaths are attributed to the metastatic spread of cancer in which cells from the primary tumor escape and relocate to distant sites. Metastasis is an intricate, poorly understood process during which genetic and phenotypic instability enables the cells to dissociate from the primary tumor, invade surrounding tissue, and escape into the bloodstream by altering expression levels of a number of protein classes (1–3). Enumeration of these CTCs has demonstrated clinical utility by predicting both progression-free survival and overall survival in patients with metastatic breast cancer (4). Multiple technologies have been developed to isolate these cells from patients for a variety of cancers using characteristics unique to epithelial cells including both cell surface expression and biophysical characteristics such as cell size. Common among these methods of CTC isolation is the use of anti-cytokeratin (CK) antibodies to visualize these cells and to verify the epithelial origin for the cells of interest (5–8). When compared to the primary and metastatic tumors, patient CTCs have been shown to retain many cytomorphic features; however, among this CTC population, a wide variation in size, shape, and nuclear to cytoplasmic (N/C) ratio was present (9). In this study, we compare total CK and AR expression as measured by indirect immunofluorescence and both cytoplasmic and nuclear cell areas of CTCs from 13 prostate cancer patient samples to the androgen sensitive LNCaP cells, a cell line derived from a metastatic lesion of human prostatic adenocarcinoma (10).
Materials and Methods
Patient recruitment
As previously reported (9, 11), blood draws from prostate cancer patients and normal blood donors (NBD) were collected into Cyto-Chex® tubes (Streck, Omaha, NE) at Scripps Clinic, USC Westside Cancer Center and TSRI Normal Blood Donor Service (for NBD) following the IRB approved protocols for each site. Stage IV prostate cancer was the only inclusion criteria for this study. Patients were included independent of treatment status. Patient samples were drawn between September 9, 2010 and April 12, 2011.
Sample processing and Imaging
Samples were processed as reported by Marrinucci et al. in this issue of Physical Biology. In brief, red blood cells are lysed in an isotonic ammonium chloride solution. Following centrifugation lysed red blood cells and other non-cellular material is discarded. Nucleated cells are resuspended and plated on custom glass slides for standard immunofluorescent staining procedures. The cells are stained with DAPI, a pan-anti-cytokeratin (epithelial cell marker) antibody cocktail that targets CKs 1,4,5,6,8,10,13,18, and 19 (Sigma-Aldrich, St. Louis, MO) and an anti-CD45 (leukocyte marker) antibody. Additionally, for this study, an anti-AR (Cell Signaling Technology, Danvers, MA) antibody was incorporated.
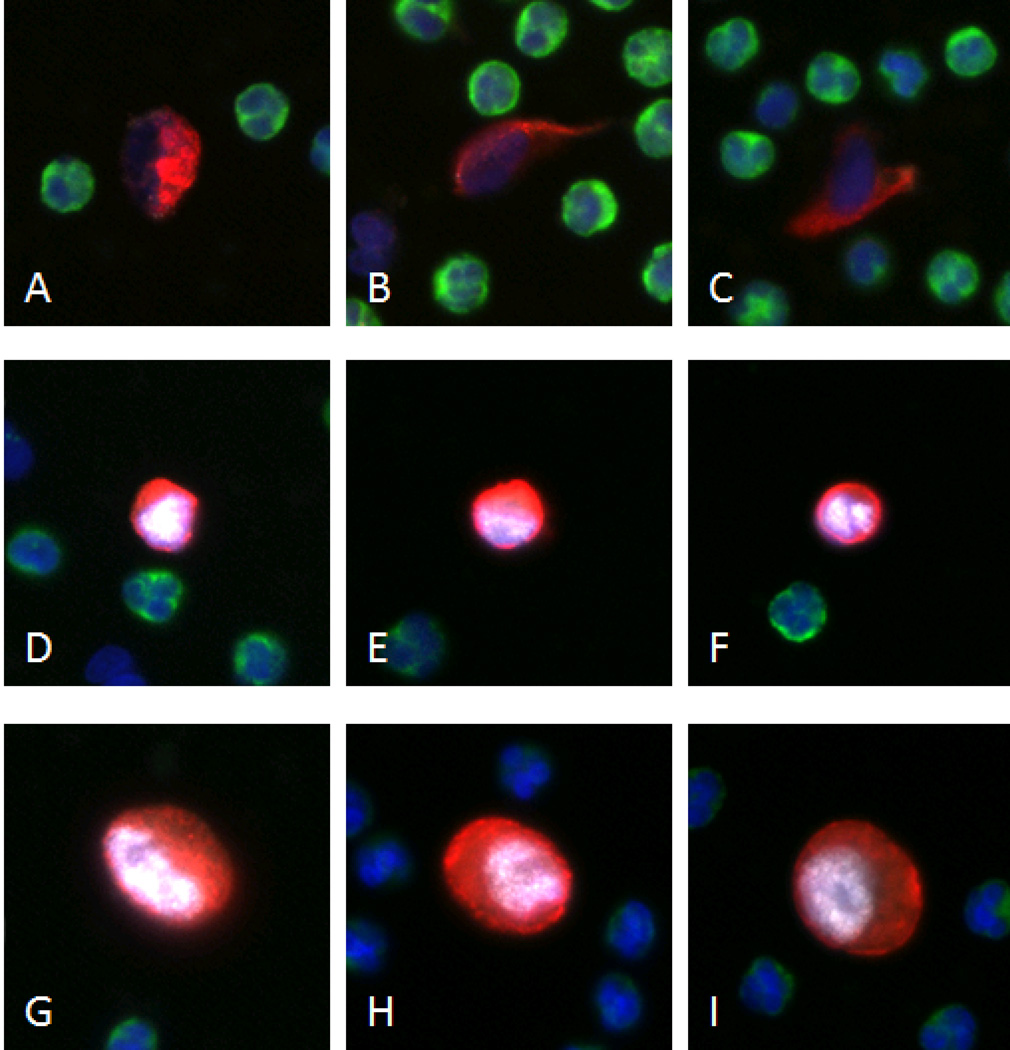
Images were taken with an automated fluorescent microscope and CTCs were classified as cells that are CK positive, CD45 negative, and demonstrate other distinct morphological characteristics such as cell and nuclear size, N/C ratio, and subcellular localization. Upon classification of a cell as a CTC, AR status was evaluated by a pathologist- trained technician. AR positive cells were classified based on nuclear localization of AR staining (Fig. 1).
Figure 1. A representative gallery of CTCs and LNCaPs.
For all cells, blue, green, red, and white represent DAPI, CD45, CK, and AR respectively. Panels A-C show CTCs classified as AR−, and panels D–F were all deemed AR+ CTCs. Images G–I represent typical LNCaP cell-line cells being always CK and AR positive.
Preparation of LNCaP cells
The prostate cancer cell line LNCaP was obtained through ATCC (Manassas, Virginia). The cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, L-glutamine (2µM), and antibiotic-antimycotic (Invitrogen 15240112). To mimic patient samples, the LNCaP cells, after removal from the culture plate with 0.25% trypsin/EDTA, were spiked into NBD blood after red blood cell lysis, to produce a ratio of 1 LNCaP cell to 1000 leukocytes. These samples were processed in an identical manner to the patient samples. Two independent LNCaP sample preparations, using blood from different NBDs and LNCaP cells from different passages, were used for cell line analysis.
Re-Imaging of candidate CTCs
Single cell measurements were performed to compare both nuclear and cytoplasmic size, CK intensities, and AR signal. CTCs and cell lines were relocated and reimaged using a macro written for ImagePro Plus (Media Cybernetics, Bethesda, MD). The cells were reimaged at fixed exposure and gain at 40× magnification on a Nikon 80i (Melville, NY) epifluorescent microscope equipped with a QImaging Retiga EXi 12-bit monochrome CCD camera (QImaging, Surrey, BC, Canada).
Data Analysis
Cells were manually segmented and measured using ImageJ (NIH, Bethesda, MD). Total cell area, nuclear area, and mean CK intensity were measured for each CTC and LNCaP cell. For AR positive CTCs and an additional 15 LNCaP cells, the nuclear AR signal was measured. Graphical representation and statistical significance determined by one-way ANOVA and Dunnett’s Multiple Comparison Test, or Mann-Whitney t-test against LNCaP cells was performed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA).
Results
For the analysis of cytokeratin levels and cytomorphic features, a total of 227 CTCs from ten patients were compared to 20 LNCaP cells. CTCs were identified in thirteen blood samples drawn from these ten patients. The number of CTCs detected ranged from 1 to 62, with a median value of 9. On average, these CTCs had a mean CK intensity of 60.43, and the standard deviation of all the CTC CK intensities was 154.36 (Table 1). The large standard deviation is the result of the skewed distribution with respect to the CK intensities. The Shapiro-Wilk normality test was administered to verify that the distribution of all CK intensities did not follow a normal distribution pattern (p<0.001) (Fig. 2). The standard deviations for CK intensities for the individual blood samples ranged from 1.55 to 295.93. Additionally, the CTCs had an average total cell area of 89.03 µm2 ± 53.77 µm2. The average nuclear area was 61.31 µm2 ± 35.99 µm2 and the cytoplasmic area was 27.72 µm2, resulting in an N/C ratio of 2.21 (Table 1).
Table 1. Data Summary for Patient Samples and LNCaPs.
The 227 patient CTCs have CK expression levels that differ from LNCaP cells. A comparison of the cytomorphic features of nuclear and cell size shows that on average, the N/C ratio of LNCaPs is less than the N/C ratio of patient samples due to the greater total cell area. The nuclear areas of the LNCaPs and CTCs are comparable.
| Patient ID | Cells Analyzed | Average Cell Size | Standard Deviation | Average Intensity | Standard Deviation | Average Nuclear Size | Standard Deviation | Cytoplasmic Area | Average N/C |
|---|---|---|---|---|---|---|---|---|---|
| Pr1 | 14 | 77.715 | 29.680 | 34.872 | 94.384 | 40.378 | 17.605 | 37.337 | 1.081 |
| Pr2-a | 1 | 115.634 | 29.399 | 39.607 | 76.027 | 0.521 | |||
| Pr2-b | 62 | 107.999 | 61.581 | 6.087 | 6.087 | 64.794 | 46.615 | 43.205 | 1.500 |
| Pr3 | 1 | 97.696 | 6.885 | 39.244 | 58.452 | 0.671 | |||
| Pr4 | 2 | 51.181 | 26.228 | 140.970 | 118.590 | 44.856 | 7.863 | 6.325 | 7.092 |
| Pr5 | 6 | 96.461 | 37.196 | 19.733 | 16.169 | 50.118 | 17.881 | 46.342 | 1.081 |
| Pr6 | 6 | 47.621 | 5.717 | 68.052 | 122.581 | 44.282 | 10.772 | 3.340 | 13.259 |
| Pr7 | 9 | 128.660 | 87.953 | 14.718 | 10.298 | 74.514 | 54.603 | 54.146 | 1.376 |
| Pr8-a | 10 | 58.053 | 19.169 | 84.837 | 94.058 | 52.366 | 52.366 | 5.687 | 9.208 |
| Pr8-b | 29 | 60.043 | 28.555 | 78.026 | 128.162 | 48.731 | 8.662 | 11.312 | 4.308 |
| Pr9 | 23 | 72.449 | 23.049 | 372.682 | 295.933 | 53.420 | 20.341 | 19.029 | 2.807 |
| Pr10-a | 2 | 104.371 | 23.552 | 5.889 | 1.559 | 50.222 | 1.962 | 54.149 | 0.927 |
| Pr10-b | 62 | 95.020 | 79.980 | 7.779 | 5.609 | 75.227 | 54.748 | 19.793 | 3.801 |
| Patient Samples | 227 | 89.032 | 53.776 | 61.762 | 154.362 | 61.313 | 35.992 | 27.718 | 2.212 |
| LNCaP Cells | 20 | 142.886 | 48.095 | 1166.464 | 306.048 | 63.129 | 18.574 | 79.756 | 0.792 |
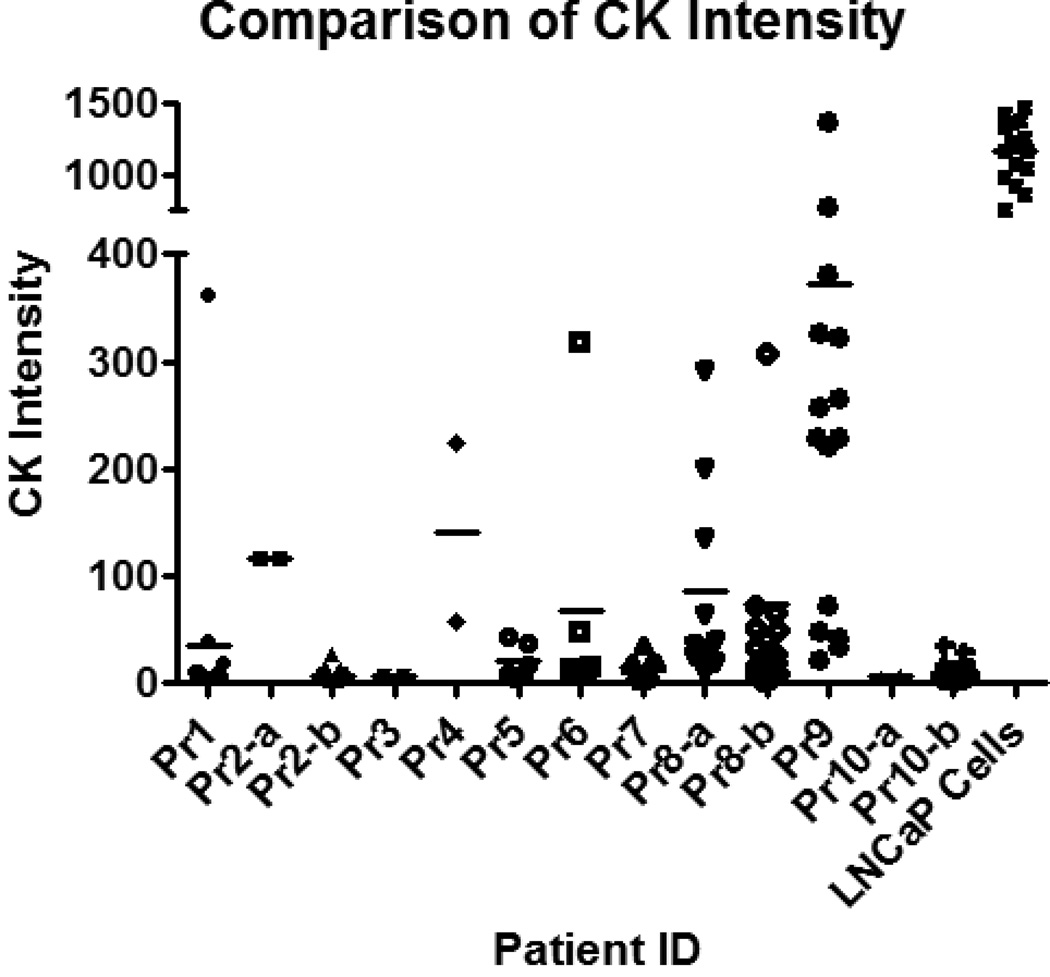
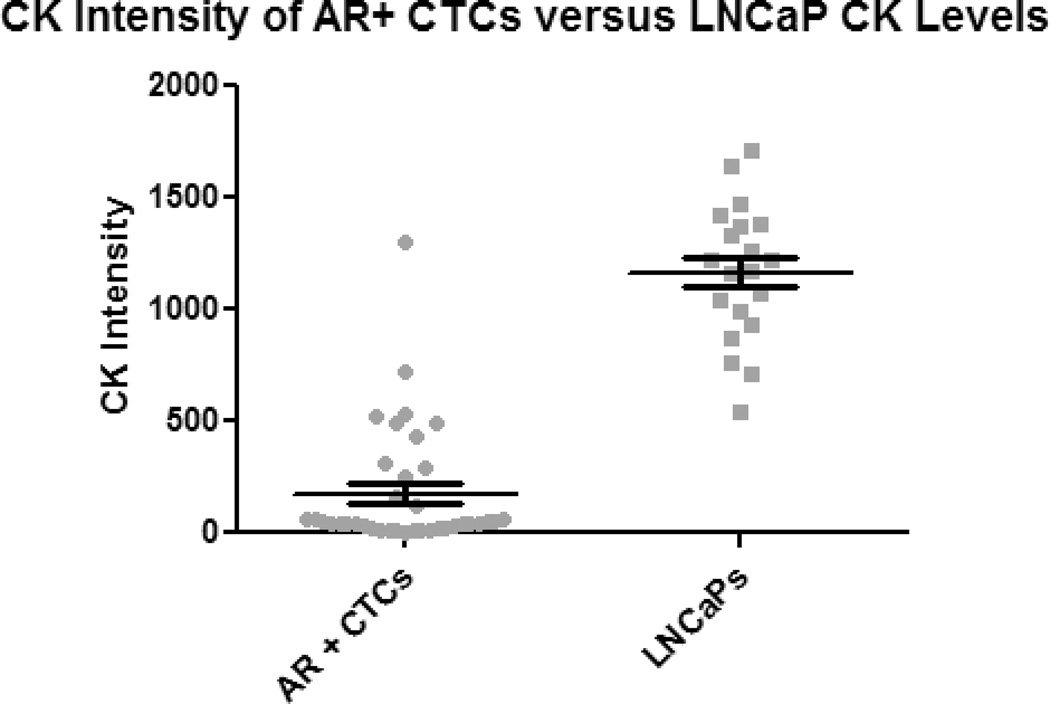
Figure 2. CK expression of CTCs is less than that of LNCaPs.
The CK intensity of each CTC and LNCaP cell is shown here. For most patients, the CK levels for all CTCs are below 500, while all LNCaP cells had intensities above 500. Using the Dunnett’s Multiple Comparison Test, the difference in CK intensity between CTCs and LNCaPs was significant (p<0.0001). Error bars represent standard error of the mean (SEM).
In contrast to the CTCs, the average CK intensity for the LNCaP cells was 1166.46 with a standard deviation of 306.05. The average total cell area was 142.89 µm2 ± 48.10 µm2, the average nuclear area was 63.13 µm2 ± 18.57 µm2, and the cytoplasmic area was 79.76 µm2 resulting in an N/C ratio for the LNCaPs of 0.79 (Table 1). The average CK intensity of the LNCaPs was 19 times greater than the CTCs, a difference that was found to be significant (p<0.0001) (Fig. 2). Additionally, the average cytoplasmic area of the LNCaPs was 2.7 times greater than CTCs, a difference that was also significant (p<0.05) (Table 1). However, the average nuclear area of the LNCaPs and CTCs was found to be similar with no significant difference between the two populations (Fig. 3).
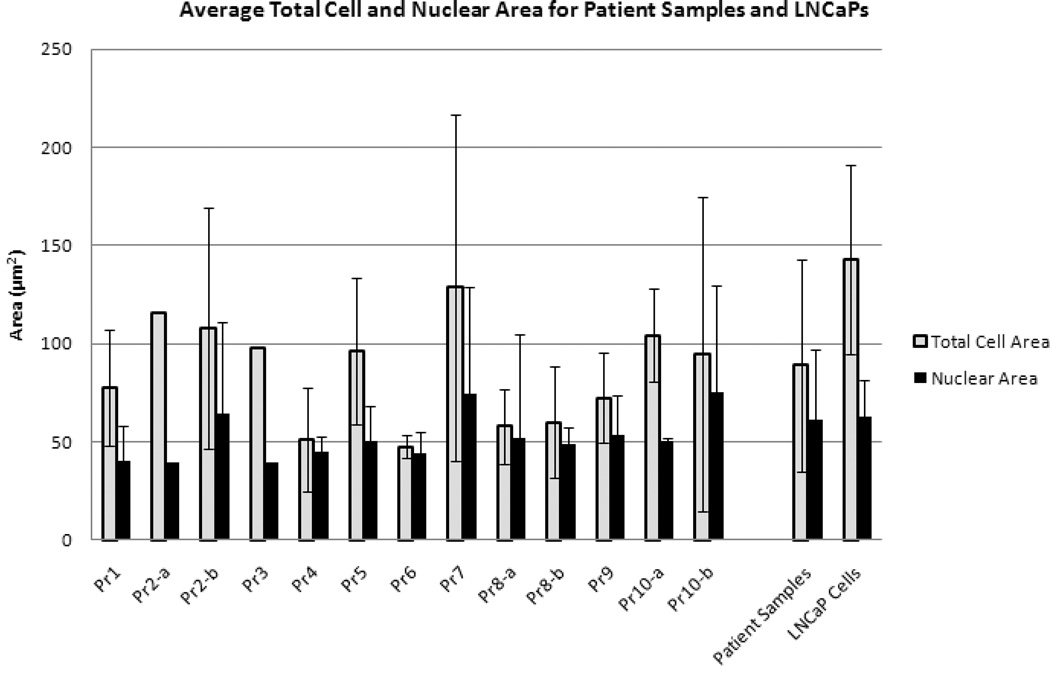
Figure 3. Comparison of Cytomorphic Features.
The average total cell and nuclear areas for each patient as well as the LNCaP cells are presented. The average nuclear area of the patient samples is similar to that of the LNCaP cells; however, LNCaP cells on average have a greater total cell area, which in turn results in a smaller N/C ratio than CTCs. Error bars represent standard deviation.
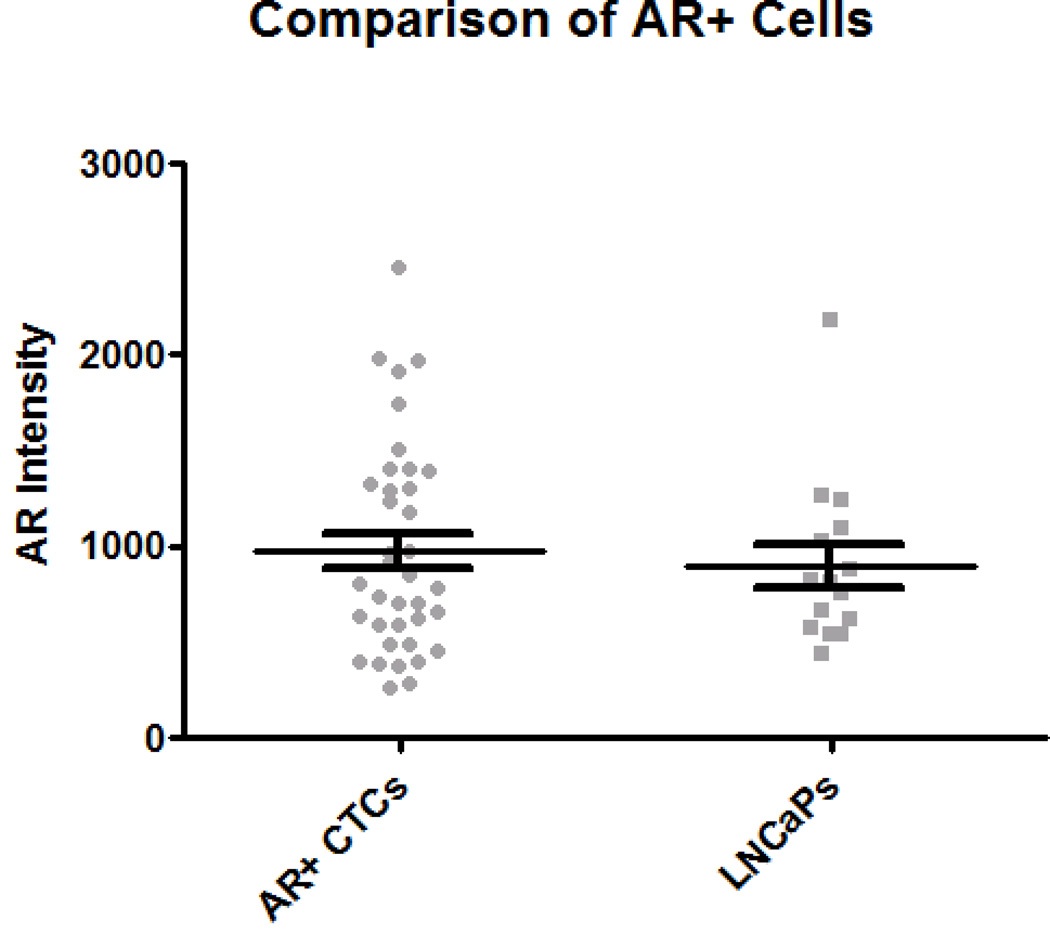
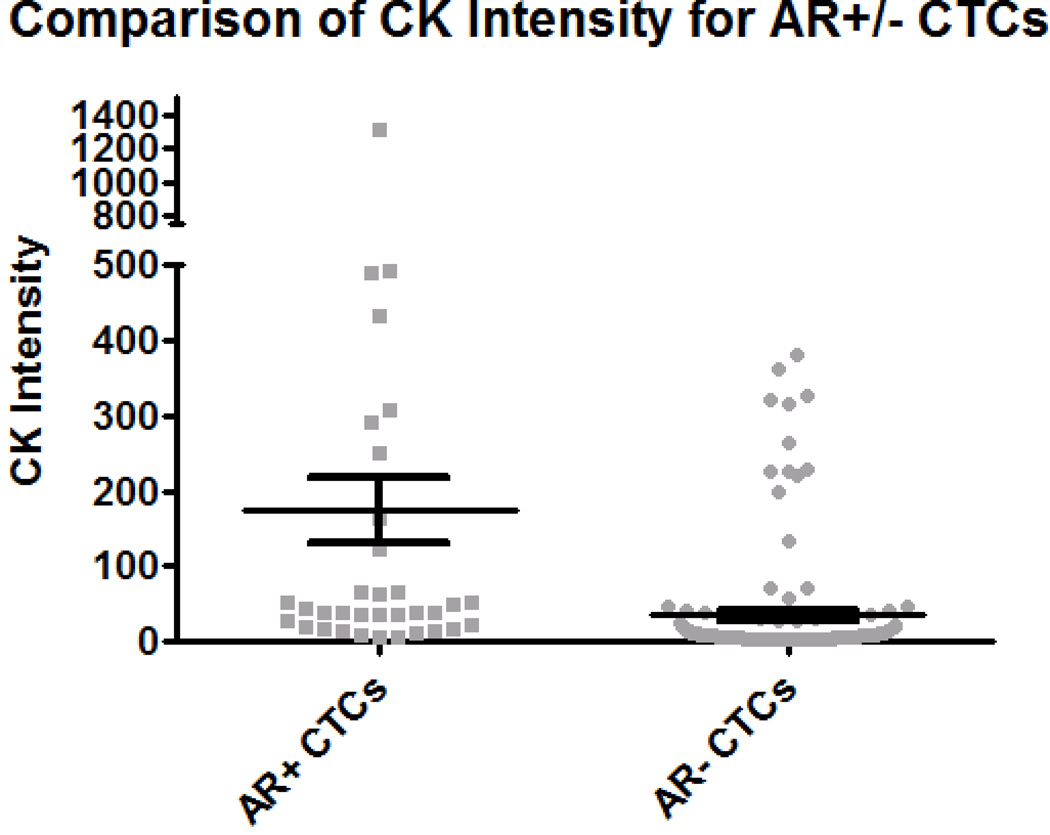
All CTCs identified in the patient samples were additionally classified as either AR positive (AR+), cells with positive nuclear signal for androgen receptor, or AR negative (AR−), cells with the absence of nuclear signal for androgen receptor (Fig. 1). 37 of the 227 (16.3%) CTCs were AR+. The 37 AR+ CTCs were identified in 3 of the 13 samples. The 37 AR+ CTCs were compared to an additional set of 15 LNCaP cells, all of which were AR+. The average AR intensity was 979.4 and 902.2 for the CTCs and LNCaPs, respectively (Table 2), which was not found to be significantly different (p=0.824) (Fig. 4A). When evaluating the stratification between AR+ and AR− CTCs, the average CK intensity between AR+ and AR− CTCs was found to be significantly different (p<0.0001) at 174.23 and 39.86, respectively (Fig. 4B). However, the difference between the average CK intensity of AR+ CTCs and LNCaPs was still found to be significant (p<0.0001) (Fig. 4C). For the AR+ CTCs, the average nuclear area was 51.88 µm2 ± 15.17µm2; the average total cell area was 56.42 µm2 ± 16.78 µm2. For the AR− CTCs, the average nuclear area was 63.15 µm2 and the average total cell area was 95.38 µm2. The N/C ratios for AR+ and AR− CTCs are 11.42 and 1.96, respectively (Table 2).
Table 2. Measurements of Cytomorphic Features and AR Intensity for AR+ Cells.
The 37 AR+ CTCs from the three draws have an average AR intensity of 979.36, an average CK intensity of 174.23, an average total cell area of 56.42 µm2 and an average nuclear area of 51.88 µm2. The N/C ratio for AR+ CTCs is 11.42. For the AR− CTCs, the average mean CK intensity is 39.86, the average total cell area is 95.38 µm2 and the average nuclear area is 63.15 µm2.
| Patient ID | AR+ Cells | Mean AR Intensity |
St. Dev. | Mean CK Intensity |
St. Dev. | Mean Total Cell Area |
Average Nuclear Area |
N/C Ratio |
|---|---|---|---|---|---|---|---|---|
| Pr8-a | 6 | 986.787 | 608.348 | 76.469 | 106.866 | 50.982 | 50.339 | 78.206 |
| Pr8-b | 23 | 1003.544 | 493.913 | 94.663 | 145.155 | 53.593 | 50.194 | 14.767 |
| Pr9 | 8 | 904.253 | 725.637 | 476.313 | 410.925 | 68.623 | 57.866 | 5.379 |
| AR+ CTCs | 37 | 979.358 | 551.818 | 174.231 | 270.578 | 56.420 | 51.876 | 11.418 |
| AR− CTCs | 190 | 39.860 | 91.202 | 95.384 | 63.151 | 1.959 |
Figure 4. Comparison of AR+ CTCs to LNCaP cells and AR− CTCs.
(A) For the 37 AR+ CTCs, the AR intensity is not significantly different than the AR intensity of the LNCaPs (p=.824). (B) The mean CK intensity for AR+ CTCs was then compared to the AR− CTCs. A significant difference was found between these two populations (p<0.0001). (C) The CK intensity for this AR+ subset of CTCs was also compared to the CK intensity of LNCaP cells. The difference was still found to be significant. Error bars represent SEM.
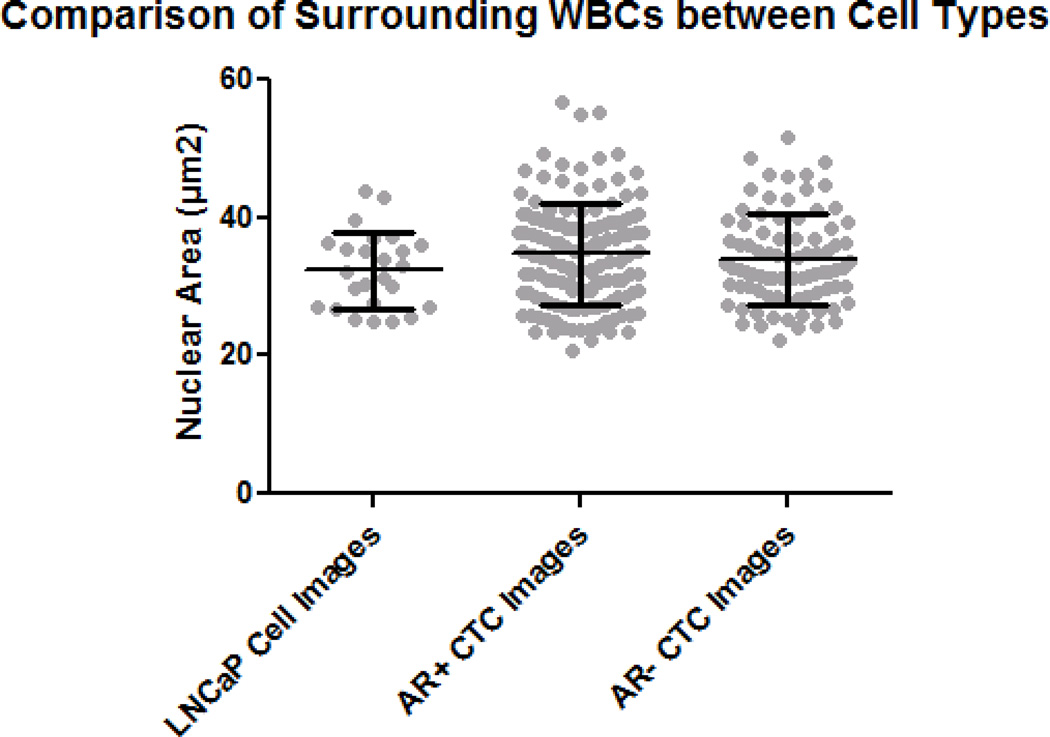
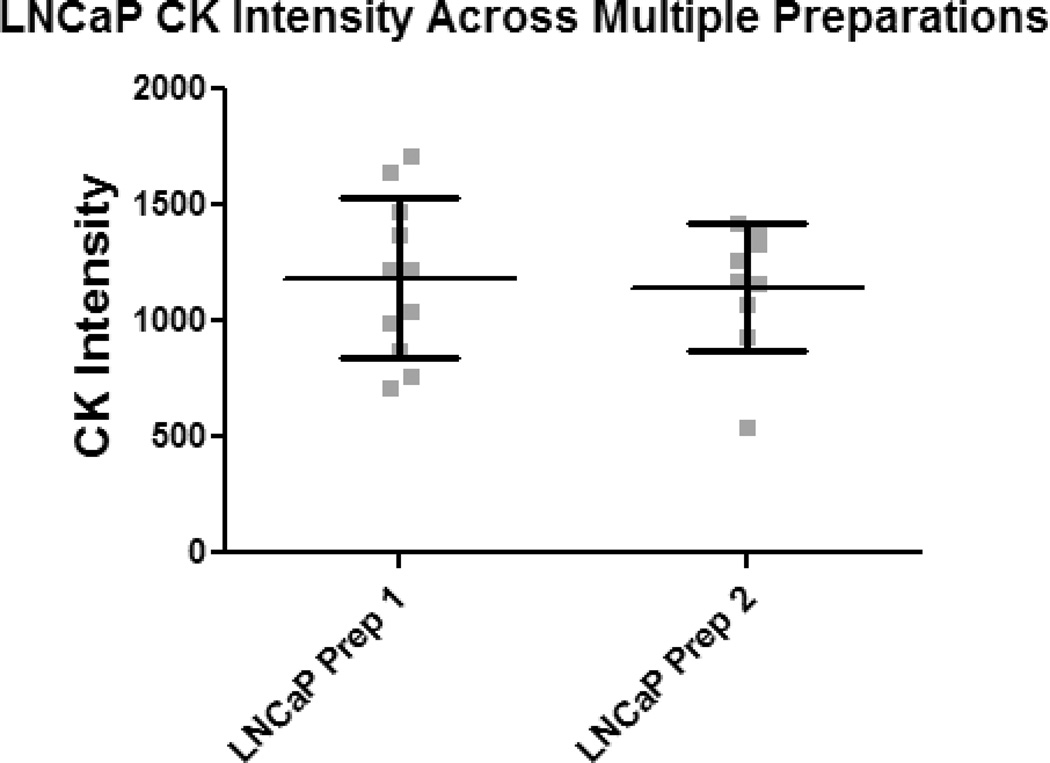
To verify the findings were not the result of preparation variability, two parameters were investigated: the white blood cell (WBC) area across multiple slides (Fig. 5A) and the CK intensity of LNCaP cells between the two preparations (Fig. 5B). Within the frame of an analyzed LNCaP or CTC, surrounding WBCs were analyzed with respect to nuclear area. No significant difference was found in the nuclear area when comparing the WBCs within the frames of AR+ CTCs, AR− CTCs, and LNCaPs (Fig. 5A). The CK intensities of LNCaPs were compared between the two preparations, and no significant difference was found (Fig. 5B).
Figure 5. Comparison of White Blood Cell (WBC) Areas and LNCaP Intensities.
(A) To ensure normalized area measurements between AR+ CTCs, AR− CTCs, and LNCaP cells, WBCs from each preparation were compared. The difference was not found to be significant. (B) To verify CK intensities were consistent between multiple preparations, the CK intensity of LNCaP cells from each preparation were compared. No significant difference was found. Error bars represent SEM.
Discussion
A comparison of both fluorescent and cytomorphic features between LNCaPs and CTCs shows similarities and significant differences between the two populations. The average CK expression level of the LNCaP cells is 19 times greater than that of the CTCs, while the average N/C ratio for CTCs is 2.8 times higher than the LNCaPs. This increase in the N/C ratio is due primarily to the larger cytoplasmic area of the LNCaPs as the two populations have near identical average nuclear area. CTCs though have greater variability with respect to the nuclear area. When analyzing the CK intensities for CTCs and LNCaPs, both populations exhibited considerable heterogeneity. This wide range of CK expression levels among the CTCs suggests that these cells may represent a heterogeneous population. This hypothesis is further supported by the comparison of AR+ and AR− CTCs. When AR+ and AR− CTCs were compared, a significant difference was found between their CK intensities. Additionally, a comparison of the cytomorphic features of these two cell types shows that on average AR+ CTCs have a greater N/C ratio than the AR− CTCs.
The reason for the difference in overall CK expression is currently unknown. Of the multiple possibilities that explain the lower CK expression in CTCs, two potential causes could be: cell lines under cell culture conditions express greater levels of protein than patient samples, or cell lines maintain expression levels similar to the primary tumors and CTCs undergo a biological change either entering or during circulation. Gene expression correlations of primary tumors and cell lines derived from those primary tumors argue against cell lines maintaining expression levels similar to primary tumors (12). However, if cell lines inherently express greater levels of protein, one would expect the AR expression to be higher in the LNCaP cells than in the CTCs. The finding that there was no significant difference between the AR intensities of LNCaPs and CTCs lends support to the notion that a biological difference exists between cell lines and CTCs with respect to the CK intensity as the difference is protein specific and not a change in global protein expression levels. Not only is there a decrease in overall CK expression, there is also a decrease in the area of the cytoplasm between LNCaPs and CTCs. In contrast, the average nuclear area for the two cell populations displayed no significant difference. These observed changes in cell size and protein expression may simply be due to the culturing conditions used for cell lines. A consistent finding in prostate cancer cells lines is that the expression level proteins change over time with response to increasing passage numbers. This is most commonly found with prostate specific antigen (PSA), where the expression level decreases over time. While considerable variation does exist across these prostate cancer cell lines with respect to the cytokeratins that are expressed, this discrepancy was not attributed to the culturing conditions. AR expression levels, however, have been shown in unique cases to be influenced by the introduction of androgens to the culturing medium (13, 14). The ability of cells to undergo phenotypic changes is not unique to cell lines. Previous research has shown that the tumor microenvironment can influence the state of the tumor cells, and as cells escape from the primary tumor, they undergo rapid phenotypic changes (15). A more accurate representation of the disease may be achieved by employing a different prostate cancer cell lines as the protein expression patterns are not always consistent across differing cell lines. For example, DU145 and LNCaPs show a different expression profile across 49 unique proteins when treated with somatostatin analogues (16). Therefore, more extensive experiments comparing additional prostate cell lines and CTCs to fresh primary tumor samples are worthy of future investigation to better understand how changes in protein expression and other cellular characteristics contribute to tumor progression and metastasis. Additionally, an alternative methodology to elucidate the contribution of cell culture to changes in protein expression would be to culture CTCs. This would enable a direct comparison of the CTCs and a CTC-derived cell line, which would provide a means of qualifying the effects of cell culture.
Two separate, although not mutually exclusive, models exist on how the cells enter circulation: active intravasation or passive shedding (17), both of which address the biological changes that CTCs may acquire in order to enter and survive circulation. For both theories, in vitro models show that tumor cells undergo phenotypic changes in order to enter the bloodstream (17). One such example of this phenotypic change is epithelial cell adhesion molecule (EpCAM) down-regulation. It is postulated that metastasis requires down-regulation of EpCAM due to its role as an adhesion molecule. This theory is supported by analysis of CTC EpCAM expression levels. CTCs have been shown to have a greater than ten-fold decrease in EpCAM expression when compared to both cell lines and carcinoma cells of primary and metastatic tissue (18). Additionally, as tumor cells progress towards a mesenchymal-like phenotype, the cytoskeleton has been shown to undergo remodeling. During this remodeling, vimentin becomes the chief intermediate filament, while CK expression is down-regulated within the cell (19). Further experiments must be performed to determine whether the lower CK expression we observed in CTCs is the result of CTCs undergoing an EMT transition by correlating the overall CK expression to known EMT markers. Another distinct possibility is that the cytoskeleton degrades over time while the cells are in circulation. Changes in CK levels relative to the primary tumor could potentially serve as a means to determine the lifetime of these cells.
In this report, we have, for the first time, directly compared the relative expression levels of proteins in cell lines to those in patient CTCs by immunofluorescence detection of overall CK and AR. We report the finding that LNCaPs have increased expression levels of CK as compared to CTCs, but have similar expression levels of AR. Additionally, we report that, on average, LNCaP cells are larger in total cell size than patient CTCs, but no significant difference exists in nuclear size; the difference in size is only in the cytoplasmic domain. The work presented here highlights some of the phenotypic variations that can be measured between cell lines and primary cells collected from cancer patients. The ability to perform and understand these measurements may be critical in understanding how to translate results from cell line data to the actual pathogenesis of cancer. From these findings, future experiments to measure the differences between other important cellular proteins in multiple cell lines versus CTCs from a variety of cancer types are currently being planned. Cell line experiments will continue to be an important tool for cancer research to determine molecular mechanisms and functionality, but CTCs can now be used to validate the clinical relevance of these molecular mechanisms and cell line models, potentially revealing important insights into the biology of cancer and metastasis.
Acknowledgements
We thank the clinical staff and patients at Scripps Clinic and USC. The work in this manuscript is supported by NCI Award U54CA143906. The content is solely the responsibility of the authors and does not represent the views of the NCI or the NIH. This is TSRI manuscript #21471.
References
- 1.Eccles SA, Welch DR. Metastasis: recent discoveries and novel treatment strategies. Lancet. 2007;369:1742–1757. doi: 10.1016/S0140-6736(07)60781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiang AC, Massagué J. Molecular Basis of Metastasis. N. Engl. J. Med. 2008;359:2814–2823. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 4.Cristofanilli M, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl. J. Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 5.Nagrath S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin HK, et al. Portable filter-based microdevice for detection and characterization of circulating tumor cells. Clin. Cancer Res. 2010;16:5011–5018. doi: 10.1158/1078-0432.CCR-10-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stott SL, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl. Acad. Sci. U S A. 2010;107:18392–18397. doi: 10.1073/pnas.1012539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu T, Lu B, Tai YC, Goldkorn A. A cancer detection platform which measures telomerase activity from live circulating tumor cells captured on a microfilter. Cancer Res. 2010;70:6420–6426. doi: 10.1158/0008-5472.CAN-10-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrinucci D, Bethel K, Lazar D, Fisher J, Huynh E, Clark P, Bruce R, Nieva J, Kuhn P. Cytomorphology of circulating colorectal tumor cells:a small case series. J. Oncol. 2010;2010:861341. doi: 10.1155/2010/861341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, Chu TM, Mirand EA, Murphy GP. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–1818. [PubMed] [Google Scholar]
- 11.Marrinucci D, Bethel K, Luttgen M, Bruce RH, Nieva J, Kuhn P. Circulating tumor cells from well-differentiated lung adenocarcinoma retain cytomorphologic features of primary tumor type. Arch. Pathol. Lab. Med. 2009;133:1468–1471. doi: 10.1043/1543-2165-133.9.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uva P, Lahm A, Sbardellati A, Grigoriadis A, Tutt A, de Rinaldis E. Comparative Membranome expression analysis in primary tumors and derived cell lines. PLoS One. 2010;5:e11742. doi: 10.1371/journal.pone.0011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sobel RE, Sadar MD. Cell lines used in prostate cancer research: a compendium of old and new lines--part 1. J. Urol. 2005;173:342–359. doi: 10.1097/01.ju.0000141580.30910.57. [DOI] [PubMed] [Google Scholar]
- 14.Sobel RE, Sadar MD. Cell lines used in prostate cancer research: a compendium of old and new lines--part 2. J Urol. 2005;173:360–372. doi: 10.1097/01.ju.0000149989.01263.dc. [DOI] [PubMed] [Google Scholar]
- 15.Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Z, Marquez M, Nilsson S, Holmberg AR. Comparison of protein expression in two prostate cancer cell-lines, LNCaP and DU145, after treatment with somatostatin. Oncol. Rep. 2009;22:1451–1458. doi: 10.3892/or_00000587. [DOI] [PubMed] [Google Scholar]
- 17.Bockhorn M, Jain RK, Munn LL. Active versus passive mechanisms in metastasis: do cancer cells crawl into vessels, or are they pushed? Lancet Oncol. 2007;8:444–448. doi: 10.1016/S1470-2045(07)70140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao CG, Chianese D, Doyle GV, Miller MC, Russell T, Sanders RA, Jr, Terstappen LW. Expression of epithelial cell adhesion molecule in carcinoma cells present in blood and primary and metastatic tumors. Int. J. Oncol. 2005;27:49–57. [PubMed] [Google Scholar]
- 19.Paccione RJ, Miyazaki H, Patel V, Waseem A, Gutkind JS, Zehner ZE, Yeudall WA. Keratin down-regulation in vimentin-positive cancer cells is reversible by vimentin RNA interference, which inhibits growth and motility. Mol. Cancer Ther. 2008;7:2894–2903. doi: 10.1158/1535-7163.MCT-08-0450. [DOI] [PubMed] [Google Scholar]