Abstract
Th1 immunity protects against tuberculosis infection in mice and humans. The widely used BCG vaccine primes CD4 and CD8 T cells through signaling mechanisms from dendritic cells and macrophages. The latter express MHC-II and MHC-I molecules through which peptides from BCG vaccine are presented to CD4 and CD8 T cells, respectively. Since BCG sequesters within a phagosome that does not fuse with lysosomes, generation of peptides within antigen-presenting cells infected with BCG occurs with reduced efficiency. We demonstrate that activation of DCs containing BCG vaccine with rapamycin leads to an enhanced ability of DC vaccines to immunize mice against tuberculosis. Coadministration of rapamycin with BCG vaccine also enhanced Th1 immunity. We propose that rapamycin-mediated increase in Th1 responses offers novel models to study mTOR-mediated regulation of immunity.
Keywords: Rapamycin, Mycobacterium tuberculosis, BCG vaccine, Mouse, mTOR, CD4, CD8 T cells, Th1 immunity
1. Introduction
Rapamycin is a well-known drug with antineoplastic properties. By repressing the mammalian target of rapamycin (mTOR), it triggers a wide variety of biochemical signaling within mammalian cells (1–4). An interesting property is the induction of autophagy (5, 6). Autophagy is a process in which a double-membrane phagophore is initiated which engulfs the cytosol and organelle. The phagophore then closes forming an autophagosome (AP) and subsequently fuses with the lysosome leading to an autophago-lysosome (APL). The degradation of cytosol and other membrane-bound vesicles or organelle within APL generates the much needed metabolites for the cell. Our initial studies have shown that the process of AP fusion with lysosome can be exploited to enhance the efficacy of vaccines for tuberculosis, where the causative pathogen hides within macrophages and dendritic cells (6). In this study, we illustrate the beneficial effects of rapamycin using a mouse model to evaluate the BCG vaccine which is used to protect against human tuberculosis.
Tuberculosis is the leading cause of death due to a single infectious agent in humans. The causative pathogen Mycobacterium tuberculosis has a unique property of sequestering within early endosome like membrane compartments or phagosomes that do not fuse with lysosomes. Interestingly, the BCG vaccine, which is derived from a similar bovine pathogen M. bovis also avoids lysosomal fusion and localizes to phagosomes that are sequestered (7–9). It is well-known that phagosome–lysosome (PL) fusion is critical for degradation of mycobacterial peptides leading to the generation of peptides which are usually sorted to the MHC-II-containing compartments (MIIC). Such peptides are ultimately loaded into MHC-II for surface presentation to the CD4 cells initiating one major arm of the Th1 immunity.
Since BCG vaccine avoids PL fusion, it was obvious that peptide presentation to T cells was likely to be defective. Indeed, in our earlier studies, we and others demonstrated such defects using a T-cell hybridoma (10, 11). We have now developed a protocol using rapamycin to induce lysosomal localization for BCG vaccine that in turn leads to enhanced degradation, peptide production, and better priming of T cells and generation of better Th1 immunity (6).
2. Materials
2.1. BCG and Reagents
Culture BCG vaccine (Pasteur strain) in 7H9 broth for 7 days and freeze log phase organisms. Before use, thaw aliquots, wash three times in PBS (3000 × g; 15 min), sonicate at 4 W using a soniprobe, and disperse suspension to match with McFarland #1 in turbidity (108 CFU/mL).
Use this as stock BCG. A single-cell (colony-forming unit, CFU) suspension of BCG for infections is made by gentle centrifugation of this suspension at 300 × g for 5 min and using supernatants that contain107 CFU/mL.
Viability of vaccine is confirmed by using live–dead stain from Invitrogen (USA). More than 90% of organisms should stain green.
2.2. H37Rv Strain of M. tuberculosis
Stock strain obtained from ATCC is cultured to log phase (7–10 days) in 7H9 broth using rotary shakers and frozen as aliquots as described above for BCG vaccine.
2.3. Rapamycin
Dissolve 1 mg of rapamycin in 100 μ L of dimethyl sulfoxide and make up to 1 mL in distilled water or as per manufacturer’s recommendation.
2.4. Mice and Dendritic Cells for Immunization
C57Bl/6 mice (M/F, 6–8 weeks of age) are sacrificed as per standard approved procedures and harvested for bone marrow from femurs and tibia. Centrifuged pellet of marrow cells are treated with a red cell lysing solution, such as ACK buffer, and the washed white cells are plated at a density of 106 per mL in 6-well TC plates using Isocove’s modification of DMEM (IDM) with 10% fetal bovine serum, penicillin and gentamicin (100 U/ mL and 50 μ g/mL, respectively), and 50 μ M 2-mercaptoethanol. Add 10 ng/mL of GM-CSF (Cell Sciences, USA) to IDM for cultures and replenish GM-CSF medium every 2–3 days.
Between days 7 and 10, harvest cells, wash, and use CD11c beads and a bead fractionator (Miltenyi Inc, USA) to obtain >95% pure CD11c-positive, CB11b-negative immature DCs.
Count and adjust to 108 cell/mL in IDM without GM-CSF. Pass through a 27-gauge needle to disperse cells and store in an ice bath.
2.5. Rapamycin Activation and Preparation of DC Vaccines
Use 4 × 2 mL aliquots of DCs kept cold in an ice bath, each with 108 cells/ mL.
To two aliquots of DCs, add rapamycin to a final 100 and 10 μ g/mL and mix cells at 37°C and 5% CO2 for 4 h. Mix also two more aliquots of DCs without the addition of rapamycin.
While cells are mixing, prepare single-cell BCG suspension as above. Remove DCs, and add 2 × 107 BCG CFU to rapamycin (10 and 100 μ g)-treated DCs and to one aliquot of untreated DCs. Do not add BCG to the fourth aliquot. Note : All DC vaccines are at 2 × 108 per 2 ml. Higher infection ratios do not make a difference as long as infection ratio does not exceed 1:5 (DC:BCG).
Mix again for 4 h at 37°C and 5% CO2. Remove DCs and wash three times with cold Hank’s balanced salt solution (HBSS) (1000 × g × 5 min) to remove unbound bacteria. Make up cells in HBSS, gently pass through a 27-gauge needle to obtain single-cell suspension, and then adjust the count of cells to 108 /mL in HBSS; keep cells cold in an ice bath.
Check cell viability using trypan blue; it should be more than 95%. (e) Inject groups of 20–25 mice per vaccine group i.p. with 100 μ L suspension of DCs containing 2 × 106 cells per mouse.
From each vaccine preparation, pellet 100 μL aliquots, lyse with 0.05% SDS, and plate the lysate dilutions on 7H11 agar media to determine colony counts (CFUs) of BCG organisms contained within the DCs vaccine inoculum.
House mice under BSL-3 conditions and provide food and water ad libitum.
3. Methods
3.1. Assay to Determine if Th1 Immune Responses Are Accelerated by Rapamycin
Seven or 14 days after vaccination, four mice per group were sacrificed and splenic T cells immunostained for surface receptors (CD4 and CD8) and intracellular staining for IFNγ following standard procedures.
T cells from four individual mice per group are acquired using a BD Facscan and analyzed using the Cellquest software. Data are represented as histograms to determine the log shift in fluorescence of T cells.
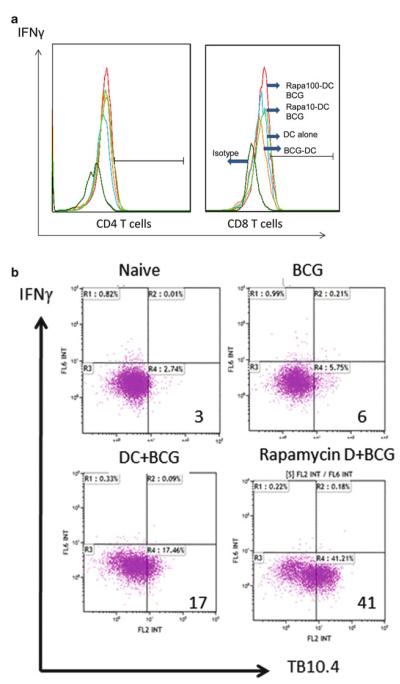
T cells from mice are also stainable using CD8 antigen-specific tetramers (TB10.4-specific tetramer obtained from NIH tetramer facility, Emory Vaccine Center, Atlanta GA, USA). Such cells are as usual analyzed in combination with intracellular IFNγ stain. Note : Use of antigen-specific tetramer stains enable more precise analysis of the CD8 memory T cells. An example of TB10.4 tetramer-stained CD8 T cells is shown in Fig. 1b.
Fig. 1.
Rapamycin treatment of dendritic cells with BCG vaccine accelerates the Th1 response in mice. C57Bl/6 mouse bone marrow-derived dendritic cells (DCs) were treated with either 10 or 100 μg of rapamycin followed by infection with Mycobacterium bovis Bacillus Calmette Guerin (BCG) bacteria for 4 h and washed. Five mice per group were injected i.p. with, 2 × 106 DCs per mouse per group as follows: (1) rapamycin-activated and BCG-infected DCs (10 or 100 μg doses), (2) untreated DCs infected with BCG, and (3) naïve DCs. An untreated group was an additional control. (a) Seven days after injection, splenic T cells were typed for CD4 and CD8 T cells secreting IFNγ after restimulation with antigen 85B, a major antigen of BCG vaccine. Flow cytometric analysis shows increased expression of IFNγ-secreting CD4 and CD8 T cells in mice given rapamycin-activated DCs containing BCG. (b) Mice were immunized as in panel (a). Two weeks later, the spleen-derived T cells were stained using a tetramer specific for BCG TB10.4 antigen (NIH Tetramer facility Emory Vaccine Center, USA). Rapamycin-activated, BCG-infected DCs induce a stronger expansion of tetramer-specific CD8 T cells compared to BCG-infected DCs alone.
3.2. Assay of Vaccine Efficacy After Rapamycin Activation of DCs
After 4 weeks of vaccination, mice in groups of 20 are exposed to an aerosol dose of a suspension of M. tuberculosis calculated to deliver 100-CFU counts per mouse using a Glas-Col (Indiana, USA) aerosol chamber. Mice are then housed in cages for another 4 weeks.
On the 4th week following aerosol challenge, the mice (five per vaccine group) were sacrificed as per standard procedures and lungs and spleens harvested individually as follows.
Lungs and spleens are homogenized in 5-mL saline with 0.05% tween 80 and then 100 μL aliquots of ten-fold dilutions are plated in replicates on 7H11 agar plates with 5 μg/mL thiophene 2-carboxylic acid hydrazide (TCH). Plates are read for CFU counts of M. tuberculosis and plotted against vaccine groups. Vaccine-induced protection is expressed as log10 reduction in CFU counts compared to controls and unvaccinated or naïve group, and significance was determined using ANOVA.
Four additional mice per group are sacrificed and T cells were purified from the organs and analyzed for IFNγ-secreting CD4 and CD8 T cells using antibody staining and flow cytometry. Note : As needed, the same pellet can also be stained for multiple cytokines using appropriate colored antibodies.
In order to confirm antigen specificity, cells were restimulated with 100 ng/mL of mycobacterial antigen 85B (available from BEI, NIH repository, MD, USA) and Elispot assay to determine IFNγ-secreting T cells. Elispot kit was obtained from either BD (USA) or Ebiosciences.
3.3. Variations of a Protocol for Rapamycin Effects In Vivo on Vaccine Efficacy
In a recent study, a low-dose administration of rapamycin given i.p. to mice was found to enhance the longevity of CD8 immune responses to viral pathogens (3). We, therefore, developed a modified protocol to demonstrate an increase in CD8 responses to BCG vaccination and M. tuberculosis infection in mice.
Mice (C57Bl/6, M/F, 6–8 weeks of age; 20 per group) are immunized with BCG given once subcutaneously at 1 × 106 CFU per mouse.
From day one post vaccination, mice are given daily intraperitoneal injections of 75 μg/kg dose of rapamycin for 30 days. Control groups received BCG vaccine alone, rapamycin alone, or left untreated as naïve.
On day 32, two days after the last rapamycin dose, mice are aerosol infected as above and protection against tuberculosis measured. Before aerosol infection, spleens and inguinal lymph nodes are evaluated for IFNγ-secreting CD8 T cells using a 4-h restimulation with phorbol myristyl acetate in the presence of brefeldin.
4. Notes
We observed earlier that rapamycin enhances the MHC-II-mediated peptide presentation in DCs and macrophages infected with BCG vaccine. Antigen-presenting cells like DCs prime CD4 and CD8 T cells through MHC-II and MHC-I in a cytokine-rich environment. We, therefore, propose that activation of DCs would enhance peptide processing within the DCs and lead to an enhancement in vaccine-induced priming and thereby efficacy.
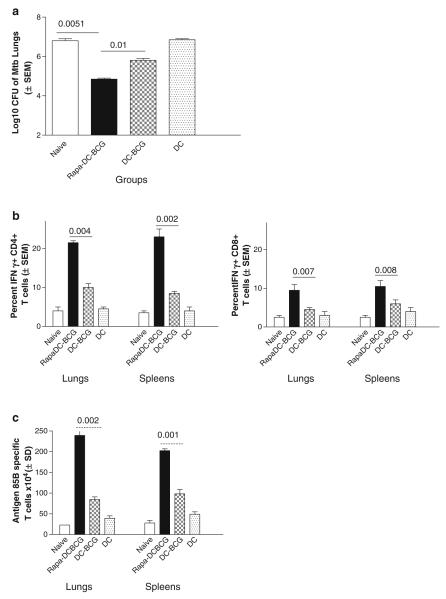
In this study, we noted that rapamycin drove an expansion of antigen-specific T cells. Figure 1 b shows the tetramer-specific CD8 T cells in response to BCG vaccination. Figure 2 c shows that the lungs and spleens of rapamycin-DC-BCG-vaccinated mice contained larger number of antigen-85B-specific T cells than comparable organs of other groups. This correlates with the hypothesis that Ag85B drives a major immune activation against tuberculosis in the lungs and that rapamycin has an amplifying response on antigen-specific T cells (4).
In this study, we have described a protocol that can be used to enhance the efficacy of the BCG vaccine in mice against tuberculosis using rapamycin-activated DCs (Figs. 1 and 2). Rapamycin has multiple effects on immune cells, including DCs, macrophages, and T cells. Therefore, a modified protocol was developed that allowed BCG antigens to be more effective in vivo when coadministered with rapamycin (Fig. 3).
Although enhanced processing of antigens in DCs is a major mechanism of rapamycin, recent studies have found that rapamycin affects mTOR signaling within T cells (4, 12, 13). Thus, we propose that when rapamycin is coadministered with BCG vaccines it can act on multiple cell populations to increase vaccine efficacy. The molecular mechanism of rapamycin in vivo needs additional investigation.
Note that all infection procedures need to be carried out under BSL-3 conditions and require approved institutional protocols.
The protocol describes measures to enhance protection against infectious tuberculosis. However, an identical protocol can also be used to measure the sterilizing effect of BCG vaccine in combination with rapamycin against an infectious challenge with live attenuated BCG vaccine. In this case, the challenge organism is marked with either green fluorescent or red fluorescent protein to enable detection and quantitation of marked BCG in organs. Such studies are useful to analyze the role of various T-cell populations that are modulated by rapamycin. We, therefore, anticipate that the models we described herein will pave the way for dissecting the role of mTOR in the regulation of T cell-mediated immunity.
Fig. 2.
Rapamycin-activated dendritic cell-BCG vaccine induces a better protection against tuberculosis in mice. C57Bl/6 mice were immunized subcutaneously with one dose of DCs (2 × 106 cells per mouse containing cell containing 1 × 106 BCG CFU) as follows. DCs were (1) preactivated with rapamycin (10 or 100 μg) and infected with BCG, (2) untreated but infected with BCG, and (3) naïve DCs. A group of mice did not receive DCs or BCG as control. Mice were analyzed as follows. (a) Mice were aerosol challenged with 100 CFU of M. tuberculosis 4 weeks after vaccination and bacterial counts of lungs determined by plating lung homogenates on 7H11 agar. Data show log10 reduction in CFUs of lungs and mice vaccinated with rapamycin-DC-BCG vaccine are protected better than mice given DC-BCG vaccine or no vaccine (five mice per group; three separate experiments; p-values determined using ANOVA shown against groups compared). (b) Lungs and spleens of mice were analyzed for Th1-type T cells using fl ow cytometry 4 weeks after aerosol challenge. Rapamycin-DC-BCG-vaccinated mice show higher levels of Th1-type CD4 (left panel) and CD8 (right panel) T cells secreting IFNγ (p-values shown against groups compared, t test). (c) Four weeks after aerosol challenge, Lungs and spleens of vaccinated mice (four per group) were analyzed for T cells specific for Ag85B using Elispot analysis. Rapamycin-DC-BCG vaccine again induces higher levels of antigen-specific T-cell responses in the lungs and spleens (p-values shown for groups compared, t test).
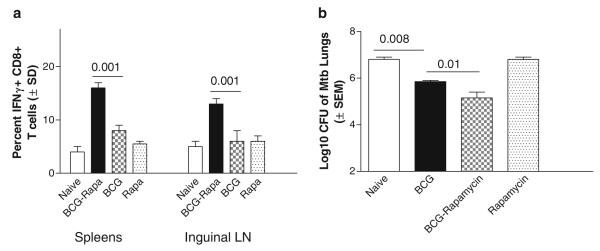
Fig. 3.
Coadministration of BCG vaccine and rapamycin induces a better protection against tuberculosis correlating with increased CD8 T-cell responses. C57Bl/6 mice were vaccinated with BCG alone or BCG vaccine followed by 30 daily injections of rapamycin given i.p. On day 32, inguinal lymph nodes and spleens were analyzed for antigen-specific Th1 T-cell responses and then aerosol challenged with M. tuberculosis to determine protection. (a) On day 32, lymphoid compartments show an increased frequency of CD8 T cells that secrete IFNγ as analyzed by fl ow cytometry (four mice per group, t test). (b) Rapamycin combination with BCG vaccine enhances protection against tuberculosis compared to BCG vaccine alone (five mice per group; two separate experiments, p-values shown for groups compared, ANOVA).
Acknowledgments
This study was supported by AI49534 and AI78420. The authors acknowledge the technical assistance of Arshad Khan, Kari Herdtner, and Devin R. Lindsey.
References
- 1.Luo H, Duguid W, Chen H, Maheu M, Wu J. The effect of rapamycin on T cell development in mice. Eur J Immunol. 1994;24:692–701. doi: 10.1002/eji.1830240331. [DOI] [PubMed] [Google Scholar]
- 2.Anderson KM, Zimring JC. Rapamycin prolongs susceptibility of responding T cells to tolerance induction by CD8+ veto cells. Transplantation. 2006;81:88–94. doi: 10.1097/01.tp.0000185302.38890.6b. [DOI] [PubMed] [Google Scholar]
- 3.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrer IR, Wagener ME, Robertson JM, Turner AP, Araki K, Ahmed R, Kirk AD, Larsen CP, Ford ML. Cutting edge: Rapamycin augments pathogen-specific but not graft-reactive CD8+ T cell responses. J Immunol. 185:2004–2008. doi: 10.4049/jimmunol.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saemann MD, Haidinger M, Hecking M, Horl WH, Weichhart T. The multifunctional role of mTOR in innate immunity: implications for transplant immunity. Am J Transplant. 2009;9:2655–2661. doi: 10.1111/j.1600-6143.2009.02832.x. [DOI] [PubMed] [Google Scholar]
- 6.Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL, Jr., Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267–276. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
- 7.Clemens DL, Horwitz MA. The Mycobacterium tuberculosis phagosome interacts with early endosomes and is accessible to exogenously administered transferrin. J Exp Med. 1996;184:1349–1355. doi: 10.1084/jem.184.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deretic V, Fratti RA. Mycobacterium tuberculosis phagosome. Mol Microbiol. 1999;31:1603–1609. doi: 10.1046/j.1365-2958.1999.01279.x. [DOI] [PubMed] [Google Scholar]
- 9.Russell DG. Mycobacterium tuberculosis: here today, and here tomorrow. Nat Rev Mol Cell Biol. 2001;2:569–577. doi: 10.1038/35085034. [DOI] [PubMed] [Google Scholar]
- 10.Singh CR, Moulton RA, Armitige LY, Bidani A, Snuggs M, Dhandayuthapani S, Hunter RL, Jagannath C. Processing and presentation of a mycobacterial antigen 85B epitope by murine macrophages is dependent on the phagosomal acquisition of vacuolar proton ATPase and in situ activation of cathepsin D. J Immunol. 2006;177:3250–3259. doi: 10.4049/jimmunol.177.5.3250. [DOI] [PubMed] [Google Scholar]
- 11.Soualhine H, Deghmane AE, Sun J, Mak K, Talal A, Av-Gay Y, Hmama Z. Mycobacterium bovis bacillus Calmette-Guerin secreting active cathepsin S stimulates expression of mature MHC class II molecules and antigen presentation in human macrophages. J Immunol. 2007;179:5137–5145. doi: 10.4049/jimmunol.179.8.5137. [DOI] [PubMed] [Google Scholar]
- 12.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sathaliyawala T, O’Gorman WE, Greter M, Bogunovic M, Konjufca V, Hou ZE, Nolan GP, Miller MJ, Merad M, Reizis B. Mammalian target of rapamycin controls dendritic cell development downstream of Flt3 ligand signaling. Immunity. 33:597–606. doi: 10.1016/j.immuni.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]