Abstract
Circulating tumor cells (CTCs) have been implicated as a population of cells that may seed metastasis and venous thromboembolism (VTE), two major causes of mortality in cancer patients. Thus far, existing CTC detection technologies have been unable to reproducibly detect CTC aggregates in order to address what contribution CTC aggregates may have on metastasis or VTE. We report here an enrichment-free immunofluorescence detection method that can reproducibly detect and enumerate homotypic CTC aggregates in patient samples. We identified CTC aggregates in 43% of 86 patient samples. The fraction of CTC aggregation was investigated in blood draws from 24 breast, 14 non-small cell lung (NSCLC), 18 pancreatic, 15 prostate stage IV cancer patients, and 15 normal blood donors (NBD). Both single CTCs and CTC aggregates were measured to determine whether differences exist in the physical characteristics of these two populations. Cells contained in CTC aggregates had less area and length, on average, than single CTCs. Nuclear to cytoplasmic (N/C) ratio between single CTCs and CTC aggregates were similar. This detection method may assist future studies in determining which population of cells is more physically likely to contribute to metastasis and VTE.
INTRODUCTION
The two major causes of cancer deaths are metastasis and venous thromboembolism (VTE) [1]. While CTCs have been implicated in metastasis, new evidence has revealed a potential role for CTC aggregates as a mode of metastasis [2]. Furthermore, evidence exists of homotypic CTC aggregation occurring at endothelium attachment sites [3]. Although cell aggregation is implicated in increasing metastatic efficiency, circumstances where CTC aggregates lead to embolus formation are also conceivable. Investigating the contribution of CTC aggregates to metastasis and VTE could provide valuable insight into the mechanisms of metastasis and VTE; however, it is first necessary to determine if CTC aggregates can be detected reproducibly in patient blood in substantial numbers. In order to identify CTC aggregates in metastatic cancer patients, we performed secondary analysis of archived data from a small cohort of cancer patients (see Marrinucci et al. in this issue of Physical Biology) using a non-enrichment rare event detection system to enumerate CTC aggregates from patient blood draws. We investigated the incidence of both single CTCs and CTC aggregates in archived data from 24 breast, 14 NSCLC, 18 pancreatic, 15 prostate cancer patients with metastatic disease, and 15 normal blood donor (NBD) patient samples. Here, a CTC aggregate is defined as two or more cytokeratin (CK) positive/CD45 negative cells with intact nuclear morphology identified by our platform as being one pixel apart or less. Furthermore, we measured basic physical attributes such as area and length of single CTCs and CTC aggregates to characterize differences in physical parameters. These parameters may have functional implications for the potential of metastasis or VTE in cancer patients which remain to be determined by future studies addressing mechanism and function.
MATERIALS AND METHODS
Patient sample collection and preparation
As previously reported [4, 5] blood draws from stage IV epithelial cancer patients and NBD were collected into Cyto-Chex® tubes (Streck, Omaha, NE) at the multiple clinical sites and Scripps Normal Blood Donor Service (for NBD) under IRB approved protocols and transported to the labs at The Scripps Physics Oncology Center.
Imaging and data analysis
CTC candidates were identified by automated microscopy as described by Marrinucci et al., in this issue. In brief, cells were identified using nuclear signal and classified based on cytokeratin (CK) positivity and CD45 positivity, for epithelial cells and WBCs, respectively. Candidate CTCs were analyzed by a hematopathologist trained technical analyst to confirm the CTC phenotype, and CTC aggregates were enumerated. Two or more CTC candidate cells separated by one pixel or less were scored as a CTC aggregate. High resolution images of CTC aggregates were taken on a Carl Zeiss LSM710 (Carl Zeiss, Jena, Germany) confocal microscope with a 63× oil immersion objective. Single CTCs and aggregates from all cancer types were automatically relocated using a customized macro written for ImagePro Plus (Media Cybernetics, Bethesda, MD) and imaged at 40× magnification on a Nikon 80i (Melville, NY) fluorescent microscope for measurements comparing nuclear and cytoplasmic characteristics. Ten WBCs in the same field of view as each CTC or CTC aggregate were measured and used for normalization to correct for localized differences in processing and imaging. Cells or aggregates for all cancer types were manually segmented and measured using ImageJ (Bethesda, MD). For length measurements, the cell or aggregate shape was fit to an ellipse, and the longest diameter of the ellipse was used for the length measurement. In the case of CTC aggregates, because of the lack of delineation of cells, entire clusters were segmented and the total length measurement was divided by the number of nuclei within the aggregate. N/C ratios were based on DAPI and CK staining, respectively. CK staining was found to delineate the cytoplasmic region of the cell as confirmed by brightfield imaging (data not shown). For physical comparison, measurements were grouped as either single CTCs or aggregates, and not separated by cancer type. This was due to loss of certain samples after archiving preventing relocation and reimaging of those events. Statistical significance of measurements was determined by Student’s t-test.
RESULTS AND DISCUSSION
Secondary analysis of archived data revealed CTC aggregates in patient samples originally planned to enumerate CTCs. We report here that CTC aggregates are reproducibly detected in peripheral blood of multiple metastatic cancer patients (Fig. 1A-D, respectively). In our study population, 54% of breast, 50% of NSCLC, 22% of pancreatic, 93% of prostate, and 0% of NBD patients had at least one CTC aggregate (Fig. 2A). Occasional CTC aggregation has been observed by others using alternative methods [6]; however, this is the first time a significant number of CTC aggregates have been reported. Interestingly, one would expect more frequent artificial CTC aggregation to be found using the enrichment-based methods due to the high collision frequencies than would be expected in our low-enrichment method. This, and the lack of observation of cell aggregates in cell line spike-in experiments (data not shown), supports the hypothesis that natural occurrence is the likely source of these aggregates. Although we only consider homotypic aggregation here, it is important to note that other entities (e.g., leukocytes and platelets) were observed associating with CTC aggregates (see Fig. 1), and may play important roles in metastasis and VTE as demonstrated in vitro [7]. Although heterotypic associations will be a consideration for future analysis, homotypic aggregation is examined for this first study to simplify the analysis.
Figure 1. CTC aggregates are reproducibly observed in multiple epithelial cancers.
CTC aggregates observed in metastatic breast (A), NSCLC (B), pancreatic (C), and prostate (D) cancer patients found by our high throughput imaging scanner were relocated and imaged by confocal microscopy for greater detail. CTCs were identified using cytokeratin antibody staining (red) with DAPI counterstain for nuclei (blue). WBCs were labeled with CD45 antibody staining (green). Lack of CD45 staining in (B) is contributed to photobleaching in a dated, archived sample. Z-projections of the images are shown here.
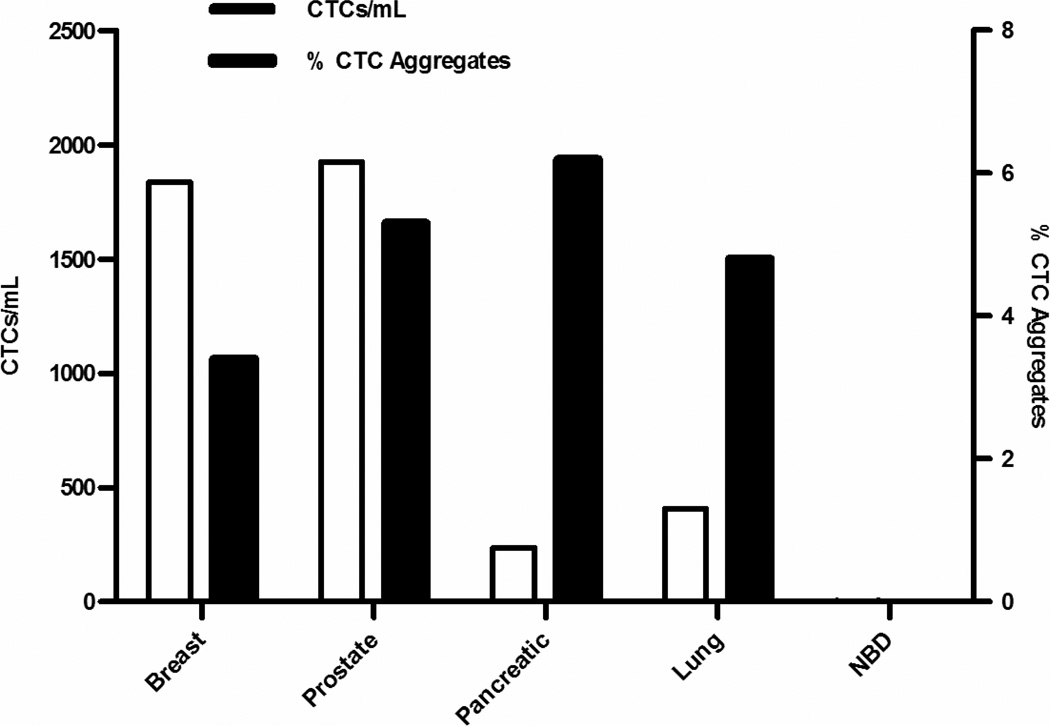
Figure 2. A large percentage of CTCs were observed in aggregates in patients.
Patient samples from 86 total patients (24 breast, 14 lung, 18 pancreatic, and 15 prostate stage IV cancer patients, and 15 NBD patients) were analyzed for single CTCs and CTC aggregates. Of the total CTCs found in all patient samples for each cancer type, 3.4% in breast, 4.8% in NSCLC, 6.2% in pancreatic, and 5.3% in prostate cancer were found in CTC aggregates. None were found in NBD patients.
We performed initial observations of CTC aggregates in patient samples to determine the prevalence in different cancer types. In 24 breast patients, 21 were found to have CTCs and 13 of those patients were found to have CTC aggregates. In 14 NSCLC patients, 13 were found to have CTCs and 7 of those patients were found to have CTC aggregates. In 18 pancreatic patients, 15 were found to have CTCs but CTC aggregates were detected in only 4 of those patients. In 15 prostate patients, 14 were found to have CTCs and 11 of those patients were found to have CTC aggregates. In 15 NDB patients, only 3 were found to have detectable CTCs and none of those patients were found to have CTC aggregates. Of the patients for each cancer type, 54% of breast, 50% of NSCLC, 22% of pancreatic, 73% of prostate, and 0% of NBDs possessed at least one CTC aggregate. Measurements of the percentage of CTCs in aggregates (that is, total number of CTCs in aggregates/total number of CTCs) found that 48.8% of breast, 52.5% of NSCLC, 4% of pancreatic, and 57.7% of prostate patient CTCs reside in aggregates. Alternatively, when CTC aggregates were measured as the percentage of the total number of events (total number of aggregates/total number of aggregates and single CTCs), 2.3% of events in breast, 16.9% in NSCLC, 1.3% in pancreatic, and 3.7% in prostate patients were aggregates. We also measured the total prevalence of CTC aggregates found in all patient samples (that is, total number of aggregates/total number of CTCs) per cancer type, which revealed that 3.4% of CTCs in breast, 4.8% in NSCLC, 6.2% in pancreatic, 5.3% in prostate cancer patient CTCs are aggregates (p=0.003; Fig. 2). Due to the study design, specific outcomes for individual patients do not exist and thus patient-specific conclusions cannot be made regarding metastasis or VTE in this patient cohort; however, the cohort population data presented here does provide some insight into one potential mechanism (e.g., aggregation) that CTCs may use for survival in metastasis and contribution to VTE.
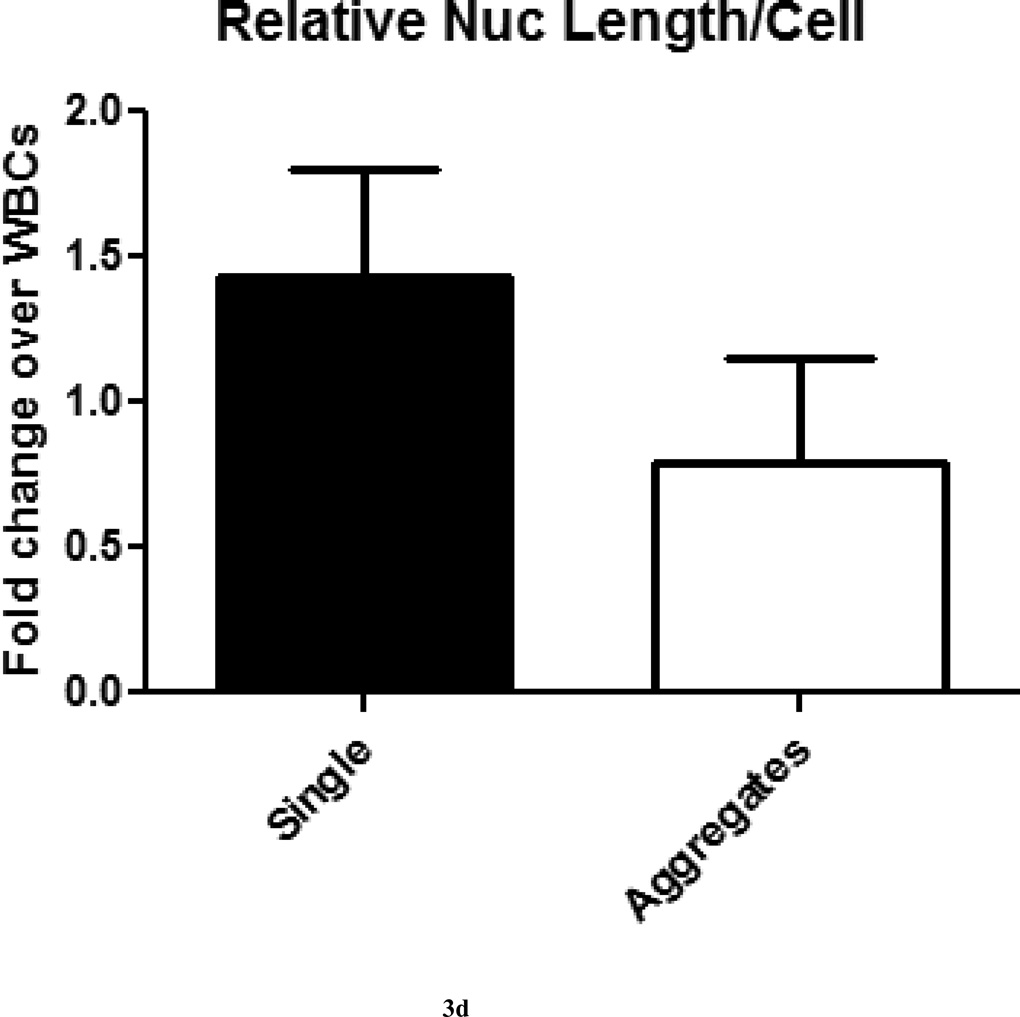
Image analysis of physical characteristics of single CTCs and CTC aggregates were made to determine differences that may functionally contribute to metastasis or VTE. Measurements were normalized to WBCs in the field of view to correct for processing and imaging variations that occur over the area of the scanned slide as described in materials and methods. On average, both single CTCs and aggregates were about two-fold larger than WBCs by cytoplasmic area on a per cell basis, but this difference was not statistically significant (p=0.057; Fig. 3A). Relative cell lengths of single CTCs were two-fold larger than WBCs, but CTCs in aggregates were approximately the same cell length as WBCs, and this difference was statistically significant (p<0.0001; Fig. 3B). Relative nuclear areas of single CTCs were only 1.5-fold larger than WBCs, while CTCs in aggregates had approximately the same nuclear area as WBCs, and this difference was statistically significant (p<0.05; Fig. 3C). Similar results were observed for relative nuclear length measurements, and this difference was statistically significant (p<0.0001; Fig. 3D). Nuclear to cytoplasmic (N/C) ratios, a measurement used routinely by pathologists for diagnoses, were found to be the same for single CTCs and CTC aggregates (2:1), and were not statistically significant (p=0.77; Fig. 3E). The observation that CTCs in aggregates were, on average, smaller than their single CTC counterparts is consistent with observations that epithelial cells in their natural environment (i.e., attached to other epithelial cells) tend to be smaller than non-attached counterparts [8–10]. Much speculation has been made on this point, mainly surrounding the consideration that epithelial cells adopt a more mesenchymal-like morphology and functionality in order to increase motility in these cells during the process of metastasis. These measurements could potentially lead to further insights, such as aiding in determination of the origins of CTC aggregates. In vitro data has shown that CTC aggregates can form in the circulation [3]; however, it is also possible that CTC aggregates separate from the tumor as an aggregate and circulate throughout the body as a single entity. Both models of CTC aggregate formation have implications for metastasis and VTE; therefore, determining the mechanism for CTC aggregate formation is critical.
Figure 3. Physical measurements of single CTCs and CTC aggregates were made to detect differences in cell and nuclear sizes.
(A) Measurements of the areas of single CTCs and CTC aggregates found both groups to be two-fold larger than surrounding WBCs in the field of view. (B) Measurements of the lengths of single CTCs and CTC aggregates found single CTCs to be approximately two-fold larger than and CTC aggregates approximately the same length as surrounding WBCs in the field of view. (C) Measurements of the nuclear areas of single CTCs and CTC aggregates found single CTCs to be approximately 1.5-fold larger than and CTC aggregates to be approximately the same nuclear area as surrounding WBCs in the field of view. (D) Measurements of the nuclear lengths of single CTCs and CTC aggregates found single CTCs to be approximately 1.5-fold larger than and CTC aggregates to be approximately 0.8-fold the nuclear length of surrounding WBCs in the field of view. (E) Nuclear to cytoplasmic (N/C) ratios of single CTCs and CTCs aggregates were not significantly different.
The results of this study establish the ability to reproducibly identify CTC aggregates in patients of multiple epithelial cancers using a low-enrichment method. These data also exemplify the type of secondary analyses that can be performed on existing data sets reported from our current protocol. Efforts are underway to further define and validate that the cytokeratin-positive cells found by our method are indeed CTCs and CTC aggregates. For example, a HER2 assay for breast cancer patients would further validate the cytokeratin staining, as well as be a clinically relevant biomarker for patient diagnoses. The same would be true for androgen receptor (AR) in prostate cancer. Information regarding CTC aggregates could potentially be used to predict risk of VTE. Since a considerable number of VTE patients are asymptomatic according to autopsy reports [11], a non-invasive method to identify patients either at risk or with pre-symptomatic VTE could be clinically useful in the cancer patient population, who are not routinely treated with anti-coagulant therapy. VTE is typically diagnosed when patients become symptomatic or as an incidental finding from imaging data; early diagnosis may aid in decreasing patient morbidity and mortality. Aggregation of CTC with other entities in the circulation (e.g., leukocytes and platelets) may also impact the physical characteristics of CTCs and aggregates and may impact mechanisms of metastasis and VTE. We hope that this initial analysis of homotypic CTC aggregation will position us to better understand the molecular connections between CTCs, metastasis, and VTE as the study continues to further characterize homotypic and heterotypic CTCs and aggregates. New study protocols are underway to determine cellular adhesion and cytoskeletal factors that may contribute to the ability of CTCs to aggregate, as well as to measure heterotypic aggregation.
ACKNOWLEDGEMENTS
We thank the clinical staff and patients at Scripps Clinic, Billings Clinic, UCSD, and UCSF. The work in this manuscript is supported by NCI Awards U54CA143906 and 5R01CA125653-04. The content is solely the responsibility of the authors and does not represent the views of the NCI or the NIH. This is TSRI manuscript number 21330.
Footnotes
Author contributions
EHC designed the study analysis and wrote the manuscript. EHC and MW performed the CTC aggregation analysis. ML and DM performed technical analysis. CY and AK developed analysis algorithms. DL processed patient samples. ES, JN, LB, AM, and MK recruited patients and provided clinical data. KB provided hematopathological analysis. KB and PK conceived and supervised the study. All authors participated in manuscript review and revision.
Disclosure of Conflicts of Interest
ML, DM, DL, JN, AK, KB, and PK are shareholders of Epic Sciences.
REFERENCES
- 1.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J. Thromb. Haemost. 2007;5:632–634. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 2.Kats-Ugurlu G, et al. Circulating tumour tissue fragments in patients with pulmonary metastasis of clear cell renal cell carcinoma. J. Pathol. 2009;219:287–293. doi: 10.1002/path.2613. [DOI] [PubMed] [Google Scholar]
- 3.Glinsky VV, Glinsky GV, Glinskii OV, Huxley VH, Turk JR, Mossine VV, Deutscher SL, Pienta KJ, Quinn TP. Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res. 2003;63:3805–3811. [PubMed] [Google Scholar]
- 4.Marrinucci D, Bethel K, Luttgen M, Bruce RH, Nieva J, Kuhn P. Circulating tumor cells from well-differentiated lung adenocarcinoma retain cytomorphologic features of primary tumor type. Arch. Pathol. Lab Med. 2009;133:1468–1471. doi: 10.1043/1543-2165-133.9.1468. http://www.archivesofpathology.org/doi/full/10.1043/1543-2165-133.9.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrinucci D, Bethel K, Lazar D, Fisher J, Huynh E, Clark P, Bruce R, Nieva J, Kuhn P. Cytomorphology of Circulating Colorectal Tumor Cells: a small case series. J. Oncol. 2010;2010:861341. doi: 10.1155/2010/861341. PMCID: PMC2810476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stott SL, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl. Acad. Sci. U.S.A. 2010;107:18392–18397. doi: 10.1073/pnas.1012539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berny-Lang MA, Aslan JE, Tormoen GW, Patel IA, Bock PE, Gruber A, McCarty OJ. Promotion of experimental thrombus formation by the procoagulant activity of breast cancer cells. Phys Biol. 2011;8:015014. doi: 10.1088/1478-3975/8/1/015014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hazan RB, Qiao R, Keren R, Badano I, Suyama K. Cadherin switch in tumor progression. Ann. N.Y. Acad. Sci. 2004;1014:155–163. doi: 10.1196/annals.1294.016. [DOI] [PubMed] [Google Scholar]
- 9.Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams Ed, Thompson EW. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J. Cell Physiol. 2007;213:374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 10.Makdissi FB, et al. Expression of E-cadherin, Snail and Hakai in epithelial cells isolated from the primary tumor and from peritumoral tissue of invasive ductal breast carcinomas Braz. J. Med. Biol. Res. 2009;42:1128–1137. doi: 10.1590/s0100-879x2009001200002. [DOI] [PubMed] [Google Scholar]
- 11.White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107:14–18. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]