Abstract
Rationale
Canonical transient receptor potential 4 (TRPC4) contributes to the molecular composition of a channel encoding for a calcium selective store operated current, ISOC, whereas Orai1 critically comprises a channel encoding for the highly selective calcium release activated calcium current, ICRAC. However, Orai1 may interact with TRPC proteins and influence their activation and permeation characteristics. Endothelium expresses both TRPC4 and Orai1, and it remains unclear as to whether Orai1 interacts with TRPC4 and contributes to calcium permeation through the TPRC4 channel.
Objective
We tested the hypothesis that Orai1 interacts with TRPC4 and contributes to the channel’s selective calcium permeation important for endothelial barrier function.
Methods and Results
A novel method to purify the endogenous TRPC4 channel and probe for functional interactions was developed, using TRPC4 binding to protein 4.1 as bait. Isolated channel complexes were conjugated to anti-TRPC protein antibodies labeled with cy3-cy5 pairs. Förster Resonance Energy Transfer among labeled subunits revealed the endogenous protein alignment. One TRPC1 and at least two TRPC4 subunits constituted the endogenous channel (TRPC1/4). Orai1 interacted with TRPC4. Conditional Orai1 knockdown reduced the probability for TRPC1/4 channel activation, and converted it from a calcium-selective to a non-selective channel, an effect that was rescued upon Orai1 re-expression. Loss of Orai1 improved endothelial cell barrier function.
Conclusion
Orai1 interacts with TRPC4 in the endogenous channel complex, where it controls TRPC1/4 activation and channel permeation characteristics, including calcium selectivity, important for control of endothelial cell barrier function.
Keywords: Store operated calcium entry; ISOC, calcium selective store operated calcium entry current; ICRAC, calcium release activated calcium current; Protein 4.1; Endothelium
Diverse environmental signals provoke transient cytosolic calcium elevations that initiate physiologically relevant cellular adaptations. Such calcium transitions occur in all eukaryotic cells, in response to chemical, mechanical, and physical first messengers1-3. In endothelium, neurohumoral inflammatory mediators induce a transient rise in cytosolic calcium that serves as a proximal signal to reorganize the cytoskeleton, disrupt cell-cell junctions, and induce intercellular gaps4-6. These gaps form a paracellular pathway for plasma-rich exudation, and delivery of immunoglobulins and anti-inflammatory molecules to sites of injury or infection.
Calcium entry across the cell membrane, and not calcium release from intracellular organelles, such as the endoplasmic reticulum, is necessary to induce endothelial cell gaps and increase permeability7. Transient receptor proteins 1, 3, 4 and 6 within the canonical subfamily (TRPC) have all been incriminated in the calcium influx that is responsible for increased permeability8-13, yet functional TRPC channels are thought to form by some combination of four subunits, and the subunit stoichiometry of an endogenously expressed TRPC channel has yet to be resolved. Recently, the TRPC4 carboxy-terminus was found to directly interact with protein 4.1, near the channel’s projected pore12, 14. Disrupting the TRPC4-protein 4.1 interaction abolished calcium entry through the TRPC4 channel, and prevented inter-endothelial cell gap formation, indicating that TRPC4 is directly tethered to the membrane skeleton as a means to localize the calcium signal to physiologically important effectors.
Both TRPC1 and TRPC4 contribute subunits to the protein 4.1-bound channel12, 15, 16. This channel encodes for a calcium selective store operated current, ISOC, in endothelia, which is small (≈1.5 pA/pF), inwardly rectifying, and possesses a reversal potential near +40 mV11, 12, 14, 17. ISOC is activated by thapsigargin, inositol 1,4,5 trisphosphate and TPEN, all of which deplete endoplasmic reticulum calcium. Questions remain as to whether ISOC is the same as, or different from, the calcium release activated calcium current, ICRAC, which displays similar biophysical properties to ISOC, although is thought to be more highly calcium selective18-20. Orai1 is a four transmembrane spanning domain protein that forms a pore through the coordination of four subunits, and encodes for ICRAC21-25. Endothelial cells express Orai118, 26, 27. Trebak and colleagues18 suggest that thapsigargin activates both ISOC and ICRAC in endothelial cells, and further work indicated that ISOC controls permeability whereas ICRAC regulates proliferation, migration and angiogenesis.
Despite the evidence that Orai1 can form a channel, it remains unclear as to whether Orai1 is sufficient to comprise the pore-forming channel accounting for ICRAC in vivo. For example, Orai1 appears to be nearly ubiquitously expressed, and not all cells that express Orai1 possess ICRAC28-30. Moreover, the Birnbaumer31, Ambudkar32, and Flockerzi28 groups have independently noted that Orai1’s anatomy is unusual for a store or receptor operated calcium channel, and recognized that it is more reminiscent of channel ancillary proteins, such as the T channel’s γ subunit27. Orai1 may be an essential subunit of TRPC channels that is required for TRPC proteins to sense calcium store depletion, and hence to be store operated32. At present this central issue remains unresolved. To address this issue, we developed a means to enrich for the endogenous TRPC4 channel from endothelial cell membrane fractions, using protein 4.1 as bait. Using purified fractions, we probed for inter-molecular interactions by Förster resonance energy transfer (FRET). Cells were engineered for the conditional expression of Orai1 shRNA, so that in the same cells, Orai1 expression could be suppressed and then rescued. Our results indicate that Orai1 constitutively interacts with TRPC4 in endothelial cells, and with TRPC1 after calcium store depletion. Furthermore, Orai1 increases the probability that TRPC4 will be activated following calcium store depletion, promotes calcium selectivity of the TRPC4 channel, and critically controls the transmembrane calcium flux that serves as a proximal signal for endothelial cell barrier disruption.
Materials and Methods
Ethical Approval
All animal use studies were approved by the University of South Alabama’s Institutional Animal Care and Use Committee.
Cell Cultures
Rat pulmonary artery (PAECs) and microvascular endothelial cells were isolated and cultured in DMEM supplemented with 10% FBS and penicillin/streptomycin using previously described methods33, 34.
Reagents
Membrane/Cytoskeleton Fractions and Immunoprecipitation
Performed as described in 12.
Preparation of Isolated Immunocomplex
SDS-PAGE and Western Blot
As described in 12.
Fluorescently Tagged Antibodies
Antibodies were tagged with the cyanine reagents cy3 and cy5 following the manufacturer’s directions (Cy3 mAb Labeling Kit #PA33001 and Cy5 mAb Labeling Kit #PA35001; GE Healthcare Bio-Sciences Corp.; Piscataway, NJ).
FRET Experiments
The FRET-based approach to determine subunit stoichiometry was adapted from 35, 36. Procedure details are presented in online supplement.
Conditional Expression of Orai1 shRNA
Four pairs of short hairpin RNA (shRNA)-encoding oligonucleotides were designed using BLOCK-iT RNAi Designer program (Invitrogen), synthesized by Integrated DNA Technologies (Coralville, IA), reconstituted in water, annealed, and cloned into BfuI-digested pMA2867; this approach was described previously37. The identity of the constructs was verified by sequencing. Lentivirus-containing supernatants were produced using standard procedures, as shown previously37. Supernatants were used to infect tetracycline-regulated (Tet-On) pulmonary artery endothelial cells, which were then selected to homogeneity using puromycin.
Whole Cell Patch Clamp Electrophysiology
Patch clamp experiments were performed as described extensively elsewhere11, 12, 14, 15, 17. Details regarding intra- and extracellular solutions are available in the online supplement.
Statistical Analysis
Results were analyzed using one-way ANOVA, where appropriate, using GraphPad Prism 4.0 software. All data represent mean + SEM. P < 0.05 is considered statistically significant for the comparisons.
Results
Protein 4.1 functions as bait for isolation of an endogenous TRPC4 channel
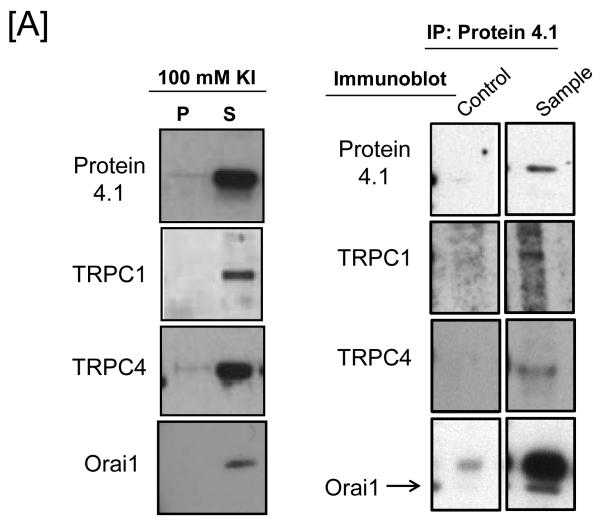
We utilized the physical interaction between TRPC4, protein 4.1 and the membrane skeleton as a way to enrich for the endogenous channel. Pulmonary artery endothelial cells were lysed and detergent extracted using octyl-ß-D-glucopyranoside. The resulting pellet fraction contained elements of the cytoskeleton and membrane skeleton, including spectrin bound to protein 4.1. In the presence of salt, protein 4.1 was displaced from the pellet fraction into a supernatant fraction with TRPC1, TRPC4 and Orai1 (Figure 1A), whereas actin and other cytoskeletal proteins remained in the pellet fraction (data not shown). Immunoprecipation of protein 4.1 and immunoblotting of TRPC proteins revealed that TRPC1, TRPC4 and Orai1 co-immunoprecipitate with protein 4.1.
Figure 1. Protein 4.1 interacts with TRPC1 and TRPC4 in vitro and in vivo.
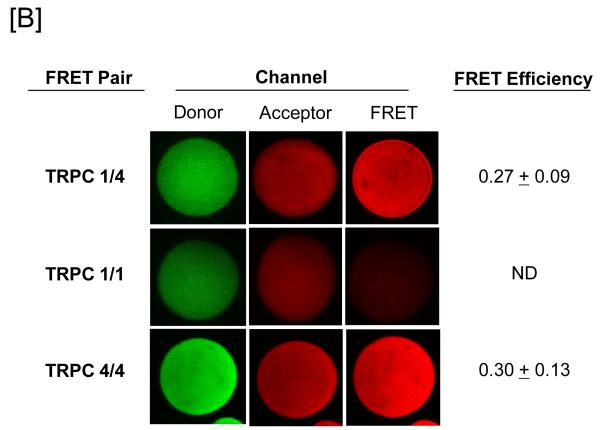
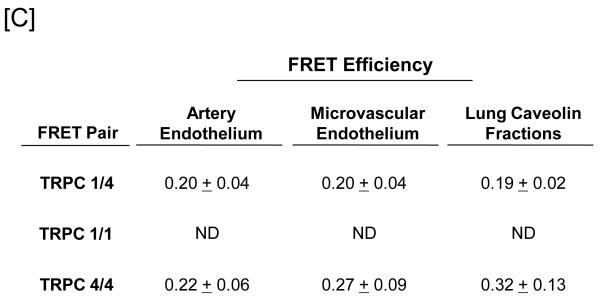
[A] Pulmonary artery endothelial cell membrane/cytoskeleton fractions were detergent extracted in the presence of 100 mM potassium iodide, KI. Pellet and supernatant fractions were separated on SDS-PAGE and immunoblot analysis performed. Protein 4.1, TRPC4, TRPC1, and Orai1 were resolved in the salt-dissociated supernatant (left panel). Protein 4.1 was immunoprecipitated and co-immunoprecipitation of TRPC4, TRPC1, and Orai1 was probed via immunoblot. TRPC4, TRPC1, and Orai1 co-immunoprecipitated with protein 4.1. [B] The protein 4.1-bound TRPC channel possesses TRPC1 and TRPC4 subunits. Protein 4.1-bound immunocomplexes containing the TRPC channel were isolated on agarose beads and treated with cy3-(donor channel) and cy5-(acceptor channel) labeled antibodies at their EC50 concentrations; a single bead is shown. FRET analyses were performed using the sensitized emission approach (FRET channel). FRET pairs refer to the cy3- and cy5-labeled proteins, respectively. FRET efficiency is derived from the summary of a minimum of 9 separate measurements. ND refers to not detected. [C] The TRPC channel FRET signal is identical in vitro and in vivo. The protein 4.1-bound immunocomplex was isolated from pulmonary artery endothelial cells, pulmonary microvascular endothelial cells and lung caveolin-enriched fractions purified from the intact pulmonary circulation. FRET efficiency is derived from the summary of a minimum of 15 separate measurements.
FRET resolves intermolecular interactions between TRPC4 and Orai1 in endothelium
We next developed a method to resolve the nature of the protein 4.1-bound TRPC4 channel purified within the salt dissociated supernatant, based upon the use of FRET as a molecular ruler38-40, to define inter-molecular interactions within a channel (Online Figure I). Protein 4.1 antibody was affixed to Protein A/agarose beads and incubated with the salt dissociated supernatant fraction, which tethered associated TRPC subunits to the beads as part of the channel complex. Our FRET approach was derived from studies resolving the GABA 35A and cyclic nucleotide gated cation channel36 stoichiometries, with the notable exception that in our system, TRPC subunits were not overexpressed. Rather, cy3 and cy5 FRET pairs were conjugated to TRPC protein antibodies and then incubated with Protein A/agarose beads containing the protein 4.1 bound TRPC channel over a range of concentrations. Fluorescence saturation binding curves were determined, and the half maximal concentration of each antibody was determined to allow for efficient FRET (Online Figure II). FRET detection occurs only when the distance between the fluorophores is <1.5 R0, where R0 is the Förster radius62,63. Using an R0 of 50 Å for the cy3-cy5 pair62,63, positive FRET reflects a distance of < 75 Å between proteins.
Since TRPC1 and TRPC4 co-immunoprecipitate50-53, and since TRPC4 appears to be required for TRPC1 membrane targeting16, we began by testing whether TRPC1 and TRPC4 directly interact. TRPC1 and TRPC4 were labeled with cy3 and cy5 conjugated antibodies, respectively, and FRET signals examined. As anticipated, strong FRET signals were observed between TRPC1 and TRPC4, indicating they directly interact within the native channel (Figure 1B).
Several control approaches were developed to ensure FRET signals are due to intra-channel subunit interactions. Dilution experiments were performed where one-fifth the amount of salt-dissociated supernatant was immunoprecipitated for protein 4.1 (Online Figure III). If the FRET measurements are non-specific, then the distance between adjacent TRPC1/4 channel complexes should increase as the sample becomes more dilute. In this instance, the FRET signal would be abolished. If the FRET measurements are specific, then the distance between individual proteins in the TRPC1/4 complex should not increase as the sample becomes more dilute. In this case, the FRET signal would be retained. To do this, FRET between cy3-labeled TRPC1 and cy5-labeled TRPC4 was tested. In undiluted samples, FRET was readily apparent between TRPC1 and TRPC4. The overall fluorescence intensity was decreased in all of the diluted samples compared to the undiluted samples. However, strong TRPC1-TRPC4 FRET signals were retained in the diluted samples (Online Figure III), demonstrating FRET detects intra-channel subunit interactions.
To confirm efficient energy transfer between cy3 and cy5 was occurring as predicted, photobleaching studies were undertaken. In this case, the cy5-labeled TRPC4 channel was irradiated to photobleach fluorescence. Such cy5 inactivation prevented energy transfer from cy3-labeled TRPC1 to cy5-labeled TRPC4. Consequently, increased cy3 fluorescence was measured, indicating the signal measured in the FRET channel is specific (Online Figure IV). Collectively, these data confirm that our FRET approach detects intra-channel subunit interactions.
To determine whether the channel possesses more than one TRPC1 subunit, TRPC1 antibody was labeled with cy3 and cy5 and FRET responses examined. FRET could not be detected under these conditions (Figure 1B). However, labeling the TRPC4 antibody with cy3 and cy5 revealed strong FRET responses, indicating more than one TRPC4 protein exists within the channel (Figure 1B).
These initial studies were conducted using pulmonary artery endothelial cells, yet considerable heterogeneity among endothelial cell phenotypes has been reported, particularly in regard to calcium signaling. To determine whether stoichiometry of the protein 4.1-bound TRPC channel is the same among endothelial cell phenotypes, FRET experiments were repeated using salt dissociated supernatants isolated from pulmonary microvascular endothelial cells (Figure 1C). The results of these experiments revealed an identical TRPC protein alignment.
Since TRPC1 and 4 proteins reside in caveolin-rich fractions41, 42, and since caveolin-rich fractions can be isolated from pulmonary endothelium in the intact circulation43, we utilized caveolin fractions obtained from an isolated perfused lung preparation to examine TRPC channel subunit alignment. These caveolin-rich fractions retain elements of the membrane skeleton, including spectrin, actin and protein 4.1. Detergent extracted caveolin fractions were prepared for FRET studies and protein-protein interactions probed. Once again, identical FRET responses were recorded (Figure 1C), indicating the channel’s subunit alignment is the same in cultured cells and in vivo.
Orai1 interacts with TRPC4 in the protein 4.1-enriched channel complex
TRPC proteins are generally believed to form non-selective cation channels. However, the protein 4.1-bound TRPC1/4 channel described presently is calcium selective11, 12, 14, 15, albeit less so than the calcium release activated calcium current, ICRAC, that is thought to be encoded by Orai121-25. Controversy remains as to whether TRPC channels interact with Orai1, and as to whether TRPC channels are activated by calcium store depletion. Orai1 has been reported to interact with TRPC1 following calcium store depletion, and this interaction may be necessary for TRPC1 to sense calcium store depletion31.
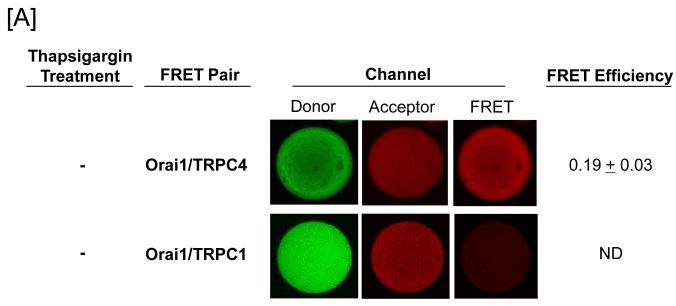
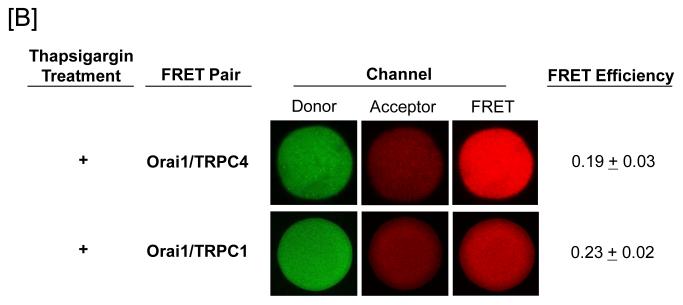
FRET was utilized to examine whether Orai1 interacts with the protein 4.1-bound TRPC1/4 channel. Whereas Orai1 was found to constitutively interact with TRPC4, it did not interact with TRPC1 (Figure 2A). We next examined whether the nature of this Orai1-TRPC4 interaction changed following channel activation. Thapsigargin was used to activate store operated calcium entry in pulmonary artery endothelial cells, and salt dissociated supernatants were prepared for FRET studies. Under these conditions, Orai1 interacted with both TRPC1 and TRPC4, generally consistent with previous observations (Figure 2B).
Figure 2. Orai1 interacts with TRPC4 in the protein 4.1-bound TRPC channel.
Protein 4.1-bound immunocomplex was isolated from pulmonary artery endothelial cells either before [A] or after [B] thapsigargin treatment (1 μM for 15 minutes). The TRPC channel was treated with cy3-(donor channel) and cy5-(acceptor channel) labeled antibodies at their EC50 concentrations. FRET analyses were performed using the sensitized emission approach (FRET channel). FRET pairs refer to the cy3- and cy5-labeled proteins, respectively. Whereas Orai1 only interacted with TRPC4 in the unstimulated state, it interacted with both TRPC1 and TRPC4 following thapsigargin treatment. FRET efficiency is derived from the summary of a minimum of 15 separate measurements. ND refers to not detected.
Orai1 impacts TRPC4 channel activation and calcium permeation
The functional significance of an Orai1-TRPC1/4 interaction is not known. We developed an approach to test whether Orai1 impacts TRPC1/4 channel activation and permeation characteristics. Four shRNA targeting Orai1 mRNA were generated and tested; one sequence was particularly effective at reducing Orai1 protein and was therefore selected for further study (Online Figure V). Pulmonary artery endothelial cells were then engineered to express the Tet-On reverse transactivator protein (rtTA) (Online Figure VI). The construct was stably introduced using a retro-lentiviral system. rtTA was followed by an internal ribosomal entry site and a fusion between enhanced green fluorescent protein (EGFP) and blasticidin resistance gene. Infected cells were selected to homogeneity using blasticidin resistance and confirmed by EGFP fluorescence. Once these cells were selected to homogeneity, they were re-infected with a second virus containing the shRNA sequence driven by doxycycline, enabling rtTA interaction with Tet-operator sequences. Cells expressing Orai1 shRNA were selected using puromycin, and confirmed by observing mCherry fluorescence upon doxycycline induction.
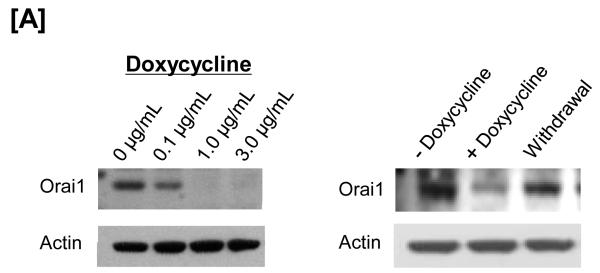
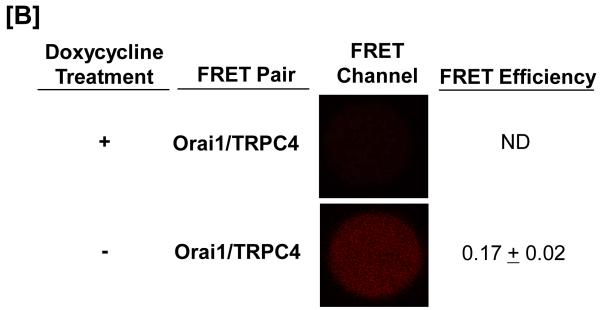
Using this system, the ability of shRNA to decrease Orai1 expression, and the time required for doxycycline withdrawal to result in Orai1 re-expression, was examined (Figure 3A). Within 72 hours of doxycycline treatment, western blot analysis revealed that Orai1 expression was nearly abolished, and within 48 hours after withdrawing doxycycline, Orai1 expression had returned to near normal levels. Doxycycline concentrations as low as 0.1 μg/mL effectively reduced Orai1, with maximal protein inhibition ranging from 1-3 μg/mL. Salt dissociated supernatant fractions were generated from these engineered cells, and FRET studies were performed. As shown previously in control cells, constitutive FRET was seen between Orai1 and TRPC4 (Figure 3B). In doxycycline-treated cells, shRNA expression abolished Orai1 fluorescence on the agarose beads; TRPC protein expression was not decreased by Orai1 knockdown. As anticipated, in the absence of Orai1 protein, an interaction between Orai1 and TRPC4 could not be detected by FRET, yet upon doxycycline withdrawal, Orai1 fluorescence was rescued, as was the Orai1-TRPC4 interaction.
Figure 3. The Orai1-TRPC4 interaction is not detectable after Orai1 mRNA is silenced using doxycycline-inducible shRNA.
The TRPC1/4 complex was purified from pulmonary artery endothelial cells. [A] Orai1 was expressed in control cells, and was resolved in the supernatant and not the pellet fraction following lysate exposure to salt (100 mM KI). Doxycycline treatment for 72-hours decreased Orai1 protein, an effect observed at concentrations as low as 0.2 μg/mL, with maximal effect seen at 1-3 μg/mL (left panel). This effect of doxycycline was reverseable 48-hours following doxycycline withrawal (right panel). [B] FRET was observed between TRPC4 and Orai1 in control cells (data not shown). Exposure to doxycycline for 72-hours abolished the Orai1-TRPC4 FRET response. Doxycycline withdrawal resulted in a recovery of Orai1-TRPC4 FRET, consistent with the re-appearance of Orai1 in the TRPC1/4 complex. FRET efficiency is derived from the summary of a minimum of 15 separate measurements. ND refers to not detected.
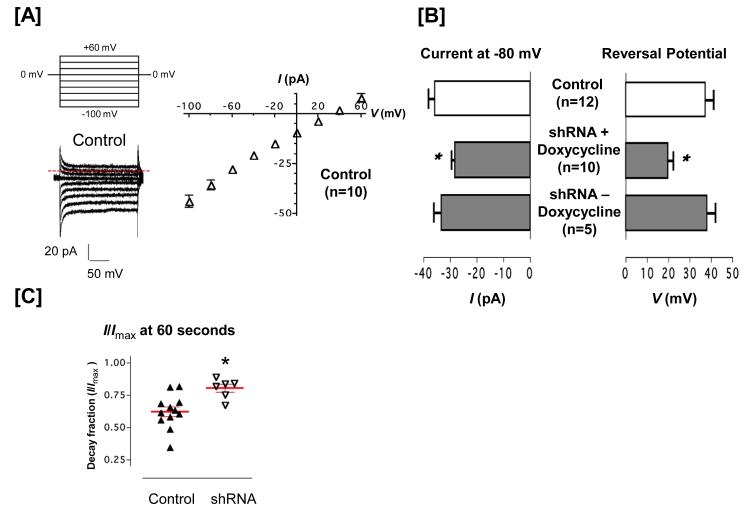
Whole cell electrophysiology experiments were performed using doxycycline naïve cells, and following doxycycline treatment and withdrawal. Thapsgargin was delivered in the patch pipette using internal solutions designed to clamp intracellular calcium at 100 nM. Under these conditions, thapsigargin induced a small, inward calcium current that displayed a reversal potential near +40 mV (Figure 4A). Eighty six percent of the control cells tested displayed the current. Four important differences were noted in cells treated with doxycycline for 72 hours to reduce Orai1 expression. First, thapsigargin activated a current in only 47% of the cells lacking Orai1, suggesting that Orai1 plays a critical role in coupling calcium store depletion to channel activation (Odds ratio = 5.25; p < 0.05 chi square). Second, of the cells that responded to thapsigargin, the current density was decreased by approximately 25% at all of the negative test potentials, although the current was not abolished, indicating Orai1 is not necessary for cation permeation through the ISOC (Figure 4B). Third, the reversal potential was left shifted to +20 mV, indicating a loss of calcium selectivity (Figure 4B). Fourth, calcium dependent inactivation was reduced (Figure 4C). The TRPC1/4 channel displays a calcium dependent inactivation, where the macroscopic current is decreased approximately 40% in sixty seconds. In cells lacking Orai1, TRPC1/4 inactivation was reduced by approximately 20%, suggesting less calcium was permeating through the channel. When doxycycline was withdrawn and Orai1 expression returned to normal levels, the thapsigargin-induced current, including the percent of cells activated, current density, and reversal potential, were normalized.
Figure 4. Orai1 regulates TRPC1/4 channel amplitude and calcium selectivity.
[A] Control pulmonary artery endothelial cells were treated with doxycycline and the TRPC1/4 current density tested. Thapsigargin (1 μM) was applied in the patch pipette, and induced a calcium selective current with a typical peak current (≈ −45 pA at −100 mV) and reversal potential (+40 mV). [B] Cells were engineered using a Tet-On system for doxycyline induction of Orai1 shRNA. Orai1 downregulation decreased the peak current and left shifted the reversal potential, effects that were reversed following doxycycline withdrawal and the re-emergence of Orai1. [C] Time-dependent inactivation of the thapsigargin-induced current was measured for 60 seconds, from the standard ramp protocol11-12, 14-15. Whereas the current density decreased in control cells, this effect was reduced in doxycycline-treated cells lacking Orai1. Following doxycycline withdrawal, the time dependent current inactivation was recovered.
We next tested the anomalous mole fraction behavior of ISOC in the presence and absence of Orai144. In cells possessing Orai1, typical anomalous mole fraction behavior was observed, where the ISOC current was greatest in 100 nmol/L extracellular calcium, reflecting sodium permeation through the TRPC1/4 channel (Figure 5A). Increasing extracellular calcium concentrations reduced this sodium current, revealing smaller calcium currents, especially at 10 mmol/L extracellular calcium. In the absence of Orai1, the sodium current was preserved while the calcium-dependent inhibition of the sodium current was nearly abolished. Orai1 did not influence the reversal potential when extracellular calcium concentrations were below 1 mmol/L, but when 10 mmol/L calcium concentrations were studied to reveal a calcium current, cells possessing Orai1 displayed a reversal potential of approximately +40 mV whereas cells lacking Orai1 had a reversal potential near +20 mV (Figure 5B). These findings suggest that Orai1 is necessary for the TRPC4-dependent ISOC to display calcium selectivity. Using this anomalous mole fraction protocol, ISOC inactivation was again tested (Figure 5C). The decay fraction was greatest in cells expressing Orai1, indicating the presence of Orai1 is an important determinant of the amount of calcium that permeates the TRPC1/4 channel.
Figure 5. Anomalous mole fraction behavior and calcium-dependent inactivation of the thapsigargin-evoked current in PAECs.
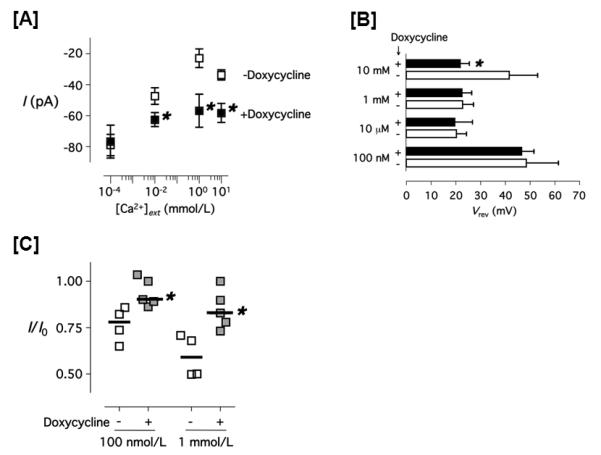
[A] The whole cell transmembrane current values of the thapsigargin-evoked current in PAECs treated with ( ) and without (
) and without ( ) doxycycline are shown. Data were collected using the stepwise voltage protocol (see Material and Methods) in 130 mmol/L extracellular Na+ and various extracellular calcium concentrations ([Ca2+]ext). Cells were clamped with the pipette containing 100 nmol/L free Ca2+ and 1 μmol/L thapsigargin, 130 mmol/L extracellular Na+ and the indicated [Ca2+]ext. [B] Reversal potential of the thapsigargin-evoked current in PAECs treated with (closed bar) and without (open bar) doxycycline in the indicated [Ca2+]ext is shown. Data were calculated using a piecewise cubic spline function. [C] The fraction decay of the thapsigargin-evoked whole cell current in PAECs treated with (
) doxycycline are shown. Data were collected using the stepwise voltage protocol (see Material and Methods) in 130 mmol/L extracellular Na+ and various extracellular calcium concentrations ([Ca2+]ext). Cells were clamped with the pipette containing 100 nmol/L free Ca2+ and 1 μmol/L thapsigargin, 130 mmol/L extracellular Na+ and the indicated [Ca2+]ext. [B] Reversal potential of the thapsigargin-evoked current in PAECs treated with (closed bar) and without (open bar) doxycycline in the indicated [Ca2+]ext is shown. Data were calculated using a piecewise cubic spline function. [C] The fraction decay of the thapsigargin-evoked whole cell current in PAECs treated with ( ) and without (
) and without ( ) doxycycline, measured for 100 seconds at −80 mV using the stepwise voltage protocol. * P < 0.05 (doxycycline-treated vs. doxycycline non-treated, Student t-test.
) doxycycline, measured for 100 seconds at −80 mV using the stepwise voltage protocol. * P < 0.05 (doxycycline-treated vs. doxycycline non-treated, Student t-test.
Orai1 contributes to the calcium entry that disrupts the endothelial cell barrier
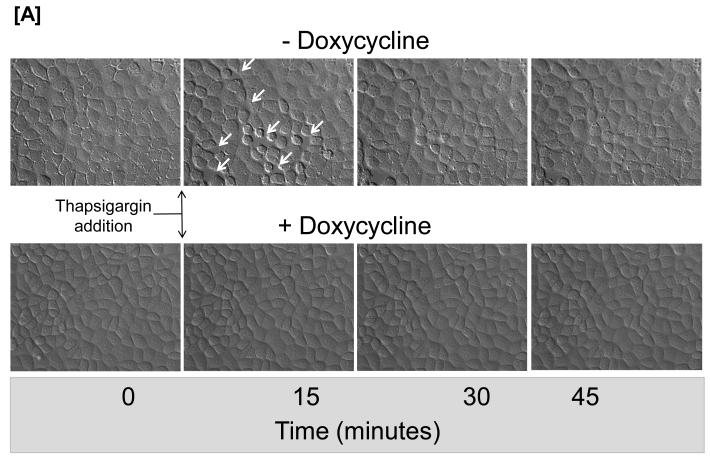
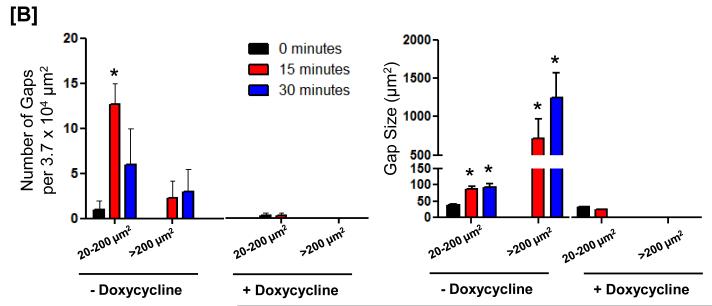
Inflammatory agonists that activate SOC entry induce gaps between adjacent endothelial cells as a paracellular pathway for exudation; TRPC1/4 localizes to junctional complexes7, 12. Here, we tested whether the presence of Orai1 contributes to endothelial cell barrier disruption. Time-lapse videomicroscopy was performed to investigate whether thapsigargin induces gaps in the presence and absence of Orai1 (Figure 6 and Online Figure VII). As previously reported, thapsigargin induced multiple small gaps ranging in size from 20-200 μm2, and fewer gaps larger than 200 μm2. Inter-endothelial cell gap formation was transient, as most gaps resealed within 30 minutes. This effect was dependent upon the presence of Orai1, as Orai1 knockdown prevented thapsigargin from inducing gaps.
Figure 6. Orai1 contributes to the calcium signal that induces inter-endothelial cell gaps.
[A] Cells engineered for conditional regulation of Orai1 shRNA were grown to confluence in the presence or absence of doxycycline. In the absence of doxycycline, thapsigargin induced the formation of transient inter-endothelial cell gaps that resealed within 45 minutes. In contrast, gaps were not visible in cells lacking Orai1 following doxycycline treatment. Data are representative of 3 experiments. [B] Analysis of number of gaps (left panel) and the gap size (right panel) reveals thapsigargin induces multiple small gaps, ranging in size from 20-200 μm, with fewer gaps larger that 200 μm. Gaps were not visible in cells following Orai1 silencing. * P < 0.05 vs. time 0.
Discussion
In this study, we illustrate the privileged inter-molecular interaction within caveolin-rich membrane domains between the protein 4.1-bound TRPC1/4 channel and Orai1. This interaction is functionally important, as Orai1 increases the probability that TRPC1/4 will be activated by calcium store depletion, and it critically influences the calcium selectivity of the TRPC1/4 channel and, hence, the calcium concentration that permeates through the channel. These results prompt further consideration of the molecular structure and function of the endothelial cell TRPC1/4 channel.
Few channels are known to bind to protein 4.145. Precedence for a role of protein 4.1 in both the scaffolding and regulation of ion channels was first vetted in neurons, where protein 4.1R was shown to co-associate with NaV1.5 channels in the Nodes of Ranvier45-49. Protein 4.1R knockout mice were later revealed to display a cardiac phenotype, with bradycardia accompanied by prolongation of the QT interval. In this case, a delay in NaV1.5 channel inactivation was reported. Similar findings were reported in TRPC4-channels, as a protein 4.1-TPRC4 interaction was shown to be essential for channel gating 12,14. There is an absolute dependence on protein 4.1-actin binding for thapsigargin to activate ISOC, and protein 4.1 in turn directly links the spectrin membrane skeleton to the TRPC4 channel. Both the introduction of a peptide targeting the conserved protein 4.1 binding domain on the TRPC4 carboxy-terminus and heterologous expression of a chimeric TRPC4 protein lacking the protein 4.1 binding domain prevent ISOC activation12. We recently recognized that Orai1 also possesses a conserved protein 4.1 binding domain (residues 24-33; TSGSRRSRRR)27. Unlike TRPC4, however, the Orai1 protein 4.1 binding domain is located in the amino-terminus; structural analysis revealed no such protein 4.1 binding domain in either Orai2 or Orai3. At this time, the function of the Orai1 protein 4.1 binding domain is unknown.
Recognition that relatively few ion channels interact with protein 4.1 prompted us to utilize protein 4.1 as bait to enrich for the endogenous TRPC4 channel, and probe for its molecular binding partners. As in earlier studies16, 50-53, we found that TRPC4 and TRPC1 co-immunoprecipate with one another. However, by using FRET as a molecular ruler we were able to identify that the TRPC4 and TRPC1 interaction is within 75 Å, indicative of direct protein-protein binding. Using this FRET approach, only one TRPC1 subunit was resolved within the complex, while at least two TRPC4 subunits were identified. Along with these six transmembrane domain proteins, Orai1 was found to interact with TRPC4. There are at least three explanations for resolving Orai1 within the TRPC4 complex. First, since Orai1 possesses a protein 4.1 binding domain it could have been selected by our use of protein 4.1 as bait, and hence, represent an independent channel that is not associated directly with TRPC4. This possibility is unlikely, as in dilution experiments – studies designed to dilute channels that are not a part of the same molecular complex – strong TRPC4-Orai1 interaction was retained. Second, Orai1 constitutes an independent channel that directly binds with the TRPC1/TRPC4 channel. This possibility has not been entirely ruled out by our studies, although as discussed below, if Orai1 is an independent channel, calcium permeation through the channel is not required to activate TRPC1/4. Third, Orai1 directly interacts with the TRPC4 protein complex; this possibility is most likely. Data to this point do not permit us to conclude whether Orai1 directly interacts with TRPC4 through membrane spanning domains, or whether it interacts with TRPC4 through protein 4.1 or another scaffolding protein. FRET studies would suggest the interaction is sufficiently close to constitute direct binding.
Orai1 is recognized as the protein responsible for the ICRAC, and data supporting this assertion are compelling21-25. Orai1 mutation, dysfunction, or suppression abolishes the ICRAC, and in cells devoid of the ICRAC, the current can be rescued by Orai1 expression. Nevertheless, ICRAC has not been measured in all of the cells that express Orai128-30, and the predicted channel membrane topology is atypical for known calcium channels28,31-32, prompting the hypothesis that Orai1 may function as an ancillary subunit for TRPC channels31-32. In some cell types, Orai1 interacts with TRPC1, or interacts with TRPC1 following calcium store depletion, suggesting it may confer sensitivity of TRPC channels to calcium store depletion32. We did not detect a constitutive interaction between Orai1 and TRPC1 in endothelium using our FRET approach as a molecular ruler, however, we did detect an Orai1-TRPC1 interaction following calcium store depletion. Our findings are consistent with the previous studies concluding that Orai1 confers calcium store depletion sensitivity to TRPC channels.
There remains some skepticism as to whether TRPC channels are indeed activated by calcium store depletion in endothelium. Although thapsigargin activates a TRPC1/4-dependent ISOC, questions persist as to whether calcium release from the endoplasmic reticulum or calcium entry through another store operated channel, such as Orai1, is sufficient to activate the channel54-59. In our studies, intracellular calcium was buffered to 100 nM, making it unlikely that calcium release from the endoplasmic reticulum activates TRPC4 channels. Moreover, Orai1 silencing did not prevent ISOC activation, suggesting that calcium permeation through an Orai1-dependent pore was not required for TRPC1/4 activation, at least not in an absolute sense, as with the protein 4.1-actin and protein 4.1-TRPC4 interactions12, 14.
Our data reveal that the Orai1-TRPC4 interaction is functionally important, however, as Orai1 silencing decreased the probability for ISOC activation. Loss of Orai1 likely decreased the coupling efficiency between stromal interaction molecule 1 (STIM1) and store operated calcium entry channels, most notably between STIM1 and Orai1 and TRPC channels32. The independent or combined roles of STIM1 and protein 4.1 in channel activation are not presently known, although their binding sites on Orai1 and TRPC proteins are distinct.
Loss of Orai1 coincided with a left shift in the reversal potential. We interpreted these data to mean that Orai1 influences the calcium selectivity of the TRPC4 channel directly, or alternatively, by contributing a calcium current that is embedded within the macroscopic current. To better understand this issue, we studied the anomalous mole fraction behavior of ISOC in the presence and absence of Orai1. Thapsigargin-induced ion permeability was measured in the absence of extracellular calcium, and then in the presence of increasing calcium concentrations. With calcium selective channels, sodium permeates through the channel in the absence of extracellular calcium44. At relatively low extracellular calcium concentrations, this sodium permeability is inhibited, and then at higher extracellular calcium concentrations a smaller, calcium current is revealed. In our studies, Orai1 right shifted the calcium current reversal potential, consistent with the idea that Orai1 contributes to calcium selectivity of the TRPC4 macroscopic current.
It will be of interest to determine how Orai1 organizes into the larger TRPC1/4 complex, and how it controls TRPC1/4 channel calcium selectivity, as our data suggest the nature of interaction between Orai1 and TRPC1/4 changes as a function of channel activation status. TRPC1/4 and Orai1 are resolved within caveolin rich membrane fractions, along with junctional complex proteins such as cadherins. These membrane regions are highly dynamic. It is presently unknown whether a larger TRPC1/4-Orai1 complex is preassembled and stably inserted into the membrane, or whether, as with K+ channels, channels or subunits of channels dynamically enter and exit the membrane60, 61.
The junctional locale of the TRPC1/4-Orai1 complex suggests its calcium signal controls endothelial cell barrier integrity. Calcium permeation through the TRPC1/4 channel has previously been incriminated in endothelial cell barrier disruption11, 12, 15. Here, however, we identify that Orai1’s regulation of the TRPC1/4 calcium selectivity is critically important. Recent work by Abdullaev and coworkers18 indicates Orai1 also controls endothelial cell proliferation. Together, these results suggest that Orai1 regulation of calcium influx disrupts the endothelial cell barrier while activating a mechanism that promotes vascular repair. Future studies will be needed to determine how the calcium signal is coordinated to regulate diverse intracellular effector mechanisms.
In summary, our studies reveal a previously unappreciated interaction between an endogenous TRPC1/4 channel and Orai1, where Orai1 constitutively interacts with TRPC4. This interaction is important for TRPC1/4 channel activation, and for the biophysical properties of the TRPC1/4 channel once it is activated, as Orai1 determines calcium selectivity of the TRPC1/4 macroscopic current and the calcium concentration permeating the channel. This tight control of calcium influx is physiologically relevant, as calcium that permeates through the TRPC1/4 channel disrupts the endothelial cell barrier.
Supplementary Material
Novelty and Significance.
What Is Known?
Transient receptor potential channel proteins TRPC1 and TRPC4 mediate endothelial cell store-operated calcium entry.
Orail is also a component of endothelial cell store-operated calcium channels.
Calcium entry through TRPC1- and TRPC4-containing channels disrupts the endothelial cell barrier, but it is not clear whether Orai1 interacts with TRPC protein to alter ion channel properties and endothelial permeability
What New Information Does This Article Contribute?
Orai1 interacts with TRPC4 and is a component of the TRPC1- and TRPC4-containing channel in endothelium, both in vitro and in vivo.
Orai1 increases calcium selectivity of, and calcium permeation through, the TRPC channel.
Orai1 is required for calcium influx through store-operated calcium channels to induce inter-endothelial cell gaps.
In acute inflammatory conditions, inflammatory mediators increase cytosolic calcium in the endothelium, which serves as a signal for reorganization and contraction of the actin cytoskeleton, loss of cell-cell apposition, and inter-endothelial cell gap formation, which increases tissue edema. This increase in cytosolic calcium is due to calcium influx across the cell membrane. While store-operated calcium channels mediate calcium entry, the molecular composition and function of endothelial cell store-operated calcium channels remain incompletely understood. Presently, we found that pulmonary artery endothelial cells express TRPC1, TRPC4, and Orai1 proteins, which co-immunoprecipitate with protein 4.1 within caveolin-rich membrane fractions. To ascertain the molecular composition of the endogenous endothelial cell store-operated calcium channel complex, we developed a novel Förster Resonance Energy Transfer approach using protein 4.1 as bait. Isolated channel complexes were conjugated to anti-TRPC protein or anti-Orai1 antibodies labeled with cy3-cy5 pairs. The protein 4.1-bound channel complex contains one TRPC1 and at least two TRPC4 subunits. Orai1 constitutively interacts with TRPC4, and with TRPC1 after the channel has been activated. The presence of Orai1 in this channel complex is necessary to optimize channel activation, and to control the amount of calcium that permeates through the TRPC channel. Moreover, the presence of Orai1 within the complex is necessary for calcium entry through the store-operated calcium channel to induce inter-endothelial cell gaps. These findings define Orai1 as an essential constituent of the endogenous endothelial cell store-operated calcium channel that controls endothelial cell permeability in the acute inflammatory response.
Acknowledgments
Sources of Funding This work was supported by HL60024 and HL66299 (T.S. and S.W.) and R00HL089361 (D.L.C).
Non-Standard Abbreviations and Acronyms
- TRPC
Transient Receptor Proteins of the Canonical Subfamily
- FRET
Förster Resonance Energy Transfer
- PAEC
Pulmonary Artery Endothelial Cells
- shRNA
Short Hairpin RNA
- EGFP
Enhanced Green Fluorescent Protein
- rtTA
Reverse Transactivator Protein
- STIM1
Stromal Interaction Molecule 1
Footnotes
Disclosures None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Nilius B, Owsianik G. Transient receptor potential channelopathies. Pflugers Arch. 2010;460:437–50. doi: 10.1007/s00424-010-0788-2. [DOI] [PubMed] [Google Scholar]
- 2.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 3.Smyth JT, Hwang SY, Tomita T, DeHaven WI, Mercer JC, Putney JW. Activation and regulation of store-operated calcium entry. J Cell Mol Med. 2010;14:2337–49. doi: 10.1111/j.1582-4934.2010.01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cioffi DL, Lowe K, Alvarez DF, Barry C, Stevens T. TRPing on the lung endothelium: calcium channels that regulate barrier function. Antioxid Redox Signal. 2009;11:765–76. doi: 10.1089/ars.2008.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cioffi DL, Stevens T. Regulation of endothelial cell barrier function by store-operated calcium entry. Microcirculation. 2006;13:709–23. doi: 10.1080/10739680600930354. [DOI] [PubMed] [Google Scholar]
- 6.Cioffi DL, Wu S, Stevens T. On the endothelial cell ISOC. Cell Calcium. 2003;33:323–36. doi: 10.1016/s0143-4160(03)00046-0. [DOI] [PubMed] [Google Scholar]
- 7.Kelly JJ, Moore TM, Babal P, Diwan AH, Stevens T, Thompson WJ. Pulmonary microvascular and macrovascular endothelial cells: differential regulation of Ca2+ and permeability. Am J Physiol. 1998;274:L810–9. doi: 10.1152/ajplung.1998.274.5.L810. [DOI] [PubMed] [Google Scholar]
- 8.Yang LX, Guo RW, Liu B, Wang XM, Qu F, Guo CM, Shi YK, Wang H. Role of TRPC1 and NF-kappaB in mediating angiotensin II-induced Ca2+ entry and endothelial hyperpermeability. Peptides. 2009;30:1368–73. doi: 10.1016/j.peptides.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Singh I, Knezevic N, Ahmmed GU, Kini V, Malik AB, Mehta D. Galphaq-TRPC6-mediated Ca2+ entry induces RhoA activation and resultant endothelial cell shape change in response to thrombin. J Biol Chem. 2007;282:7833–43. doi: 10.1074/jbc.M608288200. [DOI] [PubMed] [Google Scholar]
- 10.Paria BC, Vogel SM, Ahmmed GU, Alamgir S, Shroff J, Malik AB, Tiruppathi C. Tumor necrosis factor-alpha-induced TRPC1 expression amplifies store-operated Ca2+ influx and endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1303–13. doi: 10.1152/ajplung.00240.2004. [DOI] [PubMed] [Google Scholar]
- 11.Wu S, Cioffi EA, Alvarez D, Sayner SL, Chen H, Cioffi DL, King J, Creighton JR, Townsley M, Goodman SR, Stevens T. Essential role of a Ca2+-selective, store-operated current (ISOC) in endothelial cell permeability: determinants of the vascular leak site. Circ Res. 2005;96:856–63. doi: 10.1161/01.RES.0000163632.67282.1f. [DOI] [PubMed] [Google Scholar]
- 12.Cioffi DL, Wu S, Alexeyev M, Goodman SR, Zhu MX, Stevens T. Activation of the endothelial store-operated ISOC Ca2+ channel requires interaction of protein 4.1 with TRPC4. Circ Res. 2005;97:1164–72. doi: 10.1161/01.RES.0000193597.65217.00. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez DF, King JA, Townsley MI. Resistance to store depletion-induced endothelial injury in rat lung after chronic heart failure. Am J Respir Crit Care Med. 2005;172:1153–60. doi: 10.1164/rccm.200506-847OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu S, Sangerman J, Li M, Brough GH, Goodman SR, Stevens T. Essential control of an endothelial cell ISOC by the spectrin membrane skeleton. J Cell Biol. 2001;154:1225–33. doi: 10.1083/jcb.200106156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brough GH, Wu S, Cioffi D, Moore TM, Li M, Dean N, Stevens T. Contribution of endogenously expressed Trp1 to a Ca2+-selective, store-operated Ca2+ entry pathway. FASEB J. 2001;15:1727–38. [PubMed] [Google Scholar]
- 16.Hofmann T, Schaefer M, Schultz G, Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci U S A. 2002;99:7461–6. doi: 10.1073/pnas.102596199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu S, Chen H, Alexeyev MF, King JA, Moore TM, Stevens T, Balczon RD. Microtubule motors regulate ISOC activation necessary to increase endothelial cell permeability. J Biol Chem. 2007;282:34801–8. doi: 10.1074/jbc.M704522200. [DOI] [PubMed] [Google Scholar]
- 18.Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res. 2008;103:1289–99. doi: 10.1161/01.RES.0000338496.95579.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fierro L, Lund PE, Parekh AB. Comparison of the activation of the Ca2+ release-activated Ca2+ current ICRAC to InsP3 in Jurkat T-lymphocytes, pulmonary artery endothelia and RBL-1 cells. Pflugers Arch. 2000;440:580–7. doi: 10.1007/s004240000336. [DOI] [PubMed] [Google Scholar]
- 20.Fasolato C, Nilius B. Store depletion triggers the calcium release-activated calcium current (ICRAC) in macrovascular endothelial cells: a comparison with Jurkat and embryonic kidney cell lines. Pflugers Arch. 1998;436:69–74. doi: 10.1007/s004240050605. [DOI] [PubMed] [Google Scholar]
- 21.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–3. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–85. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 23.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci U S A. 2006;103:9357–62. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–3. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 25.Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, Kinet JP, Penner R. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–9. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Cubbon RM, Wilson LA, Amer MS, McKeown L, Hou B, Majeed Y, Tumova S, Seymour VA, Taylor H, Stacey M, O’Regan D, Foster R, Porter KE, Kearney MT, Beech DJ. Orai1 and CRAC channel dependence of VEGF-activated Ca2+ entry and endothelial tube formation. Circ Res. 2011;108:1190–8. doi: 10.1161/CIRCRESAHA.111.243352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cioffi DL, Barry C, Stevens T. Store-operated calcium entry channels in pulmonary endothelium: the emerging story of TRPCS and Orai1. Adv Exp Med Biol. 2010;661:137–54. doi: 10.1007/978-1-60761-500-2_9. [DOI] [PubMed] [Google Scholar]
- 28.Wissenbach U, Philipp SE, Gross SA, Cavalie A, Flockerzi V. Primary structure, chromosomal localization and expression in immune cells of the murine ORAI and STIM genes. Cell Calcium. 2007;42:439–46. doi: 10.1016/j.ceca.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 29.DeHaven WI, Smyth JT, Boyles RR, Putney JW., Jr. Calcium inhibition and calcium potentiation of Orai1, Orai2, and Orai3 calcium release-activated calcium channels. J Biol Chem. 2007;282:17548–56. doi: 10.1074/jbc.M611374200. [DOI] [PubMed] [Google Scholar]
- 30.Gross SA, Wissenbach U, Philipp SE, Freichel M, Cavalie A, Flockerzi V. Murine ORAI2 splice variants form functional Ca2+ release-activated Ca2+ (CRAC) channels. J Biol Chem. 2007;282:19375–84. doi: 10.1074/jbc.M701962200. [DOI] [PubMed] [Google Scholar]
- 31.Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci U S A. 2007;104:4682–7. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, Gill DL, Ambudkar IS. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem. 2007;282:9105–16. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Creighton J, Zhu B, Alexeyev M, Stevens T. Spectrin-anchored phosphodiesterase 4D4 restricts cAMP from disrupting microtubules and inducing endothelial cell gap formation. J Cell Sci. 2008;121:110–9. doi: 10.1242/jcs.011692. [DOI] [PubMed] [Google Scholar]
- 34.Prasain N, Alexeyev M, Balczon R, Stevens T. Soluble adenylyl cyclase-dependent microtubule disassembly reveals a novel mechanism of endothelial cell retraction. Am J Physiol Lung Cell Mol Physiol. 2009;297:L73–L83. doi: 10.1152/ajplung.90577.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farrar SJ, Whiting PJ, Bonnert TP, McKernan RM. Stoichiometry of a ligand-gated ion channel determined by fluorescence energy transfer. J Biol Chem. 1999;274:10100–4. doi: 10.1074/jbc.274.15.10100. [DOI] [PubMed] [Google Scholar]
- 36.Zheng J, Trudeau MC, Zagotta WN. Rod cyclic nucleotide-gated channels have a stoichiometry of three CNGA1 subunits and one CNGB1 subunit. Neuron. 2002;36:891–6. doi: 10.1016/s0896-6273(02)01099-1. [DOI] [PubMed] [Google Scholar]
- 37.Parra-Bonilla G, Alvarez DF, Al-Mehdi AB, Alexeyev M, Stevens T. Critical role for lactate dehydrogenase A in aerobic glycolysis that sustains pulmonary microvascular endothelial cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2010;299:L513–22. doi: 10.1152/ajplung.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Churchman LS, Okten Z, Rock RS, Dawson JF, Spudich JA. Single molecule high-resolution colocalization of Cy3 and Cy5 attached to macromolecules measures intramolecular distances through time. Proc Natl Acad Sci U S A. 2005;102:1419–23. doi: 10.1073/pnas.0409487102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siegel RM, Chan FK, Zacharias DA, Swofford R, Holmes KL, Tsien RY, Lenardo MJ. Measurement of molecular interactions in living cells by fluorescence resonance energy transfer between variants of the green fluorescent protein. Sci STKE. 2000;2000:pl1. doi: 10.1126/stke.2000.38.pl1. [DOI] [PubMed] [Google Scholar]
- 40.dos Remedios CG, Moens PD. Fluorescence resonance energy transfer spectroscopy is a reliable “ruler” for measuring structural changes in proteins. Dispelling the problem of the unknown orientation factor. J Struct Biol. 1995;115:175–85. doi: 10.1006/jsbi.1995.1042. [DOI] [PubMed] [Google Scholar]
- 41.Rath G, Dessy C, Feron O. Caveolae, caveolin and control of vascular tone: nitric oxide (NO) and endothelium derived hyperpolarizing factor (EDHF) regulation. J Physiol Pharmacol. 2009;60:105–9. [PubMed] [Google Scholar]
- 42.Beech DJ, Xu SZ, McHugh D, Flemming R. TRPC1 store-operated cationic channel subunit. Cell Calcium. 2003;33:433–40. doi: 10.1016/s0143-4160(03)00054-x. [DOI] [PubMed] [Google Scholar]
- 43.Schnitzer JE, Oh P, Jacobson BS, Dvorak AM. Caveolae from luminal plasmalemma of rat lung endothelium: microdomains enriched in caveolin, Ca2+-ATPase, and inositol trisphosphate receptor. Proc Natl Acad Sci U S A. 1995;92:1759–63. doi: 10.1073/pnas.92.5.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoenderop JG, Vennekens R, Muller D, Prenen J, Droogmans G, Bindels RJ, Nilius B. Function and expression of the epithelial Ca2+ channel family: comparison of mammalian ECaC1 and 2. J Physiol. 2001;537:747–61. doi: 10.1111/j.1469-7793.2001.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baines AJ, Bennett PM, Carter EW, Terracciano C. Protein 4.1 and the control of ion channels. Blood Cells Mol Dis. 2009;42:211–5. doi: 10.1016/j.bcmd.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 46.Leterrier C, Brachet A, Fache MP, Dargent B. Voltage-gated sodium channel organization in neurons: protein interactions and trafficking pathways. Neurosci Lett. 2010;486:92–100. doi: 10.1016/j.neulet.2010.08.079. [DOI] [PubMed] [Google Scholar]
- 47.Girault JA, Peles E. Development of nodes of Ranvier. Curr Opin Neurobiol. 2002;12:476–85. doi: 10.1016/s0959-4388(02)00370-7. [DOI] [PubMed] [Google Scholar]
- 48.Baines AJ. Ankyrin and the node of Ranvier. Trends Neurosci. 1990;13:119–21. doi: 10.1016/0166-2236(90)90001-q. [DOI] [PubMed] [Google Scholar]
- 49.Meves H. The gating current of the node of Ranvier. Ion Channels. 1990;2:65–121. doi: 10.1007/978-1-4615-7305-0_3. [DOI] [PubMed] [Google Scholar]
- 50.Antoniotti S, Fiorio Pla A, Barral S, Scalabrino O, Munaron L, Lovisolo D. Interaction between TRPC channel subunits in endothelial cells. J Recept Signal Transduct Res. 2006;26:225–40. doi: 10.1080/10799890600784050. [DOI] [PubMed] [Google Scholar]
- 51.Goel M, Sinkins WG, Schilling WP. Selective association of TRPC channel subunits in rat brain synaptosomes. J Biol Chem. 2002;277:48303–10. doi: 10.1074/jbc.M207882200. [DOI] [PubMed] [Google Scholar]
- 52.Sours-Brothers S, Ding M, Graham S, Ma R. Interaction between TRPC1/TRPC4 assembly and STIM1 contributes to store-operated Ca2+ entry in mesangial cells. Exp Biol Med. 2009;234:673–82. doi: 10.3181/0809-RM-279. [DOI] [PubMed] [Google Scholar]
- 53.Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem. 2003;278:39014–9. doi: 10.1074/jbc.M306705200. [DOI] [PubMed] [Google Scholar]
- 54.Cheng KT, Liu X, Ong HL, Swaim W, Ambudkar IS. Local Ca2+ entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca2+ signals required for specific cell functions. PLoS Biol. 2011;9:e1001025. doi: 10.1371/journal.pbio.1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jardin I, Lopez JJ, Salido GM, Rosado JA. Orai1 mediates the interaction between STIM1 and hTRPC1 and regulates the mode of activation of hTRPC1-forming Ca2+ channels. J Biol Chem. 2008;283:25296–304. doi: 10.1074/jbc.M802904200. [DOI] [PubMed] [Google Scholar]
- 56.Lee KP, Yuan JP, So I, Worley PF, Muallem S. STIM1-dependent and STIM1-independent function of transient receptor potential canonical (TRPC) channels tunes their store-operated mode. J Biol Chem. 2010;285:38666–73. doi: 10.1074/jbc.M110.155036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liao Y, Plummer NW, George MD, Abramowitz J, Zhu MX, Birnbaumer L. A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry. Proc Natl Acad Sci U S A. 2009;106:3202–6. doi: 10.1073/pnas.0813346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ong HL, Ambudkar IS. The dynamic complexity of the TRPC1 channelosome. Channels. 2011;5 doi: 10.4161/chan.5.5.16471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, Muallem S. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol Cell. 2008;32:439–48. doi: 10.1016/j.molcel.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Connell KM, Tamkun MM. Targeting of voltage-gated potassium channel isoforms to distinct cell surface microdomains. J Cell Sci. 2005;118:2155–66. doi: 10.1242/jcs.02348. [DOI] [PubMed] [Google Scholar]
- 61.Tamkun MM, O’Connell KM, Rolig AS. A cytoskeletal-based perimeter fence selectively corrals a sub-population of cell surface Kv2.1 channels. J Cell Sci. 2007;120:2413–23. doi: 10.1242/jcs.007351. [DOI] [PubMed] [Google Scholar]
- 62.Piston DW, Kremers G-J. Fluorescent protein FRET: the good, the bad, and the ugly. Trends Biochem Sci. 2007;32:407–414. doi: 10.1016/j.tibs.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 63.Koopmans WJA, Brehm A, Logie C, Schmidt T, van Noort J. Single-pair FRET microscopy reveals mononucleosome dynamics. J Fluoresc. 2007;17:785–795. doi: 10.1007/s10895-007-0218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.