Abstract
Sampling circulating tumor cells from peripheral blood is ideally accomplished using assays that detect high numbers of cells and preserve them for downstream characterization. We sought to evaluate a method using enrichment free fluorescent labeling of CTCs followed by automated digital microscopy in patients with non-small cell lung cancer. Twenty-eight patients with non-small cell lung cancer and hematogenously seeded metastasis were analyzed with multiple blood draws. We detected CTCs in 68% of analyzed samples and found a propensity for increased CTC detection as the disease progressed in individual patients. CTCs were present at a median concentration of 1.6 CTCs per milliliter of analyzed blood in the patient population. Higher numbers of detected CTCs were associated with an unfavorable prognosis.
Keywords: Circulating Tumor Cells, Cancer, Metastasis, prognosis, rare cell
INTRODUCTION
Circulating tumor cells (CTC) have been detected in most epithelial malignancies in which they have been investigated [1–4] detection rates have varied depending on the technology used for enumeration [5–7], however, there is consistency in the finding that they are associated with an adverse prognosis [8, 9]. Non-small cell lung cancer (NSCLC) is a particularly important disease for CTC evaluation for prognostic purposes because of the lack of a reliable protein based tumor marker [10, 11]. Characterization of tumor phenotype [12] and genotype from circulating tumor cells has the potential to offer additional prognostic information [13].
Prognostic data from CTC enumeration is only one potential for this technology. Tumors change and evolve during the natural history of the disease within an individual patient. Cancers that begin as being sensitive to a particular chemotherapy drug will become resistant to that drug over time. The ability to characterize the changes in a tumor over time requires a non-invasive method of sampling cancer cells that is sufficiently robust to identify cells in high quantities. The ideal test would be useful in a large fraction of patients and use a non-destructive methodology to permit downstream analysis of identified tumor cells.
We sought to identify the prevalence, quantity and reproducibility of CTC determination using an automated digital microscopy based technique in a population of NSCLC patients from a community cancer center. The method is one where identified CTCs remain available for downstream secondary characterization. Herein we describe the patient population, method of enumeration, and the prognostic significance of the measurement.
METHODS
This is a single institution observational clinical trial conducted at the Billings Clinic Cancer Center (Billings, Montana). All procedures were approved by the institutional review board prior to the start of the trial. Patients with histologically proven NSCLC with evidence of hematogenously seeded distant metastasis were permitted to enroll. Patients with malignant pleural effusion or thoracic nodal metastasis as the only sites of disease spread were excluded. Enrolled patients had either new metastatic disease or had progression of previously treated metastatic disease at the time of enrollment. All research subjects were able to provide informed consent and had no evidence of prior malignancy in the past 5 years aside from non-melanoma skin cancer.
Peripheral blood was collected in Cytochex BCT collection tubes (Streck Innovations, Omaha, NE) at study enrollment, at 3 weeks, as well as 3, 6, 9 and 12 months. Samples were shipped and processed at a central facility within 24 hours of collection. The majority of samples were collected just prior to administration of chemotherapy using a central venous access device.
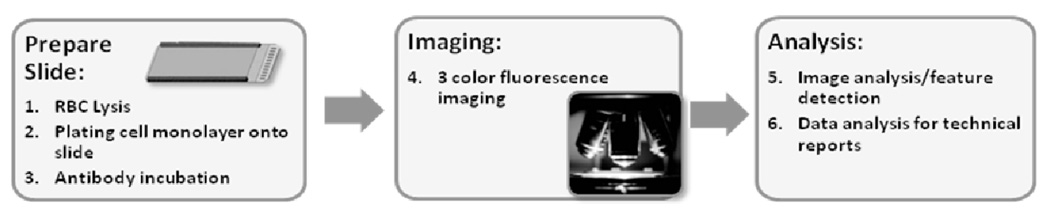
Whole blood specimens were prepared as reported in this issue of Physical Biology. In brief, samples underwent red blood cell lysis followed by monolayer preparation of all nucleated cells on custom glass substrates. After paraformaldehyde (PFA) fixation and methanol permeabilization, cells were incubated with pan anti-Cytokeratin antibodies recognizing cytokeratins 1, 4, 5, 6, 7, 8, 10, 13, 18 and 19 and a preconjugated anti-CD45 antibody followed by incubation with an Alexa 555-conjugated secondary antibody and DAPI as a nuclear stain. All nucleated cells in the specimen were imaged. Cells that were both Cytokeratin positive and CD45 negative were identified using custom computer algorithms and then subjected to technical analysis. The full process is shown schematically in Figure 1.
Figure 1.
The fluid biopsy assay begins with patient blood tubes and the removal of red blood cells (RBCs) via lysis and then plating the remaining cells onto a slide. After antibody incubation, the full active surface of each slide is imaged in 3 fluorescence channels. Image analysis is used to extract features and associated intensity and cytomorphological values for each cell and the data is reduced for evaluation by a technical analyst.
Specimens were evaluated by direct review of captured microscopic images and classified as a CTC or not based on cell morphology and immunophenotype. 47 of the 66 specimens were available for additional analysis of other cells related to CTCs but lacking essential features of a CTC based on either immunophenotype or morphology. These cells included ones in various stages of apoptosis morphologically (CTC-Ap), cells that morphologically resembled CTCs but were dim for cytokeratin (CTC-CK low) along with cells that were cytokeratin positive, but too small to be considered a classical CTC by morphologic criteria (CTC-small). Data on these types of related cellular events such as apoptotic CTCs, CTC-CK low cells and CTC-small are reported using descriptive statistics and correlated with total CTC number using Pearson’s coefficient.
CTC counts are reported per milliliter of blood specimen (CTCs/ml). The value is calculated by counting the total number of leukocytes on the glass slide used to isolate and detect CTCs and comparing it to the measured leukocytes per milliliter in the patient’s blood specimen. The ratio of counted leukocytes over the leukocytes per milliliter in the blood specimen determined the quantity of CTCs in terms of CTCs/ml. For this reason, fractional values of CTCs/ml are possible. Mean CTC counts for all measured timepoints were obtained for all patients who were also stratified into low and high CTC groups. These groups were analyzed for survival using the method of Kaplan and Meier. In patients who died while on study, the days from CTC count until patient death were recorded and specimens were grouped into high (>9 CTCs/ml), medium (2–9 CTCs/ml) and low (<2 CTCs/mL) and compared using Student’s t-test.
RESULTS
66 blood samples from 28 patients from Billings, Montana were evaluated. 31 samples were received and successfully processed within the first 30 days following patient enrollment. There were 16 samples from the 3 month timepoint and 18 samples collected 6 months or more after study initiation. The clinical features of the patient population are summarized in table 1. The median age of the population was 64 years (range 31–82). 15 men and 13 women were enrolled with 21 patients having adenocarcinomas, 5 patients having squamous carcinoma and 2 patients having non-small cell lung cancer that could not be further characterized. The most common metastatic site was a separate lung lobe (64%), followed by bone (43%), brain (32%) and lymphatics (32%). Lymphatics were considered a metastatic site only if they were outside the thoracic cavity such as cervical lymph nodes or abdominal nodes. All patients received anticancer cytotoxic chemotherapy or EGFR kinase inhibitor.
Table 1.
Clinicopathological variables
| Characteristic | n=28 |
|---|---|
| Median Age | 64 (range 31–82) |
| Gender | |
| Male | 15 (54%) |
| Female | 13 (36%) |
| Histology | |
| Adenocarcinoma | 21 (75%) |
| Squamous | 5 (18%) |
| Undifferentiated | 2 (7%) |
| Metastatic sites | |
| Adrenal | 2 (7%) |
| Bone | 12 (43%) |
| Brain | 8 (32%) |
| Liver | 7 (25%) |
| Lung | 18 (64%) |
| Lymphatic | 9 (32%) |
| Soft Tissue | 2 (7%) |
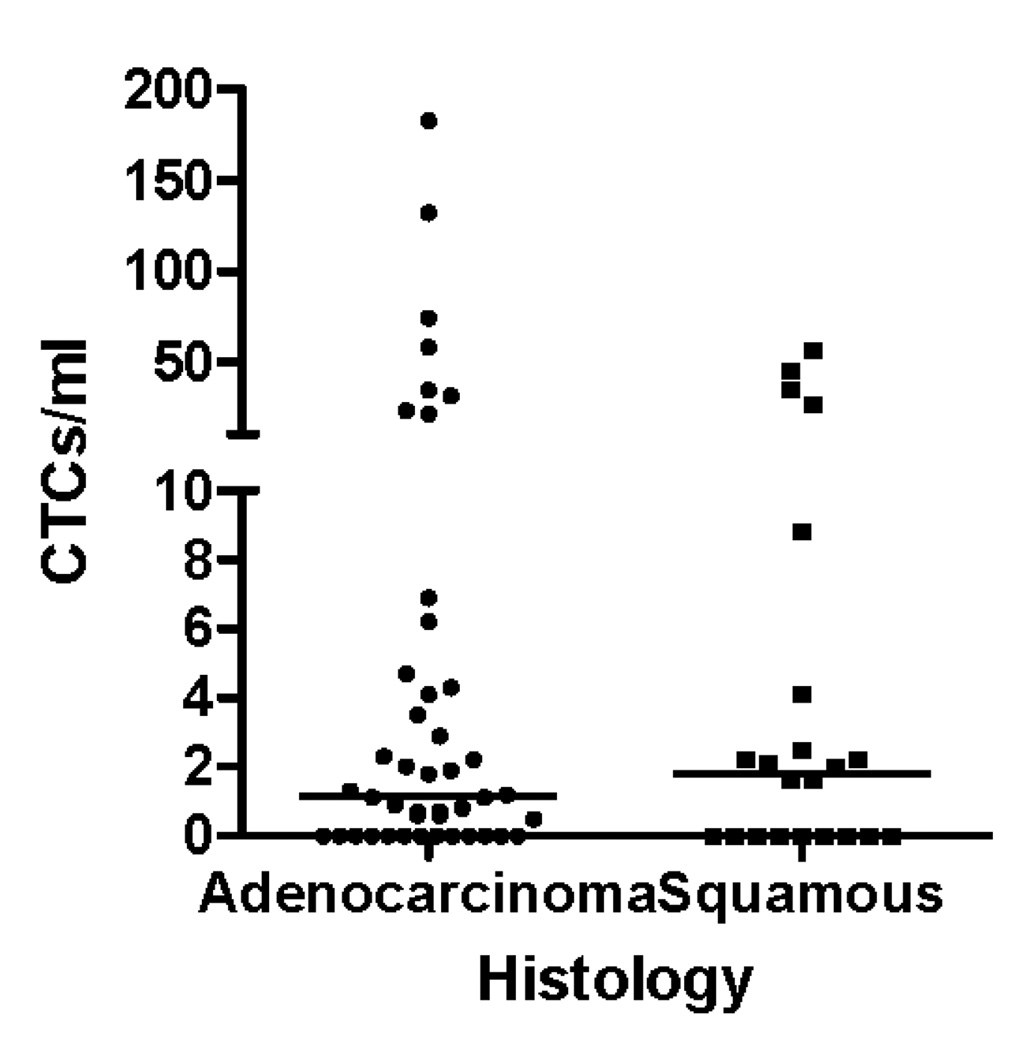
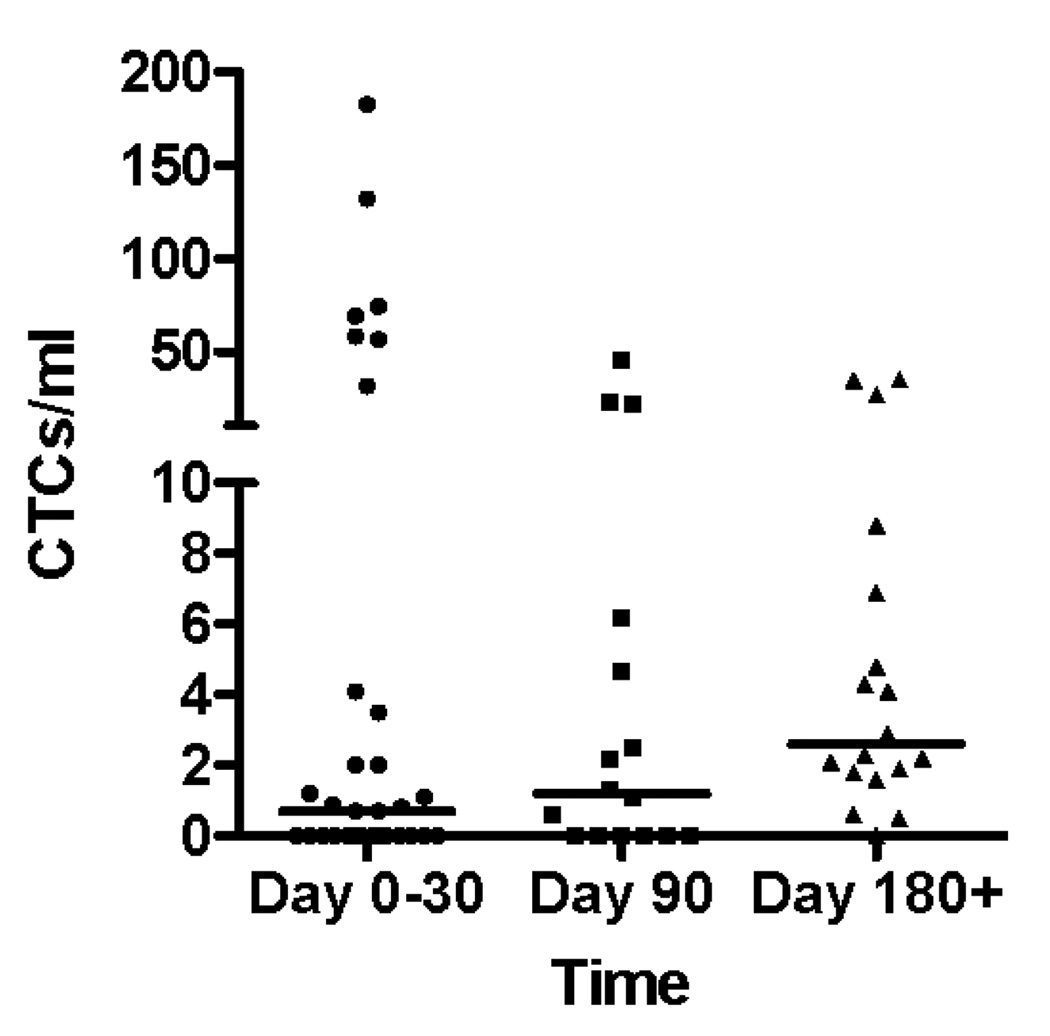
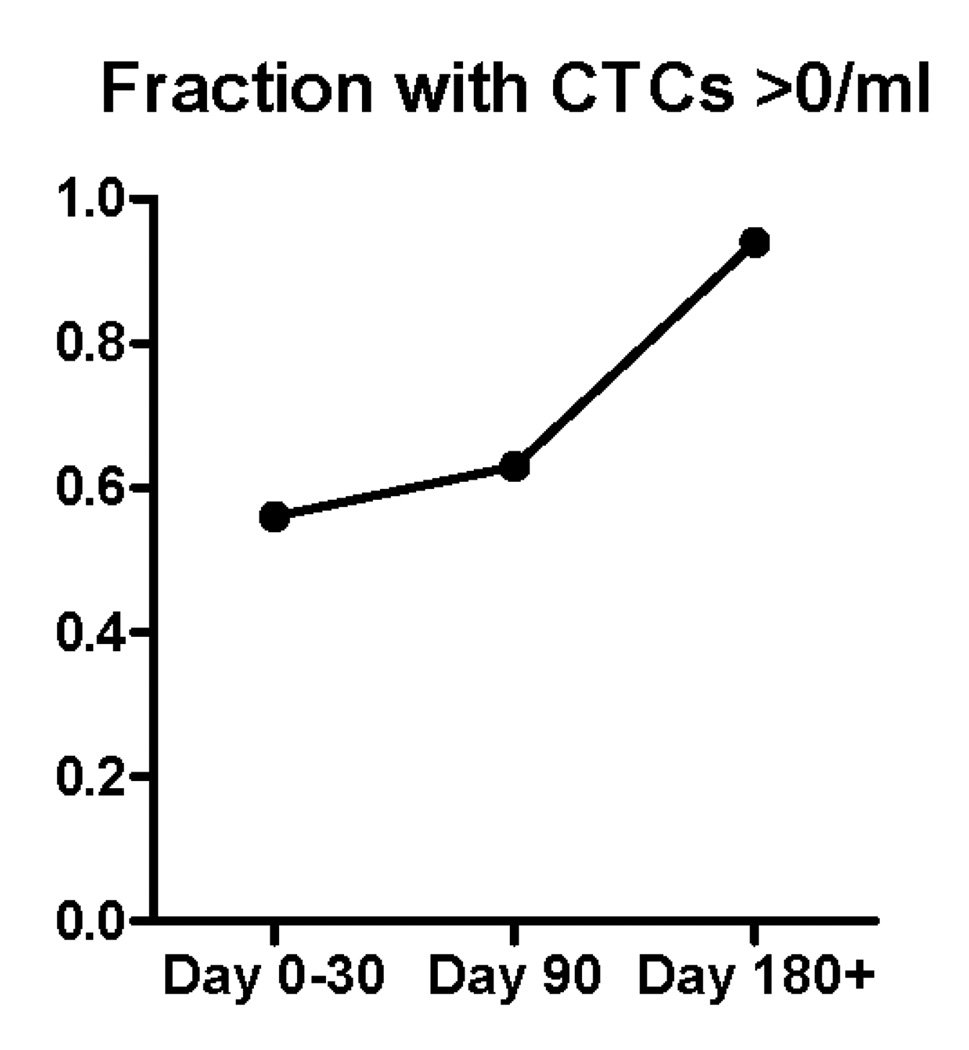
Circulating tumor cells were detected in 45 out of 66 blood specimens (68%). The CTC counts according to histologic type are shown in figure 2. There was no difference in the prevalence or mean value of circulating tumor cells in specimens from patients with squamous lung cancers compared with those patients with adenocarcinoma (Figure 2A). There was a trend toward increasing prevalence of circulating tumor cells detected in peripheral blood during the time course of the study, with 17 of 31 specimens (56%) having CTCs in the first month of enrollment (including time 0 and 3 week blood draws), 10 of 16 specimens (63%) having CTCs at the 3 month timepoint, while 17 of 18 (94%) of specimens had CTCs at 6 months and beyond. As the disease progressed the likelihood of a finding of no detectable circulating tumor cells was reduced (Figure 2C).
Figure 2.
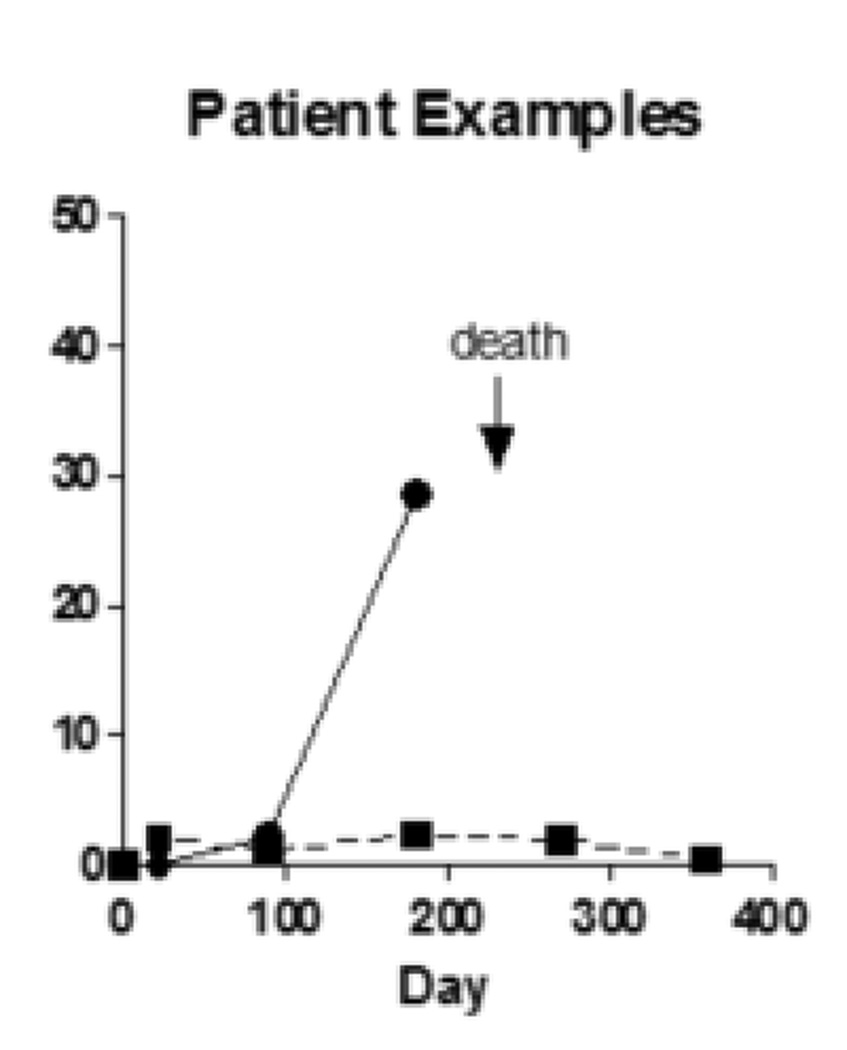
(A) CTC counts from samples from adenocarcinoma and squamous carcinoma patients do not differ according to histologic subtype of lung cancer. (B) CTC counts for the whole patient population at early, middle and late timepoints with bar representing the median CTC count at the 3 time points. (C) during the natural history of stage IV NSCLC at early, middle, and late timepoints the frequency with which patients had a non-zero value for the CTC count appeared to increase. (D) Examples of serial blood draws on two patient types. The solid line represents a patient who had undetectable CTCs early in the course of the disease, then progressed and died 45 days after the last sample showed an increase in the CTC count. The dotted line represents a patient who maintained low CTC counts and remained alive at last follow-up 345 days after the 1 year timepoint of the study.
The mean CTC count for the population was 13.4 CTCs/ml (median 1.6 CTCs/ml; range 0–182.6 CTCs/ml), with subsequent blood draws after enrollment having higher median CTC counts. We found a median 0.7CTCs/ml for samples acquired at the baseline and 3 week timepoints during days 1–30 of study participation, increasing to 1.2CTCs/ml for specimens collected at the 90 day timepoint, and a median of 2.6 CTCs/ml for samples taken at the 180 day timepoint and beyond. There was no difference in the statistical mean of the CTC count for any of these timepoints (Figure 2B). Examples of serial CTC counts from 2 patients are shown in Figure 2D. One patient, respresented by the solid line, has an undetectable CTC count at timepoints 0 and 21, at 90 days had 2.2 CTCs/mL and at 180 days had 26.6 CTCs/mL. This patient died of lung cancer 45 days after the last CTC count was measured. The second patient, represented by the dotted line had CTC counts of 0/mL, 2/mL, 1.1/mL, 2.3/mL, 1.9/mL and 0.5/mL at each successive timepoint on the study and remained alive at the last followup receiving maintenance chemotherapy 375 days after the last measured CTC count on the study.
Other Cellular Events
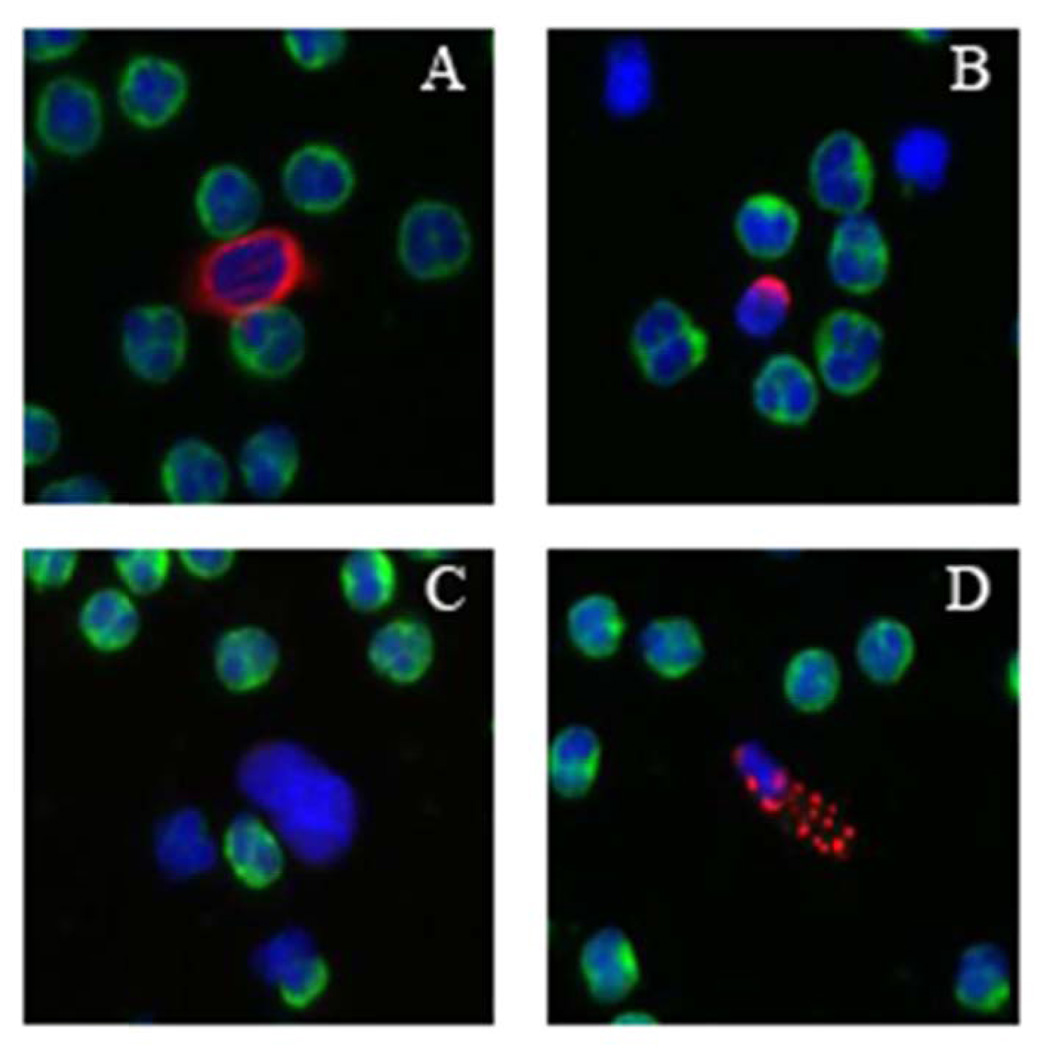
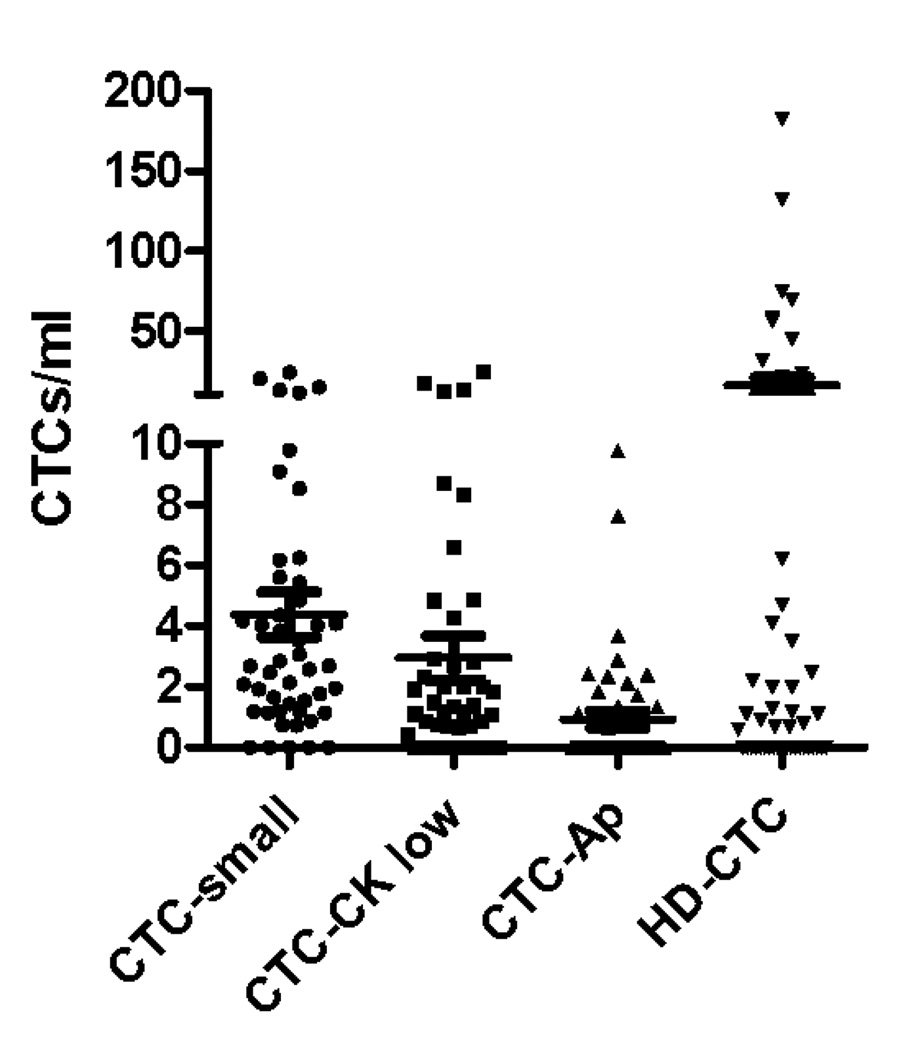
Cellular events including CTC-Ap, CTC-low CK, and CTC-small were analyzed in 47 specimens, examples of these cell types are found in figure 3B. For this subset of specimens there was a mean of 15 CTCs/ml (median 0.8 CTCs/ml; range 0–182.6) detected. There were an additional mean (SD) of 4.3 (±5.0) CTC-small cells/ml, 2.9 (±4.9) CTC-CK low and 0.9 (±1.9) CTC-Ap (Figure 3A). The CTC count did not correlate with the count of any of these other cell types. The detection of these cellular events was not related to clinical features of the patients, survival, or treatment variables.
Figure 3.
In addition to cells that are morphologically consistent with CTCs, other related cells are also detected by the assay system and are observed in lung cancer patients. (A) CTC (B) CTC-small (C) CTC-CK low (D) CTC-Ap (E) counts of the different related cells in NSCLC population of patients
CTCs and Survival
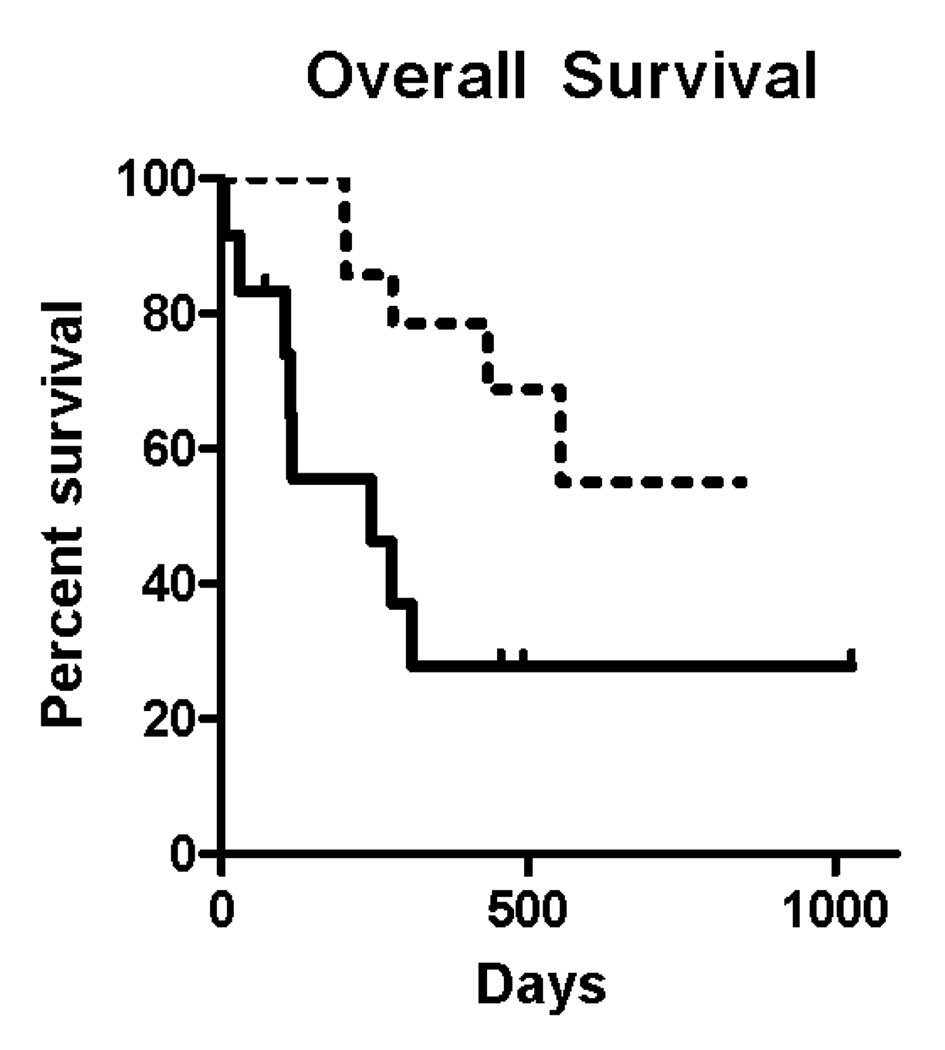
For the study population overall, including those patients who did not die while on study, the total CTC count was averaged over all draws and patients were grouped into those whose mean CTC/ml was more than 5 and less than or equal to 5. Patients with 5 or more CTCs/ml had a median survival of 244 days. The median survival was not reached in the patients with fewer than 5 CTCs at a median follow-up of 304 days. The hazard ratio for death was 4.0 in the patients with a high average CTC count relative to those patients with a lower count (Figure 4A), indicating a four-fold risk of dying at any given timepoint for those patients with higher CTC counts. These differences were statistically significant (p = 0.0084).
Figure 4.
(A) Overall survival for the entire study population based on averaging all CTC counts for each individual patients and grouping them into patients with 5 or more (solid line) and fewer than 5 CTCs/ml (dotted line). Hazard ratio for death was 4.0 (95% CI 1.242 to 13.14) comparing the group with high versus low CTC group. (B) For the subgroup of patient who died while enrolled in the study, the time in days from measurement of CTCs until death for 28 blood specimens obtained from 13 patients was recorded. The detection of 5 or more CTCs/mL was associated with a shorter period of time before death occurred, as compared with those samples where 5 or fewer CTCs/mL were observed.
Patients who died had their CTC counts analyzed in order to determine if there was a relationship between the CTC counts and the time until death occurred. At a median follow-up of 10 months, 13 of 28 enrolled patients had died. There were a total of 28 specimens collected from those 13 patients. In the subgroup of patients who died while on study, increased CTCs/ml was correlated with shortened survival time (Figure 4B). Time to death averaged 35 ±59 days for patients whose blood contained >5 CTCs/ml; by comparison, time to death was 211± 207 days when specimens had 5 or fewer CTCs/ml. There was a statistically significant difference in time to death for samples with more than 5 CTCs/ml compared with those samples with 5 or less CTCs/ml (p=0.0035).
DISCUSSION
Circulating tumor cells have been detected in the majority of epithelial tumors where they have been studied. The majority of clinical trials have used CellSearch technology which, at time of this publication, remains the only FDA approved technology that is commercially used in the United States for detection and enumeration of CTCs. As this manuscript used a non-CellSearch technology, some differences in findings are apparent and warrant discussion. Among other differences, CellSearch isolates cells that contain Epithelial Cell Adhesion molecule (Ep-CAM) on their surface, whereas the assay used here is a method that does not contain an enrichment step.
Using CellSearch, Krebs and colleagues [4] have reported results in a non-small cell lung cancer population similar to one studied here, along with results for stage III patients. Both CellSearch and the enrichment free process used in this clinical trial found CTCs in NSCLC patients. Both assays show similar hazard ratios for risk stratification of patients and identify high and low risk patient populations. There are, however, significant differences in the prevalence numbers of CTCs detected in the two assays. The CellSearch assay defines a positive test as one where there is greater > 2 CTCs/7.5 ml (0.27 CTCs/ml) of blood, and finds a positive test in 32% of stage IV patients; while the current assay defines any detectable CTC count as being of relevance and identifies a positive test 68% of the time. This difference between the two technologies is, in part, related to the ability to view high definition images with the current technology, permitting a cytomorphologic evaluation of the cells in question. Thus a single cell, cytokeratin positive, with a high nuclear cytoplasmic ratio and large overall size is reliably identified as a CTC, and considered unique from the other similar cellular events that can be seen in patient samples.
While enumeration of CTCs using this technology or others has some value in providing patients and their physicians with prognostic information, the clinical acceptance of CTC technologies in clinical practice has been limited. This is in part because CTC assay clinical trials, like this one, have shown the test to be prognostic but have failed to identify a predictive role for the assay. Results of the SWOG S0500 clinical trial are expected to determine whether clinical decision making should change on the basis of Cellsearch assay results. In the absence of a clinical trial showing predictive value for CTC enumeration, clinicians are unlikely to champion a relatively expensive assay that does not provide superior prognostic information over current protein based serum markers [14]. Were CTC assays able to provide content information, that is, information related to gene expression, mutation, or changes in cancer cells resulting from drug administration, these assays would then have a great deal of value. The unmet potential for CTC assays may be to provide clinicians and researchers with a platform by which tumor cells may be repeatedly and safely re-sampled to monitor changes in tumor genetics and cancer evolution.
The assay described herein has the potential to provide reliable identification of tumor cells in a high fraction of NSCLC patients. What is required next are the secondary assays that can inform clinicians as to the mutation status, gene, and protein expression status of these cells in order to permit enlightened decision making on the use of therapeutics. This study should provide the support and rationale for these larger undertakings.
ACKNOWLEDGEMENTS
This manuscript was supported by NIH Grants R01CA125653 and U54CA143906 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. This is manuscript #21349 from The Scripps Research Institute.
REFERENCES
- 1.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 2.Sastre J, Maestro ML, Puente J, Veganzones S, Alfonso R, Rafael S, García-Saenz JA, Vidaurreta M, Martíin M, Arroyo M, Sanz-Casla MT, Díaz-Rubio E. Circulating tumor cells in colorectal cancer: correlation with clinical and pathological variables. Ann. of Oncol. 2008;19:935–938. doi: 10.1093/annonc/mdm583. [DOI] [PubMed] [Google Scholar]
- 3.Ghossein RA, Scher HI, Gerald WL, Kelly WK, Curely T, Amsterdam A, Zhang ZF, Rosai J. Detection of circulating tumor cells in patients with localized and metastatic prostatic carcinoma: clinical implications. J. Clin. Oncol. 1995;13:1195–1200. doi: 10.1200/JCO.1995.13.5.1195. [DOI] [PubMed] [Google Scholar]
- 4.Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, Greystoke A, Ward TH, Ferraldeschi R, Hughes A, Clack G, Ranson M, Dive C, Blackhall FH. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J. Clin. Oncol. 2011;29:1556–1563. doi: 10.1200/JCO.2010.28.7045. [DOI] [PubMed] [Google Scholar]
- 5.Gervasoni A, Sandri MT, Nascimbeni R, Zorzino L, Cassatella MC, Baglioni L, Panigara S, Gervasi M, Di Lorenzo D, Parolini O. Comparison of three distinct methods for the detection of circulating tumor cells in colorectal cancer patients. Oncol. Rep. 2011;25:1669–1703. doi: 10.3892/or.2011.1231. [DOI] [PubMed] [Google Scholar]
- 6.Andreopoulou E, Yang LY, Rangel KM, Reuben JM, Hsu L, Krishnamurthy S, Valero V, Fritsche HA, Cristofanilli M. Comparison of assay methods for detection of circulating tumor cells (CTCs) in metastatic breast cancer (MBC): AdnaGen AdnaTest BreastCancer Select/Detect versus Veridex CellSearch system. Int. J. Cancer. 2011 doi: 10.1002/ijc.26111. [DOI] [PubMed] [Google Scholar]
- 7.Balic M, Dandachi N, Hofmann G, Samonigg H, Loibner H, Obwaller A, van der Kooi A, Tibbe AG, Doyle GV, Terstappen LW, Bauernhofer T. Comparison of two methods for enumerating circulating tumor cells in carcinoma patients. Cytometry. B Clin. Cytom. 2005;68:25–30. doi: 10.1002/cyto.b.20065. [DOI] [PubMed] [Google Scholar]
- 8.Zhao S, Liu Y, Zhang Q, Li H, Zhang M, Ma W, Zhao W, Wang J, Yang M. The prognostic role of circulating tumor cells (CTCs) detected by RT-PCR in breast cancer: a meta-analysis of published literature. Breast Cancer Res. Treat. 2011 Feb 11; doi: 10.1007/s10549-011-1379-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Rahbari NN, aigner M, Thorlund K, Mollberg N, Motscahll E, Jensen K, Diener MK, Büchler MW, Koch M, Weitz J. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology. 2010;138:1714–1726. doi: 10.1053/j.gastro.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Hansen M, Pedersen AG. Tumor markers in patients with lung cancer. Chest. 1986;89:219S–224S. doi: 10.1378/chest.89.4_supplement.219s. [DOI] [PubMed] [Google Scholar]
- 11.Van't Westeinde SC, van Klaveren RJ. Screening and early detection of lung cancer. Cancer J. 2011;17:3–10. doi: 10.1097/PPO.0b013e3182099319. [DOI] [PubMed] [Google Scholar]
- 12.Marrinucci D, Bethel K, Luttgen M, Bruce RH, Nieva J, Kuhn P. Circulating tumor cells from well-differentiated lung adenocarcinoma retain cytomorphologic features of primary tumor type. Arch. Pathol. Lab. Med. 2009;133:1468–1471. doi: 10.1043/1543-2165-133.9.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maheswaran S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N. Engl. J. Med. 2008;359:366–377. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bardia A, Huang P, Zhang Z, Sokoll L, Ingle JN, Carey LA, Lin NU, Nanda R, Visvanathan K, Wolff AC (on behalf of the Translational Breast Cancer Research Consortium; Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, MD; Johns Hopkins Kimmel Cancer Center, Baltimore, MD; Mayo Clinic Rochester, Rochester, MN; University of North Carolina at Chapel Hill, Chapel Hill, NC; Dana-Farber Cancer Institute, Boston, MA; The University of Chicago, Chicago, IL) Circulating tumor cell (CTC) and CA2729 as predictors of outcome in patients with metastatic breast cancer (MBC) in the prospective TBCRC 005 biomarker study. J. Clin. Oncol. 2010;28:15s. (abstr 1001) [Google Scholar]