Abstract
Background
Structural rearrangements of chromosomal band 19q13 are a non-random cytogenetic abnormality in thyroid adenomas and adenomatous goiters and lead to an expression of miRNAs of the chromosome 19 microRNA cluster C19MC. Normally, expression of these miRNAs is silenced except for embryonic stem cells and the placenta where they represent the majority of miRNAs not only in the trophoblast but also in exosomes derived from it.
Presentation of the hypothesis
We have advanced the hypothesis that as part of the feto-maternal communication miRNAs of C19MC serve immunomodulatory functions in the placenta and confer a growth advantage to thyroid nodules by protecting them against autoimmune attacks. More precisely, the exosomes containing these miRNAs may specifically target immune cells in their local environment as well as systemically by transferring their cargo to recipient cells. Within these target cells the transferred miRNAs can interact with mRNAs of the recipient cells thereby suppressing their immune-specific functions.
Testing the hypothesis
Experiments used to demonstrate the immunomodulatory capacity of placenta-derived exosomes can be modified by transfecting the target cells with those miRNAs of C19MC represented in placental exosomes.
Implications of the hypothesis
Mimics of C19MC-derived miRNAs might develop to useful drug candidates for the treatment of autoimmune disease as e.g. rheumatoid arthritis and Sjögren’s syndrome and for the prevention of transplant rejection. In case of tumor entities with elevated expression of C19MC miRNAs these miRNAs may be interesting targets for treatment with appropriate antagonists.
Keywords: MicroRNA, Chromosomal translocation, Thyroid adenoma, C19MC, Placenta, Epigenetics, Immunomodulation
Background
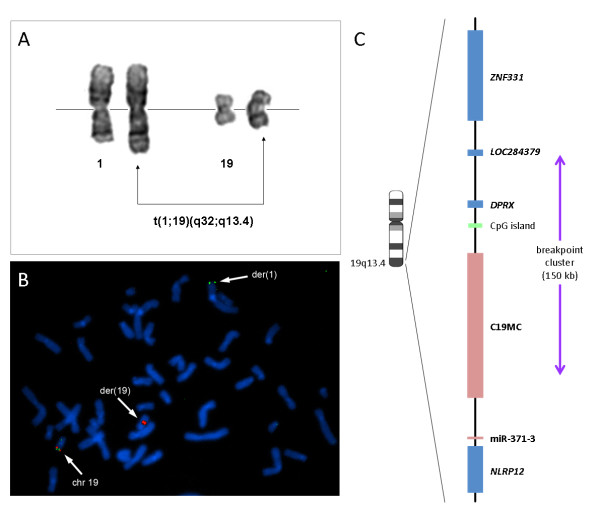
First being described in 1989 [1], structural rearrangements of chromosomal band 19q13 are a frequent non-random cytogenetic abnormality in thyroid adenomas and adenomatous goiters. By applying molecular-cytogenetic methods on established cell lines it was possible to narrow down the breakpoints to a region of about 150 kbp [2] which was later shown to harbor C19MC (chromosome 19 microRNA cluster), the largest human microRNA cluster at all [3] (Figure 1). As a rule, by the chromosomal rearrangements the microRNAs of this cluster and the neighboring miR-371-3 cluster become strongly upregulated [4]. Of these both clusters C19MC is remarkable not only because of its sheer size encoding more than 50 mature microRNAs but also because of its “young” age. The whole cluster is primate-specific [3] and thus must have evolved within a relatively short time in terms of evolution. Normally, the miRNAs of C19MC are expressed almost exclusively in embryonic stem cells [5-9] and, later during embryonic and fetal development, only in the placenta [3,10]. Luo et al. [11] demonstrated that the trophoblast secretes exosomes, i.e. small membrane microvesicles, which contain placenta-specific miRNAs. Moreover, it was demonstrated quite recently that the vast majority of miRNAs packed into placenta-derived exosomes consist of miRNAs of the C19MC cluster [12]. However, in both cases, i.e. in the placenta or after chromosomal translocations in benign thyroid nodules, the exact mechanisms by which C19MC miRNAs contribute to normal or aberrant functions, respectively, remain obscure.
Figure 1.
(A) Partial karyotype of an adenoma with an apparently balanced translocation t(1;19)(q32;q13.4) is shown as an example of adenomas with a 19q13.4 chromosomal rearrangement. (B) Metaphase of a thyroid adenoma with a t(1;19)(q32;q13.4) after FISH with dual-color break-apart rearrangement probe appropriate for the detection of 19q13.4 rearrangements. The 19q13.4 rearrangement is indicated by one single green and one single red signal each. (C) The chromosomal region 19q13.4 with the miRNA clusters C19MC and miR-371-3 (pink boxes) and surrounding protein-coding genes (blue boxes). The common breakpoint cluster of thyroid adenomas and adenomatous goiters is indicated by an arrow.
Herein, we shall advance the hypothesis that as part of the feto-maternal communication miRNAs of C19MC serve immunomodulatory functions in the placenta and, once re-expressed due to chromosomal translocations, confer a growth advantage to thyroid nodules by protecting them against autoimmune attacks.
Presentation of the hypothesis
Though cytogenetic aberrations of 19q13.4 as detected in benign thyroid lesions represent one of the most common specific chromosomal alterations in epithelial tumors at all the molecular mechanisms resulting from these frequent genomic alterations still remain obscure. As to tumorigenesis, the stimulation of invasive growth has been attributed to some miRNAs of C19MC [for review see [13] but the activation of the cluster in thyroid adenomas and nodular goiters with 19q13.4 alterations apparently does not coincide with invasive growth. Thus, it seems unlikely that in general activation of C19MC leads to invasive growth or other features characterizing malignant cells. On the other hand, presumed “physiological functions” of its miRNAs might give us a clue to understand their role in tumorigenesis. Of note, until birth expression of C19MC persists only in the placenta or, more precisely, its trophoblast [10,11], and is expressed exclusively from the paternal allele whereas the maternal allele is silenced by epigenetic modification [14].
In general, miRNAs do not necessarily exert their main functions in the cells they are expressed in since considerable amounts of miRNAs can become packed into microvesicles called exosomes. Exosomes are bioactive vesicles derived from endosomal membranes and involved in intercellular communication by their specific cargos of proteins, mRNAs, and miRNAs [for review see [15]. In previous reports placenta-derived exosomes have been demonstrated to interact with immune cells, e.g. resulting in suppression of T-cell signaling components [16,17].
Donker et al. [12] were able to demonstrate recently that the cellular miRNA composition of human primary trophoblast cells strongly resembled that of the exosomes secreted from these cells. In both cases those of C19MC represented the majority of mature miRNAs. Of note, six microRNAs of C19MC ranged among the top-ten exosomal miRNAs. Based on their findings the authors have assumed that these miRNAs “may play an important role in placental-maternal communication, possibly directing maternal adaptation to pregnancy.”
Herein, we would like to outline the hypothesis that as one major function miRNAs of C19MC prevent the embryo from being attacked by the maternal immune system. Immunologically, the embryo is considered being a semi-allograft and in case of egg donation even a full allograft [18]. Nevertheless, the embryo efficiently avoids rejection by its mother’s immune system by mechanisms that are not fully understood yet [for review see [19]. Exosomes are known to share membrane characteristics with the cells they are derived from [20]. Thus, it seems tempting to assume that they act like decoy-flairs for a jet. The exosomes can specifically target immune cells in their local environment, i.e. the decidua, as well as systemically thereby transferring their cargo when melting with the membrane of recipient cells. Within these target cells the transferred miRNAs can interact with mRNAs of the recipient cells thereby modulating post-transcriptional regulation. Non-specific systemic side effects of this mechanism may be the mild immunosuppression noted during pregnancy e.g. leading to the improvement of rheumatoid arthritis [for review see [21].
Tracing back to nodular goiters and thyroid adenomas re-expression of C19MC may protect cells against autoimmune attacks. Of note, a considerable percentage of these lesions develop after a pre-existing autoimmune disease of the thyroid.
Finally, the question arises if malignant tumors can adopt this mechanism to protect themselves. 19q13 is one of the most frequent chromosomal breakpoints identified in human tumors and even if, in particular in case of complex karyotypic aberrations, the small size of chromosome 19 may have resulted in false positive identification of this breakpoint there remain a number of tumor entities where its involvement has been identified unambiguously as e.g. hamartoma of the liver [22,23]. Also, a number of recent papers point to the role of amplification (e. g. in CNS-PNET [24] and in embryonal brain tumors with ependymoblastic multilayered rosettes [25]) or undermethylation of the C19MC locus (e. g. in hepatocellular carcinomas [26,27]) in several tumor entities.
Testing the hypothesis
There are straightforward appropriate experimental approaches to test our hypothesis. Nevertheless, these approaches are time consuming because not all miRNAs of the cluster may have the capacity to modulate immune cells and because different types of immune cells have to be tested. As a first step the same experiments used to demonstrate the immunomodulatory capacity of placenta-derived exosomes [e.g. [17] can be modified by transfecting the target cells with those miRNAs of C19MC highly represented in placental exosomes. Furthermore, it did not escape our attention that mesenchymal cells from the amniotic membrane have strong immunomodulatory properties, e.g. by actively suppressing T-cell proliferation induced by alloantigens [28]. So far, it is believed that at term only the trophoblast expresses C19MC miRNAs but if our hypothesis holds true one would expect that these amniotic-membrane derived cells do so as well.
Implications of the hypothesis
If the hypothesis holds true it will be not only relevant in terms of basic science but also for several clinical approaches. Mimics of C19MC-derived miRNAs, either encapsulated or not, might develop to useful drug candidates for the treatment of autoimmune disease as e.g. rheumatoid arthritis and Sjögren’s syndrome. Likewise, these miRNAs may prolong the maintenance of functional allografts. On the other hand, in case of tumor entities with forced expression of C19MC miRNAs their antagonists may represent interesting alternatives for targeted treatments.
Abbreviations
C19MC, chromosome 19 microRNA cluster; CNS-PNET, Central nervous system primitive neuroectodermal tumor; miRNA, microRNA.
Competing interests
The authors declare competing financial interests because the University of Bremen is currently applying for a patent claiming the use of C19MC miRNAs for immunomodulation.
Authors' contributions
Both authors have equally contributed to this hypothesis and written the manuscript. Both authors read and approved the final manuscript.
Contributor Information
Jörn Bullerdiek, Email: bullerd@uni-bremen.de.
Inga Flor, Email: iflor@uni-bremen.de.
References
- Bartnitzke S, Herrmann ME, Lobeck H, Zuschneid W, Neuhaus P, Bullerdiek J. Cytogenetic findings on eight follicular thyroid adenomas including one with a t(10;19) Canc Genet Cytogenet. 1989;39:65–68. doi: 10.1016/0165-4608(89)90230-6. [DOI] [PubMed] [Google Scholar]
- Belge G, Rippe V, Meiboom M, Drieschner N, Garcia E, Bullerdiek J. Delineation of a 150-kb breakpoint cluster in benign thyroid tumors with 19q13.4 aberrations. Cytogenet Cell Genet. 2001;93:48–51. doi: 10.1159/000056947. [DOI] [PubMed] [Google Scholar]
- Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E. et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet. 2005;37:766–770. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- Rippe V, Dittberner L, Lorenz VN, Drieschner N, Nimzyk R, Sendt W, Junker K, Belge G, Bullerdiek J. The two stem cell microRNA gene clusters C19MC and miR-371-3 are activated by specific chromosomal rearrangements in a subgroup of thyroid adenomas. PLoS One. 2010;5:e9485. doi: 10.1371/journal.pone.0009485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Wyman SK, Fritz BR, Qi J, Garg KS, Parkin RK, Kroh EM, Bendoraite A, Mitchell PS, Nelson AM. et al. MicroRNA discovery and profiling in human embryonic stem cells by deep sequencing of small RNA libraries. Stem Cell. 2008;26:2496–2505. doi: 10.1634/stemcells.2008-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent LC, Chen J, Ulitsky I, Mueller FJ, Lu C, Shamir R, Fan JB, Loring JF. Comprehensive microRNA profiling reveals a unique human embryonic stem cell signature dominated by a single seed sequence. Stem Cell. 2008;26:1506–1516. doi: 10.1634/stemcells.2007-1081. [DOI] [PubMed] [Google Scholar]
- Morin RD, O'Connor MD, Griffith M, Kuchenbauer F, Delaney A, Prabhu AL, Zhao Y, McDonald H, Zeng T, Hirst M. et al. Application of massively parallel sequencing to microRNA profiling and discovery in human embryonic stem cells. Genome Res. 2008;18:610–621. doi: 10.1101/gr.7179508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Jin P, Wang E, Marincola FM, Stroncek DF. MicroRNA and gene expression patterns in the differentiation of human embryonic stem cells. J Transl Med. 2009;7:20. doi: 10.1186/1479-5876-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Yang CS, Rana TM. Evolutionary emergence of microRNAs in human embryonic stem cells. PLoS One. 2008;3:e2820. doi: 10.1371/journal.pone.0002820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Wang YQ, Su B. Molecular evolution of a primate-specific microRNA family. Mol Biol Evol. 2008;25:1493–1502. doi: 10.1093/molbev/msn094. [DOI] [PubMed] [Google Scholar]
- Luo SS, Ishibashi O, Ishikawa G, Ishikawa T, Katayama A, Mishima T, Takizawa T, Shigihara T, Goto T, Izumi A. et al. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biol Reprod. 2009;81:717–729. doi: 10.1095/biolreprod.108.075481. [DOI] [PubMed] [Google Scholar]
- Donker RB, Mouillet JF, Chu T, Hubel CA, Stolz DB, Morelli AE, Sadovsky Y. The expression profile of C19MC microRNAs in primary human trophoblast cells and exosomes. Mol Human Reprod. 2012. “Accepted Article”. 10.1093/ molehr/gas013. [DOI] [PMC free article] [PubMed]
- Flor I, Bullerdiek J. The dark side of a success story: microRNAs of the C19MC cluster in human tumours. J Pathol. 2012. “Accepted Article”. [DOI] [PubMed]
- Noguer-Dance M, Abu-Amero S, Al-Khtib M, Lefevre A, Coullin P, Moore GE, Cavaille J. The primate-specific microRNA gene cluster (C19MC) is imprinted in the placenta. Hum Mol Genet. 2010;19:3566–3582. doi: 10.1093/hmg/ddq272. [DOI] [PubMed] [Google Scholar]
- Pant S, Hilton H, Burczynski ME. The multifaceted exosome: Biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol. 2012;83:1484–1494. doi: 10.1016/j.bcp.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DD, Akyol S, Gercel-Taylor C. Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol. 2006;176:1534–1542. doi: 10.4049/jimmunol.176.3.1534. [DOI] [PubMed] [Google Scholar]
- Sabapatha A, Gercel-Taylor C, Taylor DD. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am J Reprod Immunol. 2006;56:345–355. doi: 10.1111/j.1600-0897.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- van der Hoorn ML, Scherjon SA, Claas FH. Egg donation pregnancy as an immunological model for solid organ transplantation. Transplant Immunol. 2011;25:89–95. doi: 10.1016/j.trim.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Warning JC, McCracken SA, Morris JM. A balancing act: mechanisms by which the fetus avoids rejection by the maternal immune system. Reproduction. 2011;141:715–724. doi: 10.1530/REP-10-0360. [DOI] [PubMed] [Google Scholar]
- Mincheva-Nilsson L, Baranov V. The role of placental exosomes in reproduction. Am J Reprod Immunol. 2010;63:520–533. doi: 10.1111/j.1600-0897.2010.00822.x. [DOI] [PubMed] [Google Scholar]
- Ostensen M, Villiger PM, Forger F. Interaction of pregnancy and autoimmune rheumatic disease. Autoimmun Rev. 2012;11:A437–A446. doi: 10.1016/j.autrev.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Speleman F, De Telder V, De Potter KR, Dal Cin P, Van Daele S, Benoit Y, Leroy JG, Van den Berghe H. Cytogenetic analysis of a mesenchymal hamartoma of the liver. Canc Genet Cytogenet. 1989;40:29–32. doi: 10.1016/0165-4608(89)90142-8. [DOI] [PubMed] [Google Scholar]
- Rajaram V, Knezevich S, Bove KE, Perry A, Pfeifer JD. DNA sequence of the translocation breakpoints in undifferentiated embryonal sarcoma arising in mesenchymal hamartoma of the liver harboring the t(11;19)(q11;q13.4) translocation. Genes Chromosomes Canc. 2007;46:508–513. doi: 10.1002/gcc.20437. [DOI] [PubMed] [Google Scholar]
- Li M, Lee KF, Lu Y, Clarke I, Shih D, Eberhart C, Collins VP, Van Meter T, Picard D, Zhou L. et al. Frequent amplification of a chr19q13.41 microRNA polycistron in aggressive primitive neuroectodermal brain tumors. Canc Cell. 2009;16:533–546. doi: 10.1016/j.ccr.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobusawa S, Yokoo H, Hirato J, Kakita A, Takahashi H, Sugino T, Tasaki K, Itoh H, Hatori T, Shimoyama Y, Analysis of Chromosome 19q13.42 Amplification in Embryonal Brain Tumors with Ependymoblastic Multilayered Rosettes. Brain Pathol. 2012. “Accepted Article”. doi:10.1111/j.1750- 3639.2012.00574.x. [DOI] [PMC free article] [PubMed]
- Fornari F, Milazzo M, Chieco P, Negrini M, Marasco E, Capranico G, Mantovani V, Marinello J, Sabbioni S, Callegari E, In hepatocellular carcinoma miR-519d is upregulated by p53 and DNA hypomethylation and targets CDKN1A/p21, PTEN, AKT3 and TIMP2. J Pathol. 2012. "Accepted Article". [DOI] [PubMed]
- Augello C, Vaira V, Caruso L, Destro A, Maggioni M, Park YN, Montorsi M, Santambrogio R, Roncalli M, Bosari S. MicroRNA profiling of hepatocarcinogenesis identifies C19MC cluster as a novel prognostic biomarker in hepatocellular carcinoma. Liver Int. 2012;32:772–782. doi: 10.1111/j.1478-3231.2012.02795.x. [DOI] [PubMed] [Google Scholar]
- Wolbank S, Peterbauer A, Fahrner M, Hennerbichler S, van Griensven M, Stadler G, Redl H, Gabriel C. Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: a comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng. 2007;13:1173–1183. doi: 10.1089/ten.2006.0313. [DOI] [PubMed] [Google Scholar]