Abstract
Fidaxomicin is bactericidal against Clostridium difficile. The combined results of 8 in vitro studies of 1323 C. difficile isolates showed the minimum inhibitory concentration (MIC) range of fidaxomicin to be ≤0.001–1 μg/mL, with a maximum MIC for inhibition of 90% of organisms (MIC90) of 0.5 μg/mL. Isolates from 2 phase III clinical trials demonstrated that fidaxomicin MICs of baseline isolates did not predict clinical cure, failure, or recurrence of C. difficile infections. No resistance to fidaxomicin developed during treatment in either study, although a single strain recovered from a cured patient had an elevated MIC of 16 µg/mL at the time of recurrence. For 135 strains, OP-1118, a major metabolite, had an MIC for inhibition of 50% of organisms of 4 μg/mL and an MIC90 of 8 μg/mL. Changes in inoculum size (102–105 colony-forming units/spot) or cation concentrations of calcium or magnesium appeared to have no effect on fidaxomicin MICs. Fidaxomicin has little or no activity against gram-negative aerobes and anaerobes or yeast.
In 1991, Swanson et al [1] evaluated the in vitro activity of tiacumicin B (now fidaxomicin) isolated from the fermentation broth of Dactylosporangium aurantiacum subspecies hamdenensis [2] against Clostridium difficile. Fidaxomicin (formerly designated OPT-80 and PAR-101) has been developed for the treatment of C. difficile–associated diarrhea and is a potent new macrocyclic antibiotic that targets RNA polymerase. Fidaxomicin has a narrow spectrum of activity, with little or no activity against gram-negative aerobic and anaerobic bacteria, but demonstrates high activity against C. difficile (Table 1) [3]. Fidaxomicin reaches a high concentration in the gut with minimal systemic absorption. This article reviews and provides original data for the antimicrobial activity of fidaxomicin, including variations of test conditions and activity of its metabolite OPT-1118, as well as its kill kinetics and pharmacodynamics as related to C. difficile.
Table 1.
Antimicrobial Profile of Fidaxomicin for Various Aerobic and Anaerobic Bacteria and Yeast
| Gram-Negative Bacteria |
Gram-Positive Bacteria |
Yeast |
||||||
|---|---|---|---|---|---|---|---|---|
| Strain | ATCC No. | FDX MIC | Strain | ATCC No. | FDX MIC | Strain | ATCC No. | FDX MIC |
| Acinetobacter baumannii | 19606 | >32 | Bacillus cereus | 11778 | 1 | Yeast | ||
| Acinetobacter calcoaceticus | 23055 | 1 | B. cereus | 14579 | 1 | Candida albicans | 24433 | >64 |
| Bacteroides distasonis | 8503 | >32 | Clostridium difficile | 43255 | 0.125 | C. albicans | 90028 | >64 |
| Bacteroides fragilis | 23745 | >32 | C. difficile | 9689 | 0.06 | C. albicans | 14053 | >64 |
| B. fragilis | 25285 | >32 | C. difficile | 17857 | 0.031 | Candida krusei | 6258 | >64 |
| Bacteroides ovatus | 8483 | >32 | Clostridium perfringens | 13124 | ≤0.015 | Candida glabrata | 2001 | >64 |
| Bacteroides uniformis | 8492 | >32 | Enterococcus faecalis | 19433 | 4 | Candida lusitaniae | 66035 | >64 |
| Campylobacter jejuni | 29428 | 64 | Enterococcus faecium | 19434 | 4 | Candida parapsilosis | 22019 | >64 |
| C. jejuni | 33291 | >64 | E. faecium | 49032 | 4 | Candida tropicalis | 750 | >64 |
| C. jejuni | 49943 | 64 | E. faecium | 700221 | 4 | |||
| Citrobacter braakii | 43162 | >64 | Lactobacillus acidophilus | 4356 | >32 | |||
| Citrobacter freundii | 43864 | >64 | Lactobacillus casei | 393 | 1 | |||
| Enterobacter aerogenes | 35028 | >64 | Lactobacillus rhamnosus | 7469 | 16 | |||
| E. aerogenes | 13048 | >64 | Micrococcus luteus | 381 | ≤0.125 | |||
| Enterobacter cloacae | 49141 | >64 | M. luteus | 49732 | ≤0.125 | |||
| E. cloacae | 23355 | >32 | M. luteus | 533 | ≤0.125 | |||
| Escherichia coli | 25922 | >32 | M. luteus | 4698 | ≤0.06 | |||
| Fusobacterium nucleatum | 25586 | >32 | Peptostreptococcus anaerobius | 27337 | ≤0.06 | |||
| Haemophilus influenzae | 49247 | >32 | Peptostreptococcus (Peptoniphilus) asaccharolyticus | 29743 | 1 | |||
| Helicobacter pylori | 43504 | >32 | Peptococcus (Finegoldia) magna | 29328 | 0.5 | |||
| Klebsiella oxytoca | 43165 | >64 | Peptococcus (Micromonas) micros | 33270 | 0.125 | |||
| K. oxytoca | 49131 | >64 | Propionibacterium acnes | 11827 | 8 | |||
| Klebsiella pneumoniae | 33495 | >64 | P. acnes | 6919 | 8 | |||
| K. pneumoniae | 27736 | >64 | Staphylococcus aureus | 33591 | 8 | |||
| K. pneumoniae | 13883 | >32 | S. aureus | 25923 | 16 | |||
| Moraxella catarrhalis | 25238 | 2 | S. aureus | 29213 | 8 | |||
| M. catarrhalis | 49143 | 1 | Staphylococcus epidermidis | 12228 | 1 | |||
| Neisseria meningitidis | 13077 | 64 | S. epidermidis | 14990 | 1 | |||
| Neisseria gonorrhoeae | 19424 | 8 | Staphylococcus intermedius | 29663 | 4 | |||
| N. gonorrhoeae | 49226 | 32 | Streptococcus agalactiae | 12386 | 16 | |||
| Neisseria lactamica | 23970 | 32 | S. agalactiae | 13813 | 32 | |||
| Porphyromonas asaccharolytica | 25260 | 32 | Streptococcus pyogenes | 19615 | 16 | |||
| Prevotella loescheii | 15930 | >32 | Streptococcus pneumoniae | 49619 | >32 | |||
| Proteus mirabilis | 25933 | >64 | Streptococcus sanguinis | 10556 | 32 | |||
| P. mirabilis | 29245 | >64 | ||||||
| Proteus penneri | 33519 | >64 | ||||||
| Proteus vulgaris | 33420 | >64 | ||||||
| Pseudomonas aeruginosa | 27853 | >32 | ||||||
| Salmonella choleraesuis | 19585 | >64 | ||||||
| S. choleraesuis | 14028 | >32 | ||||||
| Serratia marcescens | 43861 | >64 | ||||||
| S. marcescens | 8100 | >32 | ||||||
| Veillonella parvula | 10790 | 32 | ||||||
Data are from [3].
Abbreviations: ATCC, American Type Culture Collection; FDX, fidaxomicin; MIC, minimum inhibitory concentration.
Comparative In Vitro Studies
Eight studies performed on strains isolated between 1983 and 2010 have reported the comparative in vitro activity of fidaxomicin against C. difficile [1, 4–10] (Table 2). A combined total of 1323 isolates were reported with a minimum inhibitory concentration (MIC) range of ≤0.001–1 μg/mL and a maximum MIC for inhibition of 90% of organisms (MIC90) of 0.5 μg/mL, which are far below the fidaxomicin levels found in feces after treatment.
Table 2.
In Vitro Activity of Fidaxomicin, Compared With Vancomycin and Metronidazole, Against Clostridium difficile Isolates From 8 Published Studies
| MIC (μg/mL) |
|||||
|---|---|---|---|---|---|
| Drug | No. of isolates | Range | MIC50 | MIC90 | [Ref] Year/sites |
| Fidaxomicin | 16 | 0.12–0.25 | 0.25 | 0.25 | [1] 1991/US |
| Vancomycin | 0.5–1 | 0.5 | 1 | ||
| Metronidazole | 0.12–0.5 | 0.25 | 0.5 | ||
| Fidaxomicin | 207 | ≤0.001–0.625 | 0.002 | 0.008 | [4] 2004/Europe |
| Vancomycin | 0.016–0.5 | 0.5 | 0.5 | ||
| Metronidazole | 0.004–0.5 | 0.06 | 0.06 | ||
| Fidaxomicin | 23 | 0.06–2 | 0.12 | 0.25 | [5] 2004/US |
| Vancomycin | 0.5–4 | 1 | 2 | ||
| Metronidazole | 0.25–1 | 0.12 | 0.25 | ||
| Fidaxomicin | 208 | 0.06–1 | 0.25 | 0.5 | [8] 2008/Canada |
| Vancomycin | 0.5–4 | 0.5 | 1 | ||
| Metronidazole | 0.25–4 | 0.5 | 1 | ||
| Fidaxomicin | 110 | 0.015–0.25 | 0.125 | 0.125 | [6] 1983–2004/US |
| Vancomycin | 0.06–4 | 1 | 1 | ||
| Metronidazole | 0.025–0.5 | 0.125 | 0.25 | ||
| Fidaxomicin | 21 | ≤0.016–0.25 | 0.016 | 0.12 | [7] 2004/US |
| Vancomycin | 0.5–2 | 1 | 2 | ||
| Metronidazole | ≤0.125–0.5 | 0.25 | 0.5 | ||
| Fidaxomicin | 38 | ≤0.008–0.25 | … | 0.125 | [9] 2004–2005/US |
| Vancomycin | 0.25–2 | … | 1 | ||
| Metronidazole | 0.25–2 | … | 1 | ||
| Fidaxomicin | 716 | ≤0.008–1 | 0.125 | 0.5 | [10] 2005–2010/US & Europe |
| Vancomycin | 0.5–8 | 1 | 2 | ||
| Metronidazole | 0.02–4 | 0.5 | 1 | ||
Abbreviations: MIC50, minimum inhibitory concentration for inhibition of 50% of organisms; MIC90, minimum inhibitory concentration for inhibition of 90% of organisms; US, United States.
Hecht et al [6] reported on the in vitro activity of fidaxomicin against 110 toxigenic C. difficile clinical isolates collected during 1983–2004 in the United States, South America, and Europe. With the use of the Clinical and Laboratory Standards Institute (CLSI) [11] supplemented Brucella agar dilution method, the fidaxomicin geometric mean MIC was 0.081 μg/mL, with a maximum MIC of 0.25 μg/mL and an MIC90 of 0.125 μg/mL. They did not note any variation of MIC related to year of isolation or restriction endonuclease analysis (REA) BI group status. A German study [4] that used the Wilkins-Chalgren broth microdilution method on isolates collected between 1986 and 2002 showed that all C. difficile strains were susceptible to ≤0.06 μg/mL of fidaxomicin and confirmed low MICs by agar dilution for a subset of isolates. A Manitoba, Canada, study [8] that used the CLSI agar dilution method on isolates collected between January and April 2007 showed that all C. difficile strains were susceptible to ≤1.0 μg/mL of fidaxomicin, with an MIC90 of 0.5 μg/mL.
Clinical Trial In Vitro Susceptibilities
Citron et al [9] reported the activity of fidaxomicin by REA type on C. difficile isolates recovered from the fidaxomicin phase II clinical trial for C. difficile infection. Thirty-eight of 49 enrolled subjects (78%) had a C. difficile organism isolated at baseline. Four subjects grew multiple colony types, with 1 of these subjects having 2 different REA-type strains. Fidaxomicin showed an MIC range of ≤0.008–0.125 μg/mL, with an MIC90 of 0.125 μg/mL. Samples from only 2 subjects who had a recurrence within 6 weeks of treatment yielded isolates with MICs within a dilution of those recovered at baseline. It was noted that the REA BI isolates had metronidazole and vancomycin, but not fidaxomicin, MIC90 values that were 2 dilutions higher than that for the non-BI strains.
Goldstein et al [10] reported the activity of fidaxomicin by REA type on 716 C. difficile isolates from 2 fidaxomicin phase III studies (Table 3). For all pretreatment isolates, the fidaxomicin MIC range was ≤0.004–1.0 μg/mL, with an MIC for inhibition of 50% of organisms (MIC50) of 0.125 μg/mL and an MIC90 of 0.25 μg/mL. Analyzed by REA type, 244 of 718 isolates (35%) were from the BI group, with MICs generally higher for all 4 drugs tested (MIC90: fidaxomicin, 0.5; vancomycin, 2.0; metronidazole, 2.0; and rifaximin >256 µg/mL) than for the other REA types. Fidaxomicin susceptibility of baseline isolates did not predict clinical cure, failure, or recurrence for fidaxomicin (baseline MIC90, 0.25 µg/mL [range, ≤0.008–1 µg/mL]). No resistance to fidaxomicin developed during treatment in either phase III study, although a single strain isolated from a cured patient had an elevated fidaxomicin MIC of 16 µg/mL at the time of recurrence.
Table 3.
Fidaxomicin-Susceptibility Profiles, by Restriction Endonuclease Analysis Group, for 716 Clostridium difficile Strains Isolated at Baseline (per protocol population) From 2 Phase III Trials
| REA Group | No. of Patients | Geometric Mean (Range) | MIC50 (μg/mL) | MIC90 (μg/mL) |
|---|---|---|---|---|
| BI | 244 | 0.18 (0.015–1) | 0.25 | 0.5 |
| BK | 12 | 0.09 (0.03–0.25) | 0.06 | 0.125 |
| CF | 7 | 0.09 (0.015–0.25) | 0.125 | 0.25 |
| DH | 4 | 0.25 (0.25–0.25) | 0.25 | 0.25 |
| G | 54 | 0.08 (0.015–0.25) | 0.06 | 0.125 |
| J | 43 | 0.02 (≤0.008–0.12) | 0.02 | 0.125 |
| Nonspecific REA | 260 | 0.08 (≤0.004–0.5) | 0.06 | 0.125 |
| K | 15 | 0.07 (0.015–0.25) | 0.06 | 0.125 |
| Y | 77 | 0.10 (0.015–0.5) | 0.125 | 0.25 |
| All strains | 716 | 0.10 (≤0.004–1) | 0.125 | 0.25 |
Copyright © American Society for Microbiology, Antimicrob Agents and Chemother 2011; 55:5194–9 [10].
Abbreviations: MIC50, minimum inhibitory concentration for inhibition of 50% of organisms; MIC90, minimum inhibitory concentration for inhibition of 90% of organisms; REA, restriction endonuclease analysis.
Results of studies by Ackermann et al [4] and Credito and Applebaum [7] showing more potent activity of fidaxomicin against C. difficile than those of Karlowsky et al [8], Hecht et al [6], and Finegold et al [5] may have been related to the inclusion of higher numbers of clones with lower MICs. Although Credito and Applebaum [7] showed an MIC90 of 0.125 μg/mL, the MIC90 reported by Ackermann et al [4] was exceptionally low (0.008 μg/mL), which could alternatively be attributed to lower viability of cells when dimethyl sulfoxide (DMSO) was used as diluent and/or to use of an anaerobic environment with a higher carbon dioxide concentration (15% vs the CLSI-recommended 4%–7%), because carbon dioxide can acidify media.
Effect of Diluent, pH, Inoculum, and Cations on Susceptibility
Babakhani et al [12] found that variations in pH affected MICs. With use of both Brucella agar dilution and broth dilution methods, fidaxomicin MICs were unchanged between pH values of 6.2 and 7.0 but increased in a linear fashion and were 8-fold higher at pH values of 7.9–8.0. The organism was shown to grow poorly at a pH of 5.0. With use of the Wilkins-Chalgren broth microdilution method, Swanson et al [1] reported that the MICs of tiacumicin B against C. difficile American Type Culture Collection (ATCC) 9689 at pH values of 6.5 and 8.0 were unchanged or only 2-fold different from MICs determined at a pH of 7.3.
The effects of inoculum concentrations of 102–105 colony-forming units/spot and of cation concentrations of calcium (at 33, 45, and 75 mg/L) or magnesium (21, 30, and 57 mg/L) were also studied [12]. Neither inoculum size nor cation concentration had an effect on fidaxomicin MICs for 2 reference C. difficile strains (ATCC 9689 and ATCC 700057) [12]. In contrast, as stated by the investigators, “vancomycin MICs increased progressively with increasing inoculum concentrations” [12, 2674–5]. Additionally, the investigators studied the effect of various commercial lots of media on MICs and reported no fidaxomicin MIC variation when tested with 3 different lots of commercially prepared supplemented Brucella agar media.
In Vitro Studies Against Enteric Flora
Ackermann et al [4] studied the activity of fidaxomicin against a limited number of eubacteria (26 isolates), lactobacilli (8), Propionibacterium acnes (16), Prevotella species (35), and Bacteroides fragilis (69) and found them generally not susceptible. MIC50 and MIC90 values were >128 μg/mL and >128 μg/mL, respectively, for B. fragilis and Prevotella species. Finegold et al [5] performed a more extensive study involving 453 intestinal bacteria and reported that streptococci, aerobic and facultative gram-negative rods, anaerobic gram-negative rods, and Clostridium ramosum were resistant, which might potentially be less disruptive to normal fecal flora. Against 50 isolates of the B. fragilis group, MIC50 was 256 μg/mL and MIC90 was >1024 μg/mL. They noted that fidaxomicin had activity against most clostridia, staphylococci, and enterococci.
Clinical results in support of these in vitro studies were seen in the fidaxomicin phase IIA dose-ranging trial, in which 30 patient stool samples cultured for normal flora [13] showed that B. fragilis group counts were not affected by increasing fidaxomicin dosages.
OP-1118 In Vitro Activity
OP-1118 is a major metabolite of fidaxomicin that also exhibits a narrow spectrum of activity. Tested in vitro by using CLSI susceptibility testing methods against 32 strains belonging to the commensal gastrointestinal flora, OP-1118 demonstrated activity against only some gram-positive organisms, with MICs 4–16-fold greater than those of fidaxomicin [14]. Similar to the parent compound, OP-1118 was not active against gram-negative bacteria.
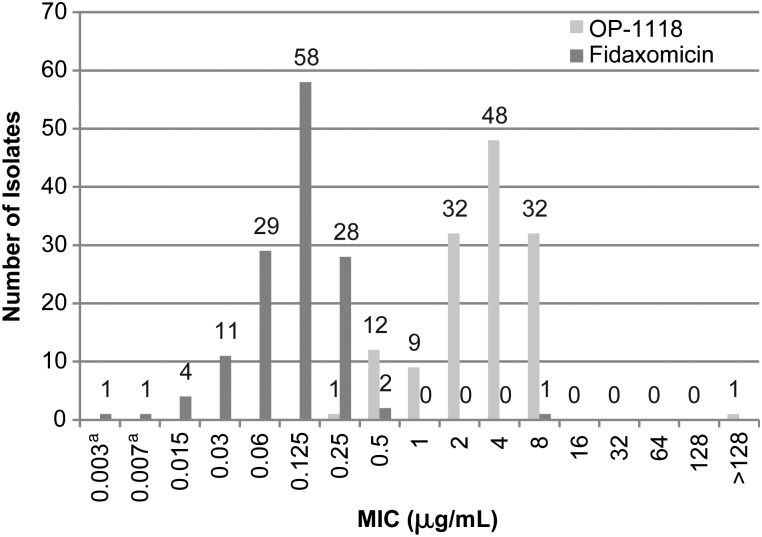
We now report previously unpublished data regarding the in vitro activity of fidaxomicin and OP-1118 against 135 clinical strains of C. difficile isolated from patients in the 004 study who were compared by using the CLSI agar dilution method in M11-A7 [11]. An inoculum of 105 colony-forming units/mL of C. difficile ATCC 700057 was included as a quality control strain. OP-1118 was dissolved and diluted in DMSO to achieve final study concentrations that ranged from 0.004 to 128 μg/mL. The MIC50 and MIC90 for OP-1118 were 4 and 8 μg/mL, compared with 0.125 and 0.25 μg/mL, respectively, for fidaxomicin (Figure 1).
Figure 1.
Minimum inhibitory concentration distribution of fidaxomicin and OPT-1118 against 135 Clostridium difficile clinical isolates from the FDX-004 [14]. aRefers to ≤0.004 μg/mL (for 0.003) and ≤0.008 μg/mL (for 0.007). Abbreviation: MIC, minimum inhibitory concentration.
Low Fecal-Binding Properties
The fecal-binding properties of fidaxomicin and OP-1118 were compared with those of vancomycin by testing their antibacterial activity in the presence or absence of 5% fecal material, using a microbroth dilution method. Similar to vancomycin, both fidaxomicin and OP-1118 demonstrated low fecal-binding properties, and their MICs against C. difficile increased only 4–8-fold in the presence of feces: the MIC of fidaxomicin increased from 0.25 to 2 μg/mL, and the MIC of OP-1118 increased from 1 to 4 μg/mL, with both increases much lower than the expected gut-level concentrations following oral administration of 400 mg/day of fidaxomicin [14].
Killing Kinetics
Fidaxomicin and its major metabolite, OP-1118, both demonstrate bactericidal activity against C. difficile strains, including the hypervirulent REA BI group strains. Exposure of C. difficile strains to fidaxomicin or OP-1118 at ≥4 times the MIC of each agent led to a ≥3 log decrease in colony-forming units in 48 hours, indicating time-dependent bactericidal activity [15]. Interestingly, fidaxomicin has been shown to be bactericidal against laboratory-generated mutant strains with reduced fidaxomicin susceptibility, indicating that with fecal concentrations that reach milligram-per-gram amounts, even mutant strains with increased fidaxomicin MICs are likely to be killed during therapy [15].
Susceptibility Breakpoints/Resistance
Results from fidaxomicin clinical trials have not demonstrated a correlation between MIC and clinical outcome [10, 16]. Although the MIC90 was shown to be 0.25 μg/mL in these trials, the highest reported MIC for wild-type isolates is 1 μg/mL. The only clinical isolate with reduced susceptibility was obtained from a subject with recurrence of disease 6 days following cure with fidaxomicin. The isolate at day 1 and the end of treatment had an MIC of 0.06 μg/mL, but the recurrence isolate demonstrated reduced susceptibility, with an MIC of 16 μg/mL, which is still less than gut-level concentrations of the drug (mean fidaxomicin and OP-1118 concentrations were reported as 1433 and 760 μg/g, respectively) [16]. The strain with reduced susceptibility has been analyzed further, and a single mutation in the β subunit of the RNA polymerase has been identified in only the isolate associated with recurrence (unpublished data). Similar mutations in the homologous positions in other bacterial species that demonstrate reduced susceptibility to lipiarmycin, a related macrocycle compound, have been reported [17, 18]. However, the functional significance of such mutations needs to be elucidated further because laboratory-generated isolates with similar mutations are rapidly killed by fidaxomicin at 4 times the MICs [15].
CONCLUSION
Fidaxomicin has excellent in vitro activity against C. difficile isolates of all REA types, including the epidemic BI strain. Resistance has not developed during therapy in clinical trials. Its lack of activity against enteric gram-negative flora should help maintain colonization resistance.
Notes
Acknowledgments. We thank Judee H. Knight and Alice E. Goldstein for various forms of assistance.
Supplement sponsorship. This article was published as part of a supplement entitled “Fidaxomicin and the Evolving Approach to the Treatment of Clostridium difficile Infection,” sponsored by Optimer Pharmaceuticals, Inc.
Potential conflicts of interest. E. J. C. G. serves on the advisory boards of Merck, Optimer, Bayer Pharmaceuticals, Theravance, BioK+, and Viropharma, Kindred Healthcare; is on the speakers bureau of Bayer, Merck, Sanofi Pasteur, and Forest Labs; and has received research grants from Merck, Schering-Plough Pharmaceuticals, Optimer Pharmaceuticals, Theravance, Cubist, Pfizer, Astellas, Cerexa, Impex Pharmaceuticals, Novexel, Novartis, Clinical Microbiology Institute, Genzyme, Nanopacific Holdings, Romark Laboratories, Viroxis, Warner Chilcott, Avidbiotics, GLSynthesis, Immunome, Toltec Pharma, and Salix Pharmaceuticals, GSK. F. B. is an employee of Optimer Pharmaceuticals. D. M. C. certifies no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Swanson RN, Hardy DJ, Shipkowitz NL, et al. In vitro and in vivo evaluation of tiacumicins B and C against Clostridium difficile. Antimicrob Agents Chemother. 1991;35:1108–11. doi: 10.1128/aac.35.6.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Theriault RJ, Karwowski JP, Jackson M, et al. Tiacumicins, a novel complex of 18-membered macrolide antibiotics. I. Taxonomy, fermentation and antibacterial activity. J Antibiot. 1987;40:567–74. doi: 10.7164/antibiotics.40.567. [DOI] [PubMed] [Google Scholar]
- 3.Babakhani FK, Robert N, Shangle S, et al. Antimicrobial activity and post-antibiotic effect of OPT-89, a new macrocyclic compound, against Clostridium difficile [abstract] Presented at: 44th Interscience Conference on Antimicrobial Agents and Chemotherapy, 30 October–2 November 2004, Washington, DC. [Google Scholar]
- 4.Ackermann G, Löffler B, Adler D, Rodloff AC. In vitro activity of OPT-80 against Clostridium difficile. Antimicrob Agents Chemother. 2004;48:2280–2. doi: 10.1128/AAC.48.6.2280-2282.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finegold SM, Molitoris D, Vaisanen ML, Song Y, Liu C, Bolaños M. In vitro activities of OPT-80 and comparator drugs against anaerobic bacteria. Antimicrob Agents Chemother. 2004;48:4898–902. doi: 10.1128/AAC.48.12.4898-4902.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hecht DW, Galang MA, Sambol SP, Osmolski JR, Johnson S, Gerding DN. In vitro activities of 15 antimicrobial agents against 110 toxigenic Clostridium difficile clinical isolates collected from 1983 to 2004. Antimicrob Agents Chemother. 2007;51:2716–9. doi: 10.1128/AAC.01623-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Credito KL, Applebaum PC. Activity of OPT-80, a novel macrocycle, compared with those of eight other agents against selected anaerobic species. Antimicrob Agents Chemother. 2004;48:4430–4. doi: 10.1128/AAC.48.11.4430-4434.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karlowsky JA, Laing NM, Zhanel GG. In vitro activity of OPT-80 tested against clinical isolates of toxin-producing Clostridium difficile. Antimicrob Agents Chemother. 2008;52:4163–5. doi: 10.1128/AAC.00476-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Citron DM, Babakhani F, Goldstein EJ, et al. Typing and susceptibility of bacterial isolates from the fidaxomicin (OPT-80) phase II study for C. difficile infection. Anaerobe. 2009;15:234–6. doi: 10.1016/j.anaerobe.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein EJ, Citron DM, Sears P, Babakhani F, Sambol SP, Gerding DN. Comparative susceptibilities of fidaxomicin (OPT-80) of isolates collected at baseline, recurrence, and failure from patients in two fidaxomicin phase III trials of C. difficile infection. Antimicrob Agents Chemother. 2011;55:5194–9. doi: 10.1128/AAC.00625-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. Methods for antimicrobial susceptibility testing of anaerobic bacteria. 7th ed. Wayne, PA: CLSI; 2007. CLSI document M11-A7. [PubMed] [Google Scholar]
- 12.Babakhani F, Seddon J, Robert N, Shue YK, Sears P. Effects of inoculum, pH, and cations on the in vitro activity of fidaxomicin (OPT-80, PAR-101) against Clostridium difficile. Antimicrob Agents Chemother. 2010;54:2674–6. doi: 10.1128/AAC.01842-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louie TJ, Emery J, Krulicki W, Bryne B, Mah M. OPT-80 eliminates Clostridium difficile and is sparing of Bacteroides species during treatment of C. difficile infection. Antimicrob Agents Chemother. 2009;53:261–3. doi: 10.1128/AAC.01443-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babakhani FK, Seddon J, Robert N, et al. Narrow spectrum activity and low fecal binding of OPT-80 and its major hydrolysis metabolite (OP-1118) [abstract E-2076] Presented at: 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, 17–20 September 2007, Chicago, Illinois. [Google Scholar]
- 15.Babakhani F, Gomez A, Robert N, Sears P. Killing kinetics of fidaxomicin and its major metabolite, OP-1118, against Clostridium difficile. J Med Microbiol. 2011;60:1213–7. doi: 10.1099/jmm.0.029470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louie T, Miller M, Donskey C, Mullane K, Goldstein EJ. Clinical outcomes, safety, and pharmacokinetics of OPT-80 in a phase 2 trial with patients with Clostridium difficile infection. Antimicrob Agents Chemother. 2009;53:223–8. doi: 10.1128/AAC.01442-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gultieri M, Tupin A, Brodolin K, Leonetti JP. Frequency and characterization of spontaneous lipiarmycin-resistant Enterococcus faecalis mutants selected in vitro. Int J Antimicrobial Agents. 2009;34:605–16. doi: 10.1016/j.ijantimicag.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Kurabachew M, Lu S, Krastel P, et al. Lipiarmycin targets RNA polymerase and has good activity against multidrug-resistant strains of Mycobacterium tuberculosis. J Antimicrob Chemother. 2008;62:713–9. doi: 10.1093/jac/dkn269. [DOI] [PubMed] [Google Scholar]