Abstract
Symptomatic recurrence of Clostridium difficile infection (CDI) occurs in approximately 20% of patients and is challenging to treat. Identifying those at high risk could allow targeted initial management and improve outcomes. Adult toxin enzyme immunoassay–positive CDI cases in a population of approximately 600 000 persons from September 2006 through December 2010 were combined with epidemiological/clinical data. The cumulative incidence of recurrence ≥14 days after the diagnosis and/or onset of first-ever CDI was estimated, treating death without recurrence as a competing risk, and predictors were identified from cause-specific proportional hazards regression models. A total of 1678 adults alive 14 days after their first CDI were included; median age was 77 years, and 1191 (78%) were inpatients. Of these, 363 (22%) experienced a recurrence ≥14 days after their first CDI, and 594 (35%) died without recurrence through March 2011. Recurrence risk was independently and significantly higher among patients admitted as emergencies, with previous gastrointestinal ward admission(s), last discharged 4–12 weeks before first diagnosis, and with CDI diagnosed at admission. Recurrence risk also increased with increasing age, previous total hours admitted, and C-reactive protein level at first CDI (all P < .05). The 4-month recurrence risk increased by approximately 5% (absolute) for every 1-point increase in a risk score based on these factors. Risk factors, including increasing age, initial disease severity, and hospital exposure, predict CDI recurrence and identify patients likely to benefit from enhanced initial CDI treatment.
Symptomatic recurrence of Clostridium difficile infection (CDI) causes significant morbidity and can prove challenging to treat effectively [1]. It also inevitably increases the risk of C. difficile transmission. Reported recurrence rates vary from 5% to 50% and typically are around 20% [2].
In a meta-analysis, recurrence risk factors included older age, use of C. difficile–provocative antibiotics after CDI diagnosis, and concomitant receipt of antacids [3]. Other factors identified in individual studies include hospital-acquired disease [4]; comorbid conditions, including severe underlying illness [5] or poor quality of life scores [6]; and previous recurrent CDI [7]. However, most existing studies are relatively small and hospital based and predate the polymerase chain reaction (PCR) ribotype 027/sequence type 1 (ST1)/North American pulsed-field gel electrophoresis type 1 (NAP-1) epidemic [3], demonstrating a need for contemporaneous large-scale population-based studies. CDI most commonly recurs within a week [8] after treatment cessation but can recur up to 6–8 weeks later [9]. However, because most studies have followed patients for only 1–2 months [3], the pattern of longer-term recurrence is unclear. Up to 50% of apparent relapses have been identified as new infections with a different strain [10–13]; uncertainty about which recurrences represent reinfection hinders the assessment of risk factors for true relapse [14].
Recent guidelines [15] outline strategies for treating recurrent CDI. However, if patients at high risk could be identified earlier, relapse might be prevented by using novel therapies [1, 16]. Therefore, the objectives of this long-term population-based study were to identify independent predictors of CDI recurrence in Oxfordshire during 2006–2010 and to confirm which predictors were related to relapses caused by the same strain by using genotyping.
METHODS
Oxford Radcliffe Hospitals (ORH) NHS Trust provides >90% of hospital care in Oxfordshire (population, approximately 600 000) and all acute services. The ORH Microbiology Laboratory tests all stool samples from the county, including those from other healthcare facilities and general practitioners (primary care). From September 2006, all unformed stools (taking the shape of the container) positive by enzyme immunoassay (EIA) for C. difficile toxins A and B (Meridian Bioscience, Cincinnati, Ohio) and with sufficient sample remaining were routinely cultured. Clostridium difficile isolates were genotyped by multilocus sequence typing (MLST) [17], typing morphologically distinct colonies separately. Infection control policy required samples from any admitted patient with diarrhea (locally defined as ≥3 unformed stools within 24 hours) to be tested for C. difficile and vancomycin treatment to be initiated empirically, continuing for 14 days if CDI is confirmed. From May 2007, all unformed stool samples from patients aged ≥65 years were routinely tested for C. difficile according to UK Department of Health policy (a mandatory test, typically not meeting the previous diarrhea definition).
Clostridium difficile MLST data were anonymously linked to information for ORH hospital admissions and discharges from April 1997 and mortality from the Infections in Oxfordshire Research Database (IORD) [18]. Admissions to other smaller hospitals in the area (including a specialist orthopedic, a psychiatric, and several community hospitals) were not included, although samples taken at these locations were identifiable. The IORD has Research Ethics Committee and UK National Information Governance Board approval.
We included the first CDI for each adult (aged ≥18 years) from September 2006 through December 2010 (when >99% of samples had been typed), if the patient had no previous CDI recorded since April 1997. Included cases therefore had ≥3 months of potential follow-up to the 1 April 2011 data cutoff. Neither symptom resolution nor antibiotic use is routinely recorded electronically and, therefore, was not available for analysis. Because (1) patients received 14 days of vancomycin treatment, (2) most symptoms resolve before 14 days in successfully treated patients, and (3) UK Department of Health guidance was not to retest patients with ongoing diarrhea (ie, without resolution) for 28 days, the primary outcome was time from first-ever CDI to the first new EIA-positive sample ≥14 days later. Analyses therefore were restricted to patients known to be alive 14 days after the first CDI. The cumulative incidence of recurrence over time was estimated using competing risks methods to account for death before recurrence. Flexible parametric models were used to estimate precisely how the unadjusted daily recurrence risk varied with time from first CDI [19]. Cause-specific proportional hazards (Cox) models were used to estimate effects of factors theoretically available at treatment initiation (demographic characteristics, sample characteristics, previous ORH hospital exposure, and previous healthcare-associated infections; details in Table 1) on recurrence risk. Independent predictors were identified using backward selection with the Akaike information criterion [20], including pairwise interactions and allowing nonlinear effects of continuous factors through fractional polynomials. Categorical effects are presented for factors with significant evidence of nonlinearity. Results from regression models for the cumulative incidence subhazard for recurrence [21] (rather than the cause-specific hazard) were similar (data not shown). In all analyses, patients not known to have died were censored at their last hospital or laboratory contact.
Table 1.
Characteristics of First Clostridium difficile Infection, September 2006–December 2010
| Levels (for Continuous Factors, Unit Increase Corresponding to the RR in the Regression Model) | No. (%) or Median (IQR) |
Unadjusted Univariable Cause-Specific Hazard Model |
||||
|---|---|---|---|---|---|---|
| Factor | Overall | With Recurrence | RR | 95% CI | Global P Value | |
| CDI, No. (%) | 1678 (100) | 393 (22) | ||||
| Demographics | ||||||
| Sex, No. (%) | Male | 711 (42) | 146 (21) | 1.00 | .36 | |
| Female | 967 (58) | 217 (22) | 1.10 | .90–1.36 | ||
| Age (y) | (/10-y older) | 77 (64–85) | 79 (71–86) | 1.23 | 1.14–1.32 | <.0001 |
| Previous hospital exposure | ||||||
| Ever previously admitted to ORH, No. (%)a | No | 230 (14) | 34 (15) | 1.00 | .11 | |
| Yes, <8-h admissions only | 108 (6) | 22 (20) | 1.13 | .74–1.75 | ||
| Yes, ≥1 admission for >8 h | 1340 (80) | 307 (23) | 1.45 | 1.02–2.07 | ||
| Last ORH admission, No. (%) | >1 y ago/never | 568 (34) | 103 (18) | 1.00 | .01 | |
| Within the last y | 1110 (66) | 260 (23) | 1.33 | 1.06–1.67 | ||
| Previous dialysis/ chemotherapy at ORH, No. (%) | No | 1486 (89) | 323 (22) | 1.00 | .97 | |
| Yes | 192 (11) | 40 (21) | 1.01 | .73–1.40 | ||
| Previously admitted to ORH GI ward, No. (%) | No | 1090 (65) | 241 (22) | 1.00 | .33 | |
| Yes | 588 (35) | 122 (21) | 0.90 | .72–1.12 | ||
| No. of previous admissions >8 h | (/5 additional >8-h admissions) | 2 (1–5) | 3 (1–5) | 1.18b | 1.03–1.36 | .02 |
| Total previous h in hospital (ORH) in admissions >8 h (h) | (/doubling of total previous h in hospital) | 366 (44–909) | 528 (122–1180) | 1.16b | 1.09–1.24 | <.0001 |
| Days since last discharged | (/additional 6 mo since last ORH discharge) | 96 (23–870) | 77 (22–472) | 0.96 | .90–1.01 | .14 |
| Discharged in last 7 d, No. (%) | No | 1567 (93) | 331 (21) | 1.00 | .07 | |
| Yes | 111 (7) | 32 (29) | 1.41 | .98–2.02 | ||
| Likely source of first infection (IDSA/SHEA), No. (%) [15] | Hospital onset, healthcare-associated | 880 (52) | 185 (21) | 1.00 | <.001 | |
| Community onset, healthcare-associated | 334 (20) | 80 (24) | 1.07 | .82–1.39 | ||
| Indeterminate | 127 (8) | 45 (35) | 1.77 | 1.27–2.45 | ||
| Community-associated | 337 (20) | 53 (16) | 0.64 | .47–.86 | ||
| Previous MRSA, No. (%) | No | 1503 (90) | 312 (21) | 1.00 | .01 | |
| Yes | 175 (10) | 51 (29) | 1.45c | 1.08–1.95 | ||
| Sample characteristics | ||||||
| Season, No. (%) | Winter (Dec/Jan/Feb) | 424 (25) | 81 (19) | 1.00 | .35 | |
| Spring (Mar/Apr/May) | 388 (23) | 86 (22) | 1.18 | .87–1.60 | ||
| Summer (Jun/Jul/Aug) | 431 (26) | 98 (23) | 1.31 | .97–1.76 | ||
| Autumn (Sep/Oct/Nov) | 435 (26) | 98 (23) | 1.20 | .89–1.61 | ||
| Calendar year | (/additional y) | 2008 (2007–2009) | 2008 (2007–2009) | 0.93 | .85–1.02 | .11 |
| Mandatory EIA test (mild diarrhea), No. (%) | No (requested by clinician) | 1404 (84) | 316 (23) | 1.00 | .01 | |
| Yes (not requested) | 274 (16) | 47 (17) | 0.68 | .50–.92 | ||
| Previous negative EIA test | Ever | 714 (43) | 187 (26) | 1.49 | 1.21–1.83 | |
| Never | 964 (57) | 176 (18) | 1.00 | <.0001 | ||
| Previous negative EIA test | In last 14 d | 452 (27) | 261 (21) | 1.11 | .88–1.40 | |
| Not in last 14 d | 1226 (73) | 102 (23) | 1.00 | .37 | ||
| Location where first sample taken | Inpatient (overnight) | 1191 (71) | 260 (21) | 1.00 | .58 | |
| Primary care | 294 (18) | 63 (22) | 0.83 | .63–1.09 | ||
| Outpatient/ED/day case | 87 (5) | 20 (23) | 0.91 | .58–1.44 | ||
| Other hospital | 106 (6) | 20 (19) | 0.88 | .56–1.39 | ||
| If inpatient at first CDI, admission specialty | Surgical | 376 (32) | 65 (17) | 1.00 | .03 | |
| Medical | 815 (68) | 195 (24) | 1.37 | 1.11–1.68 | ||
| If inpatient at first CDI, method of admission | Elective | 237 (20) | 34 (14) | 1.00 | <.0001 | |
| Emergency | 954 (80) | 226 (24) | 1.58 | 1.10–2.27 | ||
| If inpatient at first CDI, d since admitted | Within 2 d of admission | 311 (26) | 75 (24) | 1.00 | .50 | |
| >2 d after admission | 880 (74) | 185 (21) | 1.10 | .84–1.43 | ||
| If inpatient at first CDI, d since admitted | Nonlinear effectd | 8 (2–20) | 10 (2–22) | |||
| On d of admission | 128 (11) | 35 (27) | 1.51d | .99–2.30 | ||
| 1–5 d after admission | 351 (29) | 67 (19) | 1.03d | .72–1.47 | ||
| 6–14 d after admission | 311 (26) | 56 (18) | 1.00 | .03 | ||
| ≥15 d after admission | 401 (34) | 102 (25) | 1.46d | 1.06–2.03 | ||
| Biomarkerse | ||||||
| C-reactive protein (mg/L) | (/120 mg/L)f | 84 (33–156) | 97 (44–>160) | 1.69 | 1.31–2.18 | <.0001 |
| White blood cell count (×109/L) | (/10 × 109/L)f | 11.2 (7.8–16.1) | 12.2 (7.9–18.3) | 1.34 | 1.15–1.36 | <.0001 |
| Neutrophils (×109/L) | (/10 × 109/L)f | 8.9 (5.6–13.4) | 9.7 (5.7–15.4) | 1.42 | 1.20–1.69 | <.0001 |
| Lymphocytes (×109/L) | (/10 × 109/L)f | 11.0 (7.0–16.0) | 10.0 (7.0–15.0) | 0.84 | .73–.97 | .02 |
| Albumin (g/L) | (/10 g/L) | 34 (30–38) | 34 (30–37) | 0.82 | .68–1.00 | .05 |
| Urea (mmol/L) | (/5 mmol/L)f | 6.7 (4.5–11.0) | 7.3 (5.1–12.1) | 1.09 | 1.01–1.17 | .02 |
Abbreviations: CDI, Clostridium difficile infection; CI, confidence interval; ED, emergency department; EIA, enzyme immunoassay; GI, gastrointestinal; IDSA, Infectious Diseases Society of America; IQR, interquartile range; MRSA, methicillin-resistant Staphylococcus aureus; ORH, Oxford Radcliffe Hospitals; RR, relative risk; SHEA, Society for Healthcare Epidemiology of America.
a Excluding current admission if inpatient.
b Univariable model also adjusts for ever vs never previously admitted for >8 h.
c Approximate IQR.
d Significant nonlinearity (P < .0001; univariable model also adjusts for current inpatient), with greatest risk for death on day of admission, then decreasing sharply and then gradually increasing. Results presented for diagnoses made on day of admission, then approximate tertiles of days from admission to first diagnosis in inpatients.
e Imputed in the subset of 1295 (77%) patients with at least 1 of the 14 potential biomarkers available (see Methods for details). Reference ranges: C-reactive protein, 0–8 mg/L; white blood cell count, 4 – 11 × 109/L (no reference range for neutrophils or lymphocytes, which are measured as a percentage of white blood cell count); albumin, 35 – 50 g/L; urea, 2.5 – 6.7 mmol/L. None of the biomarkers not shown had an effect in univariable models (P > .1).
f Significant interaction between previous MRSA and emergency/elective admission (Table 2), such that previous MRSA increases CDI recurrence risk for emergency admissions, but decreases CDI recurrence risk for elective admissions (such patients received vancomycin and gentamicin perioperatively in place of broad-spectrum penicillin-based prophylaxis).
Baseline biomarker values were defined as the closest measurement within (−3, +1) days of the stool sample. Fourteen biomarkers available for >50% of CDI cases were considered (white blood cell count, neutrophil count, lymphocyte count, C-reactive protein level, hemoglobin level, platelet count, and sodium, potassium, creatinine, urea, albumin, alanine aminotransferase, alkaline phosphatase, and bilirubin levels). To avoid bias and/or loss of power from restricting to complete cases with all biomarkers measured, associations between biomarkers and recurrence were estimated, imputing missing values in the subset of patients with at least 1 observed baseline biomarker. As recommended, chained estimating equations [22, 23] were used to create 20 imputations on BoxCox transformed variables [24], including all cofactors listed in Table 1, allowing nonlinearity in all continuous factors with natural cubic splines (knots at 10th, 50th, and 90th percentiles [25]) and including log(recurrence time) and the censoring indicator. Standard errors were estimated across imputations using Rubin's rules.
Stata software, version 11.2 (StataCorp, College Station, Texas), was used for all analyses, which were conducted by one of the authors (A. S. W.).
RESULTS
From September 2006 through December 2010, a total of 2043 adults had their first CDI (toxin EIA-positive stool sample with no previous positive). A total of 271 (13%) patients died within 0–13 days after the EIA-positive sample, and 94 (5%) had no follow-up after 13 days, leaving 1678 (82%) alive 14 days after the first CDI for analysis of recurrence.
Recurrence Rates
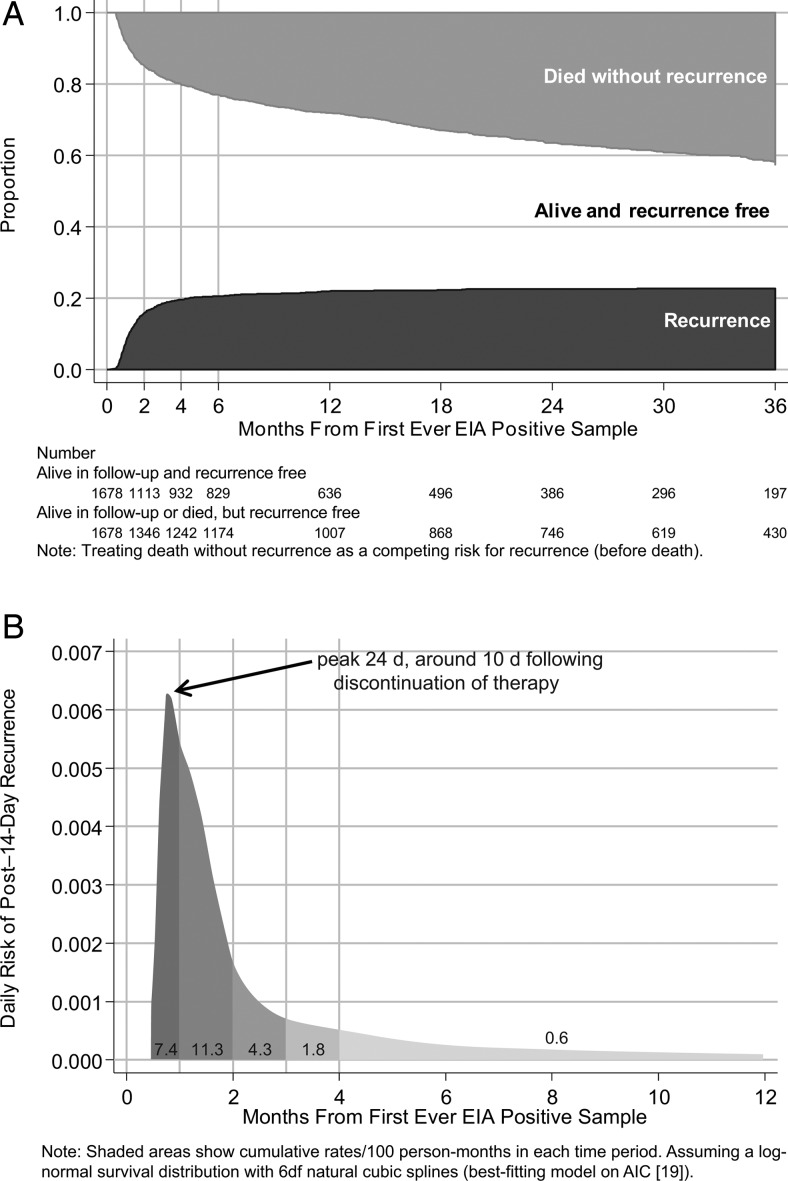
Overall, 363 of 1678 (22%) patients experienced a recurrence (new EIA-positive sample) ≥14 days after their first CDI, and 594 (35%) died without recurrence through March 2011. Median follow-up in those without recurrence was 11 months (interquartile range [IQR], 2–28 months). Cumulative incidences of recurrence increased to 7% and 16% by 1 and 2 months later, respectively (Figure 1A), corresponding to rates of 7.4 cases per 100 person-months at risk and 11.3 cases per 100 person-months at risk, respectively. Thereafter, the incidence increased more slowly to 18% and 20% by 3 and 4 months and to 22% and 23% at 1 and 2 years, respectively. The risk for recurrence was greatest 24 days after the first CDI, 10 days after cessation of treatment (Figure 1B). Of the 363 recurrences, 94 patients (26%) experienced a third CDI episode (≥14 days after the recurrence), of whom 24 had further episodes (maximum, 6) through March 2011.
Figure 1.
Time to recurrence. A, Months to new enzyme immunoassay (EIA)–positive sample or death ≥14 days after first-ever EIA-positive sample. B, Daily risk of post–14-day new EIA-positive sample. Abbreviations: AIC, Akaike information criterion; EIA, enzyme immunoassay.
Risk Factors for Recurrence
As expected, the population was predominantly older (median age, 77 years), although 302 (18%) were <60 years of age at first CDI (Table 1). A total of 1191 (71%) were inpatients (admitted a median of 8 [IQR, 2–20] days), and most (1448; 86%) had previously been admitted to ORH facilities (66% in the past year). In a multivariable model (Table 2), recurrence risk independently was significantly higher among patients admitted as emergencies, those with previous gastroenterology ward admission(s), and after diagnosis on day of admission or ≥15 days after admission and also increased with age and increasing previous total hours in the hospital (all P < .05). Risks also were higher among those for whom the last inpatient stay was 4–12 weeks before diagnosis (P = .006). Recurrence risk was independently lower among those with CDI diagnosed using a test that had not been clinically requested (a mandatory test, often for mild diarrhea; P = .07). Patients without previous hospital admissions generally had fewer other risk factors, but after adjusting for these, they had higher recurrence risks. One potential explanation, not assessable in our data, would be if such patients were more likely to be tertiary referrals with other non-ORH hospital exposure. Other factors (in particular, medical specialty, previous EIA-negative test result, number of previous admissions, and discharged in the previous 7 days) were significant in only univariable and not multivariable models (ie, were confounded with other factors).
Table 2.
Predictors of Recurrence ≥14 Days After First Clostridium difficile Infection
| All Recurrences Multivariable Cause-Specific Hazard Modela |
Shared STs Multivariable Cause-Specific Hazard Modela |
||||||
|---|---|---|---|---|---|---|---|
| Factor | Levels (Effect in Regression Model) | RR | 95% CI | Global P Value | RR | 95% CI | Global P Value |
| No. of CDI recurrences | 363 | 169 | |||||
| Age (y) | (/10-y older) | 1.16 1.57 (if previous dialysis/chemotherapy [interaction P = .02]) |
1.07 – 1.26 1.23 – 2.00 |
.0004 .0003 |
1.29 1.71 (if previous dialysis/chemotherapy) |
1.13 – 1.48 1.19 – 2.47 |
.0001 .004 |
| Total previous h in hospital (ORH) in admissions >8 h (h) | (/doubling of total previous h in hospital) | 1.29 1.12 (if emergency admission [interaction P = .002]) 1.15 (if previous GI admission [interaction P = .007]) |
1.17 – 1.42 1.03 – 1.22 1.04 – 1.28 |
<.0001 .008 .008 |
1.38 1.15 (if emergency admission) 1.24 (if previous GI admission) |
1.19 – 1.60 1.01 – 1.30 1.05 – 1.46 |
<.0001 .03 .009 |
| Ever previously admitted to ORH | No or <8-h admissions (vs yes for >8-h admission) | 1.99 | 1.20 – 3.31 | .008 | 2.57 | 1.18 – 5.62 | .02 |
| Mandatory EIA test (mild diarrhea) | Yes (vs no) | 0.74 | .53 – 1.03 | .07 | 0.67 | .41 – 1.09 | .11 |
| If inpatient at first CDI, admission method | Elective emergency | 1.00 2.79 5.10 (if previous MRSA [interaction P = .0003]) 4.74 (if previous dialysis/chemotherapy [interaction P = .04]) |
1.29 – 6.04 2.12 – 12.2 1.96 – 11.4 |
.006 .0003 .0005 |
1.00 4.85 9.52 8.79 (if previous dialysis/chemotherapy) |
1.42 – 16.6 2.42 – 37.5 2.23 – 34.6 |
.01 .001 .002 |
| Previous MRSA | Yes (vs no) | 0.45 (if not emergency) | .23 – .88 | .02 | 0.70 (if not emergency) | .31 – 1.59 | .39 |
| Previous dialysis/chemotherapy at ORH | Yes (vs no) | 0.77a (if not emergency) | .39 – 1.52 | .45 | 1.15a (if not emergency) | .46 – 2.82 | .77 |
| Previously admitted to ORH GI ward | Yes (vs no) | 2.33 | 1.13 – 4.78 | .02 | 2.10 | .71 – 6.23 | .18 |
| If inpatient at first CDI, d since admitted | On d of admission | 1.73b | .52 – 5.73 | 1.15 | .21 – 6.31 | ||
| 1 – 5 d after admission | 0.50b | .21 – 1.19 | 0.54 | .18 – 1.65 | |||
| 6 – 14 d after admission | 1.00 | .006 | 1.00 | .38 | |||
| ≥15 d after admission | 1.84b | 1.05 – 3.23 | 1.37 | .62 – 3.04 | |||
| If inpatient at first CDI, admission specialty | Medical (vs surgical) | 0.98b | .34 – 2.79 | .97 | 0.90 | .80 – 1.01 | .08 |
| Location where first CDI sample taken | Not inpatient (vs inpatient overnight) | 0.58c | .20 – 1.73 | .33 | 0.50 | .11 – 2.39 | .39 |
| Likely source of first infection (IDSA/SHEA) [15] | Hospital onset, healthcare-associated | 1.00 | .006 | 1.00 | .07 | ||
| Community onset, healthcare-associated | 1.19 | .71 – 2.00 | 1.71 | .77 – 3.79 | |||
| Indeterminate | 2.03 | 1.16 – 3.57 | 2.89 | 1.24 – 6.76 | |||
| Community-associated | 1.03 | .60 – 1.78 | 1.72 | .76 – 3.89 | |||
All other factors in Table 1 had no additional effect on CDI recurrence (P > .2). Results were similar when excluding 418 cases whose first Clostridium difficile infection was EIA positive and culture negative.
Abbreviations: CDI, Clostridium difficile infection; CI, confidence interval; EIA, enzyme immunoassay; GI, gastrointestinal; IDSA, Infectious Diseases Society of America; MRSA, methicillin-resistant Staphylococcus aureus; ORH, Oxford Radcliffe Hospitals; RR, relative risk; SHEA, Society for Healthcare Epidemiology of America; ST, sequence type.
a Effect of previous dialysis/chemotherapy estimated for a person aged 77 years; significant interaction with age (P = .03).
b RR presented for admissions to nonmedical specialties: less variation in risk for patients admitted to medical specialties (mostly also emergency admissions), with RRs of 1.21 (95% CI, .60 – 2.41), 0.96 (95% CI, .58 – 1.61), 1.00, and 1.10 (95% CI, .73 – 1.66) for first CDI diagnoses 0, 1 – 5, 6 – 14, and ≥15 days after admission, respectively (interaction P = .05).
c First CDI during inpatient admission (yes vs no) included in final model to allow those not admitted to differ from those with first CDI on day of admission.
In this multivariable model, 5 major effect modifications (interactions) were identified (Table 2): (1) increased risk at older ages was augmented in those with previous dialysis/chemotherapy, (2) increased risks associated with emergency admissions were augmented in those with previous methicillin-resistant Staphylococcus aureus (MRSA) infection or dialysis/chemotherapy, (3) recurrence risk increased less strongly with increasing previous total hours in the hospital in those admitted as emergencies and/or with previous gastroenterology ward admissions, (4) risk was significantly lower in those with previous MRSA infection and CDI in an elective admission (mostly surgical specialties; such patients received vancomycin and gentamicin perioperatively in place of broad-spectrum penicillin-based prophylaxis), and (5) recurrence risks varied less over days from admission to the first CDI diagnosis in medical than in nonmedical inpatients. No other factor listed in Table 1 had an additional effect in this multivariable model (P > .2). There also was no evidence of a trend over calendar year (P = .37) or season (P = .24).
The multivariable model in Table 2 included factors theoretically available to clinicians at treatment initiation. Of importance, after adjusting for these, higher EIA optical density of the first C. difficile test was associated with a higher risk for recurrence, comparing across absolute values (relative risk [RR], 1.20 per unit higher [95% confidence interval {CI}, 1.06–1.35]; P = .003; median, 1.6 [IQR, 0.5–>2.5]) or comparing the 37% with optical density exceeding the assay maximum (2.5) with other cases (RR, 1.33; 95% CI, 1.08–1.65; P = .008).
Biomarkers
One or more of the 14 potential biomarkers was available in 1295 patients (77%), imputing missing data in this subgroup. Only higher C-reactive protein level and higher neutrophil count at first CDI independently increased recurrence risk, but the effect was much larger for C-reactive protein level (RR, 1.45 per 120 mg/L [the IQR] higher [95% CI, 1.09–1.93]; P = .01) than for neutrophil count (RR, 1.19 per 10 × 109/L higher [95% CI, .97–1.45]; P = .09). Effects of other factors listed in Table 2 were similar after adjusting for these baseline biomarkers.
Strain-Specific Recurrence Risks
MLST was obtained for 1076 of 1678 (64%) first CDI cases: 418 (25%) samples did not yield C. difficile on culture (EIA-positive culture-negative samples), and 184 (11%) were not available for culture (28% pre– vs 4% post–September 2007 when staff cover increased). A total of 685 (64%) typed isolates belonged to phylogenetic clade 1, from 56 STs, including those corresponding to PCR ribotypes 001, 002, 005, 014, 015, 020, 072, and 106 [26]. A total of 300 typed isolates (28%) belonged to clade 2 (295 of 300 PCR ribotype 027/ST1), and the remaining 91 (8%) belonged to clades 3, 4, and 5 (Supplementary Table 1). The crude percentages with recurrence were slightly higher after initial clade 2 CDI (90 of 300; 30%), compared with clade 1 (174 of 685; 25%; χ2 P = .13). After adjusting for risk factors listed in Table 2, recurrence risk appeared to be slightly higher after clade 2, compared with 1 CDI (RR, 1.17; 95% CI, .90–1.51; P = .24), but was compatible with chance. Recurrence risk was significantly lower for EIA-positive culture-negative samples (RR [vs clade 1], 0.41 [95% CI, .29–.58]; P < .0001), supporting previous observations that this latter group likely represents false EIA-positive results [27, 28].
Relapse Versus Reinfection
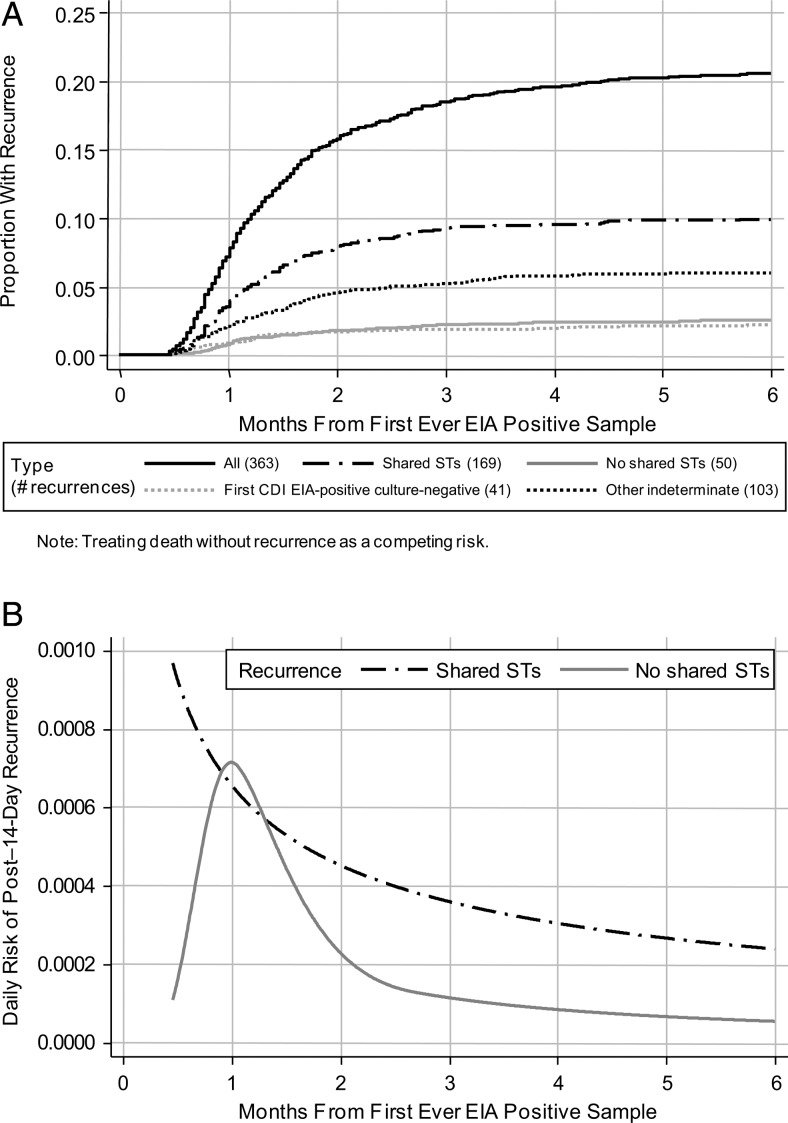
Of the 363 recurrences after the first CDI, 219 (60%) had STs determined from both episodes. Making comparisons on the basis of individual STs, not clade, 169 (77%) recurrences had the same ST as the initial episode and 50 (23%) had no STs in common (ie, were new infections). The risk for same-ST recurrence was highest 14 days after first EIA-positive sample (Figure 2), then declined slowly during the next 2–6 months. In contrast, the risk for new ST infections peaked 30 days after the first CDI and then decreased sharply; recurrence risk among the 41 patients (11%) with EIA-positive culture-negative first CDI followed a similar pattern. For the other 103 recurrences (28%; STs not determined for ≥1 isolate), risk was a mixture distribution (data not shown).
Figure 2.
Time to recurrence ≥14 days after first Clostridium difficile infection according to shared or not shared sequence types. A, Months to new enzyme immunoassay (EIA)–positive sample ≥14 days after first-ever EIA-positive sample. B, Daily risk of new post–14-day EIA-positive sample. Abbreviations: CID, Clostridium difficile infection; EIA, enzyme immunoassay; ST, sequence type.
Predictors of the 169 recurrences with shared STs were very similar to predictors of all 363 recurrences (Table 2); with relatively small numbers, power was too low to conduct separate model selection. As expected, several factors appeared to have weaker effects on the 50 recurrences with no shared STs (presumed reinfections; data not shown).
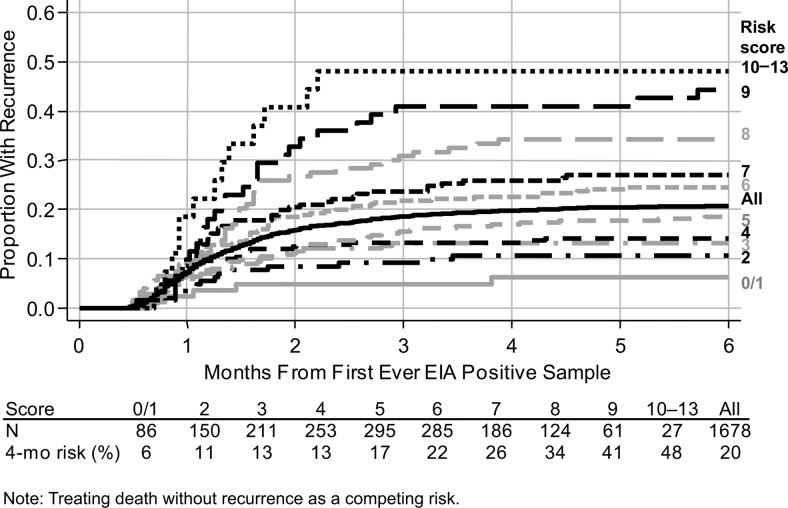
Risk Score for Recurrence Following First CDI
Because predictors of all CDI recurrences were similar to those for shared ST recurrences, we constructed an integer risk score (Table 3) based on the multivariable model for all recurrences (Table 2). The maximum possible score is 15 and minimum is −2; values of 0–13 were observed in our data set, with a median of 5 (IQR, 3–6). Models including the single risk score as a predictor provided an overall fit similar to the full Table 2 model. Four-month recurrence risk was 6%–48% across the score (Figure 3); the absolute risk of recurrence 4 months after the first CDI increased by approximately 5% for every 1-point increase in score.
Table 3.
Score to Predict Clostridium difficile Infection (CDI) Recurrence Following First-Ever CDI Diagnosis
| Factor | Scoring Criteria |
Max | Min | |||
|---|---|---|---|---|---|---|
| Patient & health status | Age (y) | 60–69 | 70–79 | ≥80 | ||
| Score | 1 | 2 | 3 | 3 | 0 | |
| Emergency admission | Any emergency admission | 1 | ||||
| AND previous MRSA+ | 1 | |||||
| AND/OR previous dialysis/chemotherapy | 1 | 3 | 0 | |||
| Severity of initial disease | Stool frequency | ≥3 unformed stools/da | 1 | |||
| Admission with CDI | Sample taken on day of inpatient admission | 1 | ||||
| C-reactive proteinb (mg/L) | <35 | 85–<160 | ≥160 | |||
| Score | −1 | 1 | 2 | 4 | −1 | |
| Past health care exposure | Type of past admission | Past gastroenterology admission | No past gastroenterology admission | |||
| Total inpatient duration before admission |
Any past admission | >2–13 wk | >13 wk | |||
| Score | 1 | 2 | 3 | 3 | 0 | |
| Antibiotic selection | (Elective admission OR community sample) AND previous MRSA isolatedc | −1 | 0 | −1 | ||
| Susceptibility to diarrhea several wk after hospital exposure | Primary CDI 4–12 wk after hospital discharged | Community sample or sample taken within ≤2 d of inpatient admission AND patient discharged from hospital 4–12 wk previously | 2 | 2 | 0 | |
| Total | 15 | −2 | ||||
Abbreviations: CDI, Clostridium difficile infection; Max, maximum; Min, minimum; MRSA, methicillin-resistant Staphylococcus aureus; MRSA+, MRSA positive.
a “Mandatory” tests in our study taken to represent mild diarrhea with <3 unformed stools per day.
b Score 0 if C-reactive protein not measured or not available at first CDI.
c Proxy for receiving vancomycin ± gentamicin rather than co-amoxiclav or other provocative antibiotics for surgical prophylaxis or other treatment.
d Infectious Diseases Society of America/Society for Healthcare Epidemiology of America indeterminate [15].
Figure 3.
Time to recurrence ≥14 days after first Clostridium difficile infection according to risk score. Abbreviation: EIA, enzyme immunoassay.
DISCUSSION
In our cohort of nearly 1700 patients surviving 2 weeks after their first CDI, 22% developed a symptomatic CDI recurrence during the 4-year study period. This is similar to commonly cited recurrence rates of approximately 20% [2] despite our considerably longer follow-up and robust design accounting for mortality. Most recurrences occurred within 2 months, but 4% of patients had recurrences at 2–4 months. The decrease in recurrence over time may reflect the eventual success of multiple treatment cycles [1, 9] or recovery from underlying illness with consequent lower exposure to antibiotics and restoration of more normal bowel flora able to suppress C. difficile growth in the colon [29]. Structural changes in bowel flora with age may impair such recovery [30]. Alternatively, because recurrence is associated with inadequate immune responses [31, 32], repeated relapse eventually may generate effective immunity, for example, to all phase variants of the cell surface–expressed proteins [33].
Two possible explanations exist for symptomatic recurrence: relapse of the same infection and reinfection. These can be distinguished, at least partially, by using strain typing data. In our study, 23% of recurrences were reinfection with a different strain, similar to the 26% reported in [14], but lower than the approximately 50% found by others [10–13]. Relapse may be more common in our cohort because of older age, compared with some other studies [11, 13]. Implementation of rigorous infection control measures [34] may also have reduced the incidence of reinfection. Of interest, reinfection with a different ST occurred most frequently 1 month after the first CDI (2–3 weeks after treatment cessation), possibly representing reexposure to C. difficile after clearance of the initial infection, whereas risks for new CDI with the same ST were greatest 14 days after the first CDI. Similar trends have been seen in other smaller studies [10, 11]. The relatively high prevalence of ST1/027/NAP-1, in 18% of cases, illustrates the difficulty distinguishing reinfection and relapse using genotyping schemes, such as MLST or ribotyping, with limited discriminatory power; the number of reinfections thus may be underestimated [15]. In the future, whole-genome sequencing may distinguish same-ST relapses from reinfections with different variants of common STs. Such distinctions are complicated further by the possibility of mixed initial or subsequent CDI; this was rarely identified in our cohort [35].
Our proposed score (Table 3) provides a summary of important risk factors for recurrence that would be present in electronic patient record systems. Several markers of underlying health status were either risk factors themselves or augmented the effect of other risk factors in our large unselected contemporaneous population, including increasing age, emergency admission, previous MRSA infection or colonization, and previous dialysis or chemotherapy. An initial CDI severe enough to be the probable cause of admission, to merit testing on the basis of clinical suspicion, or to increase levels of inflammatory markers, in particular, C-reactive protein, increased recurrence risk. We also found smaller risk increases with higher EIA optical density of the first CDI, which may reflect initial bacterial burden and/or toxin production [36]. Increasing past hospital exposure or gastrointestinal ward admission before the first CDI was also associated with recurrence. The increased recurrence risk after gastrointestinal admission requires confirmation in additional studies but may reflect increased CDI risk associated with inflammatory bowel disease [37, 38], nasogastric tube placement, possibly gastrointestinal endoscopy [39], and gastrointestinal surgery [40]. The apparent protective effect against CDI recurrence of previous MRSA infection or colonization on elective admissions is likely to reflect differences in the perioperative (and other) antibiotics received by this group, in particular, the replacement of broad-spectrum β-lactam antibiotics with glycopeptides and aminoglycosides. Conversely, previous MRSA was associated with higher recurrence risk in emergency admissions, possibly reflecting greater comorbidity in this group of patients who were also likely to receive broad-spectrum β-lactams in addition to glycopeptides. Initial CDI occurring 4–12 weeks after hospital discharge was also independently associated with recurrence. The delay in onset of the initial CDI in such cases may reflect prolonged susceptibility to the effects of hospital exposure (eg, mediated through failure of the immune system or change in bowel flora that also subsequently increases recurrence risk). Previously proposed scores for recurrence risk have been developed on much smaller hospital populations and have incorporated clinician opinion [5]. Nevertheless, further work to validate the score proposed here is essential. Of note, our risk score was constructed to capitalize on the types of data available in electronic patient records; we envisage that it could, for example, be incorporated into routine reports of positive CDI test results (if validated). This goal contrasts with other approaches to risk scoring for recurrence [5] and severity [41], which have been designed for use by clinicians at the bedside and often include assessments, such as temperature and endoscopy results not available in our study.
We did not find statistical evidence to support higher recurrence risk in clade 2 strains (almost exclusively ST1/027/NAP1), although we had low power (<40%) to detect a significant difference in recurrence rates of 25% and 30% (as observed). More than 3 times as many cases would be needed to detect such a difference with 80% power, although it might still be clinically important, because every symptomatic recurrence reflects an additional opportunity for C. difficile transmission. However, it also is clear that CDI virulence remains poorly understood, with the variable virulence of PCR ribotype 027 strains demonstrated in vivo [42] and in vitro [43, 44], suggesting that factors at levels other than strain type may play a role.
Study limitations include the lack of prescribing data, meaning that we could not directly assess the potentially important roles of antibiotics and proton pump inhibitors [3, 45]. The limited sensitivity/specificity of EIA testing [28] also has implications for assessing recurrence; the reduced recurrence rate after EIA-positive culture-negative initial test results (Figure 2) supports that these do not represent true CDI. Several factors included in our risk score may not translate to other healthcare systems and practices, particularly diarrhea severity. Investigating whether they are specific to our hospitals or reflect important underlying risk determinants is essential. Finally, the underlying electronic data sources do not capture resolution or onset of symptoms, and therefore, our assessment of recurrence is based on time since the initial diagnosis and samples that were actually submitted. A proportion of our recurrences therefore may be persistent diarrhea. However, we are unlikely to have missed new symptomatic episodes because fecal sampling was common (500–1400 EIAs/month; median, 960) with a low clinical threshold to investigate patients with diarrhea; only 7% of samples were EIA positive. This limitation also means that we are unable to distinguish between effects of previously resolved and ongoing episodes of MRSA infection/colonization or dialysis/chemotherapy in risk models.
In summary, recurrence after CDI is an important problem. Early recurrence frequently represents relapse of the same infection. Risk factors, including increasing age, severity of initial disease, and hospital exposure, can predict recurrence, in particular, relapse of the same infection, and may identify patients who would benefit from enhanced treatment of an initial CDI episode. Late recurrences highlight the ongoing need for interventions to prevent C. difficile transmission.
Supplementary Data
Notes
Acknowledgments. We thank all the people of Oxfordshire who contribute to the Infections in Oxfordshire Research Database. Research Database Team: P. Bejon, T. Berendt, C. Bunch, D. W. Crook, J. Finney, J. Gearing (community), H. Jones, L. O'Connor, T. E. A. Peto (principal investigator), J. Robinson (community), B. Shine, A. S. Walker, D. Waller, and D. Wyllie.
Financial support. This work was supported by the Oxford National Institute for Health and Research, NIHR Biomedical Research Centre, United Kingdom.
Supplement sponsorship. This article was published as part of a supplement entitled “Fidaxomicin and the Evolving Approach to the Treatment of Clostridium difficile Infection,” sponsored by Optimer Pharmaceuticals, Inc.
Potential conflicts of interest. The institution of D. W. C. and T. E. A. P. received per-case funding from Optimer Pharmaceuticals to support fidaxomicin trial patient expenses. D. W. C. and T. E. A. P. also received honoraria from Optimer Pharmaceuticals for participation in additional meetings related to investigative planning for fidaxomicin. M. H. W. has received honoraria for consultancy work, financial support to attend meetings, and research funding from bioMérieux, Optimer, Novacta, Pfizer, Summit, The Medicines Company, and Viropharma. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Johnson S. Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect. 2009;58:403–10. doi: 10.1016/j.jinf.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Aslam S, Hamill RJ, Musher DM. Treatment of Clostridium difficile-associated disease: old therapies and new strategies. Lancet Infect Dis. 2005;5:549–57. doi: 10.1016/S1473-3099(05)70215-2. [DOI] [PubMed] [Google Scholar]
- 3.Garey KW, Sethi S, Yadav Y, DuPont HL. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect. 2008;70:298–304. doi: 10.1016/j.jhin.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591–7. doi: 10.1086/430315. [DOI] [PubMed] [Google Scholar]
- 5.Hu MY, Katchar K, Kyne L, et al. Prospective derivation and validation of a clinical prediction rule for recurrent Clostridium difficile infection. Gastroenterology. 2009;136:1206–14. doi: 10.1053/j.gastro.2008.12.038. [DOI] [PubMed] [Google Scholar]
- 6.McFarland LV, Surawicz CM, Rubin M, Fekety R, Elmer GW, Greenberg RN. Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect Control Hosp Epidemiol. 1999;20:43–50. doi: 10.1086/501553. [DOI] [PubMed] [Google Scholar]
- 7.McFarland LV, Surawicz CM, Greenberg RN, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA. 1994;271:1913–8. [PubMed] [Google Scholar]
- 8.Olson MM, Shanholtzer CJ, Lee JT, Jr, Gerding DN. Ten years of prospective Clostridium difficile-associated disease surveillance and treatment at the Minneapolis VA Medical Center, 1982–1991. Infect Control Hosp Epidemiol. 1994;15:371–81. doi: 10.1086/646934. [DOI] [PubMed] [Google Scholar]
- 9.Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med. 2006;145:758–64. doi: 10.7326/0003-4819-145-10-200611210-00008. [DOI] [PubMed] [Google Scholar]
- 10.Johnson S, Adelmann A, Clabots CR, Peterson LR, Gerding DN. Recurrences of Clostridium difficile diarrhea not caused by the original infecting organism. J Infect Dis. 1989;159:340–3. doi: 10.1093/infdis/159.2.340. [DOI] [PubMed] [Google Scholar]
- 11.Barbut F, Richard A, Hamadi K, Chomette V, Burghoffer B, Petit JC. Epidemiology of recurrences or reinfections of Clostridium difficile-associated diarrhea. J Clin Microbiol. 2000;38:2386–8. doi: 10.1093/gao/9781884446054.article.t031141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilcox MH, Fawley WN, Settle CD, Davidson A. Recurrence of symptoms in Clostridium difficile infection—relapse or reinfection? J Hosp Infect. 1998;38:93–100. doi: 10.1016/s0195-6701(98)90062-7. [DOI] [PubMed] [Google Scholar]
- 13.O'Neill GL, Beaman MH, Riley TV. Relapse versus reinfection with Clostridium difficile. Epidemiol Infect. 1991;107:627–35. doi: 10.1017/s0950268800049323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Berg RJ, Ameen HA, Furusawa T, Claas EC, van der Vorm ER, Kuijper EJ. Coexistence of multiple PCR-ribotype strains of Clostridium difficile in faecal samples limits epidemiological studies. J Med Microbiol. 2005;54(Pt 2):173–9. doi: 10.1099/jmm.0.45825-0. [DOI] [PubMed] [Google Scholar]
- 15.Cohen SH, Gerding DN, Johnson S, et al. Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–55. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 16.Louie TJ, Miller MA, Mullane KM, et al. OPT-80-003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–31. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 17.Griffiths D, Fawley W, Kachrimanidou M, et al. Multilocus sequence typing of Clostridium difficile. J Clin Microbiol. 2010;48:770–8. doi: 10.1128/JCM.01796-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finney JM, Walker AS, Peto TE, Wyllie DH. An efficient record linkage scheme using graphical analysis for identifier error detection. BMC Med Inform Decis Mak. 2011;11:7. doi: 10.1186/1472-6947-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Royston P, Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21:2175–97. doi: 10.1002/sim.1203. [DOI] [PubMed] [Google Scholar]
- 20.Burnham KP, Anderson DR. Model selection and multimodel inference. 2nd ed. New York, NY: Springer; 2002. [Google Scholar]
- 21.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 22.Royston P. Multiple imputation of missing values: update of ice. Stata J. 2005;5:527–36. [Google Scholar]
- 23.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18:681–94. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 24.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hess KR. Assessing time-by-covariate interactions in proportional hazards regression models using cubic spline functions. Stat Med. 1994;13:1045–62. doi: 10.1002/sim.4780131007. [DOI] [PubMed] [Google Scholar]
- 26.Dingle KE, Griffiths D, Didelot X, et al. Clinical Clostridium difficile: clonality and pathogenicity locus diversity. PLoS One. 2011;6:e19993. doi: 10.1371/journal.pone.0019993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eastwood K, Else P, Charlett A, Wilcox M. Comparison of nine commercially available Clostridium difficile toxin detection assays, a real-time PCR assay for C. difficile tcdB, and a glutamate dehydrogenase detection assay to cytotoxin testing and cytotoxigenic culture methods. J Clin Microbiol. 2009;47:3211–7. doi: 10.1128/JCM.01082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Planche T, Aghaizu A, Holliman R, et al. Diagnosis of Clostridium difficile infection by toxin detection kits: a systematic review. Lancet Infect Dis. 2008;8:777–84. doi: 10.1016/S1473-3099(08)70233-0. [DOI] [PubMed] [Google Scholar]
- 29.Rolfe RD, Helebian S, Finegold SM. Bacterial interference between Clostridium difficile and normal fecal flora. J Infect Dis. 1981;143:470–5. doi: 10.1093/infdis/143.3.470. [DOI] [PubMed] [Google Scholar]
- 30.Claesson MJ, Cusack S, O'Sullivan O, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4586–91. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357:189–93. doi: 10.1016/S0140-6736(00)03592-3. [DOI] [PubMed] [Google Scholar]
- 32.Leav BA, Blair B, Leney M, et al. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI) Vaccine. 2010;28:965–9. doi: 10.1016/j.vaccine.2009.10.144. [DOI] [PubMed] [Google Scholar]
- 33.Emerson JE, Reynolds CB, Fagan RP, Shaw HA, Goulding D, Fairweather NF. A novel genetic switch controls phase variable expression of CwpV, a Clostridium difficile cell wall protein. Mol Microbiol. 2009;74:541–56. doi: 10.1111/j.1365-2958.2009.06812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vonberg R-P, Kuijper EJ, Wilcox MH, et al. Infection control measures to limit the spread of Clostridium difficile. Clin Microbiol Infect. 2008;14(Suppl 5):2–20. doi: 10.1111/j.1469-0691.2008.01992.x. [DOI] [PubMed] [Google Scholar]
- 35.Eyre DW, Walker AS, Griffiths D, et al. Clostridiium difficile mixed infection and reinfection. J Clin Microbiol. 2012;50:142–4. doi: 10.1128/JCM.05177-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eastwood K, Wilcox MH. Do enzyme immunoassays (EIAs) for Clostridium difficile toxins yield higher optical densities for faecal samples that are ribotype 027 culture-positive? [abstract D-126]. Presented at: 50th Interscience Conference on Antimicrobial Agents and Chemotherapy; 12–15 September 2010; Boston, Massachusetts. [Google Scholar]
- 37.Issa M, Ananthakrishnan AN, Binion DG. Clostridium difficile and inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1432–42. doi: 10.1002/ibd.20500. [DOI] [PubMed] [Google Scholar]
- 38.Kelsen JR, Kim J, Latta D, et al. Recurrence rate of Clostridium difficile infection in hospitalized pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:50–5. doi: 10.1002/ibd.21421. [DOI] [PubMed] [Google Scholar]
- 39.Selinger CP, Greer S, Sutton CJ. Is gastrointestinal endoscopy a risk factor for Clostridium difficile associated diarrhea? Am J Infect Control. 2010;38:581–2. doi: 10.1016/j.ajic.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 40.Rodrigues MA, Brady RR, Rodrigues J, Graham C, Gibb AP. Clostridium difficile infection in general surgery patients; identification of high-risk populations. Int J Surg. 2010;8:368–72. doi: 10.1016/j.ijsu.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Abou Chakra CN, Pepin J, Valiquette L. Prediction tools for unfavourable outcomes in Clostridium difficile infection: a systematic review. PLoS One. 2012;7:e30258. doi: 10.1371/journal.pone.0030258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freeman J, Bauer MP, Baines SD, et al. The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev. 2010;23:529–49. doi: 10.1128/CMR.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burns DA, Heap JT, Minton NP. The diverse sporulation characteristics of Clostridium difficile clinical isolates are not associated with type. Anaerobe. 2010;16:618–22. doi: 10.1016/j.anaerobe.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Sirard S, Valiquette L, Fortier LC. Lack of association between clinical outcome of Clostridium difficile infections, strain type, and virulence-associated phenotypes. J Clin Microbiol. 2011;49:4040–6. doi: 10.1128/JCM.05053-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JW, Lee KL, Jeong JB, et al. Proton pump inhibitors as a risk factor for recurrence of Clostridium-difficile-associated diarrhea. World J Gastroenterol. 2010;16:3573–7. doi: 10.3748/wjg.v16.i28.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.