Abstract
Our study sought to compare the strain types of Clostridium difficile causing initial and recurrent episodes of C. difficile infection (CDI) in adult patients with a first episode of CDI or 1 prior episode of CDI within the previous 90 days. Strains originated from patients who had been entered into two phase 3 randomized clinical trials of fidaxomicin versus vancomycin. Isolates of C. difficile from the initial and recurrent episodes within 28 (±2) days of cure of CDI were compared using restriction endonuclease analysis (REA) typing. Paired isolates were available from 90 of 194 (46%) patients with recurrent CDI. Patients with isolates available were significantly younger (P = .008) and more likely to be from Canadian sites (P = .0001), compared with patients without isolates. In 75 of 90 subjects (83.3%), the identical REA type strain was identified at recurrence and the initial episode (putative relapse). Early recurrences (0–14 days after treatment completion) were relapses in 86.7% and a new strain (reinfection) in 13.3%. Later recurrences (15–31 days after treatment) were relapses in 76.7% and reinfections in 23.3%. Mean time (± standard deviation) to recurrence was 12.2 (±6.4) days for relapses and 14.7 (±6.8) days for reinfections (P = .177). The most common BI/NAP1/027 group and the previous US epidemic REA group J/NAP2/001 had a significantly higher combined rate of recurrence with the same strain (relapse), compared with the other REA groups (39 of 42 [93%] vs 36 of 48 [75%], respectively; P = .023). We found a higher than historic rate of recurrent CDI caused by the same isolate as the original episode, a finding that may be related to the relatively short observation period in this study and the high frequency of isolation of epidemic strains, such as groups BI and J, for which relapse rates may be higher than for other REA groups. Caution in generalizing these observations is required, because the patients studied were younger and more likely to be from Canadian sites than were patients with recurrence who did not provide isolates.
Clinical Trials Registration. NCT00314951 and NCT00468728.
Recurrence of symptoms after effective treatment of Clostridium difficile infection (CDI) is a very common and vexing clinical problem [1]. Historically, recurrent CDI occurs in 20%–25% of patients after the initial episode but may be higher since the appearance of the epidemic strain, BI/NAP1/027 [2]. Relapse with the same strain and reinfection with a new strain have both been documented with recurrent CDI. Infection with a new strain has been reported to occur in 33%–56% of cases, but most of these studies are small or include convenience-based samples [3–7]. How often relapse and reinfection occur, the timing of the recurrence with relapse or reinfection, the relative frequency of epidemic strains, and the possibility of initial treatment influencing either outcome have not been well studied. Differentiating the nature of recurrence requires that infecting organisms be cultured and typed for both the initial and recurrent episodes of CDI. Therefore, we used data from a large, prospective, randomized, clinical treatment trial of fidaxomicin versus vancomycin, for which culture and typing data for infecting organisms were available, to better understand the epidemiology of CDI recurrences.
METHODS
Isolates for this analysis were obtained from 2 randomized, double-blind clinical trials comparing 10 days of treatment with fidaxomicin (200 mg twice daily) with treatment with vancomycin (125 mg 4 times daily) for CDI [8, 9]. Participants enrolled had diarrhea (≥3 unformed stools in a 24-hour period) and a positive stool toxin test result (C. difficile toxin A or B). Enrolled patients had either no CDI episode or only 1 episode during the previous 3 months. The primary end point was clinical cure at the end of 10 days of treatment. CDI recurrence in the 28 (±2)–day follow-up period after the end of therapy was a secondary end point. Stool specimens at study entry and the time of CDI recurrence were cultured for C. difficile at the RM Alden Research Laboratory (Culver City, California), and typing was performed on the recovered isolates using restriction endonuclease analysis (REA) at the Hines VA Microbiology Research Laboratory (Hines, Illinois). All participants who were evaluable in the clinical trials with CDI recurrence and from whom REA-typed isolates were available at study entry and at recurrence were included in this analysis.
Clostridium difficile isolates from initial and recurrent episodes were typed by REA, as described elsewhere [10]. DNA was isolated from overnight pure culture using the guanidine-EDTA-Sarkosyl method and digested with HindIII restriction enzyme. DNA fragments were separated on a 0.7% agarose gel. The REA system is divided into groups and types: groups have 90% homology in HindIII comparison and are designated by alphabetic letters, and REA types are identical and designated by Arabic numbers following the group letter(s). Images were compared with a library of REA images for C. difficile, which includes at least 110 REA groups that have been identified from a collection of >11 000 C. difficile isolates. More than 600 unique types are recognized in the library. REA is a highly discriminating typing scheme for C. difficile [11]; however, for this study, only the specific REA groups BI, J, K, G, Y, BK, CF, and DH were initially identified and the others were designated as nonspecific REA groups for the clinical trial. Final REA groups were determined from the nonspecific REA groups for isolate comparisons in this study. For the comparison of initial and recurrence isolates, an exact gel match was required to designate the recurrence isolate as a relapse.
RESULTS
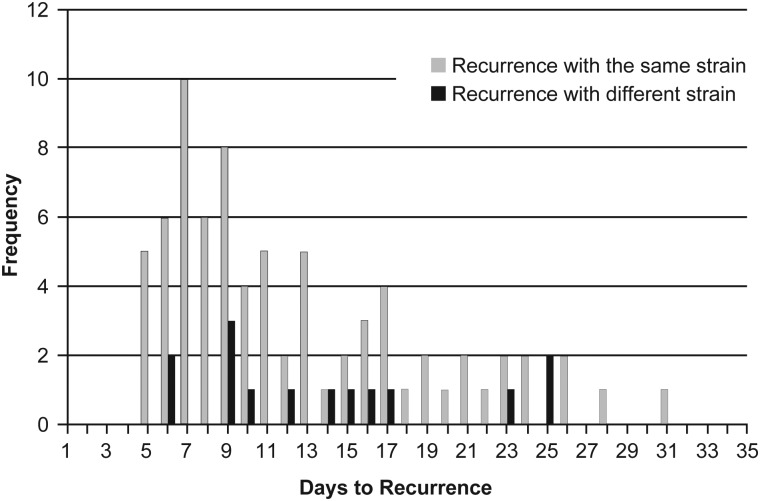
Ninety participants had recurrent CDI and had stool isolates from both initial and recurrent episodes available for typing by REA. In 75 participants (83.3%), the REA type strain identified at recurrence was identical to that identified at the initial episode. Fifteen participants (16.7%) were found to have a strain at recurrence different from the strain at the initial episode (Table 1; Figure 1).
Table 1.
Time to Recurrence of Clostridium difficile Infection With the Same or Different Strains
| Recurrence With |
|||
|---|---|---|---|
| Time Frame | Patients, No. | Same CDI Strain (No.) | Different CDI Strain (No.) |
| Full follow-up time | 90 | 75 | 15 |
| Recurrence 1–14 d after treatment | 60 | 52a | 8 |
| Recurrence 15–30 d after treatment | 30 | 23a | 7 |
| Mean time to recurrence, d (±SD) | 12.6 (±6.4) | 12.2 (±6.3)b | 14.7 (±6.8)b |
Abbreviations: CDI, Clostridium difficile infection; SD, standard deviation.
a P = .230, Pearson χ2 test comparing recurrence days 1–14 with days 15–30 for same strain.
b P = .177, t test comparing mean time to recurrence between patients with the same or different strains.
Figure 1.
Timing of recurrent Clostridium difficile infection with the same or a different strain after completion of treatment for the original episode.
In 60 participants (67.7%), the CDI recurrence episode occurred within 14 days after treatment completion (early recurrences), and 30 participants had recurrences 15 to >30 days after treatment (late recurrences). Of early recurrences, 52 of 60 (86.7%) patients experienced recurrence with the same strain, compared with 23 of 30 (76.7%) patients with late recurrences with the same strain (P = .230; Table 1).
Mean time (± standard deviation [SD]) to recurrence overall was 12.6 (±6.41) days. Mean time to recurrence for strains that remained the same at recurrence was 12.2 (±6.39) days. For recurrences with different strains, the mean time to recurrence was 14.7 (±6.80) days (P = .177) (Table 1).
The epidemic BI group was found to cause initial infection in 34 patients, 31 of whom (91.2%) experienced recurrence with the same strain, whereas 44 of 56 (78.6%) non-BI infections recurred with the same strain (P = .120) (Table 2). The BI group also caused 4 of the 15 (27%) reinfections by new recurrence strains (Table 2). In the BI group, REA type BI6/8/17 occurred more often than any other type. Twenty-four of 26 patients with initial infection due to BI6/8/17 experienced recurrence with the same strain, and 2 experienced recurrence with a different strain (P = .215, compared with all non-BI6/8/17 isolates). In addition to the BI group, commonly isolated strains belonged to the Y, J, and G groups (Table 2). The Y and G groups are well recognized as common background strains from previous studies, and the J group was epidemic in the United States during the 1990s, characterized by clindamycin resistance, and is still commonly seen in North American and European hospitals [12, 13]. Previously unidentified strains (nonspecific: those that have not yet been named or cataloged in the library) were present in the initial infection for 6 patients, recurred with the same strain in 5, and replaced a different strain in 5 cases (Table 2).
Table 2.
Prevalence of Restriction Endonuclease Analysis Groups in Clostridium difficile Infection Recurrences Due to the Same or Different Strains
| REA Group | Present in Initial Infection | Initial Infection Is First CDI for Patient | Initial Infection Is First Recurrence for Patient | Recurrence With Same Strain | Recurrence With Different Strain | Replaced a Different Strain |
|---|---|---|---|---|---|---|
| BI group | 34 | 23 | 11 | 31 | 3 | 4 |
| BI6/8/17 | 26 | 18 | 8 | 24 | 2 | 1 |
| BI group (not BI6/8/17) | 8 | 5 | 3 | 7 | 1 | 3 |
| Y group | 10 | 8 | 2 | 6 | 4 | 1 |
| J group | 8 | 8 | 0 | 8 | 0 | 1 |
| G group | 7 | 6 | 1 | 3 | 4 | 0 |
| AH group | 4 | 4 | 0 | 3 | 1 | 0 |
| L group | 3 | 3 | 0 | 3 | 0 | 1 |
| K group | 3 | 2 | 1 | 3 | 0 | 0 |
| A group | 3 | 0 | 3 | 2 | 1 | 0 |
| N group | 2 | 1 | 1 | 2 | 0 | 1 |
| CF group | 2 | 2 | 0 | 1 | 1 | 0 |
| AL group | 1 | 1 | 0 | 1 | 0 | 0 |
| BX group | 1 | 1 | 0 | 1 | 0 | 0 |
| E group | 1 | 1 | 0 | 1 | 0 | 0 |
| W group | 1 | 1 | 0 | 1 | 0 | 0 |
| AA/BK group | 1 | 0 | 1 | 1 | 0 | 0 |
| Z group | 1 | 1 | 0 | 1 | 0 | 0 |
| AV group | 1 | 1 | 0 | 1 | 0 | 0 |
| BK group | 1 | 1 | 0 | 1 | 0 | 0 |
| DH group | 0 | 0 | 0 | 0 | 0 | 2 |
| Nonspecific REA groupsa | 6 | 5 | 1 | 5 | 1 | 5 |
Abbreviations: CDI, Clostridium difficile infection; REA, restriction endonuclease analysis.
a Unable to be matched to existing REA groups.
Sixty-two patients with recurrences were treated with vancomycin, and 28 were treated with fidaxomicin. In the vancomycin treatment group, 51 patients (82.3%) experienced recurrence with the same strain and 11 had reinfection with a different strain. In the fidaxomicin group, 24 patients (85.7%) had recurrence with the same strain and 4 had recurrence with a different strain. There were no significant differences in percentage of relapses or reinfections early (days 1–14) or later (days 15–30) between vancomycin- and fidaxomicin-treated patients (Table 3). Mean times (±SD) to relapse (same strain) were 11.2 (±6.1) days for vancomycin and 14.3 (±6.2) days for fidaxomicin (P = .044). Mean times for reinfection (different strain) were 13.9 (±7.5) days for vancomycin and 16.8 (±4.6) days for fidaxomicin (P = .497).
Table 3.
Vancomycin Versus Fidaxomicin Effects on Time to Recurrence of Clostridium difficile Infection With the Same or Different Strains
| Recurrences With |
|||
|---|---|---|---|
| Treatment | Patients | Same Strain | Different Strain(s) |
| Vancomycin (no.) | 62 | 51 | 11 |
| Recurrence 1–14 d after treatment (no.) | 46 | 39 | 7 |
| Recurrence 15–30 d after treatment (no.) | 16 | 12 | 4 |
| Mean time to recurrence, d (±SD) | 11.7 (±6.4) | 11.2 (±6.1) | 13.9 (±7.5) |
| Fidaxomicin (no.) | 28 | 24 | 4 |
| Recurrence 1–14 d after treatment (no.) | 14 | 13 | 1 |
| Recurrence 15–30 d after treatment (no.) | 14 | 11 | 3 |
| Mean time to recurrence, d (±SD) | 14.7 (±6.0) | 14.3 (±6.2) | 16.8 (±4.6) |
| P valuea | .040 | .044 | .497 |
Abbreviation: SD, standard deviation.
a t Test comparing mean time to recurrence between the vancomycin- and fidaxomicin-treated groups.
Sixty-nine patients (76.7%) were treated for their first episode of CDI, whereas 21 (23.3%) were treated for their first CDI recurrence at study entry (Table 4). Recurrences with the same strain were similar in both groups: 56 of 69 (81.2%) for the first CDI episode and 19 of 21 (90.4%) for the first recurrence of CDI (P = .51). As shown in Table 2, patients with first CDI recurrence had a higher proportion of infections with the BI group (11 of 21; 52%) than did patients with a first CDI episode (23 of 69; 33%), but this was not statistically significant (P = .130). Time (±SD) to recurrence for first CDI episodes was shorter (12.0 [±6.0] days) than for first CDI recurrences (14.7 [±7.3] days) but was not statistically significant (P= .088) (Table 5). However, time (±SD) to recurrence for recurrences with the same strain was significantly shorter for patients with a first CDI episode (11.3 [±5.6] days) than for patients with a first CDI recurrence (14.9 [±7.5] days; P = .031).
Table 4.
First Clostridium difficile Infection (CDI) Episode at Study Entry Versus First Recurrence of CDI at Study Entry in Patients With Recurrence Strains Isolated
| Recurrences With |
||||
|---|---|---|---|---|
| Status at Baseline | Patients | Same Strain | Different Strain | P Value |
| First CDI episode at study entry, no. (%) | 69 (76.7) | 56 (81.2) | 13 (18.8) | |
| First CDI recurrence at study entry, no. (%) | 21 (23.3) | 19 (90.4) | 2 (9.5) | .5056a |
Abbreviation: CDI, Clostridium difficile.
a Fisher exact test comparing frequency of same strain in first CDI episode and first CDI recurrence.
Table 5.
First Clostridium difficile Infection (CDI) Episode Versus First CDI Recurrence at Study Entry; Effect on Time to Recurrence of CDI With the Same or Different Strains
| Recurrences With |
|||
|---|---|---|---|
| Parameter | Patients, No. | Same Strain | Different Strain(s) |
| First CDI episode at study entry, no. (%) | 69 | 56/69 (81.2) | 13/69 (18.8) |
| Recurrence 1–14 d after treatment, no. (%) | 48 | 41/48 (85.4) | 7/48 (14.6) |
| Recurrence 15–30 d after treatment, no. (%) | 21 | 15/21 (71.4) | 6/21 (28.6) |
| Mean time to recurrence, d (±SD) | 12.0 (±6.0) | 11.3 (±5.6) | 14.9 (±7.2) |
| First recurrence at study entry, no. (%) | 21 | 19/21 (90.5) | 2/21 (9.5) |
| Recurrence 1–14 d after treatment, no. (%) | 12 | 11/12 (91.7) | 1/12 (8.33) |
| Recurrence 15–30 d after treatment, no. (%) | 9 | 8/9 (88.9) | 1/9 (11.1) |
| Mean time to recurrence, d (±SD) | 14.7 (±7.3) | 14.9 (±7.5) | 13.0 (±5.7) |
| P valuea | .088 | .031 | .725 |
Abbreviations: CDI, Clostridium difficile; SD, standard deviation.
a t Test comparing mean time to recurrence between first CDI episode at study entry and first CDI recurrence at study entry.
A comparison of the 104 patients with recurrent CDI in these trials who did not provide paired initial and recurrence C. difficile isolates with the 90 patients from whom paired isolates were obtained is shown in Table 6. The population with isolates was significantly younger (mean age [±SD], 61.3 [±16.5] years) than the population that did not provide isolates (mean age [±SD], 67.5 [±15.7] years; P = .0081). The proportions of patients with first CDI episode and with first CDI recurrence were not significantly different in the 2 groups. However, the geographic origin of the patient specimens was significantly different, with a disproportionately higher number of patients from Canadian sites who had paired isolates provided (P = .0001) and a significantly lower number of patients from the United States and Europe who provided paired isolates (Table 6).
Table 6.
Patients With Clostridium difficile Infection (CDI) Recurrence From Whom Paired Isolates Were Obtained Versus CDI Recurrence Patients Who Did Not Have Paired Isolates Available
| Recurrences |
|||
|---|---|---|---|
| Status at Baseline | Without Isolates | With Isolates | P Value |
| Age, mean, y (±SD) | 67.5 (±15.7) | 61.3 (±16.5) | .0081a |
| First CDI episode, no. (%) | 83/104 (79.8) | 69/90 (76.7) | |
| First recurrence, no. (%) | 21/104 (20.2) | 21/90 (23.3) | .5963b |
| Canada, no. (%) | 29/104 (27.9) | 50/90 (55.6) | <.0001b |
| Europe, no. (%) | 20/104 (19.2) | 7/90 (7.8) | .0215b |
| United States, no. (%) | 55/104 (52.9) | 33/90 (36.7) | .0236b |
Abbreviations: CDI, Clostridium difficile; SD, standard deviation.
a t Test comparing mean patient age between recurrence with isolates and recurrences without isolate groups.
b Pearson χ2 test comparing patients with recurrences with isolates and patients with recurrences without isolates.
Decreased susceptibility of a C. difficile isolate to fidaxomicin that was recovered from 1 patient at the time of recurrence has previously been reported [14]. The baseline, end-of-treatment, and recurrent isolates from this patient, who had been treated with fidaxomicin, were a relatively uncommon REA group, group Z.
DISCUSSION
Our data suggest that there is a trend toward more relapses with the BI group (91.2%) overall, compared with non-BI groups. The BI6/8/17 type caused 26 of the BI group infections, is the most frequent BI type isolated from North American sites in our experience, and constitutes 3 very closely related BI types that cannot be differentiated from each other without side-by-side comparison with reference strains on the same gel, which was not performed in this analysis. The epidemic J group commonly found during the 1990s in the United States also demonstrated a high frequency of relapse, with 8 of 8 patients experiencing recurrence with the same strain. BI and J group–infected patients had relapse rates of 93%, compared with 76% for non-BI and non-J groups (P = .023).
This paired typing analysis of initial and recurrent C. difficile isolates from this large clinical trial of patients with CDI treated with fidaxomicin or vancomycin demonstrated a much higher frequency of isolation of the identical strain during recurrence (83%) than has been reported in prior studies, including one from our own laboratory [3–7]. Reasons for the difference in our findings are not readily apparent; however, epidemic strains, such as the BI and J groups, may have more recurrences with the same strain (Table 2), and 42 of the 90 (47%) CDI infections in this analysis were caused by BI or J group strains. This study also followed patients for a relatively short period of only 28 (±2) days after treatment, and it has been shown that late recurrences are more likely to be caused by strains different from the original strain causing CDI and can occur >4 weeks after treatment completion [3].
We also found that the percentage of same-strain recurrences was similar for early recurrences within 14 days (87%) and for later recurrences from days 15–30 (77%), reflecting perhaps the overall high frequency of same-strain recurrences. However, it was of interest that as early as days 5–9 after successful treatment (Figure 1), there were recurrences of CDI caused by new strains, suggesting that exogenous reinfection may occur at any time after successful treatment of CDI. Although a change of infecting strain indicates that a new exogenous infection has occurred, it is also possible that some recurrences caused by the same strain may not be relapses but could be reinfections. Patients often remain in the same contaminated environment, and recurrences with the same strain (either relapse or reinfection) may occur ≥4 weeks after successful treatment. However, because the strains are identical, they are, by default, called a relapse when they may well be reinfections with the same strain (Figure 1).
There were no significant differences in the percentage of relapses or reinfections between patients treated with vancomycin and those treated with fidaxomicin. However, the overall rates of recurrence were significantly lower for fidaxomicin in the clinical trials [8, 9]. Recurrence rates for the epidemic BI group in the full clinical trial results were not statistically different for vancomycin and fidaxomicin and were significantly higher than for non-BI isolates [8, 15].
There was no statistically significant difference in the frequency of same-strain recurrence in patients with a first episode CDI (81.2%), compared with patients with a first recurrence CDI (90.4%) (Table 4). Similar REA groups caused CDI in the first episode CDI and first recurrence CDI, with a trend toward more BI isolates in the first recurrence group. Somewhat paradoxically, the time to recurrence for relapses with the same strain was significantly shorter for patients with a first episode CDI than for patients with a first recurrence CDI (Table 5). Intuitively, one might expect patients who had already had a CDI recurrence to potentially experience recurrence faster with a relapse; however, the opposite was found in these patients.
Despite the relatively large number of paired isolates typed, the study has limitations. Less than half of all patients with CDI recurrence had paired isolates available for typing (90 of 194; 46%). Furthermore, the patients from whom paired isolates were obtained were significantly younger than their counterparts from whom isolates were not obtained. In addition, the paired isolates were geographically disproportionately from Canadian sites, with both Europe and the United States underrepresented, compared with patients who did not provide isolates (Table 6). The degree to which the data from the isolates from the tested patients represent the entire group of patients with CDI recurrence in these clinical trials is not known.
In summary, our results show a higher than expected rate of recurrent CDI caused by the same isolate as the original episode, a finding that may be related to the relatively short observation period in this study and to the high frequency of isolation of epidemic strains, such as the BI and J groups, for which relapse rates may be higher than for other REA groups. These observations may not be generalizable because patients whose paired specimens were obtained in this study were significantly younger and significantly more likely to be from Canadian study sites, compared with the patients with CDI recurrence who did not provide paired specimens.
Notes
Acknowledgments. We thank Yin Kean, PhD, for assistance with statistical analysis.
Financial support. This work was supported by Optimer Pharmaceuticals, Inc., under the National Institutes of Health (grant number R44 AI063692), and the US Department of Veterans Affairs Research Service (to S. J. and D. N. G.).
Supplement sponsorship. This article was published as part of a supplement titled “Fidaxomicin and the Evolving Approach to the Treatment of Clostridium difficile Infection,” sponsored by Optimer Pharmaceuticals, Inc.
Potential conflicts of interest. S. J. has served as a consultant for ViroPharma, Optimer, Astellas, Pfizer, Cubist, and Bio-K +. D. N. G. holds patents for the treatment and prevention of CDI licensed to ViroPharma; is a consultant for ViroPharma, Optimer, Cubist, Merck, Pfizer, TheraDoc, Astellas, BioRelix, and Actelion; and holds research grants from GOJO, Merck, Optimer, Sanofi Pasteur, Eurofins Medinet, and ViroPharma. E. J. C. G. is a member of advisory boards for Merck & Co, Optimer, Bayer Pharmaceuticals, BioK +, and Kindred Healthcare; is a speakers’ bureau member for Bayer, Merck & Co, Sanofi Pasteur, and Forest Labs; and holds research grants from Merck & Co, Schering-Plough Pharmaceuticals, Optimer Pharmaceuticals, Theravance, Cubist, Pfizer, Astellas, Cerexa, Impex Pharmaceuticals, Novexel, Novartis, Clinical Microbiology Institute, Genzyme, Nanopacific Holdings, Romark Laboratories LC, Viroxis, Warner Chilcott, Avidbiotics, GLSynthesis, Immunome, and Toltec Pharma LLC. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Johnson S. Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect. 2009;58:403–10. doi: 10.1016/j.jinf.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Pepin J, Alary ME, Valiquette L. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591–7. doi: 10.1086/430315. [DOI] [PubMed] [Google Scholar]
- 3.Johnson S, Adelmann A, Clabots CR, Peterson LR, Gerding DN. Recurrences of Clostridium difficile diarrhea not caused by the original infecting organism. J Infect Dis. 1989;159:340–3. doi: 10.1093/infdis/159.2.340. [DOI] [PubMed] [Google Scholar]
- 4.O'Neill GL, Beaman MH, Riley TV. Relapse versus reinfection with Clostridium difficile. Epidemiol Infect. 1991;107:627–35. doi: 10.1017/s0950268800049323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilcox MH, Fawley WN, Settle CD, Davidson A. Recurrence of symptoms in Clostridium difficile infection—relapse or reinfection? J Hosp Infect. 1998;38:93–100. doi: 10.1016/s0195-6701(98)90062-7. [DOI] [PubMed] [Google Scholar]
- 6.Barbut F, Richard A, Hamadi K, Chomette V, Burghoffer B, Petit JC. Epidemiology of recurrences or reinfections of Clostridium difficile-associated diarrhea. J Clin Microbiol. 2000;38:2386–8. doi: 10.1093/gao/9781884446054.article.t031141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang-Feldman Y, Mayo S, Silva J, Jr, Cohen SH. Molecular analysis of Clostridium difficile strains isolated from 18 cases of recurrent Clostridium difficile-associated diarrhea. Clin Microbiol. 2003;41:3413–4. doi: 10.1128/JCM.41.7.3413-3414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louie TJ, Miller MA, Mullane KM, et al. OPT-80-003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–31. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 9.Crook D, Weiss K, Cornely O, et al. Randomized clinical trial (RCT) in Clostridium difficile infection (CDI) confirms equivalent cure rate and lower recurrence rate of fidaxomicin (FDX) versus vancomycin (VCN) Presented at: 20th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID); 10–13 April 2010; Vienna, Austria. Abstract LB2401. [Google Scholar]
- 10.Clabots CR, Johnson S, Bettin KM, et al. Development of a rapid and efficient restriction endonuclease analysis typing system for Clostridium difficile and correlation with other typing systems. J Clin Microbiol. 1993;31:1870–5. doi: 10.1128/jcm.31.7.1870-1875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Killgore G, Thompson A, Johnson S, et al. Comparison of seven techniques for typing international epidemic strains of Clostridium difficile: restriction endonuclease analysis, pulsed-field gel electrophoresis, PCR-ribotyping, multilocus sequence typing, multilocus variable-number tandem-repeat analysis, amplified fragment length polymorphism, and surface layer protein A gene sequence typing. J Clin Microbiol. 2008;46:431–7. doi: 10.1128/JCM.01484-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belmares J, Johnson S, Parada JP, et al. Molecular epidemiology of Clostridium difficile over 10 years in a tertiary care hospital. Clin Infect Dis. 2009;49:1141–7. doi: 10.1086/605638. [DOI] [PubMed] [Google Scholar]
- 13.Johnson S, Samore MH, Farrow KA, et al. Epidemics of diarrhea caused by a clindamycin-resistant strain of Clostridium difficile in four hospitals. N Engl J Med. 1999;341:1645–51. doi: 10.1056/NEJM199911253412203. [DOI] [PubMed] [Google Scholar]
- 14.Dificid™ [prescribing Information] San Diego, California: Optimer Pharmaceuticals, Inc; 2011. NDA 201,699. 12.4: Mechanism of decreased susceptibility to fidaxomicin; p. 9. [Google Scholar]
- 15.Petrella LR, Sambol SP, Cheknis A, et al. Decreased cure and increased recurrence rate for Clostridium difficile infection caused by the epidemic C. difficile BI strain [published online ahead of print 21 May 2012] Clin Infect Dis. 2012 doi: 10.1093/cid/cis430. [DOI] [PMC free article] [PubMed] [Google Scholar]