Abstract
Two recently completed phase 3 trials (003 and 004) showed fidaxomicin to be noninferior to vancomycin for curing Clostridium difficile infection (CDI) and superior for reducing CDI recurrences. In both studies, adults with active CDI were randomized to receive blinded fidaxomicin 200 mg twice daily or vancomycin 125 mg 4 times a day for 10 days. Post hoc exploratory intent-to-treat (ITT) time-to-event analyses were undertaken on the combined study 003 and 004 data, using fixed-effects meta-analysis and Cox regression models. ITT analysis of the combined 003/004 data for 1164 patients showed that fidaxomicin reduced persistent diarrhea, recurrence, or death by 40% (95% confidence interval [CI], 26%–51%; P < .0001) compared with vancomycin through day 40. A 37% (95% CI, 2%–60%; P = .037) reduction in persistent diarrhea or death was evident through day 12 (heterogeneity P = .50 vs 13–40 days), driven by 7 (1.2%) fidaxomicin versus 17 (2.9%) vancomycin deaths at <12 days. Low albumin level, low eosinophil count, and CDI treatment preenrollment were risk factors for persistent diarrhea or death at 12 days, and CDI in the previous 3 months was a risk factor for recurrence (all P < .01). Fidaxomicin has the potential to substantially improve outcomes from CDI.
Clostridium difficile infection (CDI), mainly precipitated by antibiotic treatment, has become an increasingly severe healthcare-associated infection with markedly changed epidemiology during the past decade [1]. The emergence of a hypervirulent lineage (North American pulsed-field gel electrophoresis type 1 [NAP1]/restriction endonuclease analysis type BI [BI]/polymerase chain reaction [PCR] ribotype 027 [027]) led to steep increases in incidence, causing epidemics in hospitals [1–3], a higher proportion of severe disease [2, 3], reduced response to metronidazole treatment [4–6], and higher mortality [3, 5]. These features were first evident in North America but became prominent in Europe shortly thereafter. CDI prevention programs have become an intense focus for hospitals, with some evidence of success [7]. However, CDI treatment is an ongoing challenge as up to 20% of cases fail on currently recommended treatment with oral metronidazole or vancomycin (the only US Food and Drug Administration [FDA]–licensed therapy) [6, 8, 9]. More troubling is the high proportion of patients (20%–30%) who relapse after treatment, some repeatedly [10].
Fidaxomicin is a first-in-class macrocyclic antibiotic with advantages over other drugs used to treat CDI [11] and thus the potential to improve CDI treatment. It is more active in vitro against C. difficile strains including NAP1/BI/027, has little activity for inhibiting other bowel flora species (both in vitro and in vivo), and achieves very high fecal concentrations with minimal systemic absorption [11–16]. Fidaxomicin has been evaluated in 2 large double-blind randomized noninferiority trials (studies 003 and 004), as required for licensing. The first trial (study 003) showed fidaxomicin to be noninferior to vancomycin for cure in 629 participants, but with significantly lower recurrence rates than vancomycin for non-NAP1/BI/027 strains [17]. The second trial (004) investigating 535 patients has been recently reported and shows similar results [18].
Combining the data from both studies provides an opportunity to undertake post hoc intent-to-treat (ITT) time-to-event exploratory analyses with increased power, particularly with regard to early treatment effects, differences in treatment effects between subgroups on different outcomes, and an investigation of risk factors.
METHODS
Study Design
Both prospective, multicenter, double-blind, randomized, parallel-group trials followed the same protocol and were conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. Both study protocols and amendments were approved by institutional review boards at all centers.
Study 003 recruited from 62 sites (United States and Canada), and study 004, from 86 sites (41 from the United States and Canada and 45 from 7 European countries). In both studies, participants were eligible if they were aged ≥16 years with CDI defined as diarrhea with >3 unformed stools in the 24 hours before randomization and C. difficile toxin A, B, or both detected in stool. Participants could have received up to 4 doses, but no more than 24 hours of treatment, of vancomycin or metronidazole before randomization. Participants were excluded if they received other CDI-active antibiotics (eg, oral bacitracin, fusidic acid, or rifaximin), presented with fulminant disease (eg, toxic megacolon), and had known inflammatory bowel disease, >1 CDI episode in the previous 3 months, or previous exposure to fidaxomicin. (See [17] supplement for further exclusion criteria.) Microbiological testing for C. difficile toxin was performed at individual study sites according to their own certified testing procedures (mostly enzyme immunoassay [EIA] tests). A rapid EIA test (Meridian Bioscience, Inc, Cincinnati, Ohio) was provided by the trial; in sites where this was used, all results were confirmed by the standard laboratory test. Stools were cultured and typed as previously described [17].
After providing informed consent, participants were randomized to receive 10 days’ oral therapy as either 200 mg of fidaxomicin every 12 hours with intervening matching doses of placebo or 125 mg of vancomycin every 6 hours. Capsules containing drug or placebo were indistinguishable. Randomization was stratified according to whether the current CDI was a first (primary infection) or second (first recurrence) episode within the 3 months before enrollment and by study site.
Clinical cure was defined as resolution of diarrhea (≤3 unformed stools for 2 consecutive days) maintained for the subsequent duration of therapy with no further requirement for CDI therapy assessed 2 days after the end of the 10-day blinded treatment course (the primary posttreatment assessment). Clinical failure was defined as the persistence of diarrhea, need for additional CDI therapy, or both. Participants meeting criteria for clinical cure were followed up for 28 days after the end of treatment for a final trial assessment (36–40 days after randomization). Global cure was defined as clinical cure without subsequent recurrence, with subsequent CDI recurrence defined as the reappearance of >3 unformed stools in any 24-hour period with C. difficile toxin A or B (or both) detected and a need for CDI retreatment, and prompting a full trial assessment. “Global cure” is identical to “sustained response” as defined by the FDA.
The primary efficacy endpoint was clinical cure in the modified ITT (mITT) and per-protocol populations at the end-of-therapy assessment as previously described [17], with a 10% noninferiority margin. Secondary efficacy endpoints were CDI recurrence and global cure as defined previously. In all these prespecified analyses, early withdrawals and deaths were treated as failures (not successes). Safety was evaluated as described for study 003; results were similar (not shown).
Statistical Analysis
The primary and secondary efficacy endpoints in both studies were analyzed according to a meta-analysis of these outcomes using fixed-effect models. The endpoints were presented as the poor outcomes, namely, “no clinical cure” (converse of clinical cure), “no global cure” (converse of global cure), and “recurrence,” so that relative risks for fidaxomicin versus vancomycin of <1 consistently indicate reduced risk for poor outcome with fidaxomicin.
A post hoc exploratory time-to-event ITT analysis of the composite endpoint persistent diarrhea (equivalent to no clinical cure) or CDI recurrence or death was undertaken across both studies. This differed from the mITT analysis by only (1) including all randomized patients, censoring those who never took a dose of trial medication at 0.5 days; (2) censoring patients who were last clinically assessed before 8 days following the start of treatment, providing that they were not known to have died subsequently before 40 days from randomization (in which case their failure time was the date of death); (3) censoring patients last seen <33 days after the start of treatment, but cured at their posttreatment assessment as their last assessment (as the earliest posttreatment assessment occurred at 8 days and the FDA recommends that to be assessed as sustained response [global cure], patients should be followed up for 25 days posttreatment); and (4) considering patients who were last seen ≥33 days following treatment initiation, but were cured, as cured (ie, censored) on day 40.
Subgroup analyses of persistent diarrhea, recurrence, or death in the combined data were conducted for 6 baseline factors considered in the original study 003 publication (mild vs moderate vs severe disease [mild disease defined as 4–5 unformed stools per day or white blood cell {WBC} count ≤12 000/μL; moderate disease, as 6–9 unformed stools per day or WBC count of 12 001–15 000/μL; severe disease, as ≥10 unformed stools per day or WBC count ≥15 001/μL]), previous versus no previous CDI (in the last 3 months), strain type NAP1/BI/027 versus non-NAP1/BI/027, age <65 versus ≥65 years, inpatient versus outpatient status, and anti-CDI antibiotics (metronidazole or vancomycin) in the previous 24 hours. These were supplemented by 4 baseline biomarkers chosen to reflect underlying disease mechanisms and major morbidities (albumin <25 vs ≥25 g/L, WBC count ≤12 000 vs >12 000/μL, creatinine ≤150 vs >150 μmol/L, and hemoglobin <100 vs ≥100 g/L). Biomarker categorizations were fixed before analysis.
Last, backward selection (exit P = .1) was used to identify independent baseline predictors of persistent diarrhea, recurrence, or death in the combined data using Cox proportional hazards regression models. Factors considered were randomized group, all the factors considered for subgroup analyses as described (treating biomarkers as continuous variables truncated at the 1st and 99th percentiles to reduce the influence of outliers), plus other continuous biomarkers with P < .1 on univariate analysis (alkaline phosphatase, blood urea nitrogen [BUN], calcium, cholesterol, chloride, eosinophils, lactate dehydrogenase, urate, neutrophils, lymphocytes, creatinine clearance [estimated using the Cockcroft-Gault formula], sodium, and potassium values), and categorical variables (sex, race, and metronidazole failure); values for aspartate aminotransferase, alanine aminotransferase, bicarbonate, bilirubin, globulin, glucose, monocytes, phosphate, triglycerides, and platelets were not significant (P > .1) on univariate analyses and were not considered in backward elimination. Any factor with significant (P < .1) heterogeneity between effects in the first 0–12 days subsequently was allowed to be included in the model with this time interaction, as were any significant interactions with randomized groups (P < .1) on bivariate analyses.
RESULTS
Combined 003 and 004 Studies
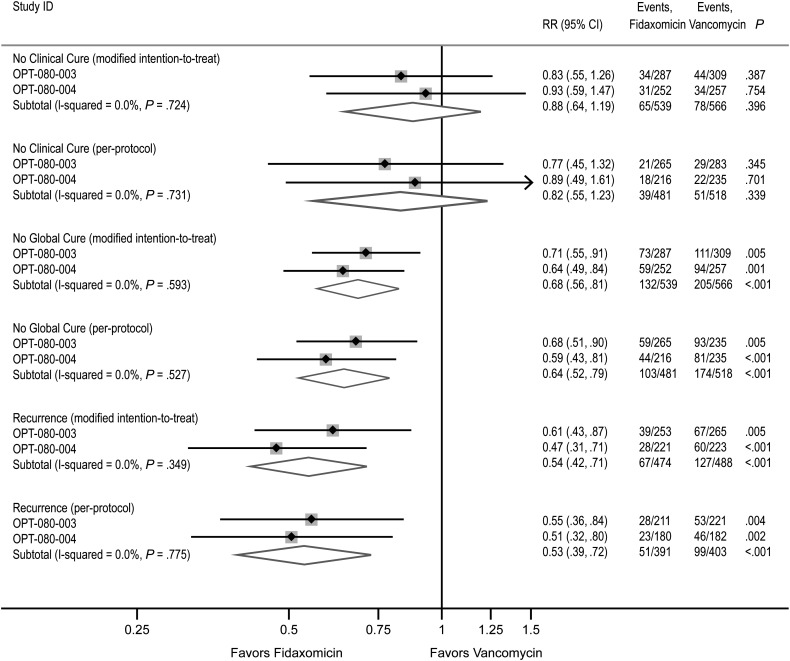
A total of 1164 participants were enrolled in either study 003 or 004 [17, 18]: there was no evidence of heterogeneity in the primary and secondary outcomes in either the mITT or per-protocol populations (P > .3, Figure 1). Overall results also demonstrated noninferiority of fidaxomicin compared with vancomycin for clinical cure and superiority of fidaxomicin over vancomycin for recurrence and global cure (P < .0001).
Figure 1.
Results of primary and secondary outcomes for studies 003 and 004. Individual results for each prespecified primary and secondary outcome are presented for studies 003 and 004 separately and are combined using fixed-effects meta-analysis in the modified intent-to-treat and per-protocol populations. The primary endpoint Clinical Cure is shown as the converse No Clinical Cure, and the secondary endpoint Global Cure, as the converse No Global Cure, so that relative risks for fidaxomicin versus vancomycin of <1 consistently indicate reduced risk for poor outcome with fidaxomicin. Abbreviations: CI, confidence interval; RR, relative risk.
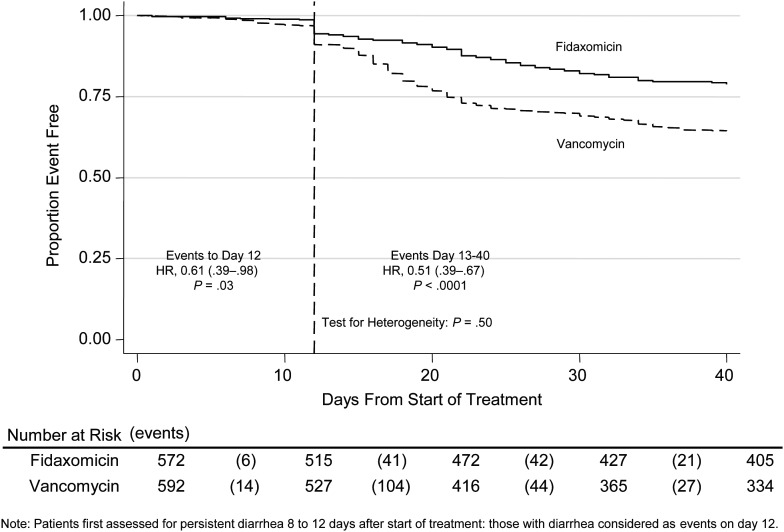
Figure 2 shows the Kaplan-Meier curve for persistent diarrhea, recurrence, or death. Of note, there was no evidence of heterogeneity in treatment effect 0–12 versus 13–40 days after randomization (P = .50), with a significant advantage evident for fidaxomicin versus vancomycin even through 0–12 days (hazard ratio [HR], 0.61 [95% CI, .39–.98]; P = .03), which was maintained over days 13–40 (HR, 0.51 [95% CI, .39–.67]; P < .0001). In contrast to the mITT and per-protocol analyses, the only patients who count as failures before 12 days in the ITT analysis are deaths occurring within 12 days of randomization, which occurred in 7 of 572 (1.2%) participants randomized to fidaxomicin versus 17 of 592 (2.9%) randomized to vancomycin (exact P = .06). The FDA requires that other participants not assessed posttreatment (early withdrawals) be treated as failures for licensing studies, even though this assumption is not necessarily conservative for the treatment effect [19–21]. A total of 55 of 592 (9.3%) vs 54 of 572 (9.4%) participants in the fidaxomicin versus vancomycin groups were early withdrawals, respectively; stated reasons were adverse events (16 vs 16), withdrawal by subject (12 vs 14), and no reason stated (27 vs 24). In the ITT analysis, these events are assumed to be uninformative with respect to the outcome. This assumption is plausible given that (1) the median day of early withdrawal was 3 (interquartile range [IQR], 2–5) before treatment response would have been expected, (2) there is no evidence that blinding was compromised, and (3) these events occurred equally in both arms at a median of 3 (IQR, 2–6) and 3 (IQR, 2–5) days for fidaxomicin and vancomycin, respectively. However, as these early withdrawals are numerically greater than the number of early deaths, counting them as failures in the FDA analysis has the potential to obscure a possible, but important, additional benefit of fidaxomicin on early deaths.
Figure 2.
Persistent diarrhea, recurrence, or death. Study treatment was administered for 10 days and clinical cure was assessed at 12 days, at which time persistent diarrhea (>3 stools/24 hours and toxin A and/or B positive or requiring anti–Clostridium difficile infection [CDI] treatment) was defined as clinical failure. The events occurring before day 12 are deaths and the step increase in events at day 12 represents cases assessed to have persistent diarrhea at the posttreatment assessment. Events from day 13 to day 40 represent CDI recurrence or deaths. Abbreviation: HR, hazard ratio.
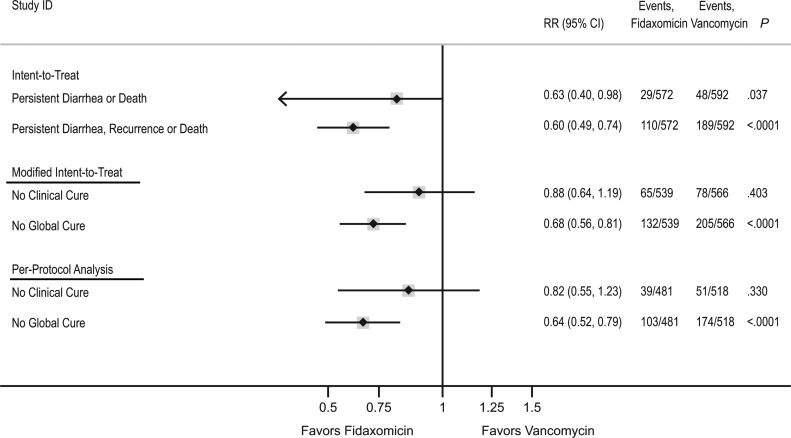
To explore the difference among the 3 analyses, Figure 3 compares the ITT analysis with the mITT and per-protocol analyses of the combined studies using fixed-effects meta-analysis. Overall, fidaxomicin reduced persistent diarrhea, recurrence, or death by 40% (95% CI, 26%–51%; P < .0001) in the ITT analysis, corresponding to a number needed to treat of 7.9 (95% CI, 5.7–12.9) in favor of fidaxomicin and similar to effects on CDI recurrence in the mITT/per-protocol populations. However, in contrast, the ITT analysis estimated a 37% reduction in persistent diarrhea or death by day 12 (95% CI, 2%–60%; P = .037), whereas mITT/per-protocol analyses of clinical failure/cure (also assessed at day 12) suggested smaller benefits associated with fidaxomicin that did not reach statistical significance (P = .40 and P = .33, respectively).
Figure 3.
Comparison of intent-to-treat (ITT), modified ITT (mITT), and per-protocol analyses of the combined 003 and 004 study populations. For the ITT analysis, persistent diarrhea or death refers to events occurring in the first 12 days of the 40-day follow-up as in the time-to-event analysis depicted for days 0–12 in the Kaplan-Meier curve. Persistent diarrhea, recurrence, or death refers to events occurring during the entire study period, days 0–40. For the mITT and per-protocol analyses, No Clinical Cure refers to the converse of Clinical Cure assessed at day 12. No Global Cure refers to the converse of Global Cure and included the 40-day follow-up. The converse of each endpoint is depicted so that relative risks for fidaxomicin versus vancomycin of <1 consistently indicate reduced risk of poor outcome with fidaxomicin. Abbreviations: CI, confidence interval; RR, relative risk.
Subgroup Analyses
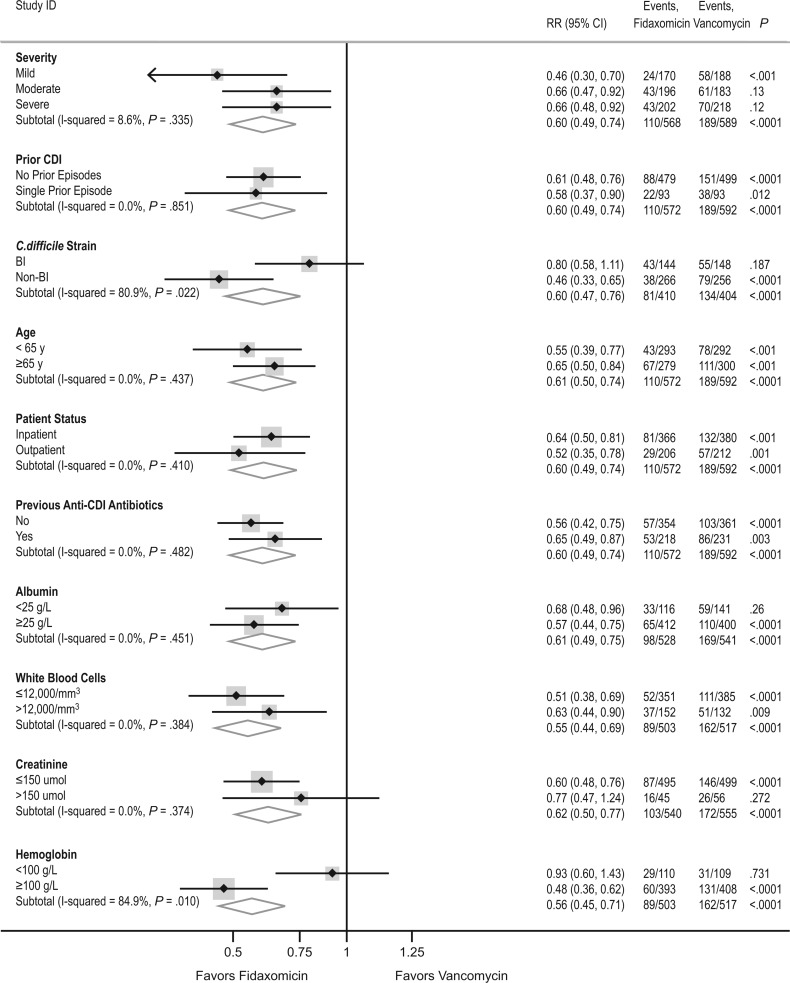
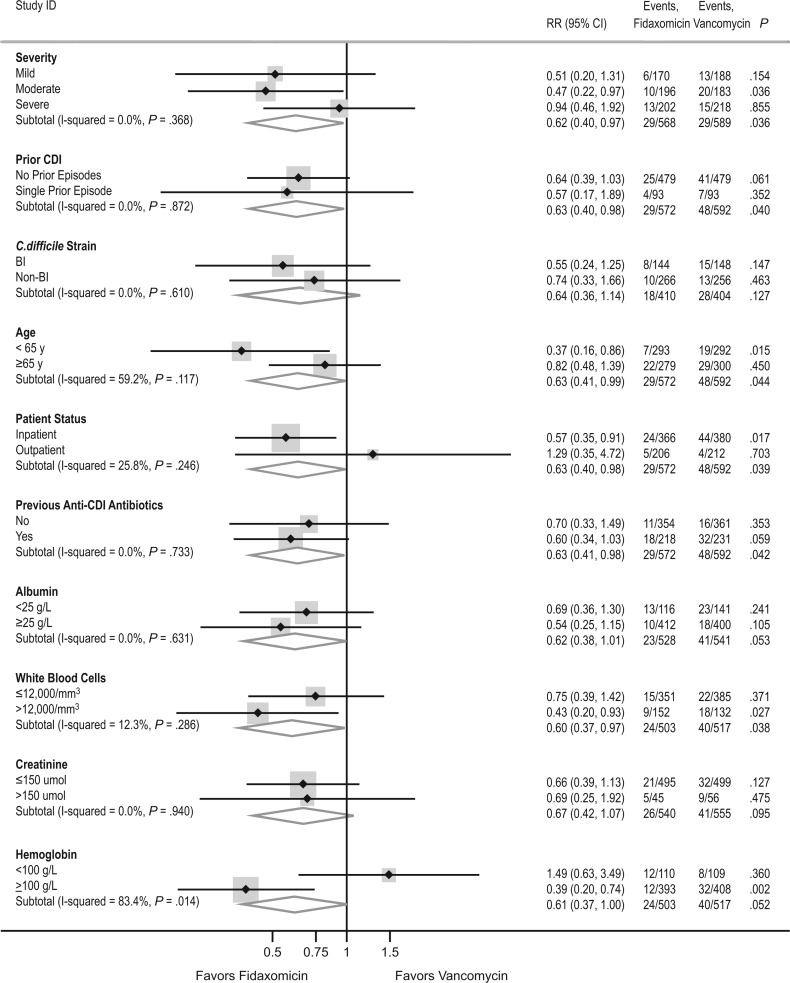
Overall, through 40 days, we found no evidence that the benefits of fidaxomicin over vancomycin varied according to disease severity, prior history of CDI, previous anti-CDI antibiotics, inpatient/outpatient status, age, baseline albumin level, or baseline creatinine level (P > .35; Figure 4). Benefits associated with fidaxomicin were significantly greater in those with non-BI/NAP1/PCR ribotype 027 strains (P = .04) and those with baseline hemoglobin level ≥100 g/L (P = .03). There was also a trend (P = .09) to somewhat greater benefits in those with WBC count ≤12 000/μL. However, of note, the data were consistent with a 22% reduction in persistent or recurrent diarrhea or death in those with BI/NAP1/PCR ribotype 027 strains, which accounted for 292 of the 814 strains assayed (36%). This did not reach statistical significance within this underpowered subgroup (95% CI, 44% reduction to 8% increase; P = .14). There was no subgroup in which fidaxomicin performed more poorly than vancomycin.
Figure 4.
Subgroup analysis for persistent diarrhea, recurrence, or death through day 40. The results depicted are from the intent-to-treat analysis and include events occurring from day 0 to day 40. Abbreviations: CDI, Clostridium difficile infection; CI, confidence interval; RR, relative risk.
Subgroup analyses restricting to the first 12 days (persistent diarrhea or death) showed similar results (Figure 5), except that there was now no evidence that the benefits associated with fidaxomicin varied according to strain type (P = .59) or WBC count (P = .40). Greater benefits from fidaxomicin in those with normal or mildly impaired hemoglobin versus moderately/severely abnormal values persisted (P = .02).
Figure 5.
Persistent diarrhea or death through day 12. The results depicted are from the intent-to-treat analysis and include events occurring from day 0 to day 12. Abbreviations: CDI, Clostridium difficile infection; CI, confidence interval; RR, relative risk.
Risk Factors
Independent predictors in the final multivariate ITT model are shown in Table 1: randomization to fidaxomicin versus vancomycin reduced the risk for persistent diarrhea, recurrence, or death by 52% (95% CI, 37%–63%; P < .0001) even after adjustment for other factors. Persistent diarrhea or death in the first 12 days was predicted most strongly by previous anti-CDI antibiotics in the 24 hours before randomization (P = .0001), and low baseline eosinophil count (P = .007) and/or albumin level (P < .0001) at randomization. Eosinophils and previous antibiotic therapy had no effect on events from days 13–40 (recurrence or death), but patients with low albumin levels at CDI diagnosis remained at increased risk for recurrence or death. Although there was no evidence that previous CDI in the last 3 months strongly affected persistent diarrhea/death in the first 12 days, it was associated with a 68% higher risk for recurrence or death (days 13–40). Patients with an elevated BUN level or lower creatinine clearance at CDI diagnosis had slightly higher risks for persisting diarrhea, recurrence, or death throughout follow-up (days 0–40). In this multivariate model, there was no evidence of increased risk for recurrence in patients with lower hemoglobin levels receiving fidaxomicin (P = .96). Strain typing information was available for only 814 (70%) of the trial participants’ isolates: effects of factors in Table 1 remained similar in the subset with strain typing available. Although BI strain per se was not associated with increased risk for persistent diarrhea, recurrence, or death (HR, 1.07 vs non-BI strains [95% CI, .73–1.56]; P = .73), the effect of fidaxomicin versus vancomycin remained larger in those with non-BI strains (HR [fidaxomicin:vancomycin], 0.30 [95% CI, .19–.46]) than BI strains (HR, 0.78 [95% CI, .51–1.19]; heterogeneity P = .002), as in the unadjusted analysis.
Table 1.
Independent Predictors of Persistent Diarrhea, Recurrence, or Death in the Intent-to-Treat Analysis
| Factor | Univariate Model |
Multivariate Model |
||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Fidaxomicin vs vancomycin | 0.54 (.42–.68) | <.0001 | 0.48 (.37–.63) | <.0001 |
| Previous anti-CDI antibioticsa in the 24 hours before randomization (yes vs no) | ||||
| Failure/death 0–12 days | 3.11 (1.95–4.97) | <.0001 | 3.11 (1.75–5.52) | .0001 |
| Recurrence/death 13–40 days | 1.24 (.95–1.62) | .12 | 1.00 (.73–1.37) | .99 |
| het P = .001 | het P = .001 | |||
| Eosinophils (/0.1 × 109/L higher) | ||||
| Failure/death 0–12 days | 0.74 (.58–.94) | .01 | 0.74 (.60–.92) | .007 |
| Recurrence/death 13–40 days | 0.99 (.90–1.09) | .78 | 0.98 (.89–1.07) | .63 |
| het P = .03 | het P = .02 | |||
| Albumin (/5 g/dL higher) | ||||
| Failure/death 0–12 days | 0.56 (.46–.68) | <.0001 | 0.64 (.51–.79) | <.0001 |
| Recurrence/death 13–40 days | 0.83 (.75–.92) | .0003 | 0.87 (.78–.98) | .02 |
| het P = .0004 | het P = .01 | |||
| Previous CDI in last 3 months (yes vs no) | ||||
| Failure/death 0–12 days | 0.84 (.44–1.58) | .58 | 0.61 (.29–1.28) | .19 |
| Recurrence/death 13–40 days | 1.52 (1.11–2.09) | .009 | 1.68 (1.19–2.37) | .003 |
| het P = .10 | het P = .02 | |||
| Creatinine clearance (/10 mL/min higher) | 0.92 (.89–.94) | <.0001 | 0.96 (.93–1.00) | .05 |
| BUN (/1 mg/dL higher) | 1.06 (1.04–1.08) | <.0001 | 1.03 (1.00–1.06) | .04 |
All models stratified by study (003 or 004). Multivariate model fitted on 994 complete cases. No additional effect of severity (P = .64); inpatient versus outpatient status (P = .65); age (P = .42); sex (P = .54); race (P = .43); metronidazole failure (P = .76); or alkaline phosphatase (P = .16), creatinine (P = .78), lactate dehydrogenase (P = .12), urate (P = .15), hemoglobin (P = .80), neutrophils (P = .13), lymphocyte (P = .93), white blood cell count (P = .25), calcium (P = .56), sodium (P = .91), potassium (P = .57), chloride (P = .82), and cholesterol levels (P = .64).
Abbreviations: BUN, blood urea nitrogen; CDI, Clostridium difficile infection; CI, confidence interval; het, heterogeneity; HR, hazard ratio; ITT, intent-to-treat.
a Anti-CDI antibiotics in this study were metronidazole and vancomycin.
DISCUSSION
In the recently reported trials (studies 003 and 004) using FDA-recommended mITT and per-protocol populations, fidaxomicin was shown to be noninferior to vancomycin for clinical cure, the primary prespecified endpoint [17, 18]. It was also shown to be superior to vancomycin for the 2 secondary endpoints, recurrence and global cure.
The antimicrobial properties of fidaxomicin have been shown to have several advantages over vancomycin in a range of settings. Fidaxomicin is bactericidal [11] and exhibits substantially higher inhibitory activity [12–16], has a more prolonged postantibiotic effect [22], and is narrower in spectrum both in vitro [12–15] and in vivo [23, 24] than vancomycin [11], which is bacteriostatic. This advantageous activity extended to the BI/NAP1/PCR ribotype 027 strains in vitro [11]. The lack of advantage of fidaxomicin observed in the mITT and per-protocol analyses of clinical cure was therefore disappointing. To further explore the relative benefits of fidaxomicin versus vancomycin, we conducted a post hoc exploratory time-to-event analysis, following the ITT analysis strategy used most commonly in nonlicensing trials.
This ITT analysis showed an overall 40% (95% CI, 26%–51%; P < .0001) reduction in persistent diarrhea, recurrence, or death during the 40-day follow-up. Interestingly, the ITT analysis through day 12 also showed a 37% (95% CI, 24%–60%; P = .037) reduction in persistent diarrhea or death, which differed from the mITT or per-protocol analyses that suggested smaller nonsignificant early benefits of fidaxomicin versus vancomycin. Furthermore, there was no evidence of heterogeneity between the beneficial effects of fidaxomicin prior to or after day 12 in the ITT analysis, supporting a consistent advantage for fidaxomicin compared with vancomycin treatment both early and late. Even though this beneficial effect is biologically plausible given the in vitro microbiological properties of fidaxomicin, the results from this post hoc exploratory analysis will need to be separately confirmed in further studies.
Subgroup analyses also supported benefits for fidaxomicin over vancomycin across all subgroups other than severe anemia (hemoglobin <100 g/L) or for BI/NAP1/PCR ribotype 027 strains, providing reassurance for its likely benefit in widespread clinical practice. There is no obvious mechanistic explanation for the lack of effect in severe anemia, although the fact that this interaction did not persist in a fully adjusted multivariate model suggests that it could be a consequence of confounding. In contrast, smaller benefits among BI/NAP1/PCR ribotype 027 strains were observed in subgroup analyses and multivariate models. The nonsignificant benefit of 22% for persistent diarrhea, recurrence, or death observed for fidaxomicin compared with vancomycin in these strains contrasts with the clear benefit evident for non-BI/NAP1/PCR ribotype 027 strains. However, given that only 292 of the 814 strains assayed were BI/NAP1/PCR ribotype 027, even the combined dataset is underpowered to conclude that fidaxomicin has no beneficial effect for treating BI/NAP1/PCR ribotype 027 strains, which was suggested in the previously reported mITT and per-protocol analyses [17]. The perils of concluding lack of benefit in underpowered subgroups of patients are well recognized in the medical literature [25, 26].
The ITT analysis was used to investigate the independent risk factors for outcomes using Cox regression. The overall benefit of fidaxomicin compared with vancomycin was clearly confirmed, even after adjustment for other important predictors. Previous vancomycin or metronidazole treatment in the 24 hours before randomization, low eosinophil count (<0.1 × 109/L), and low albumin level were independent predictors of persistent diarrhea or death in the first 12 days. Previous anti-CDI antibiotic treatment as defined here is likely to be specific to this trial design and may be an indication of cases with more severe disease receiving empiric anti-CDI antibiotics preenrollment. Low eosinophil count has not been observed as a risk factor previously, although it may reflect immunosuppression associated with, for example, steroid treatment typical of transplant or cancer patients who are recognized as being at risk for a worse outcome from CDI in some [27], but not all [28], studies. Low albumin level is a well-recognized predictor of poorer outcome [28].
The only independent factor strongly associated with longer-term recurrence or death (days 13–40) was a previous episode of CDI in the preceding 3 months, consistent with higher risks for second CDI recurrences in cases already experiencing a first recurrence [29]. Overall, a low calculated creatinine clearance or elevated BUN level was independently associated with a slightly higher risk for persistent diarrhea, recurrence, or death. Elevated creatinine level in particular has been previously reported as a risk factor for a poor outcome [5, 28]. In contrast to other well-designed studies, having adjusted for these other factors, age and WBC count (including neutrophil count) were not independently associated with poor outcome [5, 26, 28], nor was disease severity at randomization. Lack of an independent effect of age in particular is likely a consequence of baseline biomarkers lying on the causal pathway between age and poorer outcome, in other words, strong univariate associations between older age and poor outcome are explained by worse baseline biomarkers in older patients.
In contrast to a recent report, the BI/NAP1/PCR ribotype 027 strain was not associated with a poor outcome [26], possibly reflecting the inclusion of only a subset of patients with better underlying health status in these randomized trials. Although the associations found between baseline factors and outcome within this subpopulation cannot be a consequence of bias, whether our findings generalize to a wider patient pool requires confirmation in further studies.
In summary, data from the 2 licensing trials comparing fidaxomicin versus vancomycin suggest that this drug has the potential to substantially improve outcomes from this important healthcare-associated infection. Additional observational studies as it becomes more widely used in clinical practice will be important for further defining its optimal use.
Notes
Financial support. This work was supported by Optimer; the NIHR Oxford Biomedical Research Centre (to D. W. C., A. S. W., B. C. Y., N. E. S., and T. E. A. P.); and the German Federal Ministry of Research and Education (BMBF grant number 01KN1106 to O. A. C.).
Supplement sponsorship. This article was published as part of a supplement entitled “Fidaxomicin and the Evolving Approach to the Treatment of Clostridium difficile Infection,” sponsored by Optimer Pharmaceuticals, Inc.
Potential conflicts of interest. D. W. C., T. E. A. P., A. S. W., K. W., M. M., T. J. L., O. A. C., and R. E. report that their respective institutions received per-case funding from Optimer Pharmaceuticals. D. W. C., T. E. A. P., M. M., and T. J. L. received support from Optimer Pharmaceuticals for travel to meetings for the conduct of the clinical trial or presentation of the results of the clinical trial, and T. J. L. and M. M. received honoraria from Optimer Pharmaceuticals for additional meetings and related studies on fidaxomicin. In addition, M. M. receives honoraria from Actelion, Cubist, Iroko, Merck, Novartis, NuQure, Pfizer, Salix, Sanofi, and The Medicines Company, and T. J. L. receives honoraria from Merck, Cubist Pharmaceuticals, ViroPharma, Cempra, and Iroko Pharmaceuticals and is listed on a fidaxomicin patent. S. L. G. is a part-time employee of Optimer Pharmaceuticals, receiving honoraria from and owning stock options in Cempra. O. A. C. has received research grants from Actelion, Astellas, Basilea, Bayer, Biocryst, Celgene, F2G, Genzyme, Gilead, Merck/Schering, Miltenyi, Pfizer, Quintiles, and ViroPharma; is a consultant to Astellas, Basilea, F2G, Gilead, Merck/Schering, Optimer, and Pfizer; and has received lecture honoraria from Astellas, Gilead, Merck/Schering, and Pfizer. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Optimer Study 003/004 teams. In addition to the authors, the following investigators participated in the study: Humber River Regional Hospital, Toronto ON, M. Gerson; Mercury Street Medical Group, Butte MT, J. Pullman; Idaho Falls Infectious Disease, PLLC, Idaho Falls, ID, R. Nathan; Lakewood Hospital, Lakewood, OH, W. Riebel; Lakeridge Health Center, Oshawa, ON, M. Silverman; Louis Stokes VA Medical Center, Cleveland, OH, C. Donskey; Centre de Sante et des Services Sociaux de Chicoutimi, Chicoutimi, QC, D. Grimard; Remington Davis Inc, Columbus, OH, I. Baird; Missouri Baptist Medical Center, St Louis, MO, M. J. Cox; Summa Health Systems, Akron, OH, M. Tan; Infectious Diseases Minneapolis Ltd, Minneapolis, MN, C. Schrock; VA Long Beach Healthcare System, Long Beach, CA, I. L. Gordon; Erie County Medical Center, Buffalo, NY, C.-B. Hsiao; Southeast Alabama Medical Center, Dothan, AL, J. C. Jones; Harper University Hospital, Detroit, MI, G. Alangaden; St Joseph's Healthcare, Hamilton, ON, C. Lee; University of Minnesota, Minneapolis, MN, J-A. Young; Atlanta Institute for Medical Research, Decatur, GA, A. Bressler; South Jersey Infectious Disease, Somers Point, NJ, C. Lucasti; Jena, LA, R. M. Chaudhry; Royal University Hospital, Saskatoon, SK, E. Larkai; Keego Harbor MIA, Markowitz; Florida Research Network LLC, Gainesville, FL, R. G. Ashley; Morton Plant Mease Health Care Inc, Clearwater, FL, J. Berner; University of Maryland, Baltimore, MD, G. Bochicchio; Windsor Regional Hospital, Windsor, ON, A. Dhar; Lowdermilk Clinical Research, Winston-Salem, NC, T. Lowdermilk; Surrey Memorial Hospital, Surrey, BC, Y. Mirzanejad; United Medical Research, New Smyrna Beach, FL, M. Nagrani; Stamford Hospital, Stamford, CT, M. F. Parry; ID Clinical Research Ltd, Toledo, OH, L. E. Jauregui-Peredo; DiGiovanna Family Care, N. Massapequa, NY, M. DiGiovanna; Centre Hospitalier Universitaire de Quebec, Quebec City, QC, C. Dallaire; University of Virginia Health System, Charlottesville, VA, G. Donowitz; Lehigh Valley Mt Sinai Hospital, Toronto, ON, A. McGeer; East Tennessee State University, Johnson City, TN, J. Moorman; Health First Medical Group, Fort Worth, TX, M. Morrison; Cooper Hospital/University Medical Center, Camden, NJ, A. Reboli; Albany Medical Center Hospital, Albany, NY, E. Tobin; Centre Hospitalier Universitaire de Sherbrooke, Sherbrooke, QC, L. Valiquette; Christiana Care Health System, Newark, DE, A. Bacon; Hotel-Dieu de Levis, Levis, QC, R. Bourdages; St Vincent Catholic Medical Center, New York, NY, C. Carpati; Springfield Clinic, LLP, Springfield, IL, D. Graham; Newark Beth Israel Medical Center, Newark, NJ, T. Redling; Roswell Park Cancer Institute, Buffalo, NY, N. Almyroudis; Midwest Infectious Disease Specialists, Naperville, IL, J. Augustinsky; Bellam Medical Clinic, Dunnellon, FL, R. Bellam; Infectious Disease Research of Indiana, Indianapolis, IN, C. Bunce; Humility of Mary Health Partners, Youngstown, OH, A Hospital, Toronto, ON, K. Katz; KMED Research, St Clair Shores, MI, C. Ketels; University of Wisconsin, Madison, WI, D. Maki; Baystate Medical Center, Springfield, MA, P. C. Lee; AppleMed Research, Inc, Miami, FL, L. Perez-Limonte; Tequesta Research Grou Clinical Trial Links LLC, Houston, TX, K. Shivshonker; University Hospital Brugmann, Brussels, Belgium, S. L. Chérifi; O. L. Vrouwziekenhuis, Belgium, I. Demeyer; UZ Gent, Belgium, M. De Vos; Clinique Saint-Joseph, Liege, Belgium, F. Fontaine; University Hospital Saint-Pierre, Brussels, Belgium, M. Gérard; University Hospital Charleroi, Montigny-le-Tilleul, Belgium, P. Gruselle; ULB Erasmus Hospital, Brussels, Belgium, F. Jacobs; AZ Sint-Lucas, Gent, Belgium, A. Mast; Domaine Universitaire du Sart-Tilman, Liege, Belgium, M. Moutschen; ZNA Middelheim, Antwerpen, Belgium, S. Naegels; University Hospital AmbroiseParé, Mons, Belgium, C. Rossi; AZ Groeninge-Sint-Niklaas, Kortrijk, Belgium, P. Vergauwe; Raymond Poincaré Hospital, Garches, France, L. Bernard; Hôpital Nord, Amiens, France, J.-L. Dupas; Hôpital Gériatrique les Bateliers, Lille Cedex, France, F. Puisieux; Centre Hospitalier Universitaire, Caen, France; Hôpital Dron Service des Maladies Infectieuses et Tropicales, Tourcoing, France, E. Senneville; Hôpital Michallon, Grenoble, France, J.-P. Stahl; Universitätsklinikum Schleswig-Holstein, Lübeck, Germany, K. Dalhoff; Kliniken der Stadt Köln, Köln, Germany, A. J. Dormann; Krankenhaus Porz am Rhein, Köln, Germany, W. Holtmeier; Universitaetsklinikum Ulm, Ulm, Germany, P. Kern; Universitätsklinikum Magdeburg, Magdeburg, Germany, P. Malfertheiner; Klinikum der Universität Regensburg, Regenburg, Germany, B. Salzberger; Universitätsklinik Frankfurt, Frankfurt, Germany, S. Zeuzem; Universita di Torino, Turin, Italy, G. Di Perri; Struttura Complessa de Malattie Infettive, Busto Arsizio, Italy, T. Quirino; Hospital Geneal Universitario Gregorio Marañón, Madrid, Spain, E. Bouza Santiago; Hospital Ramón y Cajal, Madrid, Spain, J. Cobo Reinoso; Hospitales Universitarios Virgen del Rocio, Sevilla, Spain, M. Herrero Romeroa; Hospital Clinic, Barcelona, Spain, J. A. Martinez; Hospital Universitari Vall d'Hebron, Barcelona, Spain, A. Pahissa Berga; Orebro University Hospital, Orebro, Sweden, E. Back; Sahlgrenska University Hospital, Göteborg, Sweden, L. Hagberg; The Royal Surrey County Hospital, Guildford, UK, A. Guyot; Barnsley Hospital NHS FT, Barnsley, UK, K. Kapur; Brighton and Sussex University Hospitals NHS Trust, Brighton, UK, M. Llewelyn; Nottingham University Hospitals NHS Trust, Nottingham, UK, Y. Mahida; York Hospital, York, UK, S. J. Smale; Horton General Hospital, Banbury, UK, A. Woodhouse; Cambridge University Hospitals NHS Foundation, Cambridge, UK, J. Woodward.
References
- 1.McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433–41. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 2.Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353:2442–9. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 3.Muto CA, Pokrywka M, Shutt K, et al. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol. 2005;26:273–80. doi: 10.1086/502539. [DOI] [PubMed] [Google Scholar]
- 4.Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40:1586–90. doi: 10.1086/430311. [DOI] [PubMed] [Google Scholar]
- 5.Pepin J, Alary M-E, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591–7. doi: 10.1086/430315. [DOI] [PubMed] [Google Scholar]
- 6.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302–7. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 7.Muto CA, Blank MK, Marsh JW, et al. Control of an outbreak of infection with the hypervirulent Clostridium difficile BI strain in a university hospital using a comprehensive “bundle” approach. Clin Infect Dis. 2007;45:1266–73. doi: 10.1086/522654. [DOI] [PubMed] [Google Scholar]
- 8.Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium difficile-associated diarrhoea and colitis. Lancet. 1983;2:1043–6. doi: 10.1016/s0140-6736(83)91036-x. [DOI] [PubMed] [Google Scholar]
- 9.Wenisch C, Parschalk B, Hasenhündl M, Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22:813–8. doi: 10.1093/clinids/22.5.813. [DOI] [PubMed] [Google Scholar]
- 10.Johnson S. Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect. 2009;58:403–10. doi: 10.1016/j.jinf.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Johnson AP. Drug evaluation: OPT-80, a narrow-spectrum macrocyclic antibiotic. Curr Opin Investig Drugs. 2007;8:168–73. [PubMed] [Google Scholar]
- 12.Ackermann G, Loffler B, Adler D, Rodloff AC. In vitro activity of OPT-80 against Clostridium difficile. Antimicrob Agents Chemother. 2004;48:2280–2. doi: 10.1128/AAC.48.6.2280-2282.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Credito KL, Appelbaum PC. Activity of OPT-80, a novel macrocycle, compared with those of eight other agents against selected anaerobic species. Antimicrob Agents Chemother. 2004;48:4430–4. doi: 10.1128/AAC.48.11.4430-4434.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finegold SM, Molitoris D, Vaisanen ML, Song Y, Liu C, Bolanos M. In vitro activities of OPT-80 and comparator drugs against intestinal bacteria. Antimicrob Agents Chemother. 2004;48:4898–902. doi: 10.1128/AAC.48.12.4898-4902.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hecht DW, Galang MA, Sambol SP, Osmolski JR, Johnson S, Gerding DN. In vitro activities of 15 antimicrobial agents against 110 toxigenic Clostridium difficile clinical isolates collected from 1983 to 2004. Antimicrob Agents Chemother. 2007;51:2716–9. doi: 10.1128/AAC.01623-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karlowsky JA, Laing NM, Zhanel GG. In vitro activity of OPT-80 tested against clinical isolates of toxin-producing Clostridium difficile. Antimicrob Agents Chemother. 2008;52:4163–5. doi: 10.1128/AAC.00476-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–31. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 18.Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12:281–9. doi: 10.1016/S1473-3099(11)70374-7. [DOI] [PubMed] [Google Scholar]
- 19.Hill A, Sabin C. Designing and interpreting HIV noninferiority trials in naive and experienced patients. AIDS. 2008;22:913–21. doi: 10.1097/QAD.0b013e3282f5556d. [DOI] [PubMed] [Google Scholar]
- 20.Phillips AN, Walker AS. Drug switching and virologic-based endpoints in trials of antiretroviral drugs for HIV infection. AIDS. 2004;18:365–70. doi: 10.1097/00002030-200402200-00001. [DOI] [PubMed] [Google Scholar]
- 21.Wittkop L, Smith C, Fox Z, et al. Methodological issues in the use of composite endpoints in clinical trials: examples from the HIV field. Clin Trials. 2010;7:19–35. doi: 10.1177/1740774509356117. [DOI] [PubMed] [Google Scholar]
- 22.Babakhani F, Robert N, Shangle S, et al. Antimicrobial activity and post-antibiotic effect (PAE) of OPT-80, a new macrocyclic compound, against Clostridium difficile. Vol 44. Washington, DC: ICAAC; 2004. Abstract E-2048. [Google Scholar]
- 23.Louie TJ, Emery J, Krulicki W, Byrne B, Mah M. OPT-80 eliminates Clostridium difficile and is sparing of bacteroides species during treatment of C. difficile infection. Antimicrob Agents Chemother. 2009;53:261–3. doi: 10.1128/AAC.01443-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tannock GW, Munro K, Taylor C, et al. A new macrocyclic antibiotic, fidaxomicin (OPT-80), causes less alteration to the bowel microbiota of Clostridium difficile-infected patients than does vancomycin. Microbiology. 2010;156:3354–9. doi: 10.1099/mic.0.042010-0. [DOI] [PubMed] [Google Scholar]
- 25.Naggara O, Raymond J, Guilbert F, Altman DG. The problem of subgroup analyses: an example from a trial on ruptured intracranial aneurysms. AJNR Am J Neuroradiol. 2011;32:633–6. doi: 10.3174/ajnr.A2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller M, Gravel D, Mulvey M, et al. Health care-associated Clostridium difficile infection in Canada: patient age and infecting strain type are highly predictive of severe outcome and mortality. Clin Infect Dis. 2010;50:194–201. doi: 10.1086/649213. [DOI] [PubMed] [Google Scholar]
- 27.Dubberke ER, Sadhu J, Gatti R, et al. Severity of Clostridium difficile-associated disease (CDAD) in allogeneic stem cell transplant recipients: evaluation of a CDAD severity grading system. Infect Control Hosp Epidemiol. 2007;28:208–11. doi: 10.1086/511792. [DOI] [PubMed] [Google Scholar]
- 28.Henrich TJ, Krakower D, Bitton A, Yokoe DS. Clinical risk factors for severe Clostridium difficile-associated disease. Emerg Infect Dis. 2009;15:415–22. doi: 10.3201/eid1503.080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97:1769–75. doi: 10.1111/j.1572-0241.2002.05839.x. [DOI] [PubMed] [Google Scholar]