Abstract
There are two well characterized cannabinoid receptors (CBRs), CB1-Rs and CB2-Rs, with other candidates, such as GPR55, PPARs and vanilloid TRPV1 (VR1) receptors, which are either activated by cannabinoids and/or endocannabinoids (eCBs). The neuronal and functional expression of CB2-Rs in the brain has been much less well characterized in comparison with the expression of the ubiquitous CB1-Rs. CB2-Rs were previously thought to be predominantly expressed in immune cells in the periphery and were traditionally referred to as peripheral CB2-Rs. We and others have now demonstrated the expression of CB2-Rs in neuronal, glial and endothelial cells in the brain, and this warrants a re-evaluation of the CNS effects of CB2-Rs. In the present review we summarize our current understanding of CNR2 genomic structure, its polymorphic nature, subtype specificity, from mice to human subjects, and its variants that confer vulnerabilities to neuropsychiatric disorders beyond neuro-immuno-cannabinoid activity.
Keywords: Brain, CB2 cannabinoid receptors, CNR2, neuronal and glial distribution, neuropsychiatry
Introduction
There are two well characterized cannabinoid receptors (CBRs), CB1-R and CB2-R, with other candidates, such as GPR55, PPARs and vanilloid TRPV1 (VR1) receptors, which are either activated by cannabinoids and/or endocannabinoids. Cannabinoids are the constituents in marijuana and endocannabinoids (eCBs) are the endogenous marijuana-like substances found in animals and humans (Onaivi, 2009). The endocannabinoid system (ECS) consists of genes encoding CBRs, their endogenous ligand eCBs (anandamide, partial agonist; 2-arachidonyl glycerol, full agonist and principal eCB), and the enzymes involved in their synthesis (NAPE-PLD, PLA2, PLC, DAGL, PI-PLC and Lyso-PLC) and degradation (FAAH, MAGL) of these eCBs (Ahn et al., 2008). CBRs are abundantly distributed in the brain and peripheral tissues. However, the neuronal and functional expression of CB2-Rs in the brain has been much less well characterized in comparison with the expression of the ubiquitous CB1-Rs. Although earlier evidence suggested that CB2-Rs are present in the central nervous system (CNS) (Benito et al., 2003, 2005; Golech et al., 2004; Nunez et al., 2004; Sheng et al., 2005), they were referred to as the peripheral CBRs because many investigators were not able to detect neuronal CB2-Rs in healthy brains (Galiegue et al., 1995; Griffin et al., 1999; Ibrahim et al., 2003; Munro et al., 1993).
The functional presence of neuronal CB2-Rs in the CNS was therefore controversial (Ghose, 2009) and CB2-R has been considered as a CBR with an identity crisis (Atwood and Mackie, 2010). Nevertheless, the role of CB2-Rs in the immune system, its therapeutic promise in pain, inflammation and consequently in autoimmune and neurodegenerative disorders is receiving a great deal of attention and the subject of a number of studies and reviews (Ashton and Glass, 2007; Benito et al., 2008; Cabral et al., 2008, 2009; De Filippis et al., 2009; Ellert-Miklaszewska et al., 2007; Fernandez-Ruiz et al., 2006, 2008; Jean-Gilles et al., 2010; Lunn et al., 2008; Marriott and Huffman, 2008; Murikinati et al., 2010; Nagarkatti et al., 2009; Patel et al., 2010; Rivers and Ashton, 2010; Ruiz-Valdepenas et al., 2010; Tanasescu and Constantinescu, 2010). Therefore, as CB2-Rs are associated with immune regulation and function, it is of interest to probe the role of CB2-Rs not only in neurological disorders associated with neuroinflammation but also in neuropsychiatric disturbances. Indeed our studies provided the first evidence for neuronal CNS effects of CB2-Rs and its possible role in drug addiction, eating disorders, psychosis, depression, and autism spectrum disorders (Ishiguro et al., 2007, 2010a, 2010b; Onaivi et al., 2008a, 2008b). Previous reviews have focused on covering the evidence for the functional neuronal presence and the emerging role of brain CB2-Rs and its potential involvement in neuropsychiatric disorders (Onaivi et al., 2009; Roche and Finn, 2010). In the present review we summarize our current understanding of CNR2 genomic structure, its polymorphic nature, subtype specificity, from mice to human subjects, and its variants that confer vulnerabilities to neuropsychiatric disorders beyond neuro-immuno-cannabinoid activity.
CNR2 genomic structure and CB2-receptor sub-type specificity
The CNR2 cannabinoid gene structure has been poorly defined. However, many features of the cannabinoid CNR2 gene structure, regulation and variation are beginning to emerge with the discovery and functional identification of CB2-Rs in mammalian CNS (Brusco et al., 2008a, 2008b; Gong et al., 2006; Liu et al., 2009; Onaivi, 2006; Onaivi et al., 2006a; Van Sickle et al., 2005). This prior poor definition could be related to the previously held view that CNR2 gene and CB2-Rs were not expressed in neurons in brain but mainly in immune cells. It was therefore less investigated for CNS roles except for the association with brain cells of macrophage lineage. Recently, a number of studies from the laboratory of Gardner at the National Institute on Drug Abuse and other laboratories including that of Manzanares from Spain have mapped the cellular distribution of CNR2 gene expression in mouse brain and those over-expressing CB2 cannabinoid receptors (Garcia-Gutierrez et al., 2010). Over-expression of CB2-R in the hippocampus of transgenic CB2xP mice reduces depressive-related behaviors, such as tail suspension test, novelty-suppressed feeding test and unpredictable chronic mild stress test (Garcia-Gutierrez et al., 2010). The human CNR2 gene and its mouse and rat orthologs are located on chromosomes 1p36, 4QD3, and 5Q36, respectively. Genome-sequencing projects have also identified CNR2 genes in chimpanzee, dog, cow, chicken, amphibian, puffer fish, and zebra fish. It appears that the human, rat, mouse, and zebra fish genomes contain two isoforms of CB2-Rs that have differential distribution patterns in the brain and peripheral tissues. Interestingly the puffer fish Fugu rubripes has two CNR1 genes and one CNR2 gene in contrast to zebra fish Danio rerio that has two CNR2 genes and one CNR1 gene (McPartland et al., 2006; Rodriguez-Martin et al., 2007; Yamaguchi et al., 1996).
Human Cnr2 genomic structure and isoforms
The most striking discovery of CB2 genomic structure is species- and tissue-specific expression patterns and differences between CB2 in human and mouse (Liu et al., 2009). A novel human CB2A isoform was discovered by alignment of EST sequences and sequencing RT-PCR fragments amplified from human brain cDNA (Liu et al., 2009). The CB2A isoform is predominantly expressed in human testis and the promoter of CB2A is located 45 kb upstream of the promoter of the previously identified CB2B isoform that is predominantly expressed in spleen (Munro et al., 1993). The quantitative RT-PCR revealed that CB2A mRNA was expressed in the human brain regions of caudate, amygdala, hippocampus, cerebellum, nucleus accumbens, putamen, and cortex, with similar levels of peripheral tissue expression, such as muscle, spleen, intestine, leukocytes, and kidney, except testis expression, which is more than 100 times that of other tissues. In contrast, CB2B mRNA expression could not be detected in brain regions at a significant level and is predominantly expressed in spleen and to a lesser extent in leukocytes, muscle, intestine, liver, and heart (Liu et al., 2009). The different levels of CB2A mRNA (0–1% of testis) and the absence of CB2B mRNA in brain regions imply specific expression other than homogeneous expression in brain immune-related cells. The human CB2A and CB2B isoforms contain different 5′untranslated region (5′UTR) and their protein coding sequences are the same. The promoter region of CB2A contains CpG islands and several CCAAT boxes with a transcription factor binding site for stress response such as AP-1 (activator protein 1), HSF (heat shock factor), and STRE (stress response element). In contrast, the promoter region of CB2B contains neither CpG islands nor CCAAT boxes, but has transcription factor binding sites of GATA (GATA-binding factor), HSF, Ntx2.5 (homeo domain factor, tinman homolog) and AP-4 (activator protein 4) (TFsearch, http://molsun1.cbrc.aist.go.jp/research/db/TFSEARCH.html). It is therefore likely that human CB2A might target CB2 to specific tissues and neuron or glia cell components in response to physiological stressors.
We further analyzed human CNR2 genomic locus (1p36.1) and its neighboring genes. As shown in Figure 1, CB2A is transcribed into more than 90 kb hnRNA that is spliced into mRNA (2.37 kb) that contain exon 1A, 1B, and 3. CB2B is transcribed into 45 kb hnRNA that is spliced into CB2B mRNA (2.29 kb) that contain exon 2 and 3. Upstream of CB2A is located the PNRC2 (proline-rich nuclear receptor co-activator 2) gene, which is transcribed in the opposite direction of CB2B. With a short 5′ flanking sequence (751 bp) between CB2A and PNRC2, the promoter might co-regulate these two genes. PNRC2 plays an essential role in nonsense mediated decay (NMD) of aberrant mRNA (Cho et al., 2009), and the PNRC2 knockout mice have a higher metabolic rate and are resistant to obesity (Zhou et al., 2008). We also found a transcribed BTBD6P (BTB domain containing 6, a zinc finger protein) pseudogene in the intron 2 region between exon 1b and 2. BTBD6 plays an important role in neuronal differentiation by acting as an ubiquitination adaptor protein that targets the transcription factor PLZFA for cytoplasmic degradation (Sobieszczuk et al., 2010). More than 5 kb downstream of the CNR2 gene is located FUCA1 (fucosidase, alpha-L-1) and its mutation causes lysosomal fucose storage defect and psychomotor retardation (Kousseff et al., 1976). The functional interaction and consequences of CNR2 in close proximity with other genes remains to be determined.
Figure 1.
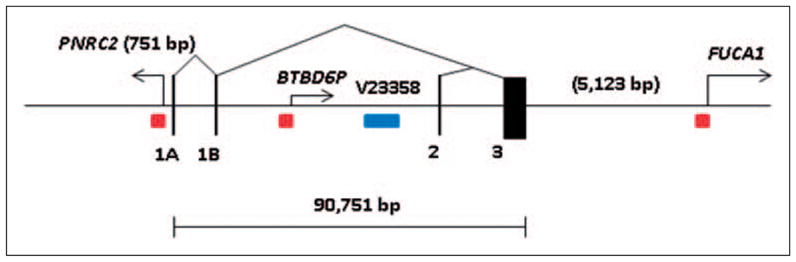
Human CB2 (CNR2, 1p36.1) genomic structure and neighboring genes. The gene size is in bp; black vertical bars represent exons; triangles represent splicing patterns, arrows represent neighboring gene transcription directions; red squares represent CpG islands; blue rectangle represents copy number variant.
The epigenetic regulation of CNR2 gene locus might play an important role in receptor regulation because CpG islands are found in the promoter regions of the four neighboring genes. The close proximity of PNRC2 and BTBD6P genes with CNR gene indicates that CB2A gene transcription might be co-regulated. In addition, there is also a copy number variant (CNV) of 2.4 kb (Zhang et al., 2006) located in intron 2 whose genetic significance awaits further investigation.
Mouse CNR2 genomic structure and isoforms
The size of mouse CB2 gene is almost four times smaller than that of human CNR2 gene, a rare case in the orthologous genes of human and mouse. Although mouse Cnr2 gene contains two promoters that transcribe mouse CB2A and CB2B isoforms, the activities of the mouse promoters deviate from those of human. Both mouse CB2A and CB2B are expressed predominantly in spleen, which is more than a hundred times the level of mouse brain regions and testis. There is no mouse testis predominant CB2 transcript. Furthermore, mouse CB2A mRNA level is five times that of CB2B mRNA. In brain stem of the C-terminus knockout mice, both CB2A and CB2B are up-regulated, which might indicate compensatory CB2 activation effects and implies brain-specific expression other than homogeneous expression in brain immune-related cells.
We further analyzed mouse Cnr2 genomic locus (4D3) and its neighboring genes. As shown in Figure 2, mouse CB2A is transcribed into about 25 kb hnRNA that is spliced into mRNA (3.88 kb) that contain exon 1 and 3. CB2B is transcribed into 8.4 kb hnRNA that is spliced into CB2B mRNA (3.91 kb) that contain exon 2 and 3. Similar to human CNR2 gene, upstream of mouse CB2A is located Pnrc2 (proline-rich nuclear receptor co-activator 2) and downstream of Cnr2 is located Fuca1 (fucosidase, alpha-L-1) gene. The CpG islands are found in the promoter regions of the Pnrc2 and Fuca1 genes, not the mouse CB2 gene. The epigenetic regulation of mouse CB2 gene is thus very different from human CB2 gene. The distance between the neighboring genes is also drastically different between mouse and human. Pnrc2 is upstream of more than 21 kb of CB2A promoter while Fuca1 is just 476 bp downstream of the coding exon. There is no transcribed Btbd6p pseudogene in the mouse CB2 locus. The promoter region of mouse CB2A does not contain CpG island and CCAAT box. However, the CB2A promoter region contains transcription factor binding sites for stress response such as AP-1, HSF, STRE, and homeo-domain factor Nkx2.5. Mouse CB2B promoter region contains not only transcription factor binding sites of AP-1, HSF, Ntx2.5 (homeo domain factor, tinman homolog) but also Nf-kB, Sox-5, and C/EBP (CCAAT enhancer binding protein). Both mouse CB2A and CB2B promoters share similar transcription factor binding sites responsive to stressors.
Figure 2.
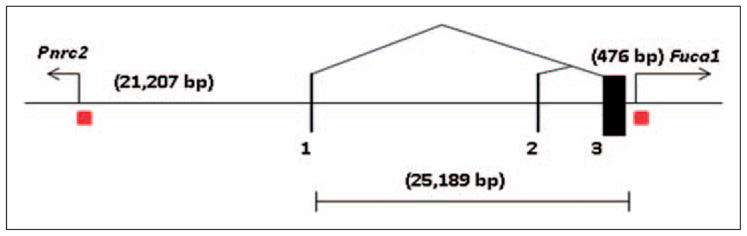
Mouse CB2 (Cnr2, 4D3) genomic structure and neighboring genes. The gene size is in bp; black vertical bars represent exons; triangles represent splicing patterns, arrows represent neighboring gene transcription directions; red squares represent CpG islands.
Comparison of human CNR2 and mouse Cnr2 gene structure and expression
Table 1 shows the genomic comparison of human CNR2 and mouse Cnr2 genes. Although these gene orthologs share molecular and functional similarities, the genomic structure and gene expression patterns are strikingly different and require special attention in developing CB2-specific agonists and antagonists in treatment of human disorders. The evolutional pace of CNR2 gene was accelerated in human with added regulatory elements such as creation of new exons, new promoter with CpG island that could be shared by PNRC2 gene, insertion of BTBD6P expressed pseudogene containing promoter of CpG island, and drastic expansion of intron sequences (Li et al., 2010). Human CNR2 gene expression could be affected by the proximity of PNRC2 and BTBD6P genes that reside only 751 bp upstream of the first exon and within intron 2 of human CNR2 gene respectively (Piontkivska et al., 2009). The expression and regulation of human CNR2 gene deviate from its ancestral counterpart, such as mouse Cnr2 gene does not have CpG island containing promoter. The distance of the mouse promoter of CB2A and CB2B is about 16 kb indicating that the two promoters are still within the same open chromatin conformation domain that confers predominant spleen expression of both isoforms. In contrast, the distance of the human promoters of CB2A and CB2B is about 46 kb, which might be located in different chromatin conformation domains that confer different tissue specific expressions (Boyle et al., 2008). Therefore, human CB2A is predominantly expressed in testis and to a much lesser extent in several brain regions. The possible DNA methylation of CpG islands and stress responsive transcription binding sites might enable human CB2A promoter to be inhibited and activated in brain regions in spatial and temporal modes and the unique 5′UTR sequence coded by human specific exon 1a and 1b might target human CB2 to specific neuronal regions such as pre- or post-synaptic structures. To investigate the brain specific and regulated expression of human CB2-R requires a highly specific CB2-R antibody that is not currently available, to our knowledge (Atwood and Mackie, 2010; Patel et al., 2010). The finding of novel human CB2-R isoforms (Liu et al., 2009) with possible brain expression and regulation has opened up a new frontier to study CB2 function in brain under normal and abnormal brain functions. Our data shows that there are two forms of the CB2-Rs in human, rat, and mouse (Liu et al., 2009) with differential subtype distribution specificities in the brain and peripheral organ tissues. The promoter-specific CB2-R isoform distribution may in part explain why CB2-Rs were previously undetectable in both human and rodent brains.
Table 1.
Genomic comparison between mouse and human CB2 genes
| Gene symbol | Isoforms | Gene size | Exons | Promoters | Promoter span | CpG islands | Amino acids | mRNA size | Tissue expression |
|---|---|---|---|---|---|---|---|---|---|
| CNR2 | hCB2A and hCB2B | 90 kb | 4 | 2 | 45,717 bp | Yes | 360 | 5.0 kb and 2.5 kb | Spleen, leukocytes, testis, muscle, brain |
| Cnr2 | mCB2A and mCB2B | 25 kb | 3 | 2 | 16,290 bp | No | 347 | 4.0 kb | Spleen, leukocytes, brain |
There are reports of endogenous and exogenous retroviral integrations found in rodent CB2 3′UTR. A frequent provirus insertion site (Evi11), about 400 bp, was found after Ca-Br-M murine leukemia virus inoculation of NIH/Swiss mice that developed malignancies (Joosten et al., 2000; Valk et al., 1999). In rat Cnr2 gene, there is a rat B2 retroposon insertion (flanked by direct repeat) into the 3′UTR region of the major coding exon in the reported rat rCB2 cDNA clone (Brown et al., 2002). This B2 insertion was also found in the NCBI rat sequence and not in the Celera rat genome sequence. We demonstrated that the Sprague-Dawley and Long Evans rats that we used in some of our studies did not contain B2 retroposon insertion (Liu et al., 2009). Since insertion of Ca-Br-M murine leukemia virus (MuLV) into mouse CB2 3′UTR sequence induced primary tumor, the B2 retroviral insertion into rat CB2 3′UTR might also contribute additional CB2-R activity. Both human FUCA1 and CNR2 genes map to 1p36 and near the common virus integration site Evi11, making CNR2 a candidate gene for viral interference (Valk et al., 1997).
Brain cannabinoid receptor genetic variation in neuropsychiatry
Cannabinoids in marijuana and eCBs acting on CBRs are important regulators of various aspects of neurophysiological, psycho-behavioral, immunological, and metabolic functions. There is an emerging understanding that brain CB2-Rs appear to be associated with vulnerability to several neuropsychiatric disorders including alcoholism, eating disorders, depression, schizophrenia, autism spectrum disorders (ASDs), and anxiety-related disorders (Ishiguro et al., 2007, 2010a, 2010b; Onaivi et al., 2008a). High comorbidity in these disorders and CB2-R function may explain certain common phenotypes in some of these neuropsychiatric disturbances. For example, common genetic contributions were suggested across alcohol and cannabis misuse (Sartor et al., 2010). Marijuana withdrawal discomfort and alcohol craving positively correlated with alcohol drinks per day in patients with marijuana abstinence (Peters and Hughes, 2010). Substance use problems, including cannabis, in adolescents with eating disorders are frequently found (Castro-Fornieles et al., 2010). Associations between marijuana use and schizophrenia have been also suggested in some studies. A dose–response relationship has been found between the amount of cannabis used in adolescence and the subsequent risk of developing schizophrenia (Andreasson et al., 1987; Zammit et al., 2002). More psychotic symptoms are experienced by schizophrenic patients who use cannabis (Leweke, 2007). Schizophrenia-like symptoms can occur in non-schizophrenic people after cannabis use (Morgan and Curran, 2008). Furthermore, there is a decrease in gray matter density in the right posterior cingulate cortex in first-episode schizophrenics who use cannabis compared with those who do not use cannabis (Bangalore et al., 2008). As a biomarker probably related to eCBs, significantly higher amounts of anandamide in the blood occur more frequently in patients with acute schizophrenia than in healthy volunteers (De Marchi et al., 2003) and significantly higher levels of anandamide are detected in the cerebrospinal fluid (CSF) of first-episode schizophrenic patients than in that of healthy volunteers (Koethe et al., 2007; Leweke et al., 1999). Another important area of growing interest is brain CBR pharmacogenomics and potential therapeutic applications targeting the endocannabinoid system, as reviewed previously (Onaivi, 2009, 2010).
Genetic polymorphisms of CNR2 gene in neuropsychiatry
Human CNR2 gene is located on chromosome 1p36, and there was no linkage with the psychiatric disorders found by previous linkage studies in the locus. The previous studies found weak linkages to alcoholism centered on chromosome 1p31 (Agrawal et al., 2008; Lappalainen et al., 2004), and a strong linkage was reported in the locus on chromosome 1p31.1 in a homogenous population from India (Holliday et al., 2009); these were not very likely to cover CNR2 locus. Furthermore, genome wide association studies (GWASs) for schizophrenia (Cichon et al., 2009; Duan et al., 2010; Holmans et al., 2009) and alcoholism (Edenberg et al., 2010; Lind et al., 2010; Treutlein et al., 2009) have been conducted worldwide. GWAS technologies can detect disease-vulnerability genes with nucleotide polymorphisms or copy number variants (deletions and duplications) in the genome. These analyses provided genetic markers for statistical evaluation for prediction of association between SNPs and disease. However, the association between CNR2 polymorphisms and the disease were unknown because GWAS datasets with Affymetrix 500K or Illumina HumanHap550 platforms did not include the SNPs of CNR2 (including rs12744386 and/or rs2501432). We have found and reported CNR2 polymorphisms to be associated with a number of disorders in Japanese subjects from our studies described below. The Japanese population is good for genetic analysis because of few stratification problems, as indicated in the study (Yamaguchi-Kabata et al., 2008). However, we have not yet confirmed a possibility for generalization of the associations to other ethnic populations.
The existence of genetic polymorphisms has been associated with CB2 function in diverse human populations, with two currently known functional polymorphisms in the CNR2 gene. One of the SNPs, rs35761398, makes the substitution of arginine at amino acid position 63 by glutamine (R63Q: two base pairs replacement polymorphism). The receptor transcribed from the gene with R63 allele appeared to have reduced responses to three CBR ligands, 2-arachidonylglyerol (2-AG), AM630, a CB2 receptor inverse agonist and JWH-015, a CB2 receptor agonist, observed in cAMP activity in cultured cells (Ishiguro et al., 2010a). Other sets of SNPs show correlation between their alleles and altered CNR2 gene expression in cis-acting fashion (for example, rs12744386C allele, effect=−0.490, H2=11.64, LOD=8.819) in lymphoblast cells. Further, in our study rs12744386 genotypes also show difference of CNR2 gene and protein expression in postmortem prefrontal cortex between CC and CT genotypes of rs12744386 and with TT genotype (Ishiguro et al., 2010a). Taken together, the low function alleles of these polymorphisms have high linkage disequilibrium (Ishiguro et al., 2010a). Thus in human populations, there could be groups with high or low CNR2 function, and other minor ones with intermediate function of the receptor. The allelic distributions in different ethnicities are similar in European and Asian but there are differences between African and others, according to the NCBI database and our study (Ishiguro et al., 2010a). We also investigated associations between psychiatric disorders and CNR2 polymorphism in Japanese populations. Interestingly, vulnerabilities to alcoholism, depression, schizophrenia, and anorexia nervosa appear to be associated with the R63Q polymorphism in the gene (Ishiguro et al., 2007, 2010a, 2010b; Onaivi et al., 2008a). The power to detect the association, if the odds ratio (OR) equals 1.2, seems to be in the 15–60 percentage range in our sample size (http://pngu.mgh.harvard.edu/~purcell/cgi-bin/cc2k.cgi). Strong association between the low function haplotype described above and schizophrenia was observed (Ishiguro et al., 2010a).
Low function of the receptor seemed to have impact on several other physical disorders, such as autoimmune disorders as well as neuropsychiatric disorders. R63 was reported to be associated with autoimmune disease (Karsak et al., 2005; Sipe et al., 2005) and with human osteoporosis (OR: 1.43 (1.07–1.92)). Sipe et al. (2005) reported its functional change from the polymorphism in the immune system in vitro. Schizophrenic patients have lower bone mass than the community population since they are young, while aging and menopausal transition effect on bone mass in the general female population cannot be seen in the schizophrenic patient group (Renn et al., 2009). Abnormalities in peripheral immune cells have been indicated in schizophrenia and numerous epidemiological studies have associated schizophrenia with autoimmunity and allergies (Muller and Schwarz, 2006; Patterson, 2009; Strous and Shoenfeld, 2006). The impact of CB2-Rs in inflammation and their role in autoimmune disorders is an area of current interest. Although the biologic and genetic mechanisms common between osteoporosis and alcoholism are not known, heavy alcohol intake and alcoholism disrupt calcium and bone homeostasis, which reduces bone mineral density and increases the incidence of fractures. Alcohol abuse has been suggested as a lifestyle factor for secondary osteoporosis (Berg et al., 2008; Malik et al., 2009; Sampson, 2002). Little has been reported about association between autoimmune disorders and alcoholism (Sammarco, 2007); however, alcohol abuse altered immune regulation leading to immunodeficiency and autoimmunity (Achur et al., 2010; Plackett and Kovacs, 2008). Such action may be mediated by the glutamatergic system (Ward et al., 2009).
Brain CB2 receptor distribution and sub-cellular localization
Some studies could not detect expression of CB2-Rs in the brain (Brown et al., 2002; Griffin et al., 1999; Munro et al., 1993) because the PCR primers may not have been specific to detect CB2-R isoforms. In addition, the specificity of the available antibodies for both CB1-Rs and CB2-Rs has also been controversial as some could not detect the native and in some cases the transfected CBR antigen, although they recognized proteins in Western blot and in immunohistochemical analysis (Grimsey et al., 2008). There are also problems with the antibodies because of the species differences between human and rodent CB2 gene (Liu et al., 2009). We have resolved some of these issues by using CB2 isoform specific TaqMan probes that could differentiate the isoform-specific expression patterns and are more sensitive and specific than the CB2 probes and primers previously used (Liu et al., 2009). The controversial CB2-R brain expression could also be due to the low expression levels of CB2A isoform in brain regions and the less specific CB2 commercial antibodies in immunohistochemical studies, especially those studies using antibodies against human hCB2 epitopes for rodent brain immunostaining. There are also problems with the use of the CB2 knockout (ko) mice (Buckley et al., 2000) in Western blots and in behavioral analysis. When we analyzed the CB2 ko mice using the three TaqMan probes against two promoters of mouse CB2 gene and the deleted part of CB2 gene, we found that the promoters of CB2-R ko mice were still active and that a CB2 truncated version was expressed, indicating that the CB2 ko mice with ablation of the C-terminal peptides of 131 amino acids was an incomplete CB2-R knockout (Liu et al., 2009). Another mouse CB2-R ko mice that has now been generated with ablation of N-terminal peptide 156 amino acid (Deltagen, Inc., San Mateo, CA) may clarify the specificity of the antibodies that were used against the N-terminal epitopes.
Table 2 shows studies documenting the expression of CB2-R mRNA and CB2-R protein in the mammalian nervous system. In demonstrating neuronal presence of CB2-Rs in vitro, Gong et al. (2006) used sequential double labeling. The hippocampal slide cultured tissue preparation was first labeled with the CB2-R antibody followed by the neuronal marker neuron-specific enolase (NSE). Immunopositive expression was detected in perikarya and in neuronal processes as well as glial cells (Gong et al., 2006). Our studies have demonstrated the expression of the CB2-R mRNA and CB2-R protein in the mammalian nervous system (Brusco et al., 2008a, 2008b; Gong et al., 2006; Liu et al., 2009; Onaivi et al., 2006a, 2006b, 2008a). Many other studies have identified brain CB2-Rs in brain stem neurons (Van Sickle et al., 2005), on neural progenitor cells of the subgranular zone of the dentate gyrus in the hippocampus (Palazuelos et al., 2006), on rat neocortical neurons (Hill et al., 2007), in the rat cerebellum and hippocampus (Suarez et al., 2008, 2009), in the thalamus (Jhaveri et al., 2008), at CNS synapses in the entorhinal cortex (Morgan et al., 2009), in primate cerebral cortex, within layers III and V on pyramidal neurons (Lanciego et al., 2010).
Table 2.
Expression of the CB2-R mRNA and CB2-R protein in the mammalian nervous system
| Area of the nervous system | Cellular type | Subcellular localization | References |
|---|---|---|---|
| Olfactory bulb and tubercle | Neurons | Gong et al., 2006 | |
| Cerebral cortex: Orbital, visual, motor and auditory cortex | Pyramidal neurons layers III and V | Gong et al., 2006 | |
| Cerebral cortex (CB2-R mRNA) | Pyramidal-like neurons layers III and V | Lanciego et al., 2010 | |
| Hippocampus: | |||
| CA2 and CA3 areas | Pyramidal neurons and interneurons | Neuronal cytoplasm and dendrites | Gong et al, 2006; Brusco et al., 2008a |
| CA1 and CA3 areas (CB2-R mRNA) | Interneuron-like cells. | Lanciego et al., 2010 | |
| Strata oriens and radiatum | Neuropil | Suarez et al., 2008, 2009 | |
| Corpus callosum | Glial cells | Gong et al., 2006 | |
| Globus pallidus (CB2-R mRNA) | PV-IR and NeuN-IR neurons | Lanciego et al., 2010 | |
| Midbrain areas: | Gong et al., 2006; Brusco et al., 2008b | ||
| Periaqueductal gray matter, paralemniscal, paratrochlear and red nuclei. | Neurons | ||
| Substantia nigra (pars reticulata) | Neurons | Dendrites, axons and axon terminals | |
| Cerebellum | Suarez et al., 2008, 2009; Baek et al., 2008 | ||
| Molecular layer | Parallel varicose fibers and neuropil | Gong et al., 2006; Baek et al., 2008; Ashton et al., 2006 | |
| Purkinje cells layer | Neurons | ||
| Granular layer | Mossy fibers and neuropil | Suarez et al., 2008, 2009; Baek et al., 2008 | |
| Brainstem nuclei | |||
| Vestibular and cochlear nuclei | Gong et al., 2006; Suarez et al., 2008 | ||
| Parvocellular reticular nucleus, spinal trigeminal tract nucleus | Gong et al., 2006 | ||
| Dorsal motor nucleus of the vagus, nucleus ambiguous and spinal trigeminal nucleus | Neurons | Van Sickle et al., 2005 | |
| Pineal gland | Pinealocytes and intrapineal nerve fibers | Koch et al., 2008 | |
| Retina (CB2-R mRNA) | Inner photoreceptor segments, inner nuclear layer, ganglion cell layer. | Lu et al., 2000 | |
| Retina (CB2-R protein) | Inner photoreceptor segments, inner nuclear layer, inner plexiform layer, ganglion cell layer | Lopez et al., 2010 | |
Using mouse and rat brains, the subcellular distribution of CB2-Rs in neuronal, endothelial and glial cells in the cortex, hippocampus and substantia nigra were shown using immunohistochemical electron microscopy (Brusco et al., 2008a, 2008b; Onaivi, 2006). Cnr2 gene and protein expression in different brain regions of Swiss ICR mice under normal conditions were demonstrated and over-expression of CB2-R in the hippocampus of transgenic CB2xP mice reduces depressive-related behaviors, such as tail suspension test, novelty-suppressed feeding test and unpredictable chronic mild stress test (Garcia-Gutierrez et al., 2010). In the rat brain study and in each region immunoperoxidase labeling for CB2-Rs was detected in neurons as well as in glial and endothelial cells. In neuronal cells, iCB2 was observed in somata and large and medium-sized dendrites. In the soma, iCB2 labeling was mainly associated with the rough endoplasmic reticulum and Golgi apparatus, suggesting its endogenous synthesis. In the dendrites, iCB2 labeling was observed in the cytoplasm and was associated with the plasma membrane near the area of synaptic contact with axon terminals, indicating a postsynaptic distribution of CB2-Rs. In iCB2 glial and endothelial cells, the labeling was also found to be associated with the plasma membrane. In the substantia nigra, some unmyelinated axons were immunoreactive for CB2-Rs, and CB2-R-labeled axon terminals were rarely found. In mice, electron micrographs from different cortical areas show dendrites with immunostaining for CB2-Rs. In some areas, axon terminals were not immunoreactive for CB2-Rs. The pattern of staining in most mouse cortical areas appeared to be mainly postsynaptic localization of CB2-Rs (Brusco et al., 2008a). Our data therefore provided the first ultrastructural evidence that CB2-Rs are mainly post-synaptic in the rat hippocampus and substantia nigra and in some cortical areas in the mouse and rat brain (Brusco et al., 2008a, 2008b; Onaivi, 2006). We cannot exclude that some of the CB2-Rs may be presynaptic (Suarez et al., 2008), just as CB1-Rs are not exclusively presynaptic, with some post-synaptic distribution reported (Ong and Mackie, 1999) in the brain.
A number of studies from mouse to human subjects, using a variety of techniques including those used in pain models, electrophysiological, brain stimulation reward paradigm, histological, immunohistochemical, electron microscopy, molecular biological, behavioral and pharmacological, pharmacological MRI, cerebral occlusion and hemicerebellectomy, transgenic and cell culture studies, show the functional presence of CB2-Rs in neural progenitor cells, neurons, glial and endothelial cells (Brusco et al., 2008a, 2008b; Chin et al., 2008; Garcia-Gutierrez et al., 2010; Palazuelos et al., 2006; Viscomi et al., 2009). Potential interactions of CB2-Rs for example, where chronic blockade of CB2-Rs or over-expression of CB2-Rs is associated with the modulation of anxiety response in the mouse model (Garcia- Gutierrez and Manzanares, 2010) have been demonstrated. Furthermore, over-expression of CB2-Rs results in neuroprotection against behavioral and neurochemical alterations induced by intracaudate administration of 6-hydroxydopamine (Ternianov et al., 2010).
Therefore the pharmacological actions at brain CB2-Rs may be more complex than previously appreciated with species and subtype differences and distribution patterns. However, the role of CB2-Rs in CNS disturbances involving neuroinflammation and neuropathic pain has been extensively reported. While the CNS presence of CB2-Rs may no longer be a debate, the neurobiological basis for CB2-R physiological activity and its interaction with or without CB1-Rs remains to be determined. However, functional interactions between forebrain CB2-R and mu-opioid receptor (MOR) was demonstrated (Paldyova et al., 2008) and CB2-R antagonist SR144528 was reported to decrease MOR expression and activation in mouse brain stem (Paldy et al., 2008). CB2-Rs in the pineal gland along with other components of the ECS may be involved in the control of pineal physiology (Koch et al., 2008). Gender-dependent changes on the expression of hippocampal CB1 and CB2-Rs were demonstrated in the early maternal deprivation model in neonatal rats (Suarez et al., 2008). While CB1-Rs remain one of the most ubiquitous G-protein coupled receptors in the mammalian brain, we have described the multifocal distribution of CB2-Rs, albeit at lower levels than the CB1-Rs in neuronal and glial processes in a number of brain areas (Gong et al., 2006). This multifocal distribution and the presence of CNS brain CB2-Rs suggest a need to re-evaluate the role of these receptors in neurotransmission.
Emerging evidence suggests that CB1-Rs and CB2-Rs modulate some of their physiological effects by acting in opposite directions. For example, it was demonstrated that CB2-Rs participate in the regulation of glucose homeostasis in rat by opposing the actions exerted through CB1-Rs (Bermudez-Silva et al., 2007). The investigators suggested that the opposing roles of CB1-Rs and CB2-Rs in glucose homeostasis were involved in the regulation of glycemia. Using CB1-R and CB2-R agonists and antagonists and their interaction, it was demonstrated and confirmed in the rat model that glucose levels remain high after stimulation of CB1-Rs (Bermudez-Silva et al., 2006) and the levels return to normal after stimulation of CB2-Rs (Bermudez-Silva et al., 2006, 2007). Using the brain stimulation reward paradigm in the rat model, such opposing effects of CB1-Rs and CB2-Rs were shown to modulate brain stimulation in opposite directions. In the study presented at the Society for Neuroscience meeting and at the International Cannabinoid Research Society meeting (Xi et al., 2009), it was reported that the brain stimulation reward (BSR) enhancing effect produced by cannabinoid is mediated by activation of brain CB1-Rs (using THC and WIN55212-2), while the brain stimulation inhibiting effect produced is mediated by activation of brain CB2-Rs.
It is important to understand the role of CB2-Rs and their gene variants in the CNS and their possible involvement in drug addiction and neuropsychiatric disorders. However, research on the involvement of CB2-Rs in neuroinflammatory conditions and in neuropathic pain has advanced more than other areas in neuropsychiatry and drug addiction. Therefore, improved information about CNR2 gene and its human variants might add to our understanding not only of the role of CB2-Rs during neuroinflammatory conditions in the CNS but also beyond neuro-immuno-cannabinoid activity.
Pharmacology of CNS effects of CB2 cannabinoid receptors
The CNS effects of CB2-Rs had been ambiguous and controversial and their role in depression and substance abuse was unknown. We therefore have conducted studies from mouse to human subjects to examine the following. 1) We investigated the involvement of CB2-Rs in alcohol preference in mice and alcoholism in a human population (Ishiguro et al., 2007); 2) we analyzed the behavioral effects of CB2 cannabinoid receptor activation and its influence on food and alcohol consumption in mice (Onaivi et al., 2008b); 3) we have described the involvement of brain neuronal CB2-Rs in the effects of drugs of abuse and in depression (Onaivi et al., 2008a). Indeed, alcoholism in humans may be caused by both genetics and environmental factors. There is a high incidence (comorbidity) of alcoholism and depression in the human population (Hasin et al., 2005). Several lines of experimental evidence support roles for the ECS in alcoholism and neuropsychiatric disorders (Basavarajappa and Hungund, 2005; Onaivi et al., 2008b; Vinod and Hungund, 2005). We therefore tested whether CB2-Rs in the CNS play a role in alcohol abuse/dependence in an animal model and then examined the association between CNR2 gene polymorphism and alcoholism in a human population. We found that mice preferring alcohol had reduced Cnr2 gene expression in the ventral midbrain whereas Cnr2 gene expression was unaltered in the ventral midbrain region of mice with little or no preference for alcohol. Treatment of mice with the CB2-R agonist JWH 015 enhanced alcohol consumption in mice subjected to chronic mild stress (CMS) and treatment with the CB2-R antagonist AM630 reduced the stress-induced increase in alcohol consumption. This CB2-R agonist or antagonist effect was absent in normal mice that were not subjected to CMS.
To further understand the physiological relevance of the expression of CB2-Rs and their gene transcripts, we examined the expression of Cnr2 gene transcripts in rodents treated with opioids, cocaine, and alcohol in comparison with control animals. Animals treated with cocaine or heroin showed increased Cnr2 gene transcripts in comparison with controls, indicating the presence of Cnr2 gene transcripts in the brain is influenced by abused substances (Ishiguro et al., 2007; Onaivi, 2006; Onaivi et al., 2008b). We utilized behavioral and molecular methods to study and determine whether there was a link between depression that may be a factor in drug/alcohol addiction and CNS CB2-Rs. First we established the use of the mouse CMS model of depression, which has been validated and is a widely used model for screening antidepressants. Briefly, the mouse CMS model measures one of the core symptoms of depression, anhedonia, a lack of pleasure. Mice were subjected daily for four weeks to CMS, and anhedonia was measured by the consumption of sucrose solution. Behavioral and rewarding effects of abused substances were determined in the CMS and control animals. The expression of CB2-Rs and their gene transcripts was compared in the brains of CMS and control animals by Western blotting and RT-PCR. CMS induced gender-specific aversions in the test of anxiety, which were blocked by WIN55212-2 and CB2-R agonist. In other studies we demonstrated that direct CB2-R antisense oligonucleotide microinjection into the mouse brain induced anxiolysis, indicating that CB2-Rs are functionally present in the brain and may influence behavior (Ishiguro et al., 2007; Onaivi, 2006; Onaivi et al., 2006a, 2008b; Uhl et al., 2006). Overall it appears that endocannabinoid activity in the nervous system may play a significant role in a number of neuropsychiatric conditions following neuroinflammation.
The genetic findings of reduced functioning of CNR2 gene associated with schizophrenia may also be supported by the findings of the pharmacological experiments using the animal model and AM630, the CB2-R antagonist in the study (Ishiguro et al., 2010a). Prepulse inhibition (PPI) is frequently used in pharmaco-behavioral studies of animal models. PPI refers to the reduction in amplitude of the startle reflex that occurs when a brief, sub-threshold stimulus immediately precedes a startle stimulus (Hoffman and Ison, 1980). Deficits in PPI are observed in several psychiatric disorders, especially in schizophrenia (Swerdlow et al., 2006), and it has been postulated that this impairment of sensori-motor gating reflects at least some portion of the cognitive dysfunction observed in patients with schizophrenia (Braff et al., 2001, 2005). The association with the cannabinoid system had been investigated and cannabidiol reverses MK-801-induced disruption of PPI in mice (Long et al., 2006). In our study, we evaluated the effect of pretreatment with AM630 on PPI, combined with MK-801 or methamphetamine treatment separately, in mice. AM630 alone did not affect PPI in mice. AM630 exacerbated MK-801 or methamphetamine induced disturbance of PPI and hyperactivity in C57BL/6JJmsSlc mice (Ishiguro et al., 2010a). When administered to mice in home cages both MK-801 and methamphetamine produced significant hyperlocomotion. Although AM630 alone did not produce significant hyperlocomotion, AM630 pretreatments significantly increased methamphetamine-induced and MK-801-induced locomotion compared with saline pretreatments. Therefore, reduced CB2-R function itself is not likely to cause schizophrenia, but it is hypothesized that, when combined with other risk factors, it could be a risk factor for schizophrenia- susceptible individuals.
There are, however, some limitations with the use of such animal models because the possible effect of AM630 on CB1 and interaction between CB1 and CB2 receptors could not be excluded. A further study using Cnr1 ko mice would be needed to explore this pharmacological possibility to clarify the functions of CB2 receptors in brain. In a recent abstract presented at the Society for Neuroscience Ortega-Alvaro et al. (2010) reported that deletion of CB2-R induces schizophrenia- related behaviors in mice, which is in agreement with the findings of Ishiguro et al. (2010a).
Comments and future directions
The clinical and functional implication of neuronal CB2-Rs in the brain will gradually become clearer because more research will certainly unravel the contribution and interaction of CB1 and CB2-Rs in neuropsychiatry beyond neuro-immuno-cannabinoid activity. The new knowledge from our data and those of other recent studies that CB2-Rs are present in the brain raises many questions about the possible roles that CB2-Rs may play in the nervous system. These results therefore extend the previous evidence that CB2-Rs are playing an important role in immune function to other putative neuronal function by their apparent presence in neuronal processes. Our studies implicate neuronal and glial CB2-Rs in the chronic mild stress model of depression, and substance abuse. With neuroinflammation known to be associated with a number of autoimmune and neurological disorders, the close association of the immune system with CB2-Rs and their functional expression in neurons warrants a re-evaluation of CB2-Rs in mental disturbances. Both CB1 and CB2 receptors seem likely to work both independently or in opposite directions and/or cooperatively in differing neuronal and/or glial cell populations to regulate important physiological activities in the central nervous system. Thus, many more studies are required to determine the exact role of CB2-Rs and the nature of their interactions with other receptors and CB1-Rs in the brain and therefore determine the therapeutic utility of CB2-R ligands in the clinic.
Acknowledgments
ESO acknowledges the Dean of William Paterson University, Dr Sandra DeYoung, for continued student worker support and the Provost Office for release time. SG was a Visiting Professor to William Paterson University and NIDA-NIH. We appreciate and thank Dr Patricia Tagliaferro for compiling Table 2 and for discussion on the distribution and subcellular localization of CB2 cannabinoid receptors.
Funding
This work was supported by William Paterson University (ESO); KAKENHI (grant numbers 20390098 and 20023006) and Imai–Kimi Memorial Stress-Related Disorders (HI); and the Intramural Research Program of the NIDA/NIH (QL).
Footnotes
Conflict of interest
None declared.
References
- Achur RN, Freeman WM, Vrana KE. Circulating cytokines as biomarkers of alcohol abuse and alcoholism. J Neuroimmune Pharmacol. 2010;5:83–91. doi: 10.1007/s11481-009-9185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Hinrichs AL, Dunn G, et al. Linkage scan for quantitative traits identifies new regions of interest for substance dependence in the Collaborative Study on the Genetics of Alcoholism (COGA) sample. Drug Alcohol Depend. 2008;93:12–20. doi: 10.1016/j.drugalcdep.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K, McKinney MK, Cravatt BF. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem Rev. 2008;108:1687–1707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasson S, Allebeck P, Engstrom A, Rydberg U. Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet. 1987;2:1483–1486. doi: 10.1016/s0140-6736(87)92620-1. [DOI] [PubMed] [Google Scholar]
- Ashton JC, Glass M. The cannabinoid CB2 receptor as a target for inflammation dependent neurodegeneration. Curr Neuropharmacol. 2007;5:73–80. doi: 10.2174/157015907780866884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton JC, Friberg D, Darlington CL, Smith PF. Expression of the cannabinoid CB2 receptor in the rat cerebellum: an immunohistochemical study. Neurosci Lett. 2006;396:113–116. doi: 10.1016/j.neulet.2005.11.038. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol. 2010;160:467–479. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek J-H, Zheng Y, Darlington CL, et al. Cannabinoid CB2 receptor expression in the rat brainstem cochlear and vestibular nuclei. Acta Otolaryngol. 2008;128:1–7. doi: 10.1080/00016480701796944. [DOI] [PubMed] [Google Scholar]
- Bangalore SS, Prasad KM, Montrose DM, Goradia DD, Diwadkar VA, Keshavan MS. Cannabis use and brain structural alterations in first episode schizophrenia – a region of interest, voxel based morphometric study. Schizophr Res. 2008;99:1–6. doi: 10.1016/j.schres.2007.11.029. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Hungund BL. Role of the endocannabinoid system in the development of tolerance to alcohol. Alcohol Alcohol. 2005;40:15–24. doi: 10.1093/alcalc/agh111. [DOI] [PubMed] [Google Scholar]
- Benito C, Nunez E, Tolon RM, et al. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J Neurosci. 2003;23:11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito C, Kim WK, Chavarria I, et al. A glial endogenous cannabinoid system is upregulated in the brains of macaques with simian immunodeficiency virus-induced encephalitis. J Neurosci. 2005;25:2530–2536. doi: 10.1523/JNEUROSCI.3923-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito C, Tolon RM, Pazos MR, et al. Cannabinoid CB2 receptors in human brain inflammation. Br J Pharmacol. 2008;153:277–285. doi: 10.1038/sj.bjp.0707505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KM, Kunins HV, Jackson JL, et al. Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med. 2008;121:406–418. doi: 10.1016/j.amjmed.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez-Silva FJ, Serrano A, Diaz-Molina FJ. Activation of cannabinoid CB1 receptors induces glucose intolerance in rats. Eur J Pharmacol. 2006;531:282–284. doi: 10.1016/j.ejphar.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Bermudez-Silva FJ, Sanchez-Vera I, Suarez J. Role of cannabinoid CB2 receptors in glucose homeostasis in rats. Eur J Pharmacol. 2007;565:207–211. doi: 10.1016/j.ejphar.2007.02.066. [DOI] [PubMed] [Google Scholar]
- Boyle AP, Davis S, Shulha HP, et al. High-resolution mapping and characterization of open chromatin across the genome. Cell. 2008;132:311–322. doi: 10.1016/j.cell.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berlin) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Braff DL, Light GA, Ellwanger J, Sprock J, Swerdlow NR. Female schizophrenia patients have prepulse inhibition deficits. Biol Psychiatry. 2005;57:817–820. doi: 10.1016/j.biopsych.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Brown SM, Wager-Miller J, Mackie K. Cloning and molecular characterization of the rat CB2 cannabinoid receptor. Biochim Biophys Acta. 2002;1576:255–264. doi: 10.1016/s0167-4781(02)00341-x. [DOI] [PubMed] [Google Scholar]
- Brusco A, Taglaiferro PA, Saez T, et al. Ultrastructural localization of neuronal brain CB2 cannabinoid receptors. Ann NY Acad Sci. 2008a;1139:450–457. doi: 10.1196/annals.1432.037. [DOI] [PubMed] [Google Scholar]
- Brusco A, Tagliaferro P, Saez T, et al. Postsynaptic localization of CB2 cannabinoid receptors in the rat hippocampus. Synapse. 2008b;62:944–949. doi: 10.1002/syn.20569. [DOI] [PubMed] [Google Scholar]
- Buckley NE, McCoy KL, Mezey E, et al. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB2 receptor. Eur J Pharmacol. 2000;396:141–149. doi: 10.1016/s0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- Cabral GA, Griffin-Thomas L. Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation. Expert Rev Mol Med. 2009;11:e3. doi: 10.1017/S1462399409000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral GA, Raborn ES, Griffin L, et al. CB2 receptors in the brain: role in central immune function. Br J Pharmacol. 2008;153:240–251. doi: 10.1038/sj.bjp.0707584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Fornieles J, Diaz R, Goti J, et al. Prevalence and factors related to substance use among adolescents with eating disorders. Eur Addict Res. 2010;16:61–68. doi: 10.1159/000268106. [DOI] [PubMed] [Google Scholar]
- Chin CL, Tovcimak AE, Hradil VP, et al. Differential effects of cannabinoid receptor agonists on regional brain activity using pharmacological MRI. Br J Pharmacol. 2008;153:367–379. doi: 10.1038/sj.bjp.0707506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Kim KM, Kim YK, et al. Human proline-rich nuclear receptor coregulatory protein 2 mediates an interaction between mRNA surveillance machinery and decapping complex. Mol Cell. 2009;33:75–86. doi: 10.1016/j.molcel.2008.11.022. [DOI] [PubMed] [Google Scholar]
- Cichon S, Craddock N, Daly M, et al. Genomewide association studies: history, rationale, and prospects for psychiatric disorders. Am J Psychiatry. 2009;166:540–556. doi: 10.1176/appi.ajp.2008.08091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis D, Steardo A, D’Amico A, et al. Differential cannabinoid receptor expression during reactive gliosis: a possible implication for a nonpsychotropic neuroprotection. Sci World J. 2009;9:229–235. doi: 10.1100/tsw.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marchi N, De Petrocellis L, Orlando P, Daniele F, Fezza F, Di Marzo V. Endocannabinoid signalling in the blood of patients with schizophrenia. Lipids Health Dis. 2003;2:5. doi: 10.1186/1476-511X-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J, Sanders AR, Gejman PV. Genome-wide approaches to schizophrenia. Brain Res Bull. 2010;00:1–00. doi: 10.1016/j.brainresbull.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Koller DL, Xuei X, et al. Genome-wide association study of alcohol dependence implicates a region on chromosome 11. Alcohol Clin Exp Res. 2010;34:840–852. doi: 10.1111/j.1530-0277.2010.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellert-Miklaszewska A, Grajkowska W, Gabrusiewicz K, et al. Distinctive pattern of cannabinoid type II (CB2) expression in adult and pediatric brain tumors. Brain Res. 2007;1137:161–169. doi: 10.1016/j.brainres.2006.12.060. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Romero J, Velasco G, et al. Cannabinoid CB2 receptor: a new target for controlling neural cell survival. Trends Pharmacol Sci. 2006;28:39–45. doi: 10.1016/j.tips.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Pazo MR, Garcia-Arencibia X, et al. Role of CB2 receptors in neuroprotective effects of cannabinoids. Mol Cell Endocrinol. 2008;286:S91–S96. doi: 10.1016/j.mce.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Gutierrez MS, Manzanares J. Overexpression of CB2 cannabinoid receptors decreased vulnerability to anxiety and impaired anxiolytic action of alprazolam in mice. J Psychopharmacol. 2010;00:1–00. doi: 10.1177/0269881110379507. [DOI] [PubMed] [Google Scholar]
- Garcia-Gutierrez MS, Perez-Ortiz JM, Gutierrez-Adan A, Manzanares J. Depression-resistant endophenotype in mice overexpressing cannabinoid CB(2) receptors. Br J Pharmacol. 2010;160:1773–1784. doi: 10.1111/j.1476-5381.2010.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose H. Cannabinoid controversy. The Scientist. 2009 Available at: http://www.the-scientist.com/news/display/55969/
- Golech SA, McCarron RM, Chen Y, et al. Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Mol Br Res. 2004;132:87–92. doi: 10.1016/j.molbrainres.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Gong J-P, Onaivi ES, Ishiguro H, et al. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Griffin G, Wray EJ, Tao Q, et al. Evaluation of the cannabinoid CB2 receptor-selective antagonist, SR144528: further evidence for CB2 receptor absence in the rat central nervous system. Eur J Pharmacol. 1999;377:117–125. doi: 10.1016/s0014-2999(99)00402-1. [DOI] [PubMed] [Google Scholar]
- Grimsey NL, Goodfellow CE, Scotter EL, et al. Specific detection of CB1 receptors; cannabinoid CB1 receptor antibodies are not all created equal. J Neurosci Methods. 2008;171:78–86. doi: 10.1016/j.jneumeth.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry. 2005;62:1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- Hill EL, Gallopin T, Ferezou I, et al. Functional CB1 receptors are broadly expressed in neocortical GABAergic and glutamatergic neurons. J Neurophysiol. 2007;97:2580–2589. doi: 10.1152/jn.00603.2006. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev. 1980;87:175–189. [PubMed] [Google Scholar]
- Holliday EG, Nyholt DR, Tirupati S, et al. Strong evidence for a novel schizophrenia risk locus on chromosome 1p31.1 in homogeneous pedigrees from Tamil Nadu, India. Am J Psychiatry. 2009;166:206–215. doi: 10.1176/appi.ajp.2008.08030442. [DOI] [PubMed] [Google Scholar]
- Holmans PA, Riley B, Pulver AE, et al. Genomewide linkage scan of schizophrenia in a large multicenter pedigree sample using single nucleotide polymorphisms. Mol Psychiatry. 2009;14:786–795. doi: 10.1038/mp.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim M, Deng H, Zvonok K, et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the brain. Proc Natl Acad Sci. 2003;100:10529–10533. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Iwasaki S, Teasenfitz L, et al. Involvement of cannabinoid CB2 receptor in alcohol preference in mice and alcoholism in humans. Pharmacogenomics J. 2007;7:380–385. doi: 10.1038/sj.tpj.6500431. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Horiuchi Y, Ishikawa M, et al. Brain cannabinoid CB2 receptor in schizophrenia. Biol Psychiatry. 2010a;67:974–982. doi: 10.1016/j.biopsych.2009.09.024. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Carpio O, Horiuchi Y, et al. A nonsynonymous polymorphism in cannabinoid CB2 receptor gene is associated with eating disorders in humans and food intake is modified in mice by its ligands. Synapse. 2010b;64:92–96. doi: 10.1002/syn.20714. [DOI] [PubMed] [Google Scholar]
- Jean-Gilles L, Gran B, Constantinescu CS. Interaction between cytokines, cannabinoids and the nervous system. Immunology. 2010;215:606–610. doi: 10.1016/j.imbio.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Jhaveri MD, Elmes SJ, Richardson D, et al. Evidence for a novel functional role of cannabinoid CB(2) receptors in the thalamus of neuropathic rats. Eur J Neurosci. 2008;27:1722–1730. doi: 10.1111/j.1460-9568.2008.06162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten M, Valk PJ, Vankan Y, et al. Phenotyping of Evi1, Evi11/Cb2, and Evi12 transformed leukemias isolated from a novel panel of cas-Br-M murine leukemia virus-infected mice. Virology. 2000;268:308–318. doi: 10.1006/viro.2000.0183. [DOI] [PubMed] [Google Scholar]
- Karsak M, Cohen-Solal M, Freudenberg J, et al. Cannabinoid receptor type 2 gene is associated with human osteoporosis. Hum Mol Genet. 2005;14:3389–3396. doi: 10.1093/hmg/ddi370. [DOI] [PubMed] [Google Scholar]
- Koch M, Habazetti I, Dehghani F, et al. The rat pineal gland comprises an endocannabinoid system. J Pineal Res. 2008;45:351–360. doi: 10.1111/j.1600-079X.2008.00597.x. [DOI] [PubMed] [Google Scholar]
- Koethe D, Llenos IC, Dulay JR, et al. Expression of CB1 cannabinoid receptor in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression. J Neural Transm. 2007;114:1055–1063. doi: 10.1007/s00702-007-0660-5. [DOI] [PubMed] [Google Scholar]
- Kousseff BG, Beratis NG, Strauss L, et al. Fucosidosis type 2. Pediatrics. 1976;57:205–213. [PubMed] [Google Scholar]
- Lanciego JL, Barroso-Chinea P, Rico AJ, et al. Expression of mRNA coding the cannabinoid receptor 2 in the pallidal complex of Macaca fascicularis. J Psychopharmacol. 2010;00:1–00. doi: 10.1177/0269881110367732. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Kranzler HR, Petrakis I, et al. Confirmation and fine mapping of the chromosome 1 alcohol dependence risk locus. Mol Psychiatry. 2004;9:312–319. doi: 10.1038/sj.mp.4001429. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Giuffrida A, Wurster U, Emrich HM, Piomelli D. Elevated endogenous cannabinoids in schizophrenia. Neuroreport. 1999;10:1665–1669. doi: 10.1097/00001756-199906030-00008. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Giuffrida A, Koethe D, et al. Anandamide levels in cerebrospinal fluid of first-episode schizophrenic patients: impact of cannabis use. Schizophr Res. 2007;94:29–36. doi: 10.1016/j.schres.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Li CY, Zhang Y, Wang Z, et al. A human-specific de novo protein-coding gene associated with human brain functions. PLoS Comput Biol. 2010;6:e1000734. doi: 10.1371/journal.pcbi.1000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind PA, Macgregor S, Vink JM, et al. A genomewide association study of nicotine and alcohol dependence in Australian and Dutch populations. Twin Res Hum Genet. 2010;13:10–29. doi: 10.1375/twin.13.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QR, Pan CH, Hishimoto A, et al. Species differences in cannabinoid receptor 2 (CNR2 gene): identification of novel human and rodent CB2 isoforms, differential tissue expression and regulation by cannabinoid receptor ligands. Genes Brain Behav. 2009;8:519–330. doi: 10.1111/j.1601-183X.2009.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long LE, Malone DT, Taylor DA. Cannabidiol reverses MK-801-induced disruption of prepulse inhibition in mice. Neuropsychopharmacology. 2006;31:795–803. doi: 10.1038/sj.npp.1300838. [DOI] [PubMed] [Google Scholar]
- Lopez EM, Tagliaferro P, Onaivi ES, Lopez-Costa JJ. Distribution of CB2 cannabinoid receptor in adult retina. Synapse. 2010 doi: 10.1002/syn.20856. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Lunn CA, Reich E-P, Fine JS, et al. Biology and therapeutic potential of cannabinoid CB2 receptor inverse agonists. Br J Pharmacol. 2008;153:226–239. doi: 10.1038/sj.bjp.0707480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik P, Gasser RW, Kemmler G, et al. Low bone mineral density and impaired bone metabolism in young alcoholic patients without liver cirrhosis: a cross-sectional study. Alcohol Clin Exp Res. 2009;33:375–381. doi: 10.1111/j.1530-0277.2008.00847.x. [DOI] [PubMed] [Google Scholar]
- Marriot KS, Huffman JW. Recent advances in the development of selective ligands for cannabinoid CB(2) receptor. Curr Top Med Chem. 2008;8:187–204. doi: 10.2174/156802608783498014. [DOI] [PubMed] [Google Scholar]
- McPartland JM, Matias I, Di Marzo V, et al. Evolutionary origins of the endocannabinoid system. Gene. 2006;370:64–74. doi: 10.1016/j.gene.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Curran HV. Effects of cannabidiol on schizophrenia- like symptoms in people who use cannabis. Br J Psychiatry. 2008;192:306–307. doi: 10.1192/bjp.bp.107.046649. [DOI] [PubMed] [Google Scholar]
- Morgan NH, Stanford IM, Woodhall GL. Functional CB2 type cannabinoid receptors at CNS synapses. Neuropharmacology. 2009;57:356–368. doi: 10.1016/j.neuropharm.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz M. Schizophrenia as an inflammation-mediated dysbalance of glutamatergic neurotransmission. Neurotox Res. 2006;10:131–148. doi: 10.1007/BF03033242. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Murikinati S, Juttler E, Keinert T, et al. Activation of cannabinoid 2 receptors protects against cerebral ischemia by inhibiting neutrophil recruitment. FASEB J. 2010;24:788–798. doi: 10.1096/fj.09-141275. [DOI] [PubMed] [Google Scholar]
- Nagarkatti P, Pandey R, Rieder SA, et al. Cannabinoids as novel anti-inflammatory drugs. Future Med Chem. 2009;1:1333–1349. doi: 10.4155/fmc.09.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez E, Benito C, Pazos MR, et al. Cannabinoid CB2 receptors are expressed by perivascular microglia cells in the human brain: an immunohistochemical study. Synapse. 2004;53:208–213. doi: 10.1002/syn.20050. [DOI] [PubMed] [Google Scholar]
- Onaivi ES. Neuropsychopharmacological evidence for the functional presence and expression of cannabinoid CB2 receptors in the brain. Neuropsychobiology. 2006;54:231–246. doi: 10.1159/000100778. [DOI] [PubMed] [Google Scholar]
- Onaivi ES. Cannabinoid receptors in brain: pharmacogenetics, neuropharmacology, neurotoxicology, and potential therapeutic applications. Int Rev Neurobiol. 2009;88:335–369. doi: 10.1016/S0074-7742(09)88012-4. [DOI] [PubMed] [Google Scholar]
- Onaivi ES. Endocannabinoid system, pharmacogenomics and response to therapy. Pharmacogenomics. 2010;11:907–910. doi: 10.2217/pgs.10.91. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann NY Acad Sci. 2006a;1074:514–536. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Patel S, et al. Methods to study the behavioral effects and expression of CB2 cannabinoid receptors and its gene transcripts in chronic mild stress model of depression. In: Onaivi ES, editor. Marijuana and Cannabinoid Research: Methods and Protocols. Totowa: Humana Press Inc; 2006b. pp. 291–298. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong J-P, et al. Functional expression of brain neuronal CB2 cannabinoid receptors are involved in the effects of drugs of abuse and in depression. Ann NY Acad Sci. 2008a;1130:434–449. doi: 10.1196/annals.1432.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Carpio O, Ishiguro H, et al. Behavioral effects of CB2 cannabinoid receptor activation and its influence on food and alcohol consumption. Ann NY Acad Sci. 2008b;1139:426–433. doi: 10.1196/annals.1432.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong WY, Mackie K. A light and electron microscopic study of the CB1 cannabinoid receptor in primate spinal cord. J Neurocytol. 1999;28:39–45. doi: 10.1023/a:1007011700677. [DOI] [PubMed] [Google Scholar]
- Ortega-Alvaro A, Aracil-Fernandez A, Garcia-Gutierrez MS, et al. Deletion of CB2 cannabinoid receptor induces schizophrenia- related behaviors in mice. Soc Neuro Abst. 2010;471:11. doi: 10.1038/npp.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazuelos J, Aguado T, Egia A, et al. Non-psychoactive CB2 cannabinoid agonists stimulate neural progenitor proliferation. FASEB J. 2006;20:E1773–E1779. doi: 10.1096/fj.06-6164fje. [DOI] [PubMed] [Google Scholar]
- Paldy E, Bereczki E, Santha M, et al. CB2 cannabinoid receptor antagonist SR144528 decreases mu-opioid receptor expression and activation in mouse brainstem: role of CB2 receptor in pain. Neurochem Int. 2008;53:309–316. doi: 10.1016/j.neuint.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Paldyova E, Bereczki E, Santha M, et al. Noladin ether, a putative endocannabinoid, inhibits mu-opoid receptor activation via CB2 cannabinoid receptors. Neurochem Int. 2008;52:321–328. doi: 10.1016/j.neuint.2007.06.033. [DOI] [PubMed] [Google Scholar]
- Patel KD, Davison JS, Pittman QJ, et al. Cannabinoid CB2 receptors in health and disease. Curr Med Chem. 2010;17:1394–1410. doi: 10.2174/092986710790980041. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Peters EN, Hughes JR. Daily marijuana users with past alcohol problems increase alcohol consumption during marijuana abstinence. Drug Alcohol Depend. 2010;106:111–118. doi: 10.1016/j.drugalcdep.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Piontkivska H, Yang MQ, Larkin DM, et al. Cross-species mapping of bidirectional promoters enables prediction of unannotated 5′ UTRs and identification of species-specific transcripts. BMC Genomics. 2009;10:189. doi: 10.1186/1471-2164-10-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plackett TP, Kovacs EJ. Acute models of ethanol exposure to mice. Methods Mol Biol. 2008;447:3–9. doi: 10.1007/978-1-59745-242-7_1. [DOI] [PubMed] [Google Scholar]
- Renn JH, Yang NP, Chueh CM, Lin CY, Lan TH, Chou P. Bone mass in schizophrenia and normal populations across different decades of life. BMC Musculoskelet Disord. 2009;10:1. doi: 10.1186/1471-2474-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers JR, Ashton JC. The development of cannabinoid CBII receptor agonists for the treatment of central neuropathies. Cent Nerv Syst Agents Med Chem. 2010;10:47–64. doi: 10.2174/187152410790780145. [DOI] [PubMed] [Google Scholar]
- Roche M, Finn DP. Brain CB2 receptors: implications for neuropsychiatric disorders. Pharmaceuticals. 2010;3:2517–2553. doi: 10.3390/ph3082517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Martin I, Herrero-Turrion MJ, Marron Fdez de Velasco E, Gonzalez-Sarmiento R, Rodriguez RE. Characterization of two duplicate zebrafish Cb2-like cannabinoid receptors. Gene. 2007;389:36–44. doi: 10.1016/j.gene.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Ruiz-Valdepenas L, Benito C, Tolon RM, et al. The endocannabinoid system and amyloid-related diseases. Exp Neurol. 2010;224:66–73. doi: 10.1016/j.expneurol.2010.03.024. [DOI] [PubMed] [Google Scholar]
- Sammarco CL. A case study: identifying alcohol abuse in multiple sclerosis. J Neurosci Nurs. 2007;39:373–376. doi: 10.1097/01376517-200712000-00008. [DOI] [PubMed] [Google Scholar]
- Sampson HW. Alcohol and other factors affecting osteoporosis risk in women. Alcohol Res Health. 2002;26:292–298. [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Grant JD, Bucholz KK, et al. Common genetic contributions to alcohol and cannabis use and dependence symptomatology. Alcohol Clin Exp Res. 2010;34:545–554. doi: 10.1111/j.1530-0277.2009.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng WS, Hu S, Min X, et al. Synthetic cannabinoid WIN55212-2 inhibits generation of inflammatory mediators by IL-IB-stimulated human astrocytes. Glia. 2005;49:211–219. doi: 10.1002/glia.20108. [DOI] [PubMed] [Google Scholar]
- Sipe JC, Arbour N, Gerber A, Beutler E. Reduced endocannabinoid immune modulation by a common cannabinoid 2 (CB2) receptor gene polymorphism: possible risk for autoimmune disorders. J Leukoc Biol. 2005;78:231–238. doi: 10.1189/jlb.0205111. [DOI] [PubMed] [Google Scholar]
- Sobieszczuk DF, Poliakov A, Xu Q, Wilkinson DG. A feedback loop mediated by degradation of an inhibitor is required to initiate neuronal differentiation. Genes Dev. 2010;242:206–218. doi: 10.1101/gad.554510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous RD, Shoenfeld Y. Schizophrenia, autoimmunity and immune system dysregulation: a comprehensive model updated and revisited. J Autoimmun. 2006;27:71–80. doi: 10.1016/j.jaut.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Suarez J, Bermudez-Silva FJ, Mackie K, et al. Immunohistochemical description of the endogenous cannabinoid system in the rat cerebellum and functionally related nuclei. J Comp Neurol. 2008;509:400–421. doi: 10.1002/cne.21774. [DOI] [PubMed] [Google Scholar]
- Suarez J, Llorente R, Romero-Zerbo SY, et al. Early maternal deprivation induces gender-dependent changes on the expression of hippocampal CB1 and CB2 cannabinoid receptors of neonatal rats. Hippocampus. 2009;19:623–632. doi: 10.1002/hipo.20537. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arch Gen Psychiatry. 2006;63:1325–1335. doi: 10.1001/archpsyc.63.12.1325. [DOI] [PubMed] [Google Scholar]
- Tanasescu R, Constantinescu CS. Cannabinoids and the immune system: an overview. Immunology. 2010;215:588–597. doi: 10.1016/j.imbio.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Ternianov A, Perez-Ortiz JM, Solesio ME, et al. Overexpression of CB2 cannabinoid receptors results in neuroprotection against behavioral and neurochemical alterations induced by intracaudate administration of 6-hydroxydopamine. Neurobiol Aging. 2010;00:1–00. doi: 10.1016/j.neurobiolaging.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Treutlein J, Cichon S, Ridinger M, et al. Genome-wide association study of alcohol dependence. Arch Gen Psychiatry. 2009;66:773–784. doi: 10.1001/archgenpsychiatry.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Ishiguro H, Onaivi ES, et al. Molecular neurobiological methods in marijuana-cannabinoid research. In: Onaivi ES, editor. Marijuana and Cannabinoid Research: Methods and Protocols. Totowa: Humana Press Inc; 2006. pp. 1–17. [DOI] [PubMed] [Google Scholar]
- Valk PJ, Hol S, Vankan Y, et al. The genes encoding the peripheral cannabinoid receptor and alpha-L-fucosidase are located near a newly identified common virus integration site, Evi11. J Virol. 1997;71:6796–6804. doi: 10.1128/jvi.71.9.6796-6804.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valk PJ, Vankan Y, Joosten M, et al. Retroviral insertions in Evi12, a novel common virus integration site upstream of Tra1/Grp94, frequently coincide with insertions in the gene encoding the peripheral cannabinoid receptor Cnr2. J Virol. 1999;73:3595–3602. doi: 10.1128/jvi.73.5.3595-3602.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Hungund BL. Endocannabinoid lipids and mediated system: implication for alcoholism and neuropsychiatric disorders. Life Sci. 2005;77:1569–1583. doi: 10.1016/j.lfs.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Viscomi MT, Oddi S, Latini L, et al. Selective CB2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the P13K/Akt pathway. J Neurosci. 2009;29:4564–4570. doi: 10.1523/JNEUROSCI.0786-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RJ, Colivicchi MA, Allen R, et al. Neuro-inflammation induced in the hippocampus of ‘binge drinking’ rats may be mediated by elevated extracellular glutamate content. J Neurochem. 2009;111:1119–1128. doi: 10.1111/j.1471-4159.2009.06389.x. [DOI] [PubMed] [Google Scholar]
- Xi Z-X, Spiller K, Gardner EL. Cannabinoid CB1 and CB2 receptors modulate brainreward function in opposite directions in rats. Soc Neuro Abst. 2009;449:13. [Google Scholar]
- Yamaguchi F, Macrae AD, Brenner S. Molecular cloning of two cannabinoid type 1-like receptor genes from the puffer fish Fugu rubribes. Genomics. 1996;35:603–605. doi: 10.1006/geno.1996.0406. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Kabata Y, Nakazono K, Takahashi A, et al. Japanese population structure, based on SNP genotypes from 7003 individuals compared to other ethnic groups: effects on population-based association studies. Am J Hum Genet. 2008;83:445–456. doi: 10.1016/j.ajhg.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ. 2002;325:1199. doi: 10.1136/bmj.325.7374.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Feuk L, Duggan GE, Khaja R, Scherer SW. Development of bioinformatics resources for display and analysis of copy number and other structural variants in the human genome. Cytogenet Genome Res. 2006;115:205–214. doi: 10.1159/000095916. [DOI] [PubMed] [Google Scholar]
- Zhou D, Shen R, Ye JJ, et al. Nuclear receptor coactivator PNRC2 regulates energy expenditure and adiposity. J Biol Chem. 2008;283:541–553. doi: 10.1074/jbc.M703234200. [DOI] [PubMed] [Google Scholar]


