Abstract
Development of post-transcriptional gene silencing (PTGS) agents for therapeutic purposes is an immense challenge in modern biology. Established technologies used to knockdown a specific target RNA and its cognate protein: antisense, ribozyme, RNAi, all conditionally depend upon an initial, critical annealing event of the PTGS ligand to a target RNA. In this review we address the nature of the bottlenecks, emphasizing the biocomplexity of target RNA structure, that currently limit PTGS therapeutic development. We briefly review existing and emerging technologies designed to release these constraints to realize the potential of PTGS agents in gene based therapies.
Keywords: gene therapy, high throughput screening, ribozyme, antisense, shRNA
Introduction to the Biocomplexity of PTGS Therapy
Biocomplexity is a new term used to represent problems in quantitative biology that embrace substantial complexity, such as understanding the mechanistic details of feedback control on a class of genes that are transcriptionally co-regulated (Nicholson, Homes, Lindon et al., 2001). The challenges to successful design of a PTGS agent, be it ribozymes (Rz), antisense (AS), or RNA interference (RNAi), necessitates address of numerous biophysical, biochemical, and cell biological aspects of both the target RNA and the ligand and their spatial and temporal interactions within a cell. PTGS agent development is a biocomplexity problem. Target mRNA is folded into secondary and tertiary structure, is coated with heterogeneous proteins, undergoes dynamic fluctuations in structure, and resides in intracellular compartments with different lifetimes (nucleus, cytoplasm, ribosomes, etc.). These factors severely constrain the locations in the RNA target that are accessible, and the range of timescales and spatial environments available for small PTGS ligand attack. It is necessary to identify stable, accessible regions for PTGS within this cellular milieu. Moreover, the PTGS ligand must present in the same locale as the target, at sufficient concentrations to allow diffusion-limited interaction, and in conformational state(s) that enable effective annealing and catalysis to achieve successful knockdown. These challenges define the slow entry of PTGS agents into the pharmaceutical market, despite their obvious clinical potential. In fact, only a single antisense agent, Vitrovene (fomiversen) (Novartis, Isis), was approved in August 1998 by the FDA for ocular use in CMV retinitis. Vitrovene (Isis-2922) is a 21-mer phosphorothioate antisense molecule that anneals to the coding region of the immediate early (IE55 gene) mRNA transcribed from the CMV genome (Anderson, Fox, Brown-Diver, et al., 1996). No ribozyme or RNAi agents are yet approved but, like antisense agents, many are in clinical trials. The therapeutic potential of PTGS agents motivates the development of high throughput screening (HTS) approaches to embrace the biocomplexity challenges presented by the target and the ligand. In this paper, we present aspects of target RNA biology that will convince the reader about the complexity of PTGS development. We then present the bottlenecks that exist in PTGS development and an overview of technologies that are emerging to deconstrain these bottlenecks. The goal is more rapid realization of efficacious and safe PTGS therapeutics for the eye or other organs. Given the complexity of the challenge of developing RNA-directed drugs it is not at all surprising that there has been slow entry into the pharmaceutical marker. It costs hundreds of millions of dollars to take a drug to clinical market. RNA-directed drugs are still largely on the horizon. Recent emergence of tools to address difficult scientific issues underlying the biocomplexity of the transcriptosome and RNA structure/function offer substantial hope that the dawn of RNA-directed drugs is visible in the near future.
PTGS Technologies
All PTGS technologies depend upon annealing between target RNA and the AS component of the PTGS ligand. AS agents are commonly built upon a deoxyribonucleic acid backbone, are transfected into cells, and are designed to bind to an accessible region of the target RNA, to promote RNaseH mediated cleavage of target and/or translational inhibit through ribosome stalling. Therefore, AS agents must recognize an accessible region and bind tightly in order to insure a long hybrid lifetime. Ribozyme agents are commonly expressed as RNA molecules from genetic templates or delivered as synthetic ribonucleotides with ribose base modifications to increase stability. Rzs must bind to an accessible region of target RNA, cleave the target RNA, and then dissociate from the target and perform the same series of reactions (enzymatic turnover) with other substrate RNA molecules. Rzs must bind to target RNA with sufficient strength to insure a hybrid lifetime that allows chemical cleavage of target RNA, but not so strongly that the product dissociation is slow and inhibits turnover (product inhibition). RNAi agents are commonly expressed as short hairpin RNAs (shRNA), or delivered as chemically stabilized short interfering RNAs (siRNAs). Expressed shRNAs are cleaved in the nucleus by Drosha endonuclease and further processed by the cytoplasmic nuclease Dicer III, to achieve a mature RNAi. The double stranded RNAi is recognized by the RNA-induced silencing complex (RISC), which selects the AS strand on the basis of designed thermodynamic end stability, and then uses this charged RISC to anneal to accessible regions of target RNAs to promote target cleavage. The RISC complex is tolerant of several mutations in the 14 nt long AS stretch that allows target recognition. In addition siRNAs can simulate microRNAs and bind to the 3′ untranslated region of mRNAs to inhibit translation. These factors are likely responsible for the plethora of off-target effects of RNAi and related toxicity (Federov, Anderson, Birmingham, et al., 2006).
Bottlenecks in Development of PTGS Therapies
There are several phases and bottlenecks in the development of PTGS agents: 1) identification of candidate molecular targets, 2) validation of targets, 3) identifying accessible regions in target RNAs, 4) identifying lead candidate PTGS agents targeting those accessible regions, 5) optimizing lead candidates, 6) preclinical testing in appropriate animal models. Each of these steps embraces biocomplexity that delays the speed at which PTGS agents are developed. We recently reported (Sullivan et al., 2007, Elsevier Conference, Retinal Degeneration and Gene Therapy) that several of these bottlenecks can be relieved with technologies emerging, in part, from work this laboratory.
Validation of Molecular Targets for PTGS Therapy
Validation of an ideal molecular target is the establishment of knowledge that chronic upregulation of a normal wild type (WT) target RNA and protein, or expression of a mutant target RNA and protein, is necessary and sufficient for the emergence of disease. Other molecules may be essential for disease to emerge and so a validated target may be necessary but not sufficient for disease. It is critical to understand that the disease emerges in time in a cellular system of gene expression. The emerging field of systems biology addresses these issues.
In an autosomal dominant retinal degeneration it is rationale to look to the mutant mRNA and protein as necessary and sufficient to initiate the disease process, but there are additional considerations. First, there is tremendous allelic and phenotypic heterogeneity in genetic retinal degenerations. Mutations in a single gene can cause multiple clinical anatomic phenotypes or a range of phenotypes within a single clinical disease classification. Consider autosomal dominant mutations in peripherin/RDS which cause several clinically distinct syndromes: retinitis pigmentosa (RP), macular dystrophy, pattern dystrophy, fundus flavimaculatus, and retinitis pigmentata albescens (Kajiwara, Hahn, Mukai, et al., 1991; Kajiwara Sandberg, Berson, et al., 1993; Nichols, Sheffield, Vandenburgh et al., 1993; Travis and Hepler, 1993; Weleber, Carr, Murphey, et al., 1993; Wells, Wroblewsky, Keen, et al., 1993). Mutations in rod opsin can cause retinitis pigmentosa, congenital stationary night blindness, or retinitis punctata albescens (see Daiger, Sullivan, Brown, et al., 2007). Phenotypic heterogeneity is ultimately a problem at the protein level of systems biology, as different mutations in even a single amino acid codon can lead to starkly different clinical diseases. Different mutations in a single gene can cause retinal degenerations with a broad range of times of onset and rates of loss. For example, autosomal dominant mutations in rod opsin can cause a broad range of phenotypes including early onset and rapidly progressive RP (e.g. C187Y), later onset and slowly progressive RP (e.g. P23H), or congenital stationary night blindness (e.g. G90D). How variation in the structural and molecular biology of such proteins contributes to the broad time scales of disease is far from understood, even for proteins such as rod rhodopsin that are well characterized.
Second, to develop PTGS therapeutics a first critical step is to determine whether a given mutation or set of mutations causes haploinsufficiency, gain-of-function, or dominant negative effects. A dominant mutation may cause loss of functional protein and the 50% of WT protein expressed from the remaining unmutated allele may be insufficient to promote cell function and vitality. Alternatively, the mutated allele may express proteins that have direct toxic effects on cell metabolism. Mutant proteins may misfold and become trapped in the endoplasmic reticulum and Golgi apparatus, where they can elicit the unfolded protein response that can lead to apoptosis (Johnson, Ward, and Kopito, 1998; Kopito, 2000; Lai, Rooney, Lee, et al., 2000; Rajan, Illing, Bence, et al., 2001; Illing, Rajan, Bence, et al., 2002; Saliba, Munro, Luthert, et al., 2002; Ron and Walter, 2007). Mutant proteins may traffic inappropriately and cause toxicity in the new cellular locales in which they take residence. Mutant proteins may have aberrant signaling properties or build aberrant macromolecular structures that create toxicity for the cell. A mutant protein could interact with a plethora of potential signaling and regulation pathways, each of which has unique input/output properties and dynamic range. Another gain of function effect of a mutant protein is called the dominant negative effect where a mutant protein acts to prevent the normal function of the WT protein. For example, the mutant protein may prevent the normal trafficking of the WT protein to the region of the cell where it is otherwise destined. Prior to development of PTGS agents for autosomal dominant diseases, consideration should be given to the nature of the cellular effects of the mutation (haploinsufficiency, gain-of-function, dominant negative). Therapy chosen should depend upon the cellular defects identified. Mutations that create haploinsufficiency (autosomal recessive, dominant negative) would benefit from WT gene reconstitution rather than attempts to silence the mutant gene or change the ratio of mutant/WT mRNAs with PTGS therapy. It is the gain-of-function mutations that can benefit from PTGS therapies. Most autosomal dominant mutations are likely to be gain-of-function mutations. Haploinsufficiency impact can be determined with heterozygous knockout mouse models for the gene in question and comparing function and cell viability with WT and knockout mice in both juvenile and older animals. Many gain-of-function effects can be determined in cell culture studies, although cell culture studies are not always reliable (e.g. Sung, Schneider, Agarwal, et al., 1991). For example, mutant human P23H expression in HEK293S cells traps in the endoplasmic reticulum and Golgi apparatus, whereas in vivo P23H traffics, at least in part, to the outer segments (Olsson, Gordon, Pawlyk, et al., 1992). Complementary studies to determine gain of function effects should be conducted in transgenic mouse models. Dominant negative effects can be demonstrated by overexpression of WT protein and determining the extent to which it ameliorates the phenotype. However, it is impractical to develop transgenic models for all mutations in a given mutation-abundant gene, to the extent that the mouse represents human biology. Correlative studies involving existing animal models and well chosen cell culture systems may aid to address this issue.
Several studies have shown the potential for use of Rz or RNAi PTGS agents for therapy of hereditary retinal degenerations when the targets are rod opsin or peripherin (e.g. Millington-Ward, O’Neill, Tuohy, et al., 1997; Lewin, Drenser, Hauswirth, et al., 1998; Sullivan, Pietras, Shin et al., 2002; Cashman, Binkley, and Kumar-Singh, 2005; Kiang, Palfi, Ader, et al., 2005). These and other studies show that PTGS agents can be efficacious to slow retinal degenerations in rodent models. Toxicity studies have not yet been conducted.
The greatest potential clinical benefit of PTGS agents exists not in autosomal dominant diseases but rather in retinal degenerations due to chronic upregulation or overexpression of WT mRNAs/proteins. Age-related macular degeneration (AMD) is an ideal model for PTGS therapy. There are validated molecular targets that underlie dry AMD pathogenesis (Petrukhin, 2007). In wet AMD due to pathological angiogenesis, anti-vascular endothelial growth factor (VEGF) therapy has established that VEGF is a suitable molecular target for PTGS therapy. VEGF is overexpressed in the emergence of choroidal neovascularization (CNV) and is essential for vascular endothelial cell replication and migration. Recent studies have developed shRNAs that are designed to knockdown VEGF mRNA and protein (Cashman, Bowman, Christofferson, et al., 2006). Ultimately, validation of PTGS agents as candidate therapies can only be determined in appropriate animal models of human disease. The target should be overexpressed in the disease, such as with VEGF and CNV, and knockdown of the target below normal levels should slow or ablate the disease process. Degenerative macular and retinal diseases emerge over time in retinal cell biological systems. A given disease may not be modulated by PTGS attack on a single target. Multiple targets are likely to have expression levels that correlate with the temporal emergence and progression of a given disease. Some targets may be upregulated and some downregulated. Multiple, validated targets may require simultaneous PTGS modulation for maximum therapeutic efficacy. Regulation of PTGS agents will likely be needed, however, to avoid toxicity and/or potential therapeutic haploinsufficiency. And, termination of the therapy should be planned, should the disease be successfully modulated. These are complex scientific and technological issues. Retinal gene therapy is a cellular systems biology problem.
Identifying Accessible Regions of Target RNAs
Accessible regions in a target mRNA must be identified before rational design of a PTGS agent can begin. This is the most difficult bottleneck in PTGS development of any kind (Rz, antisense, siRNA) because highly accessible sites are rare in any mRNA. All PTGS technologies are conditionally dependent upon a rate-limiting second-order molecular annealing event in vivo. A set of predicted secondary structures is shown for genetic disease target mRNAs (rod opsin, peripherin, bestrophin) and an mRNA involved in hypoxic retinal angiogenesis (HIF-1α) (Fig. 1). Only the minimal folding energy (MFE) structure is shown for the first 1400 nt of each mRNA. Any mRNA target is expected to be annealed into dense intramolecular secondary structure. Ideal annealing platforms are large single stranded regions that exist stably in cells. These are rare in the target mRNAs analyzed.
Figure 1.
RNA secondary structures for human disease targets. (A) rod opsin (NM_000539, 1–1400 nt), (B) peripherin (M62958.1, 1–1400 nt), (C) bestrophin (NM_004183, 1–1400 nt), and (D) HIF-1α (BC012527, 1–1400 nt). Shown are the MFE secondary structures determined by MFold. Only the first 1400 nt could be folded on version 2.3 of MFold on GCG. Note that large single stranded annealing platforms (≥ 12 nt) (labeled with an asterix *) are rare in each target (1 in RHO, 4 in RDS, 3 in BEST-1, 3 in HIF-1α). The bulk of the mRNA is folded into dense and mostly stable intramolecular secondary structure that is expected to resist annealing of PTGS agents.
There are a variety of rational and combinatorial approaches to identify accessible sites in RNAs. In a popular approach, discrete or combinatorial ODN binding is followed by RNaseH cleavage and RNA primer extension by reverse transcriptase to locate the cleavage sites (Ho. Bao, Lesher, et al., 1998; Scherr and Rossi, 1998). This approach is slow and cumbersome, gel-based, and has many pitfalls. The location of the cleavage site is not precisely known in combinatorial searches because RNaseH cleaves in the middle of the ODN: RNA hybrid region without sequence specificity. Arrays of ODNs can effectively search for accessibility sites, but this requires specialized machinery and is far from high throughput. Discrete ribozymes or combinatorial ribozyme libraries can be used to cleave RNAs with RNA extension or RT/PCR with 5′ tailing used to identify cleavage sites in procedures that are cumbersome and slow (Lieber and Strauss, 1995). Recent studies from the Clawson lab have applied in vitro Systematic Evolution of Ligands by EXponential Enrichment (SELEX) to the challenge of identifying highly efficient hammerhead ribozyme (hhRz) sites in long natively-structured RNA targets (Pan, Devlin, Kelley, et al., 2001) (Fig. 2). These approaches led to hhRzs with catalytic efficiencies (kcat/Km) on the order of 106 M−1min−1, comparable to rates observed against tiny unstructured model RNA targets. This shows that truly accessible sites can be identified. This approach can be conducted in cellular extracts where an RNA target is made more complex by heterogeneous protein coating. Combinatorial HTS tools such as SELEX that embrace molecular evolution are needed to better learn the rules of selecting regions from the folded RNA that can prove efficacious for knockdown. We are developing a SELEX system in HEK293 cells to evolve efficacious hhRzs (Butler, Misasi, and Sullivan, work in progress).
Figure 2.
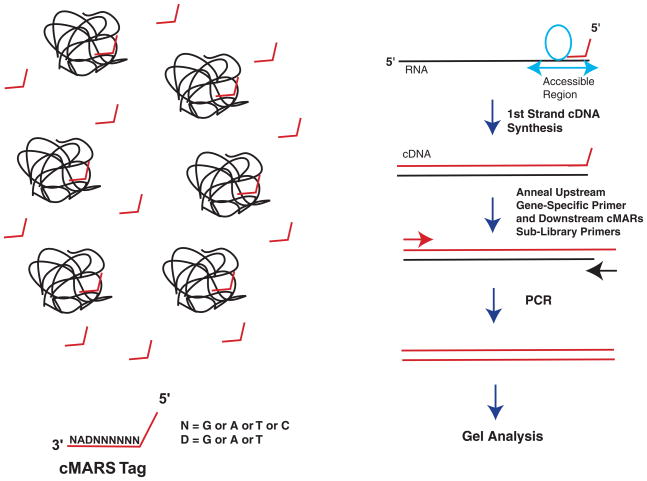
SELEX procedure to identify regions of a target mRNA that are accessible for hhRz design. A double stranded cDNA library is first generated from partially overlapping single stranded oligonucleotides and contains a randomized region with a central GA (antisense to the UC↓ of robust cleavage sites). The cDNA library and T7 RNA polymerase are used to transcribe a combinatorial guide sequence RNA library. Note that this guide sequence library is designed to anneal to accessible sites that also contain NUC↓ cleavage sites, but there is no hhRz enzyme to promote cleavage of the target RNA. The RNA guide library is then mixed with the target RNA and allowed to anneal. Guide RNAs bound to the target RNA are isolated by purification of the target RNA on nondenaturing gels. Reverse transcription is used to regenerate the single stranded cDNA and then PCR is conducted to recover and amplify a highly enriches subset of the library that contains sequences capable of annealing to target RNA. Using lower levels of target RNA the enriched library is then used to reprobe the target and the evolutionary procedure repeated several times, with lower levels of target RNA to increase stringency. Finally, PCR products are TA cloned and sequenced to identify the accessible regions of the target RNA that also contain NUC↓ cleavage sites.
Over the last two years we have completed development of three complimentary approaches that allow us to rapidly identify regions of accessibility in target mRNAs for development of hhRz or shRNA PTGS agents. Computational approaches are improving and have been effective to predict accessible sites in RNAs for PTGS agent development (Amarzguioui, Brede, Babaie, et al., 2000; Mathews, Burkard, Freier, et al., 1999; Mathews, 2006; Zuker, 2003; Ding, Chan, and Lawrence, 2004; Scherr, Rossi, Sczakiel, et al., 2000; Patzel and Sczakiel, 1998). It has become clear to us that the optimum way to employ computational tools is to use them in aggregate so that multiple parameters can be used to estimate both steric and energetic features of local RNA folding. We use a set of algorithms (MFold, SFold, and OligoWalk) to predict accessible sites in RNAs (Maksoud and Sullivan, 2003; Maksoud et al, submitted; Sullivan and Taggart, 2007; Taggart et al., in preparation). We use MFold to obtain a rigorous statistical analysis of the probabilities of sterically accessible regions in the fold. We test these outcomes with SFold which uses an independent algorithm not dependent upon free energy (ΔG) minimization to determine access probabilities. Finally we use OligoWalk to measure and map the local free energy (LFE) along the target RNA. We convolve all parameters and rank order the predicted accessible regions. We call this approach multiparameter prediction of RNA accessibility (mppRNA). mppRNA was successfully applied to two mRNA targets coding for human rod opsin and human secreted alkaline phosphatase (SEAP) (Sullivan and Taggart, 2007; Sullivan et al., in preparation). We are working to develop our convolution model to predict suitable hhRz cleavage sites by validation testing against efficacy datasets from other targets.
Recent efforts seek to identify HTS experimental systems to search for accessible sites in RNAs. mRNA Accessible Site Tagging (MAST) is another HTS approach to screen mRNAs for accessibility (Zhang, Mao, Zhou, et al., 2003). This involves a combinatorial library of AS sequences bounded by constant sequences used both to bind clamping oligonucleotides (ODNs) and for PCR amplification. With the clamping ODNs only the combinatorial (random) region is single stranded and available to anneal to target RNA. Such a MAST ODN library is bound to an RNA target that is fixed to a magnetic bead. Unbound ODNs are washed away and the bound tags amplified by PCR for cloning and sequencing. The approach has a distinct limitation in that annealing and washing temperatures are physiological, which limits stringency and specificity. Annealing sites of only 6–8 nt are identified whereas the combinatorial library has randomized regions of 18 nt. We were unsuccessful in applying the combinatorial MAST approach to screen for accessible sites in human rod opsin mRNA. However, we utilized our experience to develop gene-specific MAST (gsMAST) to test accessibility at sites in opsin mRNA predicted by mppRNA or previously tested in cultured cells (Maksoud et al., submitted) to validate this approach. gsMAST strongly confirmed accessibility at one of two sites where leads were identified in our first study (Maksoud et al., submitted) (Fig. 3). gsMAST also confirmed accessibility at most sites that were predicted by the in silico mppRNA approach (Sullivan and Taggart, 2007; Taggart and Sullivan, in preparation)
Figure 3.
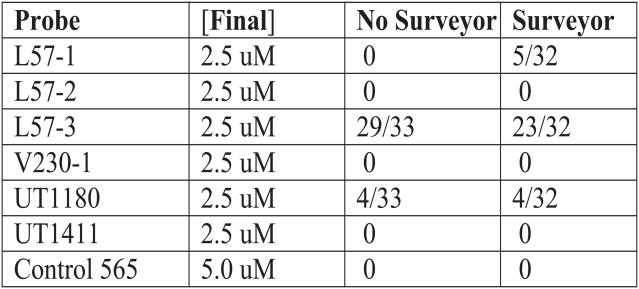
Gene-Specific mRNA Accessibility Site Tagging (gsMAST). (A) Schematic. cRNA is transcribed in vitro with biotin-UTP and attached to magnetic beads. A gsMAST probe is a clamped ODN that contain a single stranded antisense region of 18 nt (N18) that is directly complementary to a region of the target mRNA, and upstream and downstream constant regions that are clamped by small complementary ODNs. A set of such probes complementary to different regions in a target are synthesized and annealed. The mixture is then allowed to anneal to the target under competitive conditions. The target and beads are extensively washed. The annealed gsMAST tags are then displaced and amplified by PCR before cloning to obtain their sequence. The relative number of clones isolated indicates the relative probability of access of that region. (B) Table of gsMAST tag screen of full length human rod opsin mRNA. All antisense tags were designed against human opsin mRNA based upon a prior hhRz study (Maksoud et al., submitted). A control tag was designed as a sense tag to opsin. The antisense tags were present at 2.5 μM while the control tag was present at 5.0 μM. The numbers of tags of each type identified in two sequencing samples is shown. In one sample a single stranded deoxyribonuclease (Surveyor) was used to attempt a decrease of background noise, while the other simple contained no nuclease (results were comparable). The expected outcome for uniform random selection of outcomes is 4.125 for the sample without Surveyor and 4.0 for the sample with the nuclease. Chi-squared statistics were used to evaluate the outcomes of the sampling compared to the expected frequency, without or with the nuclease (without nuclease: χ2 = 183.0, critical value 12.59 (α = 0.05) with degrees of freedom = 6, p < 0.001; with nuclease: χ2 = 118.53, critical value 12.59 (α = 0.05) with 6 degrees freedom, p < 0.001). For the sample without the nuclease subset χ2 analysis showed that the nonuniformity of the sample resides in L57-3 and UT1180 (χ2 = 174.75, critical value 5.991 (α = 0.05), p < 0.001). For the sample with the nuclease subset χ2 analysis showed that the nonuniformity of the sample resides in L57-3, L57-1, and UT1180 (χ2 = 92.72, critical value 7.815 (α = 0.05), p < 0.001). The most frequent binding gsMAST tag antisense sequence is L57-3 (5′ GGTGACGTAGAGCGTGAG 3′), which binds the sense sequence of opsin mRNA 5′ CUCACGCUCUACGUCACC (264–281). The L57-1 antisense sequence (5′ GTAGAGCGTGAGGAAGTT 3′) binds the overlapping (to L57-3) sense sequence of opsin mRNA 5′ AACUUCCUCACGCUCUAC 3′ (258–275). The 3′UT1180 antisense sequence (5′ TGGCTGGGGGAAGGTGTA 3′) binds to the 3′UT region of opsin mRNA 5′ UACACCUUCCCCC AGCCA 3′ (1189–1206). The regions around 250 (33 nt, dominant substate) and 1180 (39 nt, co-dominant substate with 12 nt loop substate) have the largest predicted single-stranded annealing sites in the target. A lead hhRz candidate targets the 250 region loop, whereas knockdown in the 1180 loop occurs to a lesser level. The UT1411 site has a smaller predicted loop (14 nt) and supported lead hhRz identification. The V230-1 site has only an 8 nt loop that permits no hhRz knockdown.
Allawi, Dong, Ip, et al. (2001) described a combinatorial approach to determine accessible sites in four mRNA targets for AS and RNAi attack with randomized libraries attached to a constant upstream PCR sequence that prime first strand cDNA synthesis. We recently developed a combinatorial approach also based upon first strand cDNA synthesis, which maps hhRz cleavage sites in regions of accessibility in any RNA. We call this approach cDNA Mapping of Accessible Ribozyme Sites (cMARS). The method is fast, simple, gel-based, and quantitative. cMARS has validated sites of accessibility predicted by mppRNA and confirmed by gsMAST (Sullivan and Taggart, 2007; Taggart and Sullivan, in preparation) (Fig. 4). The existing cMARS combinatorial library can be used for HTS screening of accessibility in arbitrary mRNA targets.
Figure 4.
cDNA Mapping of Accessible Ribozyme Sites (cMARS). (A) Schematic. Numerous full length target mRNAs are present in solution. cMARS combinatorial libraries containing a constant 5′ region (for downstream PCR primer binding) is followed by a random hexamer to probe accessible regions, and followed by the antisense to NUH↓ (↓DAN) (where D (complement to H) = G, U, A). A single site is accessible and elements of the library able to anneal do so and saturate the site because the probes are in excess. cDNA synthesis from such primed sites allows PCR from any of a number of upstream probe priming sites. The size of the gel band localizes the site of annealing and the nature of D and N in each library identifies the precise hhRz site in that region. (B) Shows an agarose gel with intense bands present between 200 and 300 base pairs. G, A, T, C lanes refer to the identity of the 3′ terminal nt (N) of the hhRz (NUH↓) antisense motif sequence 5′ DAN 3′. The size of the intense bands maps them (with a single forward PCR primer) to the region around 250 nt. The two intense bands in the G lane correspond to CUH↓ cleavage motifs, the two intense bands in the A lane correspond to UUH↓ cleavage motifs, the two intense bands in the T lane correspond to AUH↓ motifs, and the fainter bands in the C lane correspond to GUH↓ cleavage motifs. There are a number of NUH↓ sites corresponding to these possibilities in the region. Absolute identity of the site is obtained by sequencing gel purified bands.
The biological nature of the target used to identify accessible sites could have a substantial impact on PTGS outcomes determined. We used in silico RNA targets, in vitro transcribed naked cRNA targets, and mRNA targets transcribed in cellulo to measure accessibility. The first two deal with a simulated or real naked RNA, while the latter is the form in which one expects the mRNA to be made in vivo. Another form of target that we have not used is an in vitro transcribed cRNA that is mixed with cellular protein extract. This latter approach adds the variables of protein RNA interaction and dynamics to screening for accessible regions. Scherr et al. (1998, 2000) used the later approach to screen for accessible sites for AS molecules. Pre-mRNAs are bound by heterogeneous nuclear proteins during synthesis. There is change in the composition of bound proteins upon pre-mRNA splicing to form a mature mRNA, during transport to the cytoplasm, and upon taking residence in the cytoplasm. Some of these proteins have structural or functional roles and some are chaperones. The extent to which the naked target cRNA is represented in the final set of mRNA structures or a singular native structure that become targets for PTGS is not known (Woodson, 2000). Our in silico approaches (mppRNA) identify high stability local secondary structures with large single stranded annealing platforms. Strong local RNA secondary structures form rapidly during transcription and are less likely to be impacted by heterogeneous or specific RNA binding proteins than are less stable regions. Use of a diverse tool set to identify PTGS targeting sites is robust, especially if positive correlation of outcomes exists among different approaches as we observe thus far.
Identifying Lead PTGS Candidates
The next major bottleneck in PTGS development is testing of many agents to identify lead candidates. PTGS cDNAs are prepared from synthetic ODNs prior to cloning in expression vectors (for Rz or RNAi). Ideally, one uses an expression vector that optimizes efficient cloning of small PTGS cDNAs. One must give strong consideration to the strength of promoter used to express both the PTGS ligand and the target RNA. These choices will have profound effect on the outcomes of efficacy testing. Different promoters have different strengths of transcriptional initiation by RNA polymerases type II or III. The concentration of PTGS agent (the enzyme [E] (for Rz) or ligand [L] (for AS and RNAi), [E] and [L] used synonymously hereafter) and the target RNA (the substrate [S]) are dictated by the relative promoter strengths and the natural decay rates of the PTGS and target RNAs. Depending upon the promoters and the half-lives of the PTGS and target RNA, in the Michaelis-Menten enzymatic sense, the [S]/[E] ratio can vary over many log orders in any given experimental paradigm. If the PTGS promoter is not strong and its RNA half-life short, it will be difficult, if not impossible, to measure target knockdown in any experimental paradigm, even if the PTGS agent was able to recognize and cleave the target with high efficiency. The useful dynamic range in any PTGS agent screening experiment must be a strongly specified variable. Once successful lead candidates have been identified one can then explore the range of [S]/[E] ratios under which efficacy remains manifest or is optimized. We have used strong CMV and Pol-III promoters to express hhRzs or shRNAs, and SV40 or CMV promoters to express target mRNAs in cells.
To test discrete PTGS agents in cultured cells there are several paradigms that might be used: P1) cotransfection of both target and PTGS expression plasmids into cells that are naïve to the expression of target and PTGS agent, P2) transfection of PTGS plasmid into a stable cell line that expresses the target mRNA and protein, P3) transfection of PTGS plasmid into a stable cell line where target cDNA expression is under tight experimental inducible control, and P4) evaluating efficacy when both the target and the PTGS agent are under inducible control in a stable cell line. While P1 is the simplest paradigm, experimental variability for two independent parameters adds to the number of experiments necessary for strong statistical testing power at chosen level of significance. P2 has the advantage that only a single PTGS plasmid is transfected, but the disadvantage is that a constitutive level of target mRNA and protein are already expressed and accumulated and cannot be attacked by the PTGS agent transcribed by the transfected plasmid (floor knockdown effect). This reduces dynamic range of the assay. A good example of this approach is the delivery of PTGS agents intended to knockdown rod opsin levels in vivo. Rhodopsin is a highly stable protein at physiological temperatures and is stable for at least 14 days in the mammalian eye. Knockdown experiments in cell culture have a practical limit of 48–72 hours before cells overgrow. One expects that the lifetime of opsin protein would far exceed the time limit of the experiment, as was previously suggested in 293S cell culture (Sung, Schneider, Agarwal, et al., 2001). All of the opsin protein already synthesized in the cell, and the opsin mRNA being transcribed and translated at the time of PTGS plasmid transfection is not subjected to PTGS agent knockdown. A 100% knockdown of target mRNA within 24 hours of transfection (unlikely) will leave at least 50% opsin protein at assay point at 48–72 hours. P2 is a low dynamic range assay unless the measured target protein has a relatively short half life. Targets with extremely short mRNA half lives (minutes) have intrinsic decay kinetics that are likely too fast for the kinetic realm of PTGS agents. P3 is a more ideal strategy but requires that stable low-off state robust-inducible cell lines be generated to control a wide dynamic range of target gene expression after the PTGS agent has been transfected. An added feature of the P3 paradigm is that the [S]/[E] ratio can be varied. [S]/[E] is expected to have a substantial impact on PTGS efficacy. In the P4 paradigm both the target and the PTGS agent are induced to express with different inducers (e.g. doxycycline, IPTG). While two gene regulated cell lines are considerably harder to realize, this formulation adds flexibility compared to P3 in that much wider range of [S]/[E] ratios are achievable.
There may be question about the validity of culture systems to test PTGS agents to identify lead candidates. PTGS occurs within the housekeeping metabolism of mammalian cell biology. Transcription, RNA folding, intracellular RNA trafficking, RNA-mediated catalysis, and ribosomal translation are expected to occur by the same mechanisms, with the same macromolecules, and on the same time scales as they would otherwise occur in the cellular target system in vivo. Therefore, efficacy outcomes of PTGS testing in cell culture expression systems are expected to be predictive of in vivo performance, especially if the tested [S]/[E] range simulates the realizable in vivo condition.
In the initial phases of lead candidate identification we recommend use of the P1 paradigm of cotransfection of both ligand and target plasmids, with appropriate transfection efficiency controls (e.g. pEGFP-N1). We chose a high ligand/target plasmid ratio when the ligand was expressed by an intragenic adenoviral VAI promoter (Pol-III) and the target was expressed by a CMV promoter (Pol-II). Under this P1 condition one expects that the PTGS ligand will be in substantial molar excess over target mRNA and colocalized with target RNA in the cytoplasmic compartment. Both conditions act to optimize annealing. The expected outcome is a bias in the [E]/[S] ratio with the PTGS agent in substantial excess over target (substrate). Under these lead screening conditions [E] ≫ [S] and with compartmental colocalization we expect that target accessibility in live cells becomes the dominant factor in testing for knockdown efficacy.
We initially recommend screening for lead PTGS candidates by the most efficient and reliable approach to measure either the target RNA or/and protein. Measures of target protein are the most efficient means to quantitate the relative knockdown of a series of PTGS agents, provided that one has a high affinity monoclonal or polyclonal antibody to use for protein detection. We initially employed western analysis to quantitate target opsin proteins co-expressed in HEK293E cells with a panel of VA1-hhRzs under paradigm P1 (Maksoud and Sullivan, 2003; Maksoud et al., submitted). With such an approach we identified two lead hhRz candidates against human rod opsin. However, we recognized a profound bottleneck in screening for lead candidates with western analysis, which is at best semi-quantitative, user-dependent, slow, and experimentally cumbersome. We then invested substantial effort to develop alternative high throughput screening to measure target protein or RNA.
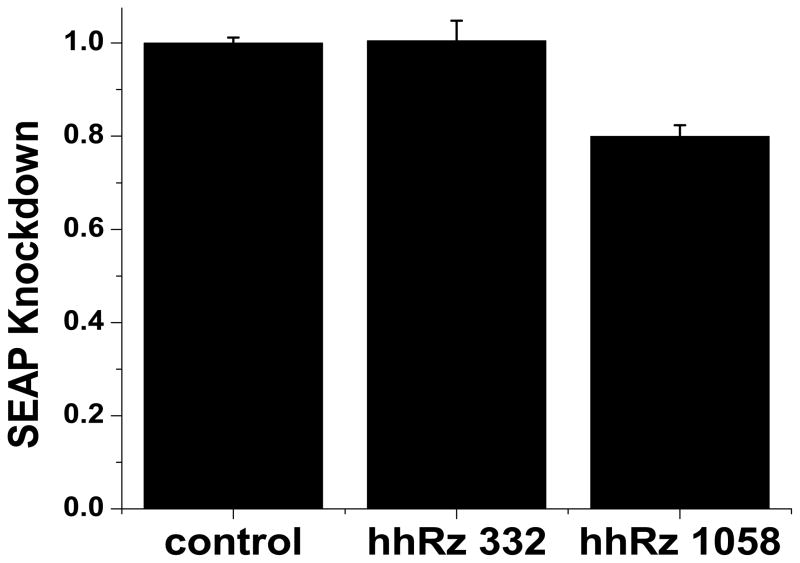
To relieve bottlenecks in screening we considered proteins that are assayed easily by HTS plate readers. We considered SEAP and various short half-life forms of EGFP (d2, d4) and decided to develop the SEAP approach because cells secrete the bulk of protein (95%) into the culture medium in proportion to steady-state mRNA levels (Berger, Hauber, Hauber, et al., 1988). This allows for live cell kinetic analyses. Fusion RNAs are useful tools for HTS of gene expression (Hüsken, Asselbergs, Kinzel, et al., 2003). One can engineer a fusion RNA between a target of interest and an easily assayed target such as SEAP or EGFP. RNA folding is known to occur cotranscriptionally and weaker tertiary RNA structure forms secondary to stronger secondary structure (Tinoco and Bustamante, 1999). Therefore, it is prudent to place the target upstream of the reporter in the fusion RNA. In this position the reporter RNA component is less likely to impact the folding of the target RNA component. To embrace the 3′UT of the target as a suitable PTGS attack site, it would be necessary to convert all in-frame stop codons in the 3′UT to coding triplets before in-frame fusion of the reporter cDNA. Or, one can place the full length unaltered target cDNA upstream of an internal ribosome entry site (IRES) element and the reporter cDNA downstream to form a bicistronic mRNA that codes for both the target protein and the reporter protein. With such a construct all four paradigms could be used to screen panels of PTGS agents. PTGS agents directed to anneal to target RNA components will promote suppression of the reporter protein fraction, which is readily and reliably assayed in a 96-well plate reader type format, and the target protein. This HTS approach has substantially relieved bottlenecks to identify and optimize lead candidate PTGS agents. Two hhRzs targeting regions of human opsin mRNA predicted by SFold to have substantial accessibility (CUA↓ 332, AUC↓ 1058) were compared for efficacy compared to control vector without hhRz cDNA in a stable cell line expressing Rho-IRES-SEAP (Fig. 5). Statistically significant KD is found for the hhRz predicted to have higher probability access. We recently used this HTS platform to identify five lead candidate hhRzs against human rod opsin mRNA (Yau and Sullivan, 2007; Kolniak, Yau, Taggart, et al., 2007; Yau and Sullivan, submitted).
Figure 5.
HTS hhRz screening. A dicistronic expression construct with built with full length human Rho cDNA (transcription start to immediately before first (dominant) polyA signal), followed by an encephalomyocarditis virus internal ribosome entry site element, followed by the cDNA for engineered human secreted alkaline phosphatase, and then a polyA signal. This construct was expressed stably in HEK293S cells. Cells were transfected with the pUC-VAI vector without hhRz cDNA (control) or with hhRz cDNAs coding for hhRzs that target NUH↓ sites at 332 and 1058 in human RHO. Extracellular SEAP was assayed 48 hours later using a HTS fluorescence microplate reader. (A) Shows two regions targeted in opsin mRNA by SFold. The blue box indicates the expected span of annealing of a 7 nt/7nt hhRz to the target. The 15 nt regions (blue boxes) embracing the 332 CUA↓ and 1058 AUC↓ cleavage sites have similar mean accessible probabilities (0.592 ± 0.095, 0.659 ± 0.081, respectively) as determined by SFold (not statistically different, p = 0.596). (B) Shows the mean levels of SEAP at 48 hours post transfection for control (pUC-VAI), pUC-VAI-hhRz-332, or pUC-VAI-hhRz-1058. One-way ANOVA demonstrated that the means were significantly different (p = 2.53 × 10−6). Post-hoc t-tests shows that there was no significant difference between control and hhRz-332 (p = 0.896) and significant differences between control and hhRz-1058 (p = 2.82 × 10−7) and between hhRz-332 and hhRz-1058 (p = 3.19 × 10−4).
Optimizing Lead Candidates
Lead PTGS candidates will likely need to be optimized, and certainly characterized under a broad range of [S]/[E] conditions. AS ODN candidates can be optimized by varying the length of the complementary region. shRNA probes will be cut by Dicer to 21 nt double stranded elements, so the only modulation possible is to create mismatches between the RNAi and the target over the region of annealing or, if synthetic siRNA is used, to use nucleotide analogues that affect binding affinity. This may or may not influence the leaving rate of cleavage product by Ago2 in RISC, because the latter ATP hydrolysis energy to aid product release (Haley and Zamore, 2004). Optimization potential is, however, very strong for Rzs. A first critical test for a lead hhRz will be to compare target knockdown by lead agents with catalytic core mutants of each of the same. Ideally, catalytic core mutations lead to complete reversal of knockdown, which indicates that the effect is purely catalytic in nature. More likely, there will be a fraction of knockdown that persists despite catalytic inactivation. This component represents an antisense effect, which is directly related to the binding energy of the hhRz AS flanks. It is possible to optimize the hhRz AS flanks with a kinetic model (Stage-Zimmermann and Uhlenbeck, 1998). In this model the AS flank lengths or composition (mismatches) can be optimized in order to maximize specificity while avoiding cleavage product inhibition (Herschlag, 1991; Bertrand, Pictet, and Grange, 1994).
Optimizing lead candidates required identification of the best enzymatic form of the hhRz. It is possible to formulate many different types of hhRz by varying the length of Stem II and its capping sequence, core nucleotides that affect catalysis, or the addition of tertiary accessory elements that are now known to affect catalytic performance (e.g. Khvorova, Lescoute, Westhof, et al., 2003). Such modifications are independent of the AS flank binding energy which is well characterized by the hhRz kinetic model. Instead, such modifications modulate hhRz catalytic rate and potentially the religation rate, which must remain low. For example, minimization of Stem II to two base pairs has been shown to speed up hhRz catalysis while expansions beyond 6 bp have been shown to inhibit catalysis (Persson, Hartmann, and Eckstein, 2002). Such rationale modifications of the hhRz can be tested against any suitable target (e.g. SEAP), and are expected to translate equivalently well to hhRzs directed to any such target. We used our HTS SEAP platform to rapidly identify the best hhRz form in a chimeric RNA (Yau and Sullivan, 2007; Yau and Sullivan, submitted). We evaluated at least ten times as many hhRz candidates in less than one half of the time when compared to classical western analysis. Screening will occur much faster with robotic tools on the platform.
Another concern for optimization of a lead candidate PTGS agent is that it is active against the native RNA that will exist in vivo. Use of fusion or bicistronic mRNAs in HTS identification of leads has a small but finite potential for the accessory RNA elements (e.g. the reporter element) to influence the folding of the true target RNA component, and thus impact PTGS knockdown. In the worst case scenario accessory elements could promote exposure of single stranded annealing platforms that are not present in the native mRNA, or promote closure of a truly accessible site(s) that would remain unidentified. A successful hhRz under such a HTS screen could flop in a native target assay or in vivo proof-of-principle test. In the best case scenario there is no influence of accessory or reporter elements on the folding of the native target RNA and the PTGS agent is expected to have equivalent performance in HTS assays and in vivo. Clearly, the outcomes could test between the extremes. This puts the experimenter back into the discouraging boat of having to conduct low throughput assays. We used our expertise in modern electro-optics (Sullivan, 1998) to develop a quantitative HTS platform to measure any native target protein (and possibly its mRNA). From research into the spectral range of cellular autofluorescence we developed a microscope-based imaging platform that measures and reliably quantifies native proteins (and we expect RNAs) normalized to both cell number and transfection efficiency in single wells of a 96-well array. A high affinity IgG antibody is needed and is usually available for disease target proteins. The antibody (or ODN) is first labeled with a high quantum efficiency, photostable, far-red fluor and binds to expressed target protein (or mRNA) in cells grown, transfected, fixed and permeabilized in a 96-well format (Butler, Kolniak, and Sullivan, 2007; Butler et al., in preparation) (Fig. 6) This HTS platform for native target protein relieves a major bottleneck in PTGS development and optimization. Robotic enhancements will speed analysis on this platform. Given appropriate antibodies, ODNs and fluors, we expect that this system will be useful for high content screening (e.g. multiple simultaneous target quantitation).
Figure 6.
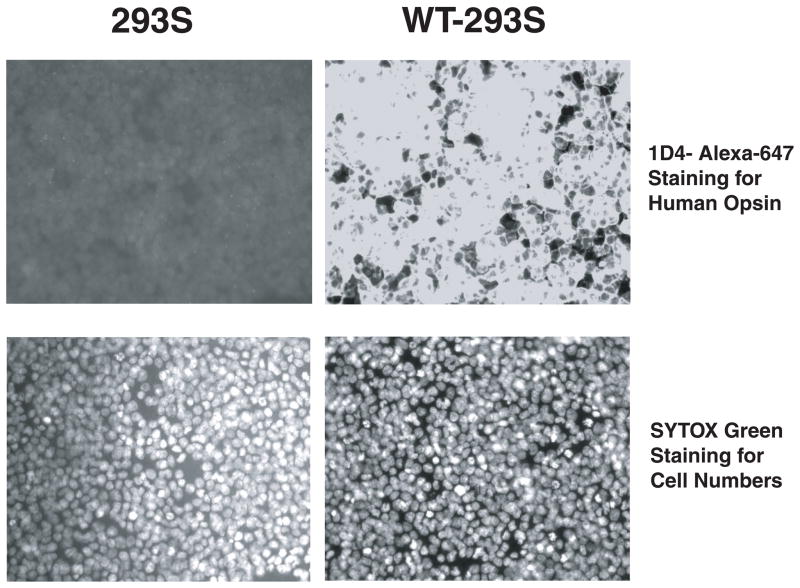
Quantitative Immunocytochemistry System. (A). HEK293S cells (no opsin) and WT-HEK293S cells (expressing 3 million copies of human opsin per cell) were fixed and permeabilized in 96-well dishes. Permeabilized cells were then exposed to an Alexa-647-labelled 1D4 mouse monoclonal IgG antibody and washed. Cells were imaged on an automated stage-controlled inverted fluorescence microscope imaging system equipped with a Hg-vapor lamp and an Alexa-647 specific dichroic cube and a high numerical aperture 20X lens. Cells were also exposed to SYTOX Green which binds avidly to nuclear DNA and can be used to count nuclei. 293S cells demonstrate only dim background non-specific and uniform staining with 1D4-Alexa647, whereas WT-HEK293S cells have intense staining. SYTOX Green labels nuclei in both lines and is used to normalize protein measures in each well to cell number. (B) Mean Alexa-647 fluorescence counts in 293S cells and WT-293S cells (± SEM). Note that the abscissa has a negative extent to demonstrate the small number of counts in the 293S category. By parametric t-test the means were significantly different (p = 2.6 × 10−6). Dynamic range is over 1000 in this experiment.
With these tools in hand we now expect to rapidly screen for accessibility sites, identify lead hhRz and shRNA candidates, and optimize these for preclinical performance. We can do this for arbitrary molecular disease targets at this time. This platform will be of substantial use for bringing HTS screening for PTGS agent development into the academic sector. This platform might be of interest to those planning development of PTGS agents against targets of ocular interest.
Finally, the optimized lead candidate should be tested in human or mammalian cultured cells to simulate the widest range of possible in vivo conditions. PTGS agent outcomes in human cultured cells are expected to simulate performance in vivo provided that the same ligand[E]/target[S] ratios are achieved. The levels of target mRNA and protein and the level of PTGS agent itself should be quantitated under a range of [E]/[S] ratios. The kinetics of knockdown of target mRNA and protein by the PTGS agent, and their intrinsic rates of degradation in the absence of PTGS agents should be measured. The cellular lifetime of the PTGS agent should also be measured. Outcomes in cells can then be compared to outcomes in vivo where the same levels should be quantified. With such robust quantitation the gene therapeutic outcomes and kinetics could be quantitatively modeled. We strongly advise use of inducible stable expression lines for the target and possibly the PTGS ligand (paradigms P3, P4). Maintaining cultured cells for substantial periods of time will promote progressive loss of unselected episomal plasmids and hence nonstationarity in the [L]/[S] ratios.
Finally, if there are several leads it is reasonable to test for additive and synergistic effects on target knockdown especially if well separated annealing sites are targeted. This is managed by P1 paradigm cotransfections. It is also feasible to examine a combined strategy of knockdown and reconstitution with an allelic variant WT expression construct in cultured cell systems, albeit with some caveats. One can separately compare the KD capacity of a hhRz or RNAi against WT and mutant targets, and the expression of an engineered resistant allelic variant WT RNA. It is more difficult to achieve this when WT, mutant and allelic variant-WT (engineered mRNA coding for WT protein that cannot be degraded by PTGS agent) constructs are expressed in the same cultured cells (Cashman et al., 2005).
Preclinical Testing of Lead Candidate PTGS Agents
Ultimately the choice of a preclinical animal model is paramount to reliably predict outcomes of a human clinical trial. There are two levels of approach in preclinical testing. The first is a proof-of-principle type of experiment in a mammalian model (generally small, e.g. mice, rats) which has a phenotype based upon expression of the target of interest, even though that mRNA target is generally not expressed from a human gene. There are many mouse transgenic models that have been engineered to express rod opsin mutant mRNAs (e.g. Olsson et al., 1992; Naash, Hollyfield, Al-Ubaidi, et al., 1993; Li, Snyder, Olsson, et al., 1996). Large scale pig transgenic (P347S, P347L rod opsin) and a naturally occurring dog (T4R rod opsin) models of RP also exist. However, when PTGS agents are developed to target non-human homologue mRNAs they may later be unable to target a human mRNA. Even in genes coding for highly conserved proteins (e.g. rod opsins) the 5′UT and 3′UT components of the mRNA are divergent and the coding region is likely to contain amino acid variations or third position codon wobble variations (degeneracy). A PTGS agent designed to target (anneal to) a particular region of the primary sequence in an animal RNA may succeed in proof-of-principle, but may encounter substantially divergent sequence in the human mRNA, and thus be clinically useless. Or, the targeted region could be sufficiently homologous to allow annealing, but in the human mRNA this particular region may not be accessible and the costly investment in PTGS development is lost. Fortunately, homology of target primary sequences across species can be checked prior to PTGS development in order to allow testing of the same agents in both small and large scale mammalian models and in human trials. A more subtle issue is that the structure of the animal mRNA, which is the primary barrier to successful annealing, is likely to be substantially different from human, even when the proteins coded are highly conserved. We tested this hypothesis through in silico folding of full-length rod opsin mRNAs from four animals: human, mouse, pig, and dog (Fig. 7). Only the coding region was folded computationally to avoid the additional complexity of 5′UT and 3′UT regions, which are known to be divergent. These mRNA segments of identical length code for highly conserved proteins (e.g. there are only 18 out of 348 amino acids that are different between mouse and human). It is clear that the MFE folding structure of the coding regions is markedly different across species. The 5′UT and 3′UT segments would add structural complexity. Therefore, even if the PTGS ligand developed for the animal mRNA has high homology to a region of the human mRNA target, this is no guarantee that the local secondary structure and accessibility will be similar between the human and animal targets. Comparison of sequence homology between human and animal targets at the primary sequence level is insufficient as a reliable predictor. At a minimum a secondary structural analysis could be conducted in silico to compare the RNA folding conformational landscape and target site accessibility at increasingly wider folding windows embracing the central region of intended annealing. However, a human mRNA is the ideal target to begin PTGS development. PTGS development can be managed in cost-effective cell culture systems that express true full-length mutant and/or WT human mRNAs and simple assays of mRNA/protein performed to measure efficacy. To consolidate the timeline and cost of PTGS development for human therapies, we propose that preclinical testing be conducted in mouse models that express human transgenes on a homozygous knockout background of the mouse homologue. It is reasonable to expect equivalent folding outcomes of a human mRNA in mouse or human photoreceptors or in other retinal cell types (e.g. RPE). There are mouse models that express human opsin mRNAs on the mouse rod opsin knockout background (Li et al., 1996; McNally, Kenna, Humphries, et al., 1999). To our knowledge mouse models of human mutant and human WT transgenes on a mouse homologue knockout background are nonexistent for other disease genes at this time. This stands as a call to interest and funding for the development of such models.
Figure 7.
RNA Folding of the Homologous Coding Regions of Opsin mRNAs. The 1047 nt long coding regions of human (Access# NM 000539.2), mouse (Access# NM 145383), dog (Access # X71380) and pig (Access# AF008947) rod opsin mRNAs were folded independently using MFold at 37°C. Regions folded were: 96–1142 (human), 79–1125 (mouse), 137–1183 (dog), and 1–1047 (pig). These regions all span from the start codon through the stop codon. The MFE state is shown for each opsin homologue.
Another issue that was recently addressed (Cashman et al., 2005) is the extent to which the plethora of single nucleotide (nt) mutations that promote human hereditary retinal and macular degenerations might promote changes in the structure of mRNA targets that could influence PTGS efficacy. We tested this hypothesis in silico. We used the full-length dominantly transcribed human rod opsin transcript (1–1532) and made the following single nt mutations for which there are (at least) murine models of disease: T17M, P23H, G90D, K296E, and P347S. WT mRNA was used as control. All mRNAs were folded using MFold and the structure of the MFE state was compared. All of the mutant mRNAs had the same global mRNA structure relative to WT mRNA folded under identical conditions (Fig. 8) (T17M and G90D not shown). Single nt mutations do not cause global changes in mRNA structure at least for this model target mRNA. Such hypotheses would be impossible to test with contemporary experimental tools.
Figure 8.
Effect of Single Nucleotide Mutations on Human Rod Opsin mRNA Folding. We generated and folded in silico five human opsin mutations for which there are current animal models and compared the folding relative to WT human opsin. P23H, K296E, and P347S are shown relative to WT. Mutations were made in the in silico target (1–1532 nt by NM 000539.2). The first 1400 nt (MFold limit) of each RNA was folded with MFold at 37°C. There are no apparent differences in the secondary structures of these minimal folding energy states, and two additional mutants (T17M, G90D) which are not shown. Note the large single stranded loop in WT and all mutant RNAs which is found by MFold in the MFE. This region has proven to be a suitable target for hhRz and RNAi knockdown.
Preclinical testing is the single remaining technological bottleneck in PTGS development. There are several procedures during PTGS testing that might have room for optimization. These include animal injections, psychophysical testing (when used), ERG testing, and histology. Highly efficient organizational schemes may be the best bottleneck relief that is possible. Substantial improvement might be made by conducting subretinal injections such that the entire retina enters into shallow detachment and nearly all photoreceptors or RPE are transduced by the vector. This would reduce difficulties in efficacy evaluations due to loss of dynamic measurement range when only a fraction of the retina is transduced, yet the total retina or RPE mass contributes to ERG measures or molecular measures of target RNA or protein. Measure of transfection efficiency in each eye for quantitative normalizations can be reliably obtained with co-expression of a fluorescent protein (e.g. EGFP). Recent studies have employed rodent retinal explants as a tool for initial testing of PTGS agents (Kiang et al., 2005). This approach has substantial potential for higher throughput testing. Finally, recent efforts suggest that ERG measurements in mice could be conducted at higher levels of throughput (Dalke, Löster, Fuchs, et al., 2004). It appears that substantial technological development could be directed to relieve bottlenecks in the final remaining area of preclinical testing.
Conclusions
The contemporary development of a successful PTGS therapeutic remains a difficult task well described by the term biocomplexity. We presented an overview of PTGS technologies. We presented emerging technologies used in this and other labs to reduce bottlenecks in development of PTGS agents for gene therapy. We expect that these tools will be useful for development of PTGS agents against other validated molecular targets in retinal, macular and ocular diseases, or in general medicine. We presented a base of knowledge to begin work, and a sense of the pitfalls. We want to encourage other investigators by providing infrastructure to proceed down these investigative paths in the interests of the patients suffering with these diseases. A rigorous review of strategies and variables in PTGS agent development will be presented elsewhere (Sullivan et al., in preparation).
Methods
RNA Secondary Structure Prediction
The secondary structure of mRNAs targets was determined with a free energy minimization algorithm (MFold, version 2.3) (Zuker, 2003). MFold was used at 37°C with 10kCal/mol window, a maximum of 99 structures, and with a difference window of 3 bp.
Acknowledgments
We thank the National Eye Institute (R01 EY13433; PI: Sullivan) for funding this work. We acknowledge support of a Research to Prevent Blindness Challenge Grant (to the Ophthalmology Department at the University at Buffalo), and a grant from the Oishei Foundation (Buffalo, NY) to the Dept of Ophthalmology at University at Buffalo. We acknowledge the strong support of the Department of Ophthalmology at University at Buffalo and the strong collegial support from our colleagues at the University at Buffalo and the Western New York Veterans Administration Healthcare System, where this work was conducted. We apologize in advance to all colleagues whose work we could not cite due to space limitations.
ABBREVIATIONS
- AMD
age-related macular degeneration
- AS
antisense
- Best-1
Best macular dystrophy gene (previously known as VMD2)
- cMARS
cDNA mapping of accessible ribozyme sites.
- CNV
choroidal neovascular membrane
- ERG
electroretinogram
- ΔG
free energy (kCal/mol)
- gsMAST
gene-specific mRNA accessible site tagging
- HEK293S
human embryonic kidney cells, suspension adapted
- hhRz
hammerhead ribozyme
- HIF-1α
hypoxia inducible factor-1α
- HTS
high throughput screening
- LFE
local folding energy
- MAST
mRNA accessible site tagging
- MFE
minimum folding energy
- mppRNA
multiparameter prediction of RNA accessibility
- nt
nucleotide
- ODN
oligodeoxynucleotide
- PTGS
post transcriptional gene silencing
- RDS
retinal degeneration slow (protein coded, peripherin)
- RHO
rod opsin
- RISC
RNA-inducing silencing complex
- RNAi
RNA interference
- Rz
ribozyme
- RP
retinitis pigmentosa
- SEAP
secreted alkaline phosphatase
- shRNA
short hairpin RNA
- siRNA
short interfering RNA
- VEGF
vascular endothelial growth factor
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allawi HT, Dong F, Ip HS, Neri BP, Lyamichev VI. Mapping of RNA accessible sites by extension of random oligonucleotide libraries with reverse transcriptase. RNA. 2001;7:314–327. doi: 10.1017/s1355838201001698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarzguioui M, Brede G, Babaie E, Grøtli M, Sproat B, Prydz H. Secondary structure prediction and in vitro accessibility of mRNA as tools in the selection of target sites for ribozymes. Nucleic Acids Research. 2000;28:4113–4124. doi: 10.1093/nar/28.21.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KP, Fox MC, Brown-Driver V, Martin MJ, Azad RF. Inhibition of cytomegalovirus immediate-early gene expression by an antisense oligonucleotide complementary to immediate-early RNA. Antimicrobial Agents and Chemotherapy. 1996;40:2004–2011. doi: 10.1128/aac.40.9.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J, Hauber J, Hauber R, Geiger R, Cullen BR. Secreted placental alkaline phosphatase: a powerful new quantitative indicator of gene expression in eukaryotic cells. Gene. 1988;66:1–10. doi: 10.1016/0378-1119(88)90219-3. [DOI] [PubMed] [Google Scholar]
- Bertrand E, Pictet R, Grange T. Can hammerhead ribozymes be efficient tools to inactivate gene function? Nucleic Acids Research. 1994;22:293–300. doi: 10.1093/nar/22.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MC, Kolniak T, Sullivan JM. Microscope-based high throughput cell protein quantitation for therapeutics development. Investigative Ophthalmology & Visual Science. 2007;48:4608, B445. [Google Scholar]
- Cashman SM, Binkley EA, Kumar-Singh R. Towards mutation-independent silencing of genes involved in retinal degeneration by RNA interference. Gene Therapy. 2005;12:1223–1228. doi: 10.1038/sj.gt.3302512. [DOI] [PubMed] [Google Scholar]
- Cashman SM, Bowman L, Christofferson J, Kumar-Singh R. Inhibition of choroidal neovascularization by adenovirus-mediated delivery of short hairpin RNAs targeting VEGF as a potential therapy for AMD. Investigative Ophthalmology & Visual Science. 2006;47(6):3496–3504. doi: 10.1167/iovs.05-1610. [DOI] [PubMed] [Google Scholar]
- Daiger SP, Sullivan LS, Bowne SJ, Rossiter BJF. RetNet Retinal information network. 2007 http://www.sph.uth.tmc.edu/retnet/(last update 5/21/2007)
- Dalke C, Löster J, Fuchs H, Gailus-Durner V, Soewarto D, Favor J, Neuhäuser-Klaus A, Pretsch W, Gekeler F, Sbinoda K, Zrenner E, Mitinger T, Hrabé de Angelis M, Graw J. Electroretinography as a screening method for mutations causing retinal dysfunction in mice. Investigative Ophthalmology & Visual Science. 2004;45:601–609. doi: 10.1167/iovs.03-0561. [DOI] [PubMed] [Google Scholar]
- Ding Y, Chan CY, Lawrence CE. Sfold web server for statistical folding and rational design of nucleic acids. Nucleic Acids Research. 2004;32:W135–141. doi: 10.1093/nar/gkh449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov Y, Anderson EM, Birmingham A, Reynolds A, Karpilow J, Robinson K, Leake D, Marshall WS, Khvorova A. Off-target effects by siRNA can induce toxic phenotype. RNA. 2006;12:1188–1196. doi: 10.1261/rna.28106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley B, Zamore PD. Kinetic analysis of the RNAi enzyme complex. Nature Structural Molecular Biology. 2004;11:599–606. doi: 10.1038/nsmb780. [DOI] [PubMed] [Google Scholar]
- Herschlag D. Implications of ribozyme kinetics for targeting the cleavage of specific RNA molecules in vivo: more isn’t always better. Proceedings of the National Academy of Sciences USA. 1991;88:6921–6925. doi: 10.1073/pnas.88.16.6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SP, Bao Y, Lesher T, Malhotra R, Ma LY, Fluharty SJ, Sakal RR. Mapping of RNA accessible sites for antisense experiments with oligonucleotide libraries. Nature Biotechnology. 1998;16:56–63. doi: 10.1038/nbt0198-59. [DOI] [PubMed] [Google Scholar]
- Hüsken D, Asselbergs F, Kinzel B, Natt F, Weiler J, Martin P, Häner R, Hall J. mRNA fusion constructs serve in a general cell-based assay to profile oligonucleotide activity. Nucleic Acids Research. 2003;31:e102. doi: 10.1093/nar/gng103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illing ME, Rajan RS, Bence NF, Kopito RR. A rhodopsin mutant linked to retinitis pigmentosa is prone to aggregate and interacts with the ubiquitin proteasome system. J Biol Chem. 2002;277:34150–34160. doi: 10.1074/jbc.M204955200. [DOI] [PubMed] [Google Scholar]
- Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara K, Hahn LB, Mukai S, Travis GH, et al. Mutations in the human retinal degeneration slow gene in autosomal dominant retinitis pigmentosa. Nature. 1991;354:480–483. doi: 10.1038/354480a0. [DOI] [PubMed] [Google Scholar]
- Kajiwara K, Sandberg MA, Berson EL, Dryja TP. A null mutation in the human peripherin/RDS gene in a familiy with autosomal dominant retinitis punctata albescens. Nat Genet. 1993;3:208–212. doi: 10.1038/ng0393-208. [DOI] [PubMed] [Google Scholar]
- Kopito RR. Aggresomes: inclusion bodies and protein aggregation. Trends Cell Biol. 2000;10:524–530. doi: 10.1016/s0962-8924(00)01852-3. [DOI] [PubMed] [Google Scholar]
- Khvorova A, Lescoute A, Westhof E, Jayasena SD. Sequence elements outside the hammerhead ribozyme catalytic core enable intracellular activity. Nature Structural Biology. 2003;10:708–712. doi: 10.1038/nsb959. [DOI] [PubMed] [Google Scholar]
- Kiang A-S, Palfi A, Ader M, Kenna PF, Millington-Ward S, Clark G, Kennan A, O’Reilly M, Tam LC, Aherne A, McNally N, Humphries P, Farrar GJ. Toward a gene therapy for dominant disease: validation of an RNA interference-based mutation-independent approach. Molecular Therapy. 2005;12:555–561. doi: 10.1016/j.ymthe.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Kolniak T, Yau EH, Taggart RT, Sullivan JM. Identification of lead candidate ribozymes for human rod opsin therapeutics. Investigative Ophthalmology & Visual Science. 2007;48:1682, B498. [Google Scholar]
- Lai E, Teodoro T, Volchuk A. Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology (Bethesda) 2007;22:193–201. doi: 10.1152/physiol.00050.2006. [DOI] [PubMed] [Google Scholar]
- Lewin AS, Drenser KA, Hauswirth WW, Nishikawa S, Yasamura D, Flannery JG, LaVail MM. Ribozyme rescue of photoreceptor cells in transgenic rat model of autosomal dominant retinitis pigmentosa. Nature Medicine. 1998;4:967–971. doi: 10.1038/nm0898-967. [DOI] [PubMed] [Google Scholar]
- Li T, Snyder WK, Olsson JE, Dryja TP. Transgenic mice carrying the dominant rhodopsin mutation P347S: evidence for defective vectorial transport of rhodopsin to the outer segments. Proceedings of the National Academy of Sciences USA. 1996;93:14176–14181. doi: 10.1073/pnas.93.24.14176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber A, Strauss M. Selection of efficient cleavage sites in target RNAs by using a ribozyme expression library. Molecular and Cellular Biology. 1995;15:540–551. doi: 10.1128/mcb.15.1.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksoud HEBA, Sullivan JM. Knockdown ribozyme development for adRP gene therapy. Investigative Ophthalmology & Visual Science. 2003;44:S2340. [Google Scholar]
- Mathews DH, Burkard ME, Freier SM, Wyatt JR, Turner DH. Predicting oligonucleotide affinity to nucleic acid targets. RNA. 1999;5:1458–1469. doi: 10.1017/s1355838299991148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews DH. Revolutions in RNA secondary structure prediction. Journal of Molecular Biology. 2006;359:526–532. doi: 10.1016/j.jmb.2006.01.067. [DOI] [PubMed] [Google Scholar]
- McNally N, Kenna P, Humphries MM, Hobson AH, Khan NW, Bush RA, Sieving PA, Humphries P, Farrar GJ. Structural and functional rescue of murine rod photoreceptors by human rhodopsin transgene. Human Molecular Genetics. 1999;8:1309–1312. doi: 10.1093/hmg/8.7.1309. [DOI] [PubMed] [Google Scholar]
- Millington-Ward S, O’Neill B, Tuohy G, Al-Jandel N, Kiang AS, Kenna PF, Palfi A, Hayden P, Mansergh F, Kennan A, Humphries P, Farrar GJ. Strategems in vitro for gene therapies directed to dominant mutations. Human Molecular Genetics. 1997;6:1415–1426. doi: 10.1093/hmg/6.9.1415. [DOI] [PubMed] [Google Scholar]
- Naash MI, Hollyfield JG, Al-Ubaidi MR, Baehr W. Simulation of human autosomal dominant retinitis pigmentosa in transgenic mice expressing a mutated murine opsin gene. Proceedings of the National Academy of Sciences USA. 1993;90:5499–5503. doi: 10.1073/pnas.90.12.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols BE, Sheffield VC, Vandenburgh K, Drack AV, et al. Butterfly-shaped pigment dystrophy of the fovea caused by a point mutation in codon 167 of the RDS gene. Nat Genet. 1993;3:202–207. doi: 10.1038/ng0393-202. [DOI] [PubMed] [Google Scholar]
- Nicholson JK, Holmes E, Lindon JC, Wilson ID. The challenges of modeling mammalian biocomplexity. Nat Biotechnol. 2004;22:1268–1274. doi: 10.1038/nbt1015. [DOI] [PubMed] [Google Scholar]
- Olsson JE, Gordon JW, Pawlyk BS, Roof D, Hayes A, Molday RS, Mukai S, Cowley GS, Berson EL, Dryja TP. Transgenic mice with a rhodopsin mutation (Pro23His): a mouse model of autosomal dominant retinitis pigmentosa. Neuron. 1992;9:815–830. doi: 10.1016/0896-6273(92)90236-7. [DOI] [PubMed] [Google Scholar]
- Pan W-H, Devlin HF, Kelley C, Isom HC, Clawson GA. A selection system for identifying accessible sites in target RNAs. RNA. 2001;7:610–621. doi: 10.1017/s1355838201001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzel V, Sczakiel G. Theoretical design of antisense RNA structures substantially improves annealing kinetics and efficacy in human cells. Nature Biotechnology. 1998;16:64–68. doi: 10.1038/nbt0198-64. [DOI] [PubMed] [Google Scholar]
- Persson T, Hartmann RK, Eckstein F. Selection of hammerhead ribozyme variants with low Mg2+ requirement: importance of stem-loop II. Chembiochemistry. 2002;3:1066–1071. doi: 10.1002/1439-7633(20021104)3:11<1066::AID-CBIC1066>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Petrukhin K. New therapeutic targets in atrophic age-related macular degeneration. Expert Opinion on Therapeutic Targets. 2007;11:625–639. doi: 10.1517/14728222.11.5.625. [DOI] [PubMed] [Google Scholar]
- Rajan RS, Illing ME, Bence NF, Kopito RR. Specificity in intracellular protein aggregation and inclusion body formation. Proc Natl Acad Sci USA. 2001;98:13060–13065. doi: 10.1073/pnas.181479798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Saliba RS, Munro PM, Luthert PJ, Cheetham ME. The cellular fate of mutant rhodopsin: quality control, degradation and aggresome formation. J Cell Sci. 2002;115:2907–2918. doi: 10.1242/jcs.115.14.2907. [DOI] [PubMed] [Google Scholar]
- Scherr M, Rossi JJ. Rapid determination and quantitation of the accessibility to native RNAs by antisense oligodeoxynucleotides in murine cell extracts. Nucleic Acids Research. 1998;26:5079–5085. doi: 10.1093/nar/26.22.5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherr M, Rossi JJ, Sczakiel G, Patzel V. RNA accessibility prediction: a theoretical approach is consistent with experimental studies in cell extracts. Nucleic Acids Research. 2000;28:2455–2461. doi: 10.1093/nar/28.13.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stage-Zimmermann TK, Uhlenbeck OC. Hammerhead ribozyme kinetics. RNA. 1998;4:875–889. doi: 10.1017/s1355838298980876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM. Low-cost monochromatic microsecond flash microbeam apparatus for single-cell photolysis of rhodopsin or other photolabile pigments. Review of Scientific Instruments. 1998;69:527–539. [Google Scholar]
- Sullivan JM, Pietras KM, Shin BJ, Misasi JN. Hammerhead ribozymes designed to cleave all human rod opsin mRNAs which cause autosomal dominant retinitis pigmentosa. Molecular Vision. 2002;8:102–113. [PubMed] [Google Scholar]
- Sullivan JM, Taggart RT. Novel and enhanced approaches to determine local mRNA accessibility. Investigative Ophthalmology & Visual Sciences. 2007;48:4605, B442. [Google Scholar]
- Sung C-H, Schneider B, Agarwal N, Papermaster DS, Nathans J. Functional heterogeneity of mutant rhodopsins responsible for autosomal dominant retinitis pigmentosa. Proceedings of the National Academy of Sciences USA. 1991;88:8840–8844. doi: 10.1073/pnas.88.19.8840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I, Jr, Bustamante C. How RNA folds. Journal of Molecular Biology. 1999;293:271–281. doi: 10.1006/jmbi.1999.3001. [DOI] [PubMed] [Google Scholar]
- Travis GH, Hepler JE. A medley of retinal dystrophies. Nat Genet. 1993;3:191–192. doi: 10.1038/ng0393-191. [DOI] [PubMed] [Google Scholar]
- Weleber RG, Carr RE, Murphey WH, Sheffield VC, Stone EM. Phenotypic variation including retinitis pigmentosa, pattern dystrophy, and fundus flavimaculatus in a single family with a deletion of codon 153 or 154 of the peripherin/RDS gene. Arch Ophthalmol. 1993;111:1531–1542. doi: 10.1001/archopht.1993.01090110097033. [DOI] [PubMed] [Google Scholar]
- Wells J, Wroblewski J, Keen J, Inglehearn C, et al. Mutations in the human retinal degeneration slow (RDS) gene can cause either retinitis pigmentosa or macular dystrophy. Nat Genet. 1993;3:213–218. doi: 10.1038/ng0393-213. [DOI] [PubMed] [Google Scholar]
- Woodson SA. Recent insights on RNA folding mechanisms from catalytic RNA. CMLS Cell Mol Life Sci. 2000;57:796–808. doi: 10.1007/s000180050042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau EH, Sullivan JM. High throughput cellular screening for ribozyme development against arbitrary mRNA targets. Investigative Ophthalmology & Visual Science. 2007;48:1681, B497. [Google Scholar]
- Zhang H-Y, Mao J, Zhou D, Xu Y, Thonberg H, Liang Z, Wahlestedt C. mRNA accessible site tagging (MAST): a novel high throughput method for selecting effective antisense oligonucleotides. Nucleic Acids Research. 2003;31:e72. doi: 10.1093/nar/gng072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Research. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]