Abstract
There is a great clinical need for tissue engineered blood vessels that could be used to replace or bypass damaged arteries. The success of such grafts will depend strongly on their ability to mimic the cellular and matrix organization found in native arteries, but currently available cell scaffolds such as electrospun fibers or hydrogels lack the ability to simultaneously encapsulate and align cells. Our laboratory has recently developed liquid crystalline solutions of peptide amphiphile nanofibers that form aligned domains at exceedingly low concentrations (<1wt%), and can be trapped as gels with macroscopic alignment using low shear rates and ionic crosslinking. We describe here the use of these systems to fabricate tubes with macroscopic circumferential alignment and demonstrate their potential as arterial cell scaffolds. The nanofibers in these tubes were circumferentially aligned by applying small amounts of shear in a custom built flow chamber prior to gelation. Small angle X-ray scattering confirmed that the direction of nanofiber alignment was the same as the direction of shear flow. We also show the encapsulation of smooth muscle cells during the fabrication process without compromising cell viability. After two days in culture the encapsulated cells oriented their long axis in the direction of nanofiber alignment thus mimicking the circumferential alignment seen in native arteries. Cell density roughly doubled after 12 days demonstrating the scaffold’s ability to facilitate necessary graft maturation. Since these nanofiber gels are composed of >99% water by weight, the cells have abundant room for proliferation and remodeling. In contrast to previously reported arterial cell scaffolds, this new material can encapsulate cells and direct cellular organization without the requirement of external stimuli or gel compaction.
1. Introduction
Heart disease is an unsolved problem accounting for over 30% all US deaths in recent years, and it is most often caused by damaged or weakened coronary arteries.[1] In such cases the affected blood vessels can be bypassed to restore blood supply to cardiac tissue. Synthetic materials have poor patency when used to bypass small diameter blood vessels (>5mm) and autologous grafts are in short supply.[2][3] Therefore, there is a critical need for tissue engineered blood vessels that can be used to replace damaged and blocked arteries. After the pioneering work of Weinberg and Bell[4], a significant focus of vascular engineering has been the development of methods that mimic the native microscopic organization found in arteries.[5–10] The functions of arteries are dependent upon their cellular organization, and are known to fail when this organization is not present.[11][12] The key feature of arterial microarchitecture is the alignment of smooth muscle cells (SMCs) with their long axis extending in the circumferential direction in the medial layer.[13] Vasoactivity, the constriction or dilation of blood vessels, is controlled by the contractile force produced by circumferentially aligned SMCs, and the durable mechanical properties of arteries can be attributed to the circumferential alignment of SMCs and their fibrous extracellular matrix (ECM). Therefore, it has been established that the circumferential alignment of contractile SMCs is necessary for the successful design of artificial blood vessels.[10]
One of the first and most widely researched techniques used to align SMCs within vascular grafts was first suggested by L’Heureux et al[14] using a collagen gel (and later fibrin gel) compacted around a non-adhesive mandrel.[15][5] While this method induces significant cellular alignment, it has inherent drawbacks such as the use of natural biopolymers that are known to influence cell behavior. For example, encapsulation of SMCs within collagen gels is known to inhibit the cellular production of elastin, a vital ECM component in arteries.[10][16] Other strategies have yielded similar cellular alignment via electrospinning of biocompatible polymers[6][7][17]. Macroscopic tubes can be made with highly aligned fibers using a rotating rod as the electrospinning target. However, the extremely high shear forces and organic solvents used during electrospinning can significantly damage cells and therefore they cannot be encapsulated into materials during the fabrication procedure. Instead cells must be seeded onto the surface of these tubes post-fabrication and allowed to infiltrate as the construct degrades. The infiltration of cells lengthens the maturation time of the graft, and the polymer degradation products will often negatively affect cell behavior.[18] Regardless of material, the application of a pulsed pressure on tubular scaffolds has been shown to preferentially aligned cells in the circumferential direction.[9][19] However, problems can arise due to mechanical stimulation causing SMCs to differentiate thus reducing their ECM production and proliferation capacity. Therefore an ideal scaffold for arterial tissue engineering would template the circumferential alignment of encapsulated SMCs while also displaying a select set of bioactive cues to induce a specific cell behavior. In this context, synthetic self-assembling fibrous materials offer a promising alternative to both electrospinning and the common biopolymers used in tissue engineering.
Over the past decade Stupp and co-workers have developed a class of peptide amphiphile (PA) molecules that self-assemble into high aspect ratio supramolecular nanostructures resembling ECM fibrils.[20][21] The PA nanofibers typically have diameters of approximately 6–10 nanometers and can be microns in length. These PA molecules can be modified with cell signaling amino acid sequences that, after self-assembly, are displayed in high density on the surface of nanofibers.[22] Previous research has investigated these signal-displaying nanofibers and demonstrated their ability to promote processes such as cell proliferation[23], cell adhesion [24][25], angiogenesis[26], axon elongation [27][28], bone regeneration [29], and for rational delivery of growth factors for cartilage regeneration[30] and islet transplantation[31].
We have shown recently that heating and cooling of certain PA solutions leads to a process of fusion and then rupture of two-dimensional plaques into fiber bundles approximately 40 nm in diameter.[32] In that work, it was observed that solutions of these fiber bundles form lyotropic liquid crystals at exceedingly low concentrations, <1 wt %. Using the low strain forces produced by dragging PA solutions from a pipette tip by hand (1–4 s−1) it was possible to align these fiber bundles over macroscopic lengths and trap them in the aligned state through crosslinking by divalent ions. The procedure of pipetting and gelling an orientationally monodomain gel was found to be gentle enough to accommodate living cells suspended in solution. Once the matrix was gelled the cells responded to the anisotropy of fiber alignment by elongating processes parallel to the fiber bundles via contact guidance.
We investigate here the fabrication of tubular gels with the liquid crystalline PA nanofiber bundles and their potential use as cell scaffolds for tissue engineered arteries. Using small angle x-ray scattering (SAXS) and birefringent microscopy we have characterized the alignment of the nanofibers in these tubes following application of a circumferential strain prior to gelling. We have also encapsulated SMCs during fabrication of the tubular structures and measure their survival and proliferation in culture. Fluorescent microscopy and Fast Fourier Transform image analysis was used to investigate the cells’ orientational response to the aligned matrix. The goal of this research was to develop a cell scaffold with the ability to template the alignment of encapsulated vascular cells and thus mimic the organization of native arteries. Such a scaffold could ultimately lead to a vasoactive graft capable of replacing damaged or weakened arteries.
2. Materials and Methods
2.1 Synthesis, purification, and solution preparation of peptide amphiphiles
The PA molecule used for this study, C16-V3A3E3(NH2), was synthesized by standard solid-phase Fmoc chemistry on a CS Bio automated peptide synthesizer. Fmoc-protected amino acids, MBHA rink amide resin and HBTU were purchased from NovaBiochem and all reagents were purchased from Mallinckrodt. The resulting product was purified using standard reversed-phase high performance liquid chromatography. The PA was dialyzed against deionized water using 500 MWCO dialysis tubing and isolated by lyophilization. The purity and accurate molar mass for each PA was verified using liquid chromatography/mass spectrometry on an electrospray ionization quadruple time-of-flight mass spectrometer.
PA solutions were prepared at 10 mg/mL or 12 mg/mL in Tris buffer and pH adjusted using 1 M NaOH to pH 7.2. These solutions were sonicated for 15 minutes, annealed at 80°C for 30 minutes, and slowly cooled overnight to room temperature. As previously described, these processing conditions create a liquid crystalline solution of PA nanofiber bundles[28].
2.2 Shear Flow Chamber
Custom made glass tubes were fabricated with inside diameters of 4 mm and lengths of approximately 6 cm (see Figure 1). The glass tubes were designed with three O-ring grooves for isolation of PA solution and stabilizing the inner rotating rod during fabrication. The glass tube was fixed to a modified 50 mL Falcon tube cap. When the stainless steel rod (3mm OD) was inserted into the custom glass tube it formed a water-tight seal with the O-rings completely isolating the annular gap. The annular gap measured 0.5 mm with a volume of approximately 275 µL. These dimensions were chosen to mimic the approximate size of the adult coronary artery medial layer.
Figure 1.
Fabrication device with resulting macroscopic tubular gel. (A) Empty shear chamber assembled and (B) PA solution loaded in shear chamber. For better visual effect, 0.02 wt% of pyrenebutyric acid was mixed evenly into the 10 mg/mL PA solution, and the photo was taken under UV light. (C) Fabrication procedure showing the inner rod’s combined rotation and retraction movement allowing the Ca2+ solution to flow into the lumen of the tube. (D) Macroscopic photo of final PA tube retaining its tubular shape.
2.3 Tube Fabrication Procedure
The tubular constructs were made by sequentially shearing the PA solution then gelling through exposure to Ca2+ ions. The previously describe PA solution at 10mg/mL with suspended cells when specified was injected via an 18 gauge needle into the annular gap of the shearing chamber. The sheaing chamber with the inserted steel rod was fitted onto a 50 mL falcon tube filled with 160 mM NaCl, 15 mM CaCl2, and then the entire device was loaded onto a modified metal lathe (Central Machinery 7 inch×12 inch Precision Mini Lathe). The rotating chuck gripped the inner steel rod while the glass tube was fixed to the translational stage. The modified metal lathe was set to rotate at 116±5 RPMs for 10 seconds then the translational stage was engaged. This retracted the inner rod from the glass tube at a rate of 0.42±0.02 cm/s with constant rotation. As the inner rod retracts from the glass chamber the salt solution (160 mM NaCl, 15 mM CaCl2) flowed into the glass tube, illustrated in Figure 1C, thus gelling the cell suspension in the shape of a tube. This setup ensured that the PA solution is gelled immediately after the cessation of the applied shear force. When the inner rod was completely removed from the glass tube the resulting tubular gel was carefully extracted under sterile conditions and place in culture media when encapsulating cells. This fabrication procedure can be completed in less than one minute. Two types of tubes were prepared: one prepared with the previously described shearing process and the other with the same method but without rotational shear. The non-sheared sample was prepared by retracting the inner rod at an extremely slow rate of approximately 6 mm/min but without rotation resulting in a strain rate less than 1 s−1. These control samples are referred to as “non-aligned”, and samples prepared at the normal rotation of 116 RPM are referred to as “aligned.”
2.4 X-Ray Diffraction
SAXS measurements were performed using beam line 5ID-D, in the DuPont-Northwestern-Dow Collaborative Access team (DND-CAT) Synchrotron Research Center at the Advanced Photon Source, Argonne National Laboratory. An energy of 15 keV corresponding to a wavelength 0.083 nm was selected using a double-crystal monochromator. The data was collected using a CCD detector (MAR) positioned 245 cm behind the sample. The scattering intensity was recorded in the interval 0.008< q < 0.25 A−1 with an exposure time of 4 seconds. Samples were placed in a water-filled customized sample holder made from aluminum and mica sheets. Five 2-D SAXS images were averaged to produce one 2-D image.
2.5 Cell Culture and Cell Solutions
Human coronary artery smooth muscle cells (Invitrogen) were purchased at passage 3 and maintained in Media231 (Invitrogen) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 5% smooth muscle growth supplement (Invitrogen) at 37°C and 5% CO2. Media was changed every 3 days. The cells were expanded to 90 – 100% confluence prior to trypsinization. For construct preparation 250 µL of 12 mg/mL PA solution was mixed with 9×105 SMCs dispersed in 50 µL Tris buffer, bringing the final concentration to 10 mg/mL PA and 3×106 cells/mL.
2.6 Assessment of Cell Damage
A trypan blue exclusion analysis was used to determine cell viability before and after exposure to the tube fabrication procedure. The PA solutions with suspended SMCs were tested at each step of the fabrication process for membrane integrity. The cell suspension was mixed with trypan blue in a 1:9 volumetric ratio, and then placed between two microscope slides with a 40 µm gap distance. Randomly chosen areas of 1223µm × 917 µm were imaged using the Nikon Eclipse TE200 Inverted Microscope. Cell images were visually counted for live and dead cells indicated by color. Data was expressed as dead cells per total cells for every image.
2.7 SEM, Birefringence, and Fluorescent Imaging
Samples imaged using SEM were fixed in 3% gluteraldehyde for 20 minutes, and dehydrate in a series of EtOH washes. Samples were then critically point dried, mounted, and coated with 5 nm of osmium before imaging using a LEO Gemini 1525 sFEG SEM. The fiber alignment was visualized using birefringence in 1 mm segments cut perpendicular to the tube’s long axis, and laid face down in a glass dish filled with water. The sample was then examined between two perpendicular light polarizers using an inverted Nikon Eclipse TE200 Inverted Microscope. For fluorescent imaging, tubular constructs were stained with CalceinAM (Molecular Probes) immediately following culture according to the distributor’s protocol allowing for the visualization of living cells throughout the construct. For F-actin staining, Alexa Fluor® 568 phalloidin (Molecular Probes) was used according to the distributor’s protocol. A Nikon Eclipse TE200 Inverted Microscope was used for all fluorescent imaging.
2.8 Cellular Alignment Quantification
Cell orientation was calculated from F-actin stained images of SMCs at the focal plane 100 µm into the lumen surface of tubes. Fast Fourier Transform (FFT) images were produced from the F-actin stained images using ImageJ.[33] Similar anisotropic analyses based on Fourier-transformed images have been applied to scanning electron microscopy, confocal images, and second harmonic generation.[34][35] The Fourier-transformed images were analyzed in MATLAB to quantify the anisotropy in each FFT image in the form of an anisotropy factor (AF) along with the direction of anisotropy. These MATLAB calculations were originally developed to quantify anisotropy in 2D small angle X-ray scattering images.[36] AF values range between 0 and 1, where 0 represents completely random orientation and 1 represents perfect alignment. The direction of alignment is calculated as an angle ranging between 0 and 180°, where 0° is the circumferential direction and 90° is the longitudinal axis of the tube observed from the lumen surface.
3. Results and Discussion
3.1 Nanofiber Alignment
The shear force applied during fabrication was expected to align PA nanofibers in the direction of fluid motion, and figure 2 shows the representative fiber alignment observed by SEM on the inner surface of the tubes. PA fibers and fiber bundles can be seen ranging from 8 to 50 nm in diameter. Aligned samples contained highly oriented fibers previously observed within macroscopic PA strings.[28] The alignment was also visualized using polarized optical microscopy, and segments cut into 1 mm thick rings were observed to be highly birefringent (see Figure 3). In this cross-polarizer setup, all fiber domains oriented vertically and horizontally should appear dark, while diagonally oriented fiber domains should be bright. The aligned sample shows a birefringent pattern that indicates a large amount of fiber orientation in the circumferential direction. As evidenced by the uniform brightness, the alignment appears consistent through the thickness of the wall suggesting that the same alignment observed on the surface via SEM persists throughout the bulk of the gel. Comparing the aligned and non-aligned samples, it can be seen that non-aligned samples contained more disorganized domains, and even the brightest portions of the non-aligned sample are much fainter than those in aligned sample suggesting less fiber alignment. This result was not surprising considering the non-aligned tube was exposed to a relatively small strain force (< 1 s−1) during fabrication in order to minimize any macroscopically induced alignment.
Figure 2.
SEM of the inner surface of PA tube. (A) The aligned sample shows noticeably more aligned nanofibers than (B) the non-aligned sample.
Figure 3.
PA tube cross-section visualized under polarized light. In this set up nanofiber domains oriented diagonally appear bright while horizontally oriented and disorganized domains appear dark. The aligned sample shows the expected light-dark pattern expected for circumferentially aligned nanofibers.
Small angle X-ray scattering (SAXS) was used to characterize the orientation of fibers. Tube constructs were sectioned down the long axis and mounted in the beam path either as a whole tube or a half tube. The sample positions are shown by the insets in Figures 4A–C, where the X-ray beam path is going into the plane of the page. As expected non-aligned samples revealed isotropic scattering patterns. On the other hand, the 2D SAXS pattern of aligned samples revealed a high degree of anisotropy, as indicated by the elongated shape of the scattering pattern. A plot of intensity versus azimuthal angle shows the elongated SAXS pattern as a peak indicating the angle perpendicular to fiber orientation, i.e., a peak occurring at 90° corresponds to fiber alignment at 0°. It is obvious from this data that the fiber orientation is not perfectly circumferential because the aligned half tube shows a peak occurring at an angle offset from 90°. Instead, the orientation spirals up the tube at a certain pitch angle illustrated in Figure 4E. The pitch angle is defined as the angle between the fiber orientation and the circumferential direction, i.e. pitch angles of 0° and 90° correspond to perfect circumferential and longitudinal orientation, respectively. It is possible to determine the pitch angle from the azimuthal plot of the aligned half tube, however, the accuracy is limited by spatial control of sample positioning. As explained below, in order to obtain an accurate measurement of the pitch angle the whole tube must be probed and the corresponding SAXS pattern must be deconvoluted into its two component patterns.
Figure 4.
SAXS patterns of PA tubes. (A) Non-aligned half tube, (B) Aligned half tube, (C) Aligned whole tube. The orientation of samples is show in the inserts of A, B, and C, where the X-ray beam passes into the plane of the picture. (D) Azimuthal scan of intensity in A, B, and C. The black arrows designate the two peaks pointed out by the white arrows in the SAXS pattern. (E) Nanofiber orientation in aligned tube suggested by SAXS experiments.
To measure the direction of fiber alignment the whole tube construct was probed in a setup allowing the incident beam to pass through both the front and back walls of the tube. Therefore, the resulting diffraction pattern contained features from the fiber orientation in both walls. Consequently, the azimuthal intensity scan revealed two distinct peaks (Figure 4D), and this scan was deconvoluted into the component peaks of each wall, thus revealing the angle of fiber orientation. Due to the geometry of a tube with a spiral pattern shown in Figure 4E the pitch angle can be calculated as half the angle between the front and back wall fiber orientations.
This difference allows for an accurate measurement of the pitch angle, since the orientation of the tube does not influence the calculations. The pitch angle found via this method was 13.5°±1.4°, which closely matches the pitch angle predicted by the shear flow calculations discussed in the following paragraph. The direction of PA solution flow is a result of both the rotation and retraction of the inner rod resulting in a helical flow pattern. The angle of the flow direction in reference to the tube’s circumferential direction is referred to as the pitch angle, which can be predicted based on the vector sum of strains produced by the rod’s rotation and retraction motion. These strain rates were calculated by assuming a simplified parallel plate geometry and no slip boundary conditions,
where Vs and d are the velocity at the mandrel surface and annular gap distance, respectively. For the specified fabrication conditions the rotational and retraction strains were 36.4 s−1 and 8.4 s−1, respectively, and assuming these flows are perpendicular results in a vector sum of 37.4 s−1 with a pitch angle of 13°. The SAXS measurements show that the PA nanofibers do indeed orient in the direction of fluid flow when being sheared.
These constructs were designed to mimic both geometry and fiber orientation found within native arteries. It is well established that the collagen fibers existing in the arterial medial layer have a preferential alignment in the circumferential direction; however, this is only the average fiber orientation over the entire medial layer. It has been observed via confocal microscopy and X-ray diffraction that collagen fibers within discrete medial lamellar units have pitch angles as large as 20°.[12][37] Mathematical simulations of collagen fibers arranged in such helical orientations show much greater compliance values than if they are arranged with perfect circumferential orientation.[38] Matching the compliance of vascular grafts to that of native arteries is especially important because it is known that grafting blood vessels with mismatched compliance values will lead to hyperplasia at the site of anastomosis and eventual graft failure.[2] It was for these reasons that our PA tubular constructs were designed with a mid-ranged pitch angle found in native arterial collagen fibers, 13.5°. However, constructs of other pitch angles can easily be made simply by changing the relative speeds of mandrel rotation and retraction during the fabrication procedure. There is also the possibility of further modifying these tubes by the addition of more PA gel layers containing fibers with alternating orientations, thus creating a crosshatched pattern similar to human arteries.[39]
3.2 Cell Incorporation
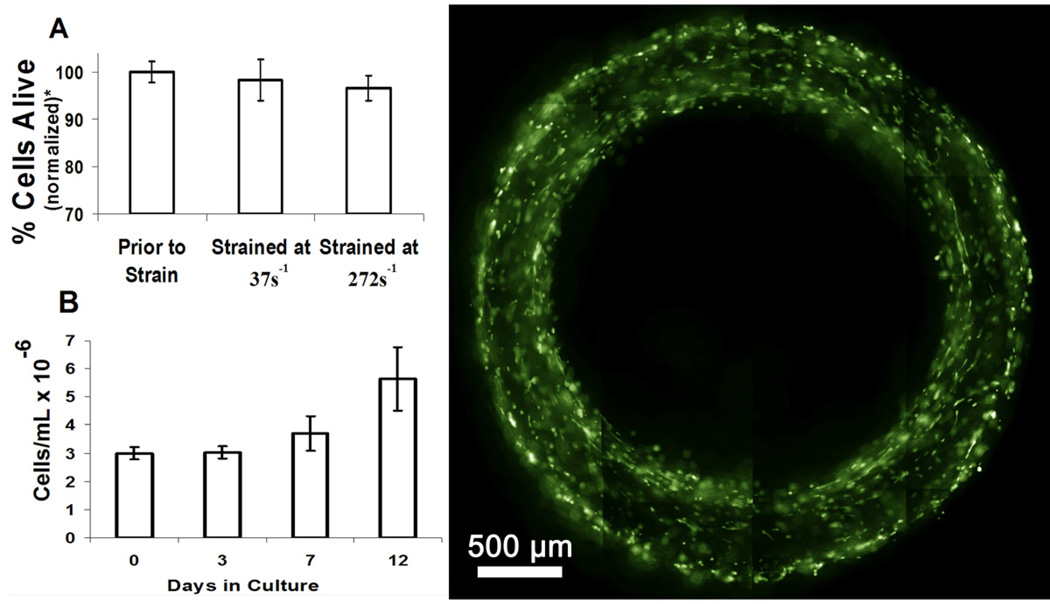
For this approach to be suitable for creating vascular grafts, facile incorporation of vascular cells into the scaffold during processing will be required. However, the fabrication process will cause cells dispersed in the PA solution to experience significant shear forces, which could damage the cells. Trypan blue was used to assess cell damage as it only permeates cells with compromised membranes. PA/cell suspensions were prepared and an aliquot of the solution was taken before and after the shearing process. The data in Figure 5A shows no significant difference between sheared and non-sheared cell suspensions even when using very high strain rates greater than those used for normal processing conditions. Using the PA solution viscosity of 0.015 Pa·s measured at 37 s−1, and assuming spherical cells with laminar flow we calculated that our fabrication procedure would apply a maximum hydrodynamic stress of 0.7 Pa on the suspended SMCs for just a few seconds. Thus the absence of damage is not surprising given previous results in which mammalian cells were found to experience significant damage accompanied by apoptosis only after exposure to hydrodynamic stress greater than 1 Pa for over an hour.[40]
Figure 5.
(A) Trypan blue measurements of living cells before and after fabrication. Fabrication is normally performed at 37s−1, but 272s−1 was also done to maximize the possible shear induced death. *Values normalized to “Prior to Strain”. (B) Cell content of PA gel during cell culture measured by DNA assay. (C) Cells within aligned PA tube visualized by live fluorescence stain after 7 days in culture. All error bars represent standard deviation.
Electrospinning is another technique that can be used to fabricate tubular scaffolds with circumferentially aligned nanofibers, but it is not compatible with living cells.[6][7][16] One attempt to electrospin cells within a compound cone set-up resulted in a 30% reduction in cell viability which was attributed to the strong shear flow experienced by cells when spun into fibers.[41] Other attempts have been made to directly incorporate cells into the electrospinning process by simultaneously electrospraying concentrated cell solutions.[42] This technique was accomplished without compromising cell viability, however, the effects of residual polymer solvent on cell behavior remain an important concern along with the fact that constructs over 500 µm thick can take hours to fabricate. The fabrication conditions of our PA tubular constructs are mild enough to incorporate cells with fabrication times lasting less than 20 seconds. This allows our constructs to have a very high and homogeneous initial cell density reducing the maturation time required to develop the graft properties necessary for implantation.
An important consideration for any new tissue engineering technique that encapsulates living cells is the mass transfer limitations imposed by the geometry and composition of the cell scaffold. More specifically, sufficient transport of O2 into hydrogels is a major design criterion that must be considered, because hypoxic conditions will limit the ability of encapsulated cells to proliferate and produce ECM. A qualitative assessment of cell viability was performed at day 7 of culture by fluorescently staining living cells in a cross section of the tubular construct. This allowed the visualization of living cells in the center of the tube wall where the lowest concentrations of O2 are expected to occur. The calcein staining shown in Figure 5C reveals a high density of living cells throughout the thickness of the construct suggesting this material allows for sufficient transport of essential nutrients and O2 required for cell survival. Staining for dead cells using ethidium homodimer is not shown because of high background fluorescence due to its binding affinity to PA nanofibers; however, observations confirmed that virtually every visible cell was also positive for the calcien live-stain.
Calcein staining revealed that seven day cultured samples appeared to have cell densities similar to freshly seeded tubes. Therefore, a DNA assay was performed to investigate the proliferative capacity of SMCs encapsulated in PA nanofiber gels over a twelve-day period. PA solutions containing cells were sheared and then pipetted and gelled in 24-well culture plates, rather than formed into tubes. This was done to maintain a well-defined volume necessary for the accurate assessment DNA content. However, all samples were exposed to the same shear forces and gelling conditions as the tubular constructs. The results in Figure 5B show that by day 12 enough proliferation occurred to double the initial cellular concentration.
3.3 Cellular Alignment
Images of the tube surface at day 12 of culture revealed that many SMCs had migrated to the surface (see Figure 6). We observed a very distinct difference in the morphology of cells cultured in aligned tubes compared to non-aligned tubes. The aligned tubes showed dense monolayers of cells with elongated features oriented in the circumferential direction. In contrast, the non-aligned tubes showed monolayers of cells with few elongated features having no consistent directionality.
Figure 6.
SEM of the inner surface of cell seeded PA tubes after 12 days in culture. (A) Aligned tube shows that SMCs migrated to the surface and organized with elongated features parallel for nanofiber alignment. (B) The non-aligned sample did not display much organization of SMCs.
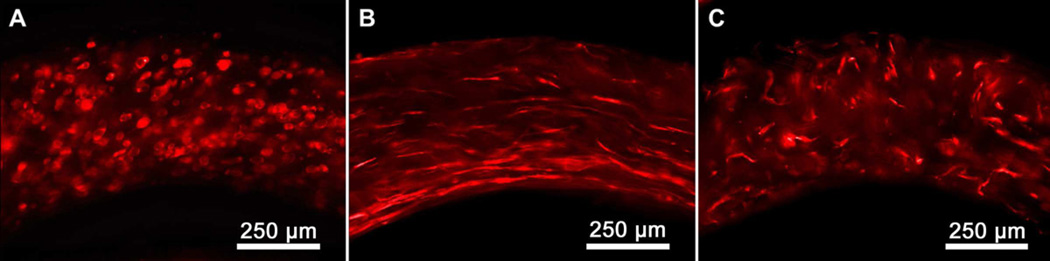
Tubular gel constructs were sufficiently rigid to allow sectioning with tweezers and a 100 µm thick razor blade. Sections were cut perpendicular to the tube’s long axis creating small rings that were laid with the cut surface down on glass slides. These samples were stained with fluorescently labeled phalloidin allowing for visualization of the cells’ F-actin filaments. These cross-sectional fluorescence images revealed the stark contrast in the cellular alignment between aligned and non-aligned tubes (see Figure 7). Aligned samples taken at day 4 showed that almost all cells had aligned in the circumferential direction resembling histological cross sections of native arterial medial layers. The non-aligned samples did not contain cells with a preferred orientation relative to the circumferential direction; however, the cells did show a normal elongated morphology.
Figure 7.
Cellular alignment viewed from cross-section of PA tubes stained with Alexa Fluor® 568 phalloidin. (A) Aligned tube at day 0, (B) Aligned tube at day 4, (C) Non-aligned tube at day 4.
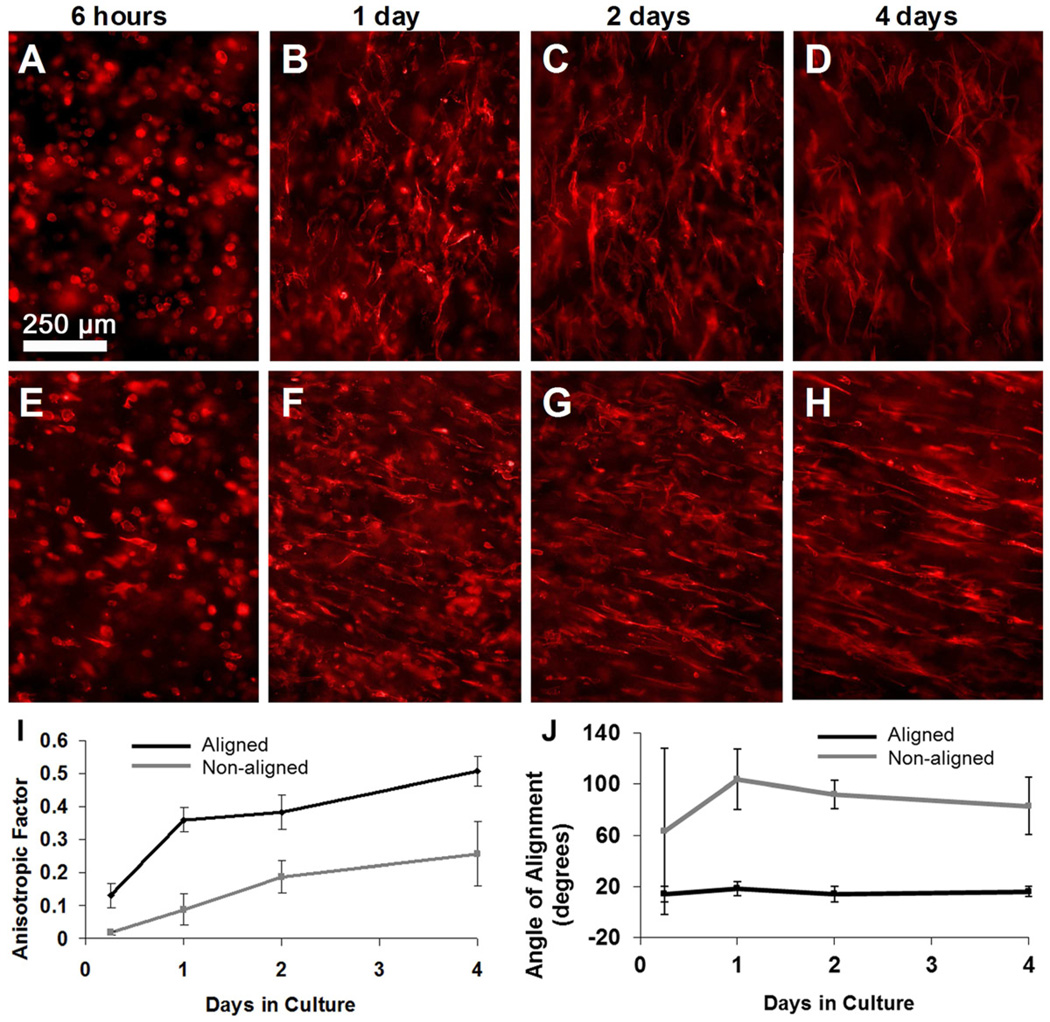
Anisotropic factors (AF) were calculated from fluorescently stained images as described in section 2.8. Cells cultured over a four-day period showed large changes in the cellular alignment and obvious differences between aligned and non-aligned constructs. During the entire four-day culture the aligned samples contained cells with significantly more alignment than non-aligned samples. The greatest alignment was observed in samples at day four, shown in Figure 8H with AF=0.45, where greater than 90% of the cells can be seen elongated in one direction. Non-aligned constructs had much less alignment shown by their AF values, 0.23±0.08 at day four. Based on visual observations we assume an AF value of approximately 0.4 or higher indicates a high degree of aligned cells.
Figure 8.
Cellular alignment viewed from inner surface with the tube’s long axis in the vertical direction; Non-aligned samples (A–D), Aligned samples (E–H). FFT analysis was used to measure anisotropy from fluorescent images. (I) Anisotropic Values from Aligned and non-aligned samples remain statistically different for the entire culture period. (J) The orientation of cells was measured as the angle made between the cells’ long axis and the tube’s circumferential direction. All error bars represent standard deviation.
The direction of cell orientation was also calculated from fluorescently stained images using the previously described FFT analysis. Cell orientation angles were defined as the angle between the cells’ long axis and the circumferential direction, where 0° and 90° correspond to the tube’s circumferential and longitudinal direction, respectively. Within only six hours in culture, cells within aligned tubes had begun extending their filopodia in a consistent direction as indicated by the low standard deviation of the alignment angle, 14.4 ±6.1°. Cells within aligned tubes oriented within a narrow range of directors for the entire four-day culture and stabilized at 15.5 ±4.2° by the fourth day. These cellular alignment angles are consistent with the angle of nanofiber alignment (13.5 ±1.4°) measured by SAXS as described in section 3.1.
We expected that there would be no consistent cellular orientation in non-aligned constructs because the fabrication process was performed without using rotation and the inner rod was retracted at an extremely slow velocity (6 mm/min) in an effort to minimize any shear forces that would align nanofibers. However, it appears that there was some nanofiber alignment induced during this process, because by day four cells cultured in non-aligned constructs did align at 83.0±22.4°, which is essentially the longitudinal direction of the tube. Even though the standard deviation is high, an overall random cell orientation would have a deviation spanning the entire range of orientation angles, as found with non-aligned samples at the first time point. We believe the slow retraction of the inner rod during fabrication did produce some nanofiber alignment after all. Recalling the SAXS azimuthal scan of the non-aligned construct (Figure 4D), it can be seen that a minor dip in intensity occurs at approximately 90°, indicating that there may indeed be a slight fiber orientation preference in the longitudinal direction. This result is surprising as it shows that PA nanofibers can be aligned with much smaller strains than previously reported, 1 – 4s−1. [28].
The results reported here show that PA nanofibers can be used to mimic the geometry and circumferential ECM cell alignment found in native arteries. This alignment is essential for tissue engineered blood vessels to perform their biologic functions in vivo. Therefore, PA nanofiber gels offer a promising alternative to isotropic gels typically used to create tissue engineered blood vessels, such as collagen and fibrin, which require anisotropic strain during culture to produce cellular alignment.[9][17] Using our technique we were able to create tubular constructs containing cells with a helical alignment mimicking native arterial cell alignment. Such helical patterns are not attainable using one the most popular approaches to circumferential alignment first suggested by L’Heureux[13] in which tube shaped hydrogels containing SMCs are compacted around a non-adhesive mandrel.[5][14] PA gels have the ability to direct cellular alignment without the use of external stimuli or compaction giving them a distinct advantage. Using an external force to promote cell orientation in 3D constructs is currently limited to simple macroscopic shapes, because creating directionally complex compaction or mechanical forces on the microscale is not currently feasible. Moreover, an external stimulus requires sophisticated macroscale manipulation systems that are costly and difficult to scale up. On the other hand, the self-assembling and liquid crystalline PA gels studied here induce cell alignment through contact guidance with the nanofibers, organized spatially by the applied fluid flow prior to gelation. Therefore, highly complex cell alignment patterns and shapes could be created using these systems via microfluidic shear flow.
One of the biggest challenges in vascular tissue engineering is creating vessels with a high degree of internal alignment without sacrificing robust mechanical properties. Previous work on collagen and fibrin gels has shown that insufficient mechanical parameters can be attributed to poor arrangement of ECM fibrils and the inability of seeded cells to remodel the dense matrix of proteins.[9][17][43][44] Such tubular gels often contract to 10% of their original volume creating an extremely dense matrix (>15 mg/mL biopolymer) that can inhibit SMCs from breaking down and forming new ECM fibrils.[45] Because of this, an alternative approach using cell sheets has gained much attention within the past decade.[46–48] By culturing confluent layers of cells and then rolling them into tubes, constructs can be made with burst pressures comparable to native blood vessels, but these vessels contain cells and ECM with little circumferential alignment, limiting the vasoactivity and compliance of the final graft.[44] A construct created with the aligned PA gels studied here could potentially integrate both strong mechanics and a high degree of cellular alignment. Our PA gels showed no contraction during the culture period and therefore retain the low concentrations of scaffold material. Using a vascular graft templated by the mostly aqueous PA construct (99% by weight water) allows the seeded SMCs to easily remodel their nanofiber environment by producing the essential mechanically robust ECM components like collagen and elastin. The nanofiber environment imparts minimal strength to the construct and merely serves as a template to direct the arrangement of proliferating cells during the culture period. Furthermore, tubes can be formed with high densities of cells that become extremely aligned, and this should ultimately lead to a vasoactive graft.
Another important feature of the PA molecules used here is the possibility of adding bioactive signals at the termini of peptides, thus allowing biological signaling to further control SMC behavior. Our group has previously designed a number of bioactive PAs for regenerative medicine, including some with the ability to specifically bind growth factors,[27][49]promote differentiation,[20] and accelerate angiogenesis,[50] among others. This feature would allow for the design of bioactive nanofiber matrices that can be tailored to elicit specific cell responses desired for arterial morphogenesis while templating the spatial organization of the developing tissue. Such a bioactive matrix is beyond the scope of this paper, but is currently being investigated by our laboratory.
Conclusions
An arterial tubular scaffold of bundled peptide nanofibers with macroscopic circumferential alignment can be formed with minimal strain using an aqueous liquid crystalline solution. The low strain and gelation of the tube with simple electrolytes allows three-dimensional encapsulation of human artery cells within the scaffold which spontaneously acquire circumferential alignment guided by the fibers. Unlike gel compaction, electrospining, or use of mechanical stimuli to generate scaffold alignment, the self-assembly approach described here not only allows cells to survive during scaffold formation but also allows formation of more complex patterns such as the spiraling tube alignment which more closely resembles the alignment found in the matrix of native arteries. These findings support the potential of these systems as artificial matrices to direct growth of highly organized tissue engineered blood vessels to replace damaged arteries.
Acknowledgments
This work was funded by NIH-NHLBI grants 1P01HL108795 and 2R01HL053354-13. The authors are grateful to Prof. Wesley Burghardt and Saswati Pujari for providing the MATLAB code for orientational anisotropy calculations. The authors are also thankful to Dr. Steve Weigand from Argonne National Laboratory for assistance with x-ray measurements, Dr. Liam Palmer for useful discussions, Mark Seniw for 3D graphic illustrations, and Dr. Matthew Webber of the author’s laboratory for assistance with DNA quantification. Portions of this work were performed at the EPIC imaging facilities at Northwestern University and the DuPont-Northwestern-Dow Collaborative Access Team (DND-CAT) located at Sector 5 of the Advanced Photon Source (APS). DND-CAT is supported by E.I. DuPont de Nemours & Co., The Dow Chemical Company and Northwestern University. Use of the APS, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the U.S. DOE under Contract No. DE-AC02-06CH11357.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM. Heart Disease and Stroke Statistics–2011 Update: A Report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kannan RY, Salacinski HJ, Butler PE, Hamilton G, Seifalian AM. Current status of prosthetic bypass grafts: a review. J Biomed Mater Res B. 2005;74(1):570–581. doi: 10.1002/jbm.b.30247. [DOI] [PubMed] [Google Scholar]
- 3.Lith RV, Ameer GA. Biohybrid Strategies for Vascular Grafts. In: Pallua N, Suscheck CV, editors. Tissue Engineering: From Lab to Clinic. Springer: 2011. pp. 279–316. [Google Scholar]
- 4.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 5.Barocas VH, Girton TS, Tranquillo RT. Engineered alignment in media equivalents: magnetic prealignment and mandrel compaction. J Biomech Eng. 1998;120:660–666. doi: 10.1115/1.2834759. [DOI] [PubMed] [Google Scholar]
- 6.Xua CY, Inaic R, Kotakib M, Ramakrishna S. Aligned biodegradable nanofibrous structure: a potential scaffold for blood vessel engineering. Biomaterials. 2004;25(5):877–886. doi: 10.1016/s0142-9612(03)00593-3. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Y, Cao Y, Pan J, Liu Y. Macro-alignment of electrospun fibers for vascular tissue engineering. J Biomed Mater Res B. 2010;92(2):508–516. doi: 10.1002/jbm.b.31544. [DOI] [PubMed] [Google Scholar]
- 8.Yost M, Baicu C, Stonerock C, Goodwin R, Price R, Davis J, Evans H, et al. A novel tubular scaffold for cardiovascular tissue engineering. Tissue Eng. 2004;10(1–2):273–284. doi: 10.1089/107632704322791916. [DOI] [PubMed] [Google Scholar]
- 9.Mironova V, Kasyanova V, Zheng XS, Eisenberga C, Eisenberga L, Gondad S, Truska T, Markwalda RR, Prestwich GD. Fabrication of tubular tissue constructs by centrifugal casting of cells suspended in an in situ crosslinkable hyaluronan-gelatin hydrogel. Biomaterials. 2005;26:7628–7635. doi: 10.1016/j.biomaterials.2005.05.061. [DOI] [PubMed] [Google Scholar]
- 10.Seliktar D, Black RA, Vito RP, Nerem RM. Dynamic mechanical conditioning of collagen-gel blood vessel constructs induces remodeling in vitro. Ann Biomed Eng. 2000;28:351–362. doi: 10.1114/1.275. [DOI] [PubMed] [Google Scholar]
- 11.Chan-Park MB, Shen JY, Cao Y, Xiong Y, Liu Y, Rayatpisheh S, Kang GC, Greisler HP. Biomimetic control of vascular smooth muscle cell morphology and phenotype for functional tissue-engineered small-diameter blood vessels. J Biomed Mater Res A. 2009;88(4):1104–1121. doi: 10.1002/jbm.a.32318. [DOI] [PubMed] [Google Scholar]
- 12.Mulvany MJ, Aalkjaer C. Structure and Function of Small Arteries. Phys Rev. 1990;70(4):921–961. doi: 10.1152/physrev.1990.70.4.921. [DOI] [PubMed] [Google Scholar]
- 13.O’Connell MK, Murthy S, Phan S, Xu C, Buchanan J, Spilker R, Dalman RL, Zarins CK, Denk W, Taylor CA. The three-dimensional micro- and nanostructure of the aortic medial lamellar unit measured using 3D confocal and electron microscopy imaging. Matrix Biology. 2008;27:171–181. doi: 10.1016/j.matbio.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.L’Heureux N, Germain L, Labbe R, Auger FA. In vitro construction of a human blood vessel from cultured vascular cells: a morphologic study. J Vascular Surg. 1993;17:499–509. doi: 10.1067/mva.1993.38251. [DOI] [PubMed] [Google Scholar]
- 15.Grassl ED, Oegema TR, Tranquillo RT. Fibrin as an alternative biopolymer to type-I collagen for the fabrication of a media equivalent. J Biomed Mater Res. 2002;60(4):607–612. doi: 10.1002/jbm.10107. [DOI] [PubMed] [Google Scholar]
- 16.Long JL, Tranquillo RT. Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biology. 2003;22:339–350. doi: 10.1016/s0945-053x(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 17.Uttayarat P, Perets A, Li M, Pimton P, Stachelek SJ, Alferiev I, Composto RJ, Levy RJ, Lelkes PI. Micropatterning of three-dimensional electrospun polyurethane vascular grafts. Acta Biomater. 2010;6(11):4229–4237. doi: 10.1016/j.actbio.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Sung H, Meredith C, Johnson C, Galis Z. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials. 2004;25:5735–5742. doi: 10.1016/j.biomaterials.2004.01.066. [DOI] [PubMed] [Google Scholar]
- 19.Schutte S, Chen Z, Brockbank K, Nerem RM. Cyclic Strain Improves Strength and Function. Tissue Eng Part A. 2010;16(10):3149–3157. doi: 10.1089/ten.TEA.2010.0009. [DOI] [PubMed] [Google Scholar]
- 20.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294:1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 21.Webber MJ, Kessler JA, Stupp SI. Emerging peptide nanomedicine to regenerate tissues and organs. J Intern Med. 2010;267(1):71–88. doi: 10.1111/j.1365-2796.2009.02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 23.Webber MJ, Tongers J, Renault M, Roncalli JG, Losordo DW, Stupp SI. Development of bioactive peptide amphiphiles for therapeutic cell delivery. Acta Biomater. 2010;6(1):3–11. doi: 10.1016/j.actbio.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrington D, Cheng E, Guler M, Lee L, Donovan J, Claussen R, Stupp SI. Branched peptide-amphiphiles as self-assembling coatings for tissue engineering scaffolds. J Biomed Mater Res A. 2006;78a(1):157–167. doi: 10.1002/jbm.a.30718. [DOI] [PubMed] [Google Scholar]
- 25.Storrie H, Guler m, Abu-Amara S, Volberg T, Rao M, Geiger B, Stupp SI. Supramolecular crafting of cell adhesion. Biomaterials. 2007;28:4608–4618. doi: 10.1016/j.biomaterials.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 26.Chow L, Bitton R, Webber M, Carvajal D, Shull K, Sharma A, Stupp SI. A bioactive self-assembled membrane to promote angiogenesis. Biomaterials. 2011;32(6):1574–1582. doi: 10.1016/j.biomaterials.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tysseling-Mattiace M, Sahni V, Niece K, Birch D, Czeisler C, Fehlings M, Stupp SI, Kessler JA. Self-Assembling Nanofibers Inhibit Glial Scar Formation and Promote Axon Elongation after Spinal Cord Injury. J Neurosci. 2008;28(14):3814–3823. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldberger JE, Berns EJ, Bitton R, Newcomb CJ, Stupp SI. Electrostatic control of bioactivity. Angew Chem Int Edit. 2011;50(28):6292–6295. doi: 10.1002/anie.201100202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mata A, Geng Y, Henrikson K, Aparicio C, Stock S, Satcher R, Stupp SI. Bone regeneration mediated by biomimetic mineralization of a nanofiber matrix. Biomaterials. 2010;31(8):6004–6012. doi: 10.1016/j.biomaterials.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah RM, Shah NA, Del Rosario L, Hsieh C, Nuber G, Stupp SI. Supramolecular Design of Self-Assembling Nanofibers for Cartilage Regeneration. Proc Natl Acad Sci. 2010;107(8):3293–3298. doi: 10.1073/pnas.0906501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.JC, Wang LJ, Chow L, Kaufman D, Stupp SI. Growth Factor Delivery from Self-Assembling Nanofibers to Facilitate Islet Transplantation. Transplantation. 2008;86(3):478–481. doi: 10.1097/TP.0b013e3181806d9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang S, Greenfield M, Mata A, Palmer LC, Bitton R, Mantei JR, Aparicio C, Cruz MO, Stupp SI. A self-assembly pathway to aligned monodomain gels. Nature Mater. 2010;9(7):594–601. doi: 10.1038/nmat2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasband WS. ImageJ, U. S. National Institutes of Health. Bethesda, Maryland USA: 1997–2011. http://imagej.nih.gov/ij/, [Google Scholar]
- 34.Yoshigi M, Karnik S, Li DY, Clark EB, Yost HJ. Quantitative analysis of cytoskeletal remodeling in vascular smooth muscle cells during phenotypic modulation. Comput in Card. 2000;27:205–206. [Google Scholar]
- 35.Bowles RD, Williams RM, Zipfel WR, Bonassar LJ. Self-assembly of aligned tissue-engineered annulus fibrosus and intervertebral disc composite via collagen gel contraction. Tissue Eng Part A. 2010;16(4):1339–1348. doi: 10.1089/ten.tea.2009.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pujari S, Rahatekar SS, Gilman JW, Koziol KK, Windle AH, Burghardt WR. Orientation dynamics in multiwalled carbon nanotube dispersions under shear flow. J Chem Phys. 2009;130(21):214903. doi: 10.1063/1.3139446. [DOI] [PubMed] [Google Scholar]
- 37.Canham PB, Finlay HM, Boughner DR. Contrasting structure of the saphenous vein and internal mammary artery used as coronary bypass vessels. Cardiovas Res. 1997;34(3):557–567. doi: 10.1016/s0008-6363(97)00056-4. [DOI] [PubMed] [Google Scholar]
- 38.Gasser TC, Ogden RW, Holzapfel G. Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J R Soc Interface. 2006;3(6):15–35. doi: 10.1098/rsif.2005.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis EC. Smooth muscle cell to elastic lamina connections in developing mouse aorta: Role in aortic medial organization. Lab Invest. 1993;68(1):89–99. [PubMed] [Google Scholar]
- 40.Tanzeglock T, Soos M, Stephanopoulos G, Morbidelli M. Induction of mammalian cell death by simple shear and extensional flows. Biotechnol Bioeng. 2009;104(2):360–370. doi: 10.1002/bit.22405. [DOI] [PubMed] [Google Scholar]
- 41.Townsend-Nicholson A, Jayasinghe SN. Cell electrospinning: a unique biotechnique for encapsulating living organisms for generating active biological microthreads/scaffolds. Biomacromolecules. 2006;7(12):3364–3369. doi: 10.1021/bm060649h. [DOI] [PubMed] [Google Scholar]
- 42.Stankus JJ, Guan J, Fujimoto K, Wagner WR. Microintegrating smooth muscle cells into a biodegradable, elastomeric fiber matrix. Biomaterials. 2006;27(5):735–744. doi: 10.1016/j.biomaterials.2005.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swartz DD, Russell JA, Andreadis ST. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am J Physiol Heart Circ Physiol. 2005;288:1451–1460. doi: 10.1152/ajpheart.00479.2004. [DOI] [PubMed] [Google Scholar]
- 44.Rowe SL, Stegemann JP. Influence of thrombin concentration on the mechanical and morphological properties of cell seeded fibrin hydrogels. Acta Biomater. 2007;3:59–67. doi: 10.1016/j.actbio.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stegemann JP, Kaszuba SN, Rowe SL. Review: advances in vascular tissue engineering using protein-based biomaterials. Tissue Eng. 2007;13(11):2601–2613. doi: 10.1089/ten.2007.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.L’Heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue engineered human blood vessel. FASEB J. 1998;12:47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 47.Konig G, McAllister TN, Dusserre N, Garrido S, Iyican C, Marini A, Fiorillo A, et al. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials. 2009;30(8):1542–1550. doi: 10.1016/j.biomaterials.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gauvin R, Ahsan T, Larouche D, Lévesque P, Dubé J, Auger F, Nerem RM, Germain L. A novel single-step self-assembly approach for the fabrication of tissue-engineered vascular constructs. Tissue Eng Part A. 2010;16(5):1737–1747. doi: 10.1089/ten.TEA.2009.0313. [DOI] [PubMed] [Google Scholar]
- 49.Chow LW, Wang L, Kaufman DB, Stupp SI. Self-assembling nanostructures to deliver angiogenic factors to pancreatic islets. Biomaterials. 2010;31(24):6154–6161. doi: 10.1016/j.biomaterials.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghanaati S, Webber MJ, Unger RE, Orth C, Hulvat JF, Kiehna SE, Barbeck M, Rasic A, Stupp SI, Kirkpatrick CJ. Dynamic in vivo biocompatibility of angiogenic peptide amphiphile nanofibers. Biomaterials. 2009;30(31):6202–6212. doi: 10.1016/j.biomaterials.2009.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]