Abstract
Fruit-feeding butterflies can experience a more nutrient rich adult diet than nectar-feeding species, and can be expected to use these nutrients for egg production. Here we compare life span, and reproduction parameters of wild-caught females of large and long-lived species on either a sucrose or a mashed banana diet. With small sample sizes per species, but rich longitudinal data for each individual, we examined the longitudinal reproduction pattern, egg size and hatchability of these butterflies in captivity. Diet significantly affected mortality in captivity in a time-dependent manner. On average, we found that butterflies fed mashed banana laid 1.855 times more eggs than those fed sugar. They laid significantly more eggs when they laid and conserved egg size with age while butterflies fed sucrose showed significantly declining egg sizes. Egg hatchability was not significantly affected by diet. Long pre-oviposition periods, significantly smaller first eggs, and absence of age at capture effects on intensity of reproduction indicate low reproduction rates in the field that are due to low food availability. With our small sample sizes, we did not detect significant differences between the species in their response to the diet treatments.
Keywords: Aging, Egg size, Egg hatchability, Reproduction, Wing-wear
1. Introduction
1.1. Diet and evolution of extended life span
Adult food quality has been implicated in the evolution of extended longevity in holometabolous insects, because the ability to use adult nutrition for reproduction would relieve limits imposed by nutrients carried over from the larval stage on potential reproductive output and thus length of reproduction period (Carey, 2001; Dunlap-Pianka et al., 1977; Gilbert and Jervis, 1998). Lepidoptera are among the short-lived insect orders (Carey, 2001), and in most representatives, nitrogen sources carried over from the larval diet place an upper limit on female reproduction, as the adult diet provides sugar, but limited (sources of) amino acids (Boggs, 1997; O’Brien et al., 2004; Stjernholm and Karlsson, 2006). While in nectar-feeding Lepidoptera, amino acids in the adult diet can result in increases in reproduction when larvae had fed on nitrogen poor plants (Mevi-Schutz and Erhardt, 2003, 2005), in tropical pollen-feeding Heliconius butterflies a greater dependence on pollen and spermatophore derived amino acids has been detected (Boggs and Gilbert, 1979; Dunlap-Pianka, 1979; Gilbert, 1972; O’Brien et al., 2003). This nutritional strategy could remove the upper limit on reproduction imposed by reliance on stored nutrients and, therefore, facilitate the evolution of longer reproductive life spans (Dunlap-Pianka et al., 1977).
Fruit-feeding butterflies can obtain similarly long life spans (Molleman et al., 2007), but the few studies available on dietary effects on female reproduction in fruit-feeding butterflies found no (Bauerfeind and Fischer, 2005; Bauerfeind et al., 2007; Braby and Jones, 1995; Molleman et al., 2008b) or small (Bauerfeind et al., 2007) increases in egg numbers when butterflies were fed fruits or amino acids were added to a sugar solution. Some positive effects of nutrients other than sugar were recorded for egg size in Mycalesis terminus (Braby and Jones, 1995) and egg size and hatchability in Bicyclus anynana (Bauerfeind et al., 2007). These experiments were done on small satyrines. In our previous experiment on the larger Charaxes fulvescens, the phenotypic response to diet appeared complex and hard to ascertain due to extensive variation between and within individuals in daily oviposition, and an experimental design that introduced confounding environmental and seasonal effects. Nevertheless, some evidence for improved reproduction was found in banana, and amino acid fed cohorts compared to the sucrose cohort (Molleman et al., 2008a).
1.2. Phenotypic diet effects on longevity
Diet can affect life span of an individual directly or via its effect on reproduction (Beck, 2007; Carey et al., 2008a; Crawford et al., 2007; Flatt et al., 2005; Mair et al., 2005; Min et al., 2007; Min and Tatar, 2006; Piper, 2005; Romeis and Wackers, 2002; Tatar et al., 2001; Weithoff, 2007). Therefore, the phenotypic effects of diet on longevity and reproduction have to be integrated to gain insight into diet effects on life span evolution. When amino acids are included in a sugar solution, life spans of fruit-feeding butterflies can be longer in males (Beck, 2007) or shorter in females (Molleman et al., 2008b), through unknown mechanisms. Life span and reproduction may show a correlated response to diet, as we detected a life span cost of reproduction in B. anynana (Molleman et al., 2008b). However, such an effect was not discovered in C. fulvescens (Molleman et al., 2008a).
1.3. Use of field captured individuals of unknown age
This study uses longitudinal data on wild-caught individuals of unknown age to tests hypotheses on the effects of diet on life span and reproduction. This procedure has three advantages. Firstly, we can gain insight into life-history trajectories in the wild. The concept of using randomly selected wild-caught individuals builds on a new paradigm for deriving information on life-history trajectories in the wild from individuals of unknown age (Carey et al., 2008b; Crespin et al., 2006; Molleman et al., 2008a; Müller et al., 2004, 2007; Müller and Zhang, 2005). These studies focus mainly on survival and age distribution estimation, but also include aspects of reproduction, dietary restriction, and wear. Secondly, we are sure to use relevant larval nutrition. Larval diet quality has been shown to affect adult food preferences and life history responses to amino acids in the adult diet in nectar-feeding butterflies (Mevi-Schutz and Erhardt, 2003, 2005; Mevi-Schutz et al., 2003). Therefore, laboratory reared butterflies may not always represent the right context for experiments with adult diet. Thirdly, it opens up opportunities to use species that are not well suited for laboratory rearing.
In insect research, damage of wings is often used to assess either predation pressure or age (e.g. Benson, 1972; Ide, 2006; Shapiro, 1974). However, this remains problematic because insects can suffer injuries at any age and can also be handled by birds without showing beak marks (Wourms and Wasserman, 1985), thus generating extensive variation in wing-wear within age classes, and causing a weak correlation with age. In addition, the consequences of damage for survival have rarely been studied in wild insects (Bateman and Fleming, 2005, 2006; Hedenstrom et al., 2001; Maginnis, 2006). Because impairments usually have negative consequences for survival (Carey et al., 2007), heavily worn individuals can be expected to be under-represented in a population, independent of age. Scale loss is considered to correlate most with age in butterflies (Kemp, 2001). The small scales on the butterfly wing give it its color and may be loosed gradually during its life and be less sensitive to random traumatic events such as attacks by predators, although significant scale loss may occur during entrapment in and escape from spider webs (Eisner et al., 1964).
By bringing butterflies with different degrees of wing-wear into captivity and comparing remaining life span in captivity, age may be inferred from wear, if (1) the particular form of wear does not affect survival in captivity and (2) survival decreases with age. The first assumption may be rarely met as experimental removal of legs reduced life span in captivity in Drosophila melanogaster (Carey et al., 2007), and clipping of wings extended life span in captive house flies (Vergrugge, 2005). Intuitively, removal of legs or wings of small Diptera will affect their movement patterns in captivity, while for scale loss in butterflies no such effect would be expected, and since butterflies have little use of flight inside small cages this may also be true for other mild forms of wing-wear. To explore the prognostic value of wing-wear for remaining life-span, and to account for age variation among captured females, we recorded scale loss and other forms of wear and impairments in wild-caught individuals and used wear as a factor in all our analyses.
1.4. Aims
To further elucidate any potential for evolution of extended longevity in fruit-feeding butterflies based on the use of adult diet derived nutrients for reproduction, we performed an experiment with some of the most long-lived butterfly species and collected information on daily oviposition, egg size and hatchability. We took detailed notes on wing-wear and impairments upon capture to explore the potential for aging butterflies. With small sample size per species, but rich longitudinal data for each individual, we examined the longitudinal reproduction pattern, egg size and hatchability of these butterflies in captivity.
2. Material and methods
2.1. Study organisms
Butterflies of the African genus Euphaedra (Nymphalidae) are understory butterflies that are often observed feeding on fallen fruits and there are some observations of Euphaedra species feeding on rotting wood or sap oozing from tree roots (Hecq, 1997; Molleman and Hecq, 2005; Molleman et al., 2005b). Even-though they are strong flyers, they do not typically disperse widely and this contributed to the discovery of their extraordinary long life spans (Molleman et al., 2007). Euphaedra eggs are dome shaped (halved ball). E. medon Thurau 1903, is the most abundant Euphaedra (Molleman et al., 2006), and is sexually dimorphic. Its host-plant is a small vine, Paullinia pinata (Sapindaceae) and its longevity record is 293 days (10 months). E. alacris (or E. rattrayi) Sharpe 1904 has the second longest longevity record with 253 days (9 months) and its host-plant is Aphania (Sapindaceae). Both sexes of E. harpalyce Talbot 1929 resemble E. medon females, but they are larger. They have several host-plants; Blighia, Aphania and Pancovia (Sapindaceae).
2.2. Experimental methods
Butterflies were collected from Kibale National Park in April–May 2007 (E. medon and E. alacris were all collected during the first 2 weeks) using traps baited with fermenting banana, regardless of wing-wear, and kept singly in cages at Makerere University Biological Field Station. The locally manufactured cages were cylindrical (30 cm height, 21 cm width), made of fine mesh and 2 metal rings and fitted with a zipper. Butterflies and cages were numbered and hung in a room with four mesh covered windows (allowing ventilation and indirect sunlight) and a high (3–4 m) wooden ceiling under a tin roof at Makerere University Biological Field Station.
The wing damage and other impairments (Table 1) were scored using experience from ongoing field research on impairments, and individuals were randomly assigned to either a mashed banana or a 10% sugar solution diet. The mashed banana was prepared by slicing and mashing up two hands of bananas (about 30 bananas) in a clean bucket using a piece of wood. Bananas are known to contain about 1 g of protein and 14 g of sugar per 100 g (NutritionData, 2008), and the sugar composition is very variable with about 50% sucrose, 25% glucose and 25% fructose (Molleman et al., 2008b). The sugar solution was made of locally obtained cane sugar and boiled rainwater. Banana was presented on a plastic dish and replaced twice a week and sugar solution was presented in a small vial mounted on a piece of cardboard, topped up daily and refreshed once every other week. Water was presented as wetted tissue paper on a plastic dish inside the cages. The tissue paper and mashed banana was moistened daily by spraying water and paper was replaced weekly.
Table 1.
Summary information on sample sizes, life spans, wear/impairments, and reproduction parameters of wild-caught fruit-feeding butterflies in captivity.
| Species
|
||||||
|---|---|---|---|---|---|---|
|
E. alacris
|
E. harpalyce
|
E. medon
|
||||
| Sugara | Bananaa | Sugara | Bananaa | Sugara | Bananaa | |
| Life span | ||||||
| N | 19 | 22 | 13 | 28 | 18 | 21 |
| Number of censored | 4 | 0 | 2 | 1 | 3 | 1 |
| Aver. wing length (cm) | 4.8 | 4.8 | 5.1 | 5.2 | 4.1 | 4.2 |
| Median life span (days) | 29 | 32 | 25 | 22 | 31.5 | 39 |
| Maximum life span | 50 | 83 | 47 | 62 | 59 | 80 |
| Average wear | ||||||
| Wing missing (%) | 1.017 | 2.434 | 5.251 | 2.732 | 1.044 | 1.037 |
| Number of tears | 0.088 | 0.087 | 0.179 | 0.262 | 0.148 | 0.111 |
| Scale loss (%) | 3.684 | 5.304 | 9.231 | 9.536 | 4.556 | 4.238 |
| Reproduction | ||||||
| N fertile | 17 | 19 | 11 | 23 | 16 | 21 |
| Number of censored | 2 | 0 | 2 | 1 | 2 | 1 |
| Average egg diameter | 1.8 | 1.79 | 1.88 | 1.89 | 1.35 | 1.35 |
| Mean number of eggs per day when >0 | 5.76 | 6.79 | 9.82 | 9.65 | 9.25 | 14.9 |
| Max number of eggs per day when >0 | 14 | 23 | 19 | 34 | 21 | 36 |
| Mean total number of eggs | 21.88 | 34.32 | 42.18 | 61.3 | 55.13 | 115.9 |
| Max total number of eggs | 76 | 172 | 98 | 253 | 108 | 322 |
Diet.
Females were presented with a cutting of their respective host-plants with two mature leaves (Blighia for E. harpalyce) which was replaced every day. Small leaf discs with the eggs were cut out (to avoid molding) and placed in a plastic container together with any detached eggs that were laid elsewhere. For each individual, the diameter of up to four eggs for each day it laid was measured by overlaying them with a 0.5 mm grid that was printed on a transparent sheet and using a binocular microscope. All eggs were left to hatch and the number of caterpillars was counted daily, 6–14 days after the eggs were laid. Butterflies were monitored daily until death.
2.3. Statistical methods for data analyses
Due to the limited sample size, we carefully included the results with p-value smaller than 0.1 and interpreted them as marginally significant. Diet was included in all statistical analyses regardless of significance, because it is the factor of primary interest. Wing impairment measurements, such as scale loss, average of number of tears on four wings and average missing percentage of four wings, were included as covariates if they significantly affected the response variables. Butterflies laying no eggs in captivity were referred to as ‘infertile’ and the 0–1 coded infertility was treated as a covariate in analyzing life span. Five out of one hundred and seven fertile butterflies, which were most likely relatively young (with scale loss less than 5%) and laid more than 10 eggs in captivity, hatched none of their eggs. These are suspected to be unmated. Hence we had excluded them and did a sensitivity analysis for reproduction data. The result was similar and, therefore, not reported here. Censored butterflies were included in all statistical models assuming non-informative right censoring.
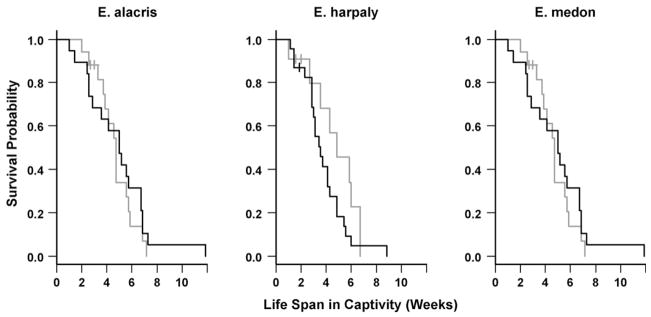
Species specific diet effects on life span in captivity were first visually examined with Kaplan–Meier plots (Fig. 1). Subsequently, a time-dependent Cox model (Cox, 1972, 1975) on life span in captivity was used to quantify any diet effect after adjusting other covariates.
Fig. 1.
Kaplan–Meier plots for wild-caught female E. alacris, E. harpalyce and E. medon with butterflies fed sugar (grey) and mashed banana (black).
Fertility (probability of being fertile) was modeled through a logistic regression. Then we excluded infertile butterflies in the analysis of reproduction. Both overall reproduction in captivity, measured by total number of eggs, and intensity of reproduction, measured by the number of eggs divided by the number of days on which eggs were laid (not from first to last reproduction), are of interest and were modeled using a Generalized Linear Model (GLM) with Poisson distribution.
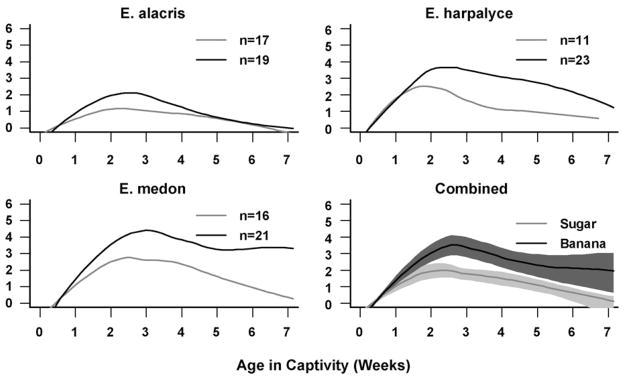
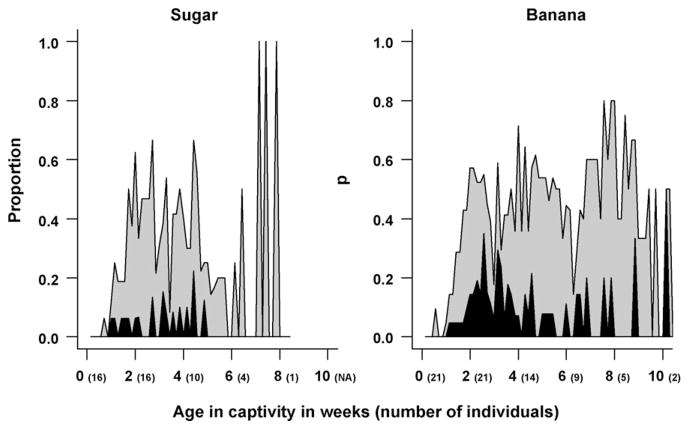
With rich longitudinal reproduction data, we also investigated the reproduction pattern over time. Nonparametric smoothed mean curves were created for each diet (Fig. 3) to visualize this longitudinal pattern of reproduction in captivity. The smoothed mean curves were created using the “loess” function in R, which used a local polynomial smoothing technique, and the 95% point-wise bootstrap confidence intervals from combined data were derived from the 1000 bootstrap samples. A daily parity plot, which represents raw egg-laying over time, is reported for E. Medon as an example (Fig. 4).
Fig. 3.
Longitudinal egg reproduction of butterflies in sugar and banana cohorts. The smoothed mean curves were created from the raw data of all species combined using the “loess” function in R, which is a local polynomial smoothing technique, and the 95% point-wise bootstrap confidence intervals for combined species were derived from the 1000 bootstrap samples.
Fig. 4.
Daily parity plots for wild-caught E. medon fed sugar (left) and mashed banana (right), where black regions represent the proportion of butterflies laying more than 10 eggs on a day, grey 1–10 eggs, and white region 0 eggs.
Plots of the egg size data showed a decrease over time in the sugar cohort. Hence, we used a linear mixed effects model with species, diet, and day as covariates to investigate the egg diameter changes over time. Random intercepts and slopes were used to accommodate individual-specific patterns. Wing impairment measurements were insignificant and hence excluded from the model (Table 6).
Table 6.
Parameter estimation of linear mixed-effects model for the size of eggs of females captured form the wild held in captivity on either sugar or banana diets where E. medon fed with banana is treated as baseline group.
| Covariance parameter estimates | |
|---|---|
| Covariance parameter | Estimate × 10−2 |
| Var (intercept bi0) | 0.438 |
| Covariance (intercept bi0, slope bi1) | −0.020 |
| Var (slope bi1) | 0.001 |
| Var (error) | 1.003 |
| Estimate | Standard error | t-Value | Pr > |t| | 95% confidence interval | ||
|---|---|---|---|---|---|---|
| Parameter estimation for fixed effects | ||||||
| Intercept | 1.350 | 0.018 | 73.01 | <.0001 | 1.313 | 1.387 |
| Sugar | 0.015 | 0.022 | 0.67 | 0.505 | −0.028 | 0.058 |
| E. alacris | 0.455 | 0.026 | 17.41 | <.0001 | 0.404 | 0.506 |
| E. harpalyce | 0.581 | 0.025 | 23.33 | <.0001 | 0.532 | 0.630 |
| Day | 0.002 | 0.001 | 1.50 | 0.139 | −0.001 | 0.004 |
| Day × sugar | −0.003 | 0.002 | −2.09 | 0.037 | −0.006 | −0.0002 |
| Day × E. alacris | 0.002 | 0.002 | 1.16 | 0.246 | −0.002 | 0.006 |
| Day × E. harpalyce | −0.003 | 0.002 | −1.88 | 0.061 | −0.006 | 0.001 |
| DF numerator | DF denominator | F-value | Pr > F | |
|---|---|---|---|---|
| Type III tests of fixed effects | ||||
| Sugar | 1 | 699 | 0.44 | 0.505 |
| Species | 2 | 699 | 306.77 | <.0001 |
| Day | 1 | 73 | 2.23 | 0.140 |
| Day × sugar | 1 | 699 | 4.36 | 0.037 |
| Day × species | 2 | 699 | 3.63 | 0.027 |
The mathematical model is: Sizei(Dayj) = β0 + β1Sugar + β2E. alacris + β3E. har palyce + β4Dayj + β5SugarDayj + β6E. alacrisDayj + β7E. har palyceDayj + bi0 + bi1Dayj+ errorij, ere individual-specific intercept bi0 and individual-specific slope bi1are jointly normally distributed with mean 0 and variance Σ. Errors are assumed to be independent of random effects and normally distributed. There is an interaction between diet and time as the egg size in sugar cohorts decreases significantly different from that in the banana cohorts. The bold values signify p-value.
3. Results
3.1. Impairments
Only one butterfly was missing a leg (left hind) and none had broken antennas. This individual did not form an outlier and was retained in the data set. Wing-wear was summarized as average missing percentage of surface of four wings, average number of tears of four wings and percent scale loss (Table 1). Together with the average of wing length, which represents the body size of butterflies within a species, these wing traits were included in the following analyses of longevity and fecundity. Percent scale loss differed significantly between the species (ANOVA; F2,21 = 12.28, p < 0.0001), with E. harpalyce having more scale loss at capture than E. medon and E. alacris.
3.2. Life span
Eleven out of one hundred and twenty-one butterflies were censored as large ants attacked butterflies after finishing the sugar in the sugar cohort, and few individuals escaped. Escape was either noted (known date) or inferred later (last day of egg-laying used as censored time of death). Comparing with butterflies fed sugar, the ones fed banana were at a marginally lower survival rate at first and a significantly higher rate after a month in captivity (Fig. 1). This is corroborated by the significant interaction between diet and days in captivity in the Cox model (Table 2). Other prognostic factors satisfied the proportional hazard assumption and were included without interaction with time. E. harpalyce lived marginally significantly shorter than E. alacris and E. medon. The percent scale loss at capture (possible proxy for age) was associated with marginally higher mortality rate as we expected. Infertile butterflies also lived significantly shorter than fertile butterflies with hazard ratio 5.026 (with 95% confidence interval [2.501, 10.100]), after adjusting species, diet and wing-wear. Those infertile butterflies might be simply older than the others, have temperaments that are incompatible with caged life (Molleman et al., 2005a), or have biological defects.
Table 2.
Life span in captivity: parameter estimation in the Cox model for wild-caught fruit-feeding butterflies in captivity held on sugar or banana diets. Hazard ratios show the mortality compared to E. medon fed sugar. E. harpalyce had a significantly lower survival probability than E. medon and E. alacris. Butterflies that did not lay eggs lived shorter than those that did. More scale loss was also associated with lower survival rates.
| Estimate | Standard error | χ2 | Pr > χ2 | Hazard ratio | |
|---|---|---|---|---|---|
| Banana | 1.051 | 0.550 | 3.648 | 0.056 | 2.860 |
| Banana × t | −0.036 | 0.017 | 4.514 | 0.034 | 0.965 |
| E. alacris | 0.200 | 0.243 | 0.675 | 0.411 | 1.221 |
| E. harpalyce | 0.490 | 0.265 | 3.412 | 0.065 | 1.632 |
| Infertile | 1.615 | 0.356 | 20.566 | <0.001 | 5.026 |
| Scale loss | 0.033 | 0.017 | 3.773 | 0.052 | 1.033 |
The bold values signify p-value.
3.3. Fertility
One hundred and seven out of 121 butterflies laid eggs in captivity and the other 15 were classified as infertile (no eggs). The estimated odds ratios with 95% confidence intervals are reported in Table 3. Diet had no significant effect on fertility (laying egg(s) or not), and we found no differences among the species. Butterflies with more tears tended to be infertile. To avoid this confounding factor, infertile butterflies were excluded from the following analyses of reproduction.
Table 3.
Estimated odds ratios of being infertile for wild-caught fruit-feeding butterflies held in captivity on sugar or banana diets, where E. medon fed sugar is treated as the baseline group. Results are significant when the confidence interval does not contain 1. The species had similar probabilities of being infertile and diet did not significantly affect fertility.
| Point estimate | 95% Wald confidence interval | ||
|---|---|---|---|
| Banana | 0.776 | 0.232 | 2.598 |
| E. alacris | 3.181 | 0.533 | 18.986 |
| E. harpalyce | 2.907 | 0.509 | 16.599 |
| Tears | 10.079 | 1.646 | 61.711 |
3.4. Reproduction
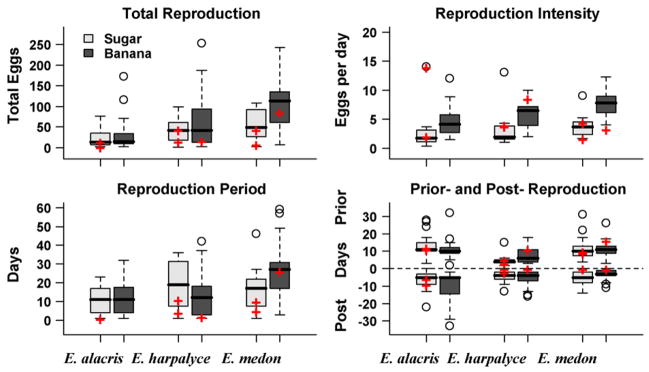
Butterflies fed banana laid significantly more eggs than those fed sugar (Table 4), which is mainly due to banana fed butterflies laying significantly more eggs when they laid any, but they also laid eggs non-significantly more often and over a non-significantly longer period (Fig. 2). Graphical representations of egg laying patterns also show that banana fed butterflies laid eggs more often and in larger numbers per day than the sucrose cohorts and continued to reproduce intensively with progressing age (Figs. 3 and 4). Over all species, the banana fed butterflies laid 1.855 (exponential of 0.618 reported in Table 4) times more eggs than those fed sucrose, which is about double. There were significant differences among the species in egg numbers: E. medon laid more (but smaller) eggs than E. alacris and E. harpalyce. The diet effect was not significantly different across the species. Individuals with more scale loss laid significantly fewer eggs (and no other wing-wear measurements were significant). This is not surprising as butterflies with more scale loss lived shorter in captivity and thus had shorter reproduction periods.
Table 4.
Parameter estimation of Generalized Linear Model (Poisson distribution) for modeling the total reproduction of wild-caught fruit-feeding butterflies held in captivity on sugar or banana diets, where E. medon fed sugar is treated as the baseline group. Overall, butterflies fed banana laid 1.855 times (exponential of 0.618) more eggs than those fed sugar. E. alacris and E. harpalyce laid fewer eggs than E. medon, and individuals with more scale loss laid fewer eggs.
| Parameter | DF | Estimate | StandError | 95% CI | Pr > Chisq | |
|---|---|---|---|---|---|---|
| Intercept | 1 | 4.443 | 0.030 | 4.384 | 4.502 | <0.001 |
| Banana | 1 | 0.618 | 0.029 | 0.561 | 0.675 | <0.001 |
| E. alacris | 1 | −1.135 | 0.036 | −1.206 | −1.064 | <0.001 |
| E. harpalyce | 1 | −0.364 | 0.031 | −0.424 | −0.303 | <0.001 |
| Scale loss | 1 | −0.070 | 0.004 | −0.077 | −0.063 | <0.001 |
Fig. 2.
Reproduction of wild-caught female butterflies in captivity. The median is indicated by the solid line in each box, whose lower and upper bounds are the first and third quartiles. The difference between the first and third quartiles is referred as inter quartile range (IQR). Data points which are 1.5 IQR smaller than the first quartile or 1.5 IQR bigger than the third quartile are indicated by circles and referred to as outliers. A whisker connects the boundary of the box with the largest value which is not an outlier. Censored observations are indicated with plus signs.
We found no significant diet effect on the number of days an individual laid eggs. Compared to E. medon, E. alacris had significantly fewer days of reproduction in captivity. More scale loss is associated with fewer reproductive days in captivity; however, other wing-wear measurements were not significant. Similar results were obtained for the length of the reproduction period (bottom left of Fig. 2).
Reproduction intensity is also a good measure for diet effect on reproduction which is less affected by life span. On average, butterflies fed banana produce 1.269 more eggs per day (on which eggs were laid) than those fed sugar (Table 5). Scale loss had no significant effect on intensity of reproduction, bearing out that age and intensity of reproduction are not strongly related. Compared to E. medon, E. alacris and E. harpalyce laid fewer eggs per day.
Table 5.
Parameter estimation of Generalized Linear Model (Poisson distribution) for modeling the intensity of reproduction of wild-caught fruit-feeding butterflies held in captivity on sugar or banana diets, where E. medon fed sugar is treated as the baseline group. Overall, butterflies fed banana laid 1.269 times more eggs than those fed sugar on days that they laid any.
| Parameter | DF | Estimate | StandError | 95% CI | Pr > Chisq | |
|---|---|---|---|---|---|---|
| Intercept | 1 | 1.847 | 0.105 | 1.638 | 2.049 | <0.001 |
| Banana | 1 | 0.238 | 0.100 | 0.044 | 0.436 | 0.017 |
| E. alacris | 1 | −0.486 | 0.120 | −0.723 | −0.253 | <0.001 |
| E. harpalyce | 1 | −0.259 | 0.121 | −0.499 | −0.023 | 0.033 |
| Scale loss | 1 | −0.004 | 0.010 | −0.024 | 0.016 | 0.725 |
Prior reproduction period was defined as the time from the day of capture to the day before the first egg is collected. Similarly, post-reproduction period was the period between the day after the last egg is collected and death or censoring. The length of prior and post-reproduction period is shown in the bottom right of Fig. 2, where post-reproduction days are shown in negative direction. We did not find significant diet and wing-wear effects on prior and post-reproduction periods. E. harpalyce started laying eggs in captivity significantly earlier (with p-value < 0.0001), and E. alacris had longer post-reproduction periods (with p-value = 0.002) than E. medon.
3.5. Egg size
The eggs that were laid on the first day of oviposition tended to be smaller than subsequent eggs (with p-value < 0.0001) in both diet groups. Hence we excluded those very first eggs in the following analysis. Wing-wear measurements were not significant and excluded from the model (Table 6). On average, butterflies fed banana laid similar sized eggs as those fed sugar at the beginning. However, the size of eggs in the sugar cohort decreased over time, which was not the case in the banana cohorts. The difference between the two slopes was 0.003 with p-value 0.037. Furthermore, an individual’s intercept negatively correlates with its slope. This shows a regression phenomenon that butterflies laying larger eggs at the beginning tended to have a more decreasing or less increasing egg size later. E. harpalyce laid the biggest eggs, and E. medon laid significantly smaller eggs than E. alacris (Tables 1 and 6). The rate of change in egg size over time was also significantly different from species to species (Table 6).
3.6. Hatchability
Hatchability was defined as the percentage of hatched eggs among the collected eggs for each butterfly. The banana cohort was not significantly different from the sugar cohort, and none of the wing-wear measurements were significant, neither was the size of wings. E. alacris had a significantly lower overall hatching rate than E. medon and E. harpalyce. We found no significant trend in egg hatchability over time.
4. Discussion
This is the first study showing large effects (1.855 fold increase) of enriched adult diet on fruit-feeding butterfly reproduction. This may not rival the effect sizes found for some pollen-feeding Heliconius species that lay eggs over longer periods when provided with pollen. However, we observed short post-reproductive life spans in these fruit-feeding butterflies (except perhaps E. alacris) and little evidence for a decrease in intensity of reproduction with age in banana fed cohorts. Possibly, captive conditions reduced longevity, and thus limited the leverage of nutrients in the banana for increasing total reproduction. In addition, the size of the cages and the amount and quality of host-plant can have reduced reproduction rate in some or all of the species. The results for these large and long-lived fruit-feeding butterfly species contrast with findings for smaller species in which at most small effects of enriched diet were noted. The most positive effect was found in experiments with B. anynana in which butterflies fed banana laid about 1.4 times more eggs than those fed sucrose (Bauerfeind et al., 2007; Geister et al., 2008), while no significant effect was found when a banana diet was compared with a sugar solution mimicking the sugar composition in banana in an earlier experiment (first reported in Molleman, 2004; Molleman et al., 2008b), and no such response was recorded when amino acids or other nutrients were added to a sugar solution (Bauerfeind and Fischer, 2005; Bauerfeind et al., 2007; Molleman et al., 2008b). Because different sugars can affect butterfly reproduction and longevity differently (Erhardt, 1991; Romeis and Wackers, 2002; Rusterholz and Erhardt, 1997), the seemingly conflicting results found in these similar experiments with B. anynana suggest that sugar composition or (uncontrolled) food intake was (partly) responsible for the differences in egg numbers found between banana and sucrose diets. Fruit sugars are often dominated by sucrose and fruit-feeding birds are adapted to pass food quickly to avoid osmotic problems associated with sucrose intake. This problem is, however, avoided by hummingbirds which have highly efficient sucrase (Delrio et al., 1992). Therefore, while flowers may gear their nectar offerings to the sugar preferences of their pollinators (Baker and Baker, 1990), fruit-feeding butterflies can be expected to be adapted to a high proportion of sucrose in the fruits that are geared toward seed dispersal (Baker et al., 1998). The sugar composition of foods is a potentially important, but often overlooked factor in this type of studies (Rusterholz and Erhardt, 2000).
In an earlier experiment with C. fulvescens, which included amino acids added to a sucrose solution as one of the diet treatments, we found some evidence for positive effects of amino acids on reproduction (Molleman et al., 2008a). Because of the almost two-fold diet effect found in our study and the longitudinal patterns observed (Figs. 3 and 4), we suspect that these larger fruit-feeding butterflies responded to (sources of) amino acids and possibly other nutrients in their adult diet.
The sugar solution was topped up daily and could have become more concentrated as water evaporated, but could also have absorbed water from humid air, and in addition, microorganisms could have effected its composition. The mashed banana treatment had similar issues as it could also dry out (despite daily moistening) and decay. We do not expect decay to be a major issue because experiments with B. anynana showed that yeast and decay of banana do not affect the reproduction of females (Bauerfeind et al., 2007; Molleman et al., 2008b). Because females were not able to mate during this experiment they did not receive new sperm or nuptial gifts and this may have reduced lifespan and reproduction in our experiment (Boggs and Gilbert, 1979).
Our methods for measuring egg size were crude compared to those in earlier studies (Fischer et al., 2002), but they were still useful, partly because these butterflies laid relatively large eggs. Our results corroborate that fruit-feeding butterflies maintain egg size when fed fruits but not on sugar only diets (Bauerfeind et al., 2007; Braby and Jones, 1995). We found differences between the species in egg size and egg size change over time which may have to do with host-plant leave-thickness (e.g. Paulinia leaves on which small eggs are laid seem much more delicate than Aphania leaves on which large eggs are laid), as the ability of newly emerged caterpillars to chew their host-plant seems to be an important factor in the evolution of egg size in butterflies (Fischer et al., 2002; GarciaBarros and Munguira, 1997). Even-though egg hatchability can be expected to correlate with egg size, this parameter did not reveal differences between the diet cohorts. However, the power of this analysis was limited by the nature of the data that often took the shape of 1 (all eggs of that day hatched) or 0 (no eggs hatched).
By using individuals directly from the field there is more variation in our study populations than would exist in laboratory populations. This includes both genetic variation which would be lost during adaptation to laboratory rearing and variation caused by pre-capture life experience (e.g. pre-capture diet, life span, copulation(s), and reproduction). Most of this variation will be age-related and any estimates of age at capture are important covariates in our analyses.
Our results showed shorter survival associated with more wing-wear at capture, which implies either the impairment effect on survival in cages or aging in the wild (survival decreases with age). The latter may be more likely as scale loss can hardly be expected to affect performance and butterflies have little use for flight in these cages. Hence the prognostic power of wing-wear measurement on remaining life span in captivity supports the usage of impairment measurements as an estimate of age at the cohort level. The remaining life span in captivity is predicted by scale loss and this may be interpreted as measure of age corroborating earlier finding that scale loss is best correlated with butterfly age (Ehrlich and Gilbert, 1973; Kemp, 2001). (The number of tears in the wings was a predictor of infertility in our data.) Our results then show that older butterflies lay eggs at similar rates as younger butterflies since wing-wear measures did not significantly correlate with the intensity of reproduction. If reproduction rates in the wild are low as suggested by our data (see below), the pre-capture reproduction may fade in comparison to the captive reproduction. This may explain why for both diet treatments, life span in captivity was affected by age at capture (as estimated by scale loss), while reproduction rate was not, even if these butterflies partly rely on stored nutrients for reproduction. Wing length was not a significant factor in any of our analyses, even though size is often an important predictor of reproductive potential in insects. This result may be due to limited within species variation in wing-length and small sample sizes.
Ultimately, we would like to elucidate life-history trajectories of fruit-feeding butterflies in the wild. Therefore, we will need to know the food quality and quantity that these butterflies experience in the wild and how that affects their reproduction and survival. Some data on food quality of these butterflies have been collected (Molleman et al., 2005b), but the effects of these particular fruits on life-history remain to be measured. Our results and those of an earlier experiment with C. fulvescens (Molleman et al., 2008a) provide three indications that these butterflies are dietary restricted and may have low reproduction rates in the wild: (1) they typically have prior reproduction periods of at least a week (Fig. 2); (2) the first eggs laid in captivity are significantly smaller than subsequent eggs, indicating some physiological shift towards egg-laying and (3) intensity of reproduction in both diet cohorts was not affected by wing-wear measures that can be interpreted as surrogates of age at capture (elaborated above).
With butterflies being dependent on adult nutrition for reproduction and adult food being scarce in their environment, fruiting phenology may be an important factor in the population dynamics and temporal abundance patterns of fruit-feeding butterflies. Therefore, more insight into the role of adult nutrition in fruit-feeding butterfly life-history could be relevant to conservation in the face of climate change and associated changes in fruiting phenology (Chapman et al., 2005), and loss of fruiting-tree seed dispersers (Chapman and Onderdonk, 1998; Maisels et al., 2001; Wang et al., 2007). However, while fruiting phenology is readily measurable, potential other foods (such as tree sap and honey dew) are more elusive (Uehara-Prado et al., 2007), and direct measures of the nutritional state of butterflies in the wild may be needed. Prior oviposition time of wild-caught females might be useable as such. The potential importance of insight into butterfly nutrition for understanding population dynamics was shown in studies on temperate nectar-feeding butterflies which showed that semi-starvation impacts fecundity directly (Boggs and Ross, 1993), and this translates to the field (Murphy, 1983; Boggs pers. comm.).
With small sample sizes per species we cannot make detailed claims on the effects of diet on particular species. The response to diet did not significantly differ among the species in our experiment, but we did find significant differences among the species in life span and reproduction parameters. These inter-species differences can be interpreted as differences among the species that reflect their ecology and life-history in the wild (e.g. larval diet), differential suitability for captivity in the small cages, or differences among the species in the age distribution of the populations they were drawn from. The latter is indicated by the significantly different scale loss among the species: being higher in E. harpalyce than in E. medon and E. alacris. Thus, the adult abundance of E. harpalyce could have been in a decline phase with more aged individuals and less young recruits, while the E. medon population was growing and had a relatively high proportion of young animals. However, such assertions need to be tested against animals of known age as species can loose scales at different rates which are or are not proportional to their longevity. Even though we found no significant differences in diet effect between the species, inter-species differences were numerous and large enough to demonstrate the inappropriateness of generalizing from a single model species to even closely related taxa.
5. Conclusions
Large and long-lived fruit-feeding butterflies of the genus Euphaedra benefit from a banana-compared to a sugar diet because they lay significantly more eggs when they lay, lay eggs non-significantly more often, and over a non-significantly longer period, resulting in a doubling of reproduction, while survival is slightly reduced during the first 30 days in captivity. Therefore, use of adult diet derived nutrients other than sugar could have played a role in the evolution of extended longevity in fruit-feeding butterflies. The remaining life span in captivity of wild-caught females is predicted by scale loss and, therefore, this may be interpreted as a surrogate of age. We noted important differences between the species that emphasize the danger of generalizing from a small number of model species.
Acknowledgments
We thank Boniface Balyeganira, Harriet Kesiime, Christopher Aliganyira, Francis Katuramu Kaywanii, John Koojo, Mary Alum, and Malgorzata Arlet for their invaluable assistance in the field and in the laboratory and Francis Akoch Edigu for data entry. Paul Brakefield and Bas Zwaan have been involved in our previous efforts to show diet effects on reproduction in fruit-feeding butterflies and their past contributions have been important to this manuscript. We are grateful to Carol Boggs and anonymous reviewers who provided helpful comments on an earlier version of this manuscript. This study was conducted with kind permission of the Uganda Wildlife Authority (U.W.A.) and the Ugandan National Council for Science and Technology (U.N.C.S.T.). The funding was provided by the National Institute on Aging (PO1 AG022500-01 and PO1 AG608761-10 to JRC).
References
- Baker HG, Baker I. The predictive value of nectar chemistry to the recognition of pollinator types. Israel Journal of Botany. 1990;39:157–166. [Google Scholar]
- Baker HG, Baker I, Hodges SA. Sugar composition of nectars and fruits consumed by birds and bats in the tropics and subtropics. Biotropica. 1998;30:559–586. [Google Scholar]
- Bateman PW, Fleming PA. Direct and indirect costs of limb autotomy in field crickets, Gryllus bimaculatus. Animal Behaviour. 2005;69:151–159. [Google Scholar]
- Bateman PW, Fleming PA. Increased susceptibility to predation for autotomized house crickets (Acheta domestica) Ethology. 2006;112:670–677. [Google Scholar]
- Bauerfeind SS, Fischer K. Effects of adult-derived carbohydrates, amino acids and micronutrients on female reproduction in a fruit-feeding butterfly. Journal of Insect Physiology. 2005;51:545–554. doi: 10.1016/j.jinsphys.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Bauerfeind SS, Fischer K, Hartstein S, Janowitz S, Martin-Creuzburg D. Effects of adult nutrition on female reproduction in a fruit-feeding butterfly: the role of fruit decay and dietary lipids. Journal of Insect Physiology. 2007;53:964–973. doi: 10.1016/j.jinsphys.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Beck J. The importance of amino acids in the adult diet of male tropical rainforest butterflies. Oecologia. 2007;151:741–747. doi: 10.1007/s00442-006-0613-y. [DOI] [PubMed] [Google Scholar]
- Benson WW. Natural selection for Müllerian mimicry in Heliconius erato in Costa Rica. Science. 1972;176:936–939. doi: 10.1126/science.176.4037.936. [DOI] [PubMed] [Google Scholar]
- Boggs CL. Reproductive allocation from reserves and income in butterfly species with differing adult diets. Ecology. 1997;11:181–191. [Google Scholar]
- Boggs CL, Gilbert LE. Male contribution to egg-production in butterflies: evidence for transfer of nutrients at mating. Science. 1979;206:83–84. doi: 10.1126/science.206.4414.83. [DOI] [PubMed] [Google Scholar]
- Boggs CL, Ross CL. The effect of adult food limitation on life-history traits in Speyeria mormonia (Lepidoptera, Nymphalidae) Ecology. 1993;9:433–441. [Google Scholar]
- Braby MF, Jones RE. Reproductive patterns and resource-allocation in tropical butterflies: influence of adult diet and seasonal phenotype on fecundity, longevity and egg size. Oikos. 1995;72:189–204. [Google Scholar]
- Carey JR. Insect biodemography. Annual Review of Entomology. 2001;46:79–110. doi: 10.1146/annurev.ento.46.1.79. [DOI] [PubMed] [Google Scholar]
- Carey JR, Pinter-Wollman N, Wyman M, Müller HG, Molleman F, Zhang N. A search for principles of disability using experimental impairment of Drosophila melanogaster. Experimental Gerontology. 2007;42:166–172. doi: 10.1016/j.exger.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR, Harshman L, Liedo P, Müller HG, Wang JL, Zhang Z. Longevity-fertility trade-offs in the tephritid fruit fly, Anastrepha ludens, across dietary restriction gradients. Aging Cell. 2008a;7:470–477. doi: 10.1111/j.1474-9726.2008.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR, Papadopoulos NT, Müller HG, Katsoyannos BI, Kouloussis NA, Wang JL, Wachter K, Yu W, Liedo P. Age structure changes and extraordinary lifespan in wild medfly populations. Aging Cell. 2008b;7:426–437. doi: 10.1111/j.1474-9726.2008.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman CA, Onderdonk DA. Forests without primates: primate-plant codependency. American Journal of Primatology. 1998;15:127–141. doi: 10.1002/(SICI)1098-2345(1998)45:1<127::AID-AJP9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Chapman LJ, Struhsaker TT, Zanne AE, Clark CJ, Poulsen JR. A long-term evaluation of fruiting phenology: importance of climate change. Journal of Tropical Ecology. 2005;21:31–45. [Google Scholar]
- Cox DR. Regression models and life-tables. Journal of Royal Statistical Society, Series B. 1972;34:187–220. [Google Scholar]
- Cox DR. Partial likelihood. Biometrika. 1975;62:264–276. [Google Scholar]
- Crawford D, Libina N, Kenyon C. Caenorhabditis elegans integrates food and reproductive signals in lifespan determination. Aging Cell. 2007;6:715–721. doi: 10.1111/j.1474-9726.2007.00327.x. [DOI] [PubMed] [Google Scholar]
- Crespin L, Harris MP, Lebreton JD, Wanless S. Increased adult mortality and reduced breeding success with age in a population of common guillemot Uria aalge using marked birds of unknown age. Journal of Avian Biology. 2006;37:273–282. [Google Scholar]
- Delrio CM, Baker HG, Baker I. Ecological and evolutionary implications of digestive processes: bird preferences and the sugar constituents of floral nectar and fruit pulp. Experientia. 1992;7:544–550. [Google Scholar]
- Dunlap-Pianka HL. Ovarian dynamics in Heliconius butterflies: correlations among daily oviposition rates, egg weights, and quantitative aspects of oogenesis. Journal of Insect Physiology. 1979;25:741–749. [Google Scholar]
- Dunlap-Pianka HL, Boggs CL, Gilbert LE. Ovarian dynamics in Heliconiine butterflies: programmed senescence versus eternal youth. Science. 1977;197:487–490. doi: 10.1126/science.197.4302.487. [DOI] [PubMed] [Google Scholar]
- Ehrlich PR, Gilbert LE. Population structure and dynamics of the tropical butterfly Helicoinius ethilla. Biotropica. 1973;5:69–82. [Google Scholar]
- Eisner T, Alsop R, Ettershank G. Adhesiveness of spider silk. Science. 1964;146:1058–1061. doi: 10.1126/science.146.3647.1058. [DOI] [PubMed] [Google Scholar]
- Erhardt A. Nectar sugar and amino-acid preferences of Battus philenor (Lepidoptera, Papilionidae) Ecological Entomology. 1991;16:425–434. [Google Scholar]
- Fischer K, Zwaan BJ, Brakefield PM. How does egg size relate to body size in butterflies? Oecologia. 2002;131:375–379. doi: 10.1007/s00442-002-0913-9. [DOI] [PubMed] [Google Scholar]
- Flatt T, Tu MP, Tatar M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. Bioessays. 2005;27:999–1010. doi: 10.1002/bies.20290. [DOI] [PubMed] [Google Scholar]
- GarciaBarros E, Munguira ML. Uncertain branch lengths, taxonomic sampling error, and the egg to body size allometry in temperate butterflies (Lepidoptera) Biological Journal of the Linnean Society. 1997;61:201–221. [Google Scholar]
- Geister TL, Lorenz MW, Hoffmann KH, Fischer K. Adult nutrition and butterfly fitness: effects of diet quality on reproductive output, egg composition, and egg hatching success. Frontiers in Zoology. 2008;5:10. doi: 10.1186/1742-9994-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert LE. Pollen feeding and reproductive biology of Heliconius butterflies. Proceedings of the National Academy of Science of the United States of America. 1972;69:1403–1407. doi: 10.1073/pnas.69.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert F, Jervis M. Functional, evolutionary and ecological aspects of feeding-related mouthpart specializations in parasitoid flies. Biological Journal of the Linnean Society. 1998;63:495–535. [Google Scholar]
- Hecq J. Euphaedra. Lambillionea, Union de Entomologistes Belges; Tervuren: 1997. [Google Scholar]
- Hedenstrom A, Ellington CP, Wolf TJ. Wing wear, aerodynamics and flight energetics in bumblebees (Bombus terrestris): an experimental study. Functional Ecology. 2001;15:417–422. [Google Scholar]
- Ide JY. Sexual and seasonal differences in the frequency of beak marks on the wings of two Lethe butterflies. Ecological Research. 2006;21:453–459. [Google Scholar]
- Kemp DJ. Age-related site fidelity in the territorial butterfly Hypolimnas bolina (L.) (Lepidoptera: Nymphalidae) Australian Journal of Entomology. 2001;4:65–68. [Google Scholar]
- Maginnis TL. The costs of autotomy and regeneration in animals: a review and framework for future research. Behavioral Ecology. 2006;17:857–872. [Google Scholar]
- Mair W, Piper MDW, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. Public Library of Science. 2005;3:1–7. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisels F, Keming E, Kemei M, Toh C. The extirpation of large mammals and implications for montane forest conservation: the case of the Kilum-Ijim Forest, North-west Province, Cameroon. Oryx. 2001;10:322–331. [Google Scholar]
- Mevi-Schutz J, Erhardt A. Larval nutrition affects female nectar amino acid preference in the map butterfly (Araschnia levana) Ecology. 2003;84:2788–2794. [Google Scholar]
- Mevi-Schutz J, Erhardt A. Amino acids in nectar enhance butterfly fecundity: a long-awaited link. American Naturalist. 2005;165:411–419. doi: 10.1086/429150. [DOI] [PubMed] [Google Scholar]
- Mevi-Schutz J, Goverde M, Erhardt A. Effects of fertilization and elevated CO2 on larval food and butterfly nectar amino acid preference in Coenonympha pamphilus L. Behavioral Ecology and Sociobiology. 2003;54:36–43. [Google Scholar]
- Min KJ, Tatar M. Restriction of amino acids extends lifespan in Drosophila melanogaster. Mechanisms of Ageing and Development. 2006;127:643–646. doi: 10.1016/j.mad.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Min KJ, Flatt T, Kulaots I, Tatar M. Counting calories in Drosophila diet restriction. Experimental Gerontology. 2007;42:247–251. doi: 10.1016/j.exger.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molleman F. PhD: Evolutionary Biology. Leiden University; Leiden: 2004. Patterns of biodiversity and life history in fruit-feeding butterflies; p. 157. [Google Scholar]
- Molleman F, Hecq J. Host plant records and photographs of immature Euphaedra from Kibale National Park, Uganda. Lambillionea CV. 2005:423–429. [Google Scholar]
- Molleman F, Krenn HW, Van Alphen ME, Brakefleld PM, Devries PJ, Zwaan BJ. Food intake of fruit-feeding butterflies: evidence for adaptive variation in proboscis morphology. Biological Journal of the Linnean Society. 2005a;86:333–343. [Google Scholar]
- Molleman F, van Alphen ME, Brakefield PM, Zwaan BJ. Preferences and food quality of fruit-feeding butterflies in Kibale Forest, Uganda. Biotropica. 2005b;37:657–663. [Google Scholar]
- Molleman F, Kop A, Brakefield PM, De Vries PJ, Zwaan BJ. Vertical and temporal patterns of biodiversity of fruit-feeding butterflies in a tropical forest in Uganda. Biodiversity and Conservation. 2006;15:107–121. [Google Scholar]
- Molleman F, Zwaan BJ, Brakefield PM, Carey JR. Extraordinary long life spans in fruit-feeding butterflies can provide window on evolution of life span and aging. Experimental Gerontology. 2007;42:472–482. doi: 10.1016/j.exger.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molleman F, Ding J, Wang J-L, Brakefield PM, Carey JR, Zwaan BJ. Adult diet affects life span and reproduction of the fruit-feeding butterfly Charaxes fulvescens. Entomologia Experimentalis Et Applicata. 2008a doi: 10.1111/j.1570-7458.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molleman F, Ding J, Wang JL, Brakefield PM, Carey JR, Zwaan BJ. Amino acid sources in the adult diet do not affect life span and fecundity in the fruit-feeding butterfly Bicyclus anynana. Ecological Entomology. 2008b;33:429–438. doi: 10.1111/j.1365-2311.2008.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller HG, Zhang Y. Time-varying functional regression for predicting remaining lifetime distributions from longitudinal trajectories. Biometrics. 2005;59:676–685. doi: 10.1111/j.1541-0420.2005.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller HG, Wang JL, Carey JR, Caswell-Chen EP, Chen C, Papadopoulos N, Yao F. Demographic window to aging in the wild: constructing life tables and estimating survival functions from marked individuals of unknown age. Aging Cell. 2004;3:125–131. doi: 10.1111/j.1474-9728.2004.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller HG, Wang JL, Yu W, Delaigle A, Carey JR. Survival in the wild via residual demography. Theoretical Population Biology. 2007;72:513–522. doi: 10.1016/j.tpb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD. The role of adult feeding in egg-production and population-dynamics of the checkerspot butterfly Euphydryas editha. Oecologia. 1983;56:257–263. doi: 10.1007/BF00379699. [DOI] [PubMed] [Google Scholar]
- NutritionData. Nutrition facts: know what you eat (USDA’s national nutrient database for standard reference) 2008 http://www.nutritiondata.com/facts-C00001-01c20Tm.html.
- O’Brien DM, Boggs CL, Fogel ML. Pollen feeding in the butterfly Heliconius charitonia: isotopic evidence for essential amino acid transfer from pollen to eggs. Proceedings of the Royal Society of London Series B-Biological Sciences. 2003;270:2631–2636. doi: 10.1098/rspb.2003.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien DM, Boggs CL, Fogel ML. Making eggs from nectar: the role of life history and dietary carbon turnover in butterfly reproductive resource allocation. 2004;105:279. [Google Scholar]; Oikos. 107:222–222. [Google Scholar]
- Piper MDW. Counting the calories: the role of specific nutrients in extension of life span by food restriction. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2005;60:549–555. doi: 10.1093/gerona/60.5.549. [DOI] [PubMed] [Google Scholar]
- Romeis J, Wackers FL. Nutritional suitability of individual carbohydrates and amino acids for adult Pieris brassicae. Physiological Entomology. 2002;9:148–156. [Google Scholar]
- Rusterholz HP, Erhardt A. Preferences for nectar sugars in the peacock butterfly, Inachis io. Ecological Entomology. 1997;22:220–224. [Google Scholar]
- Rusterholz HP, Erhardt A. Can nectar properties explain sex-specific flower preferences in the Adonis Blue butterfly Lysandra bellargus? Ecological Entomology. 2000;10:81–90. [Google Scholar]
- Shapiro AM. Beak-mark frequency as an index of seasonal predation intensity on common butterflies. American Naturalist. 1974;108:229–232. [Google Scholar]
- Stjernholm F, Karlsson B. Reproductive expenditure affects utilization of thoracic and abdominal resources in male Pieris napi butterflies. Functional Ecology. 2006;20:442–448. [Google Scholar]
- Tatar M, Chien SA, Priest NK. Negligible senescence during reproductive dormancy in Drosophila melanogaster. American Naturalist. 2001;158:248–258. doi: 10.1086/321320. [DOI] [PubMed] [Google Scholar]
- Uehara-Prado M, Brown KS, Freitas AVL. Species richness, composition and abundance of fruit-feeding butterflies in the Brazilian Atlantic Forest: comparison between a fragmented and a continuous landscape. Global Ecology and Biogeography. 2007;16:43–54. [Google Scholar]
- Vergrugge LM. In: Flies without wings: frailty and Longevity. Carey JR, Robine J-M, Michel J-P, Christen Y, editors. Springer–Verlag; Berlin: 2005. pp. 67–82. [Google Scholar]
- Wang BC, Sork VL, Leong MT, Smith TB. Hunting of mammals reduces seed removal and dispersal of the afrotropical tree Antrocaryon klaineanum (Anacardiaceae) Biotropica. 2007;39:340–347. [Google Scholar]
- Weithoff G. Dietary restriction in two rotifer species: the effect of the length of food deprivation on life span and reproduction. Oecologia. 2007;153:303–308. doi: 10.1007/s00442-007-0739-6. [DOI] [PubMed] [Google Scholar]
- Wourms MK, Wasserman FE. Bird predation on Lepidoptera and the reliability of beak-marks in determining predation pressure. Journal of the Lepidopterists’ Society. 1985;39:239–261. [Google Scholar]