Abstract
The Aurora-A kinase gene is frequently amplified and/or over-expressed in a variety of human cancers, leading to major efforts to develop therapeutic agents targeting this pathway. Here we demonstrate that Aurora-A is targeted for ubiquitination and subsequent degradation by the F-box protein FBXW7 in a process that is regulated by GSK3β. Using a series of truncated Aurora-A proteins and site directed mutagenesis, we identified distinct FBXW7 and GSK3β binding sites in Aurora-A. Mutation of critical residues in either site substantially disrupts degradation of Aurora-A. Furthermore, we show that loss of Pten results in the stabilization of Aurora-A by attenuating FBXW7-dependent degradation of Aurora-A through the AKT/GSK3β pathway. Moreover, radiation-induced tumor latency is significantly shortened in Fbxw7+/− Pten+/− mice as compared to either Fbxw7+/− or Pten+/− mice, indicating that Fbxw7 and Pten appear to cooperate in suppressing tumorigenesis. Our results establish a novel posttranslational regulatory network in which the Pten and Fbxw7 pathways appear to converge on the regulation of Aurora-A level.
Keywords: Aurora-A, Fbxw7, Pten, tumorigenesis, Ubiquitination
Introduction
Aurora-A kinase, a key regulator of chromosome segregation and cytokinesis (1), is frequently amplified in a variety of human carcinomas, including breast, colorectal, pancreatic, and bladder cancers (2-5). Overexpression of Aurora-A in tumors is correlated with clinically aggressive disease (6). A wealth of functional data exists showing that overexpression of Aurora-A leads to centrosome amplification, chromosomal instability and oncogenic transformation (5, 7). Furthermore, overexpression of Aurora-A in transgenic mouse models result in the development of mammary gland tumors (8). Taken together, these data indicate that Aurora-A possesses oncogenic activity and may be a valuable therapeutic target in cancer therapy (6, 9). Consequently, several small-molecule inhibitors of Aurora-A kinase have been developed and are currently undergoing clinical trials.
FBXW7 is a p53-dependent tumor suppressor that undergoes deletion and/or mutation in a number of human neoplasms, including those from the breast, colon, endometrium, stomach, lung, ovary, pancreas, and prostate (10). The FBXW7 gene encodes an F-box protein essential for ubiquitination of several well-defined oncoproteins including c-Myc (11), Cyclin E (12), Notch (13), c-Jun (14), and mammalian target of rapamycin (mTor) (15). Previous studies have shown that FBXW7 binds Aurora-A in cell lines (16), and that depletion of Fbxw7 results in increased Aurora-A expression (17); however, the mechanism by which Fbxw7 controls Aurora-A level remains unknown. In the present study, we show that Fbxw7 physically binds Aurora-A and facilitates the ubiquitination and degradation of Aurora-A through the proteasome pathway. Furthermore, we demonstrate that this process occurs in a Gsk3β-dependent manner, similar to what has been observed for several oncoproteins that undergo FBXW7-mediated ubiquitination.
Prior studies indicate that GSK3β is inactivated through phosphorylation at the Ser-9 position (18), which occurs through PI3K/AKT signaling. Since the PI3K/AKT pathway is crucial in tumorigenesis and is regulated by the tumor suppressor PTEN, we further investigated whether PTEN is able to regulate Aurora-A via the PI3K/AKT/GSK3β pathway. Our results reveal that similar to Gsk3β inhibition, downregulation of Pten by shRNA increased the level and half-life of Aurora-A by blocking its ubiqutination. Finally, we show that the deletion of one copy of either Fbxw7 or Pten leads to the increase in protein levels of Aurora-A in vivo, and mice heterozygous for both Fbxw7 and Pten (Pten+/−Fbxw7+/−) display significantly shortened latency for tumor development when compared to either Pten+/− mice or Fbxw7+/− mice, indicating that simultaneous loss of Pten and Fbxw7 has a synergic effect in tumorigenesis. In summary, our data establish a novel regulatory pathway in which interactions between Fbxw7, Pten and Gsk3β ultimately regulate Aurora-A levels in a post-translational manner. We also demonstrate that disruption of multiple components of this regulatory pathway can accelerate tumor development.
Materials and Methods
Cells and cell culture
NIH3T3 (mouse embryonic fibroblast), C5N (mouse keratinocyte cell line) and HEK293T were maintained in Dulbecco’s Modified Eagle Medium (Invitrogen) supplemented with 10% fetal calf serum (Invitrogen). HCT116 and HCT116 FBXW7−/− cell lines were a gift from Dr. Bert Vogelstein.
Antibodies and Reagents
The antibodies and reagents used were from the following sources: anti-PTEN rabbit polyclonal (1:1,000), anti-Akt rabbit polyclonal (1:1,000), anti-phospho-Akt (Ser473) rabbit polyclonal (1:1,000), anti-Gsk3β rabbit polyclonal (1:1,000), and anti-phospho-Gsk3β (Ser9) rabbit polyclonal (1:1,000) antibody were from Cell Signaling; anti-Aurora-A (IAK1) mouse monoclonal antibody (1:2,000) was from BD Sciences; anti-Flag-M2 Affinity gel, anti-HA-Affinity gel, and anti-β-actin mouse monoclonal antibody (1:5,000) were from Sigma; cycloheximide (C4859) was from Sigma; MG132 and Gsk3β inhibitor VIII were from Calbiochem.
Plasmids construction and site-directed mutagenesis
pCG-N-HA-FBXW7 was a gift from Prof. K.I. Nakayama, and pCG-N-HA-Ubiquitin was a gift from Dr. O. Tetsu. Full-length and truncated Aurora-A were amplified by PCR and subcloned into Bgl II/Xha I sites of p3xFlag-CMV-10 (Sigma). The Aurora-A mutants (T217A/E221A, S226A/E230A, S245A, S387A, and S245A/S387A) were generated by site-directed mutagenesis (Stratagen). The primers for constructing and mutagenesis were listed in Supplementary Table 1. All the constructs were validated by DNA sequencing.
shRNA constructs and retrovirus production
A shRNA with high Pten knockdown efficiency was cloned into pSuper.Retro-puro (Oligoengine). The shRNA sequence for Pten is 5′-GACCATAACCCACCACAGC-3′. NIH3T3 and C5N cell lines were infected with high-titer retroviral stocks produced by the transient transfection of 293T ecotropic Phoenix cells. After infection with the pSuper.Retro.puro retrovirus, which allowed the expression of the shRNA, cells were selected with 1-2 μg ml of puromycin in the culture medium. pSuper.Retro.puro vector without shRNA was used as control.
Immunoblotting and immunoprecipitation
Cells were collected by washing with cold PBS at 4°C. Total protein extracts were prepared from cells and normal and tumor tissues with RIPA lysis buffer (1% Triton X-100, 0.1% SDS, 50 mM Tris pH 7.5, 150 mM NaCl, 0.5% sodium deoxycholate,10 mM NaF) supplemented with 2 mM PMSF, 2 mM Na3VO4, and complete protease inhibitors (Roche). The lysates were fractionated by SDS-PAGE and electoblotted on to PDVF membrane (Millipore). The membranes were then incubated for 1 hr in TBS-Tween-20 (0.1%) containing 5% nonfat skim milk, then with primary antibodies for 1 hr at RT or overnight at 4°C, and the target antigens were identified with the appropriate horseradish peroxidase-labeled secondary antibody (Amersham) in the presence of SuperSignal West Pico Chemoluminiscent substrate (Pierce).
For immunoprecipitation, 293T cells were transiently transfected using lipofectamine 2000™ with p3xFlag-Aurora-A and pCGN-HA-Fbxw7. Forty-eight hours after transfection, MG132 (10 μM) and Gsk3β inhibitor (25 μM) were added to the media. Six hours later, cells were lysed on ice in NG lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 10% Glycerol, 1% NP-40, 10 mM NaF, 2 mM PMSF, 2 mM Na3VO4, plus “complete protease inhibitors” (Roche). The lysates were incubated with anti-Flag-M2 affinity gel (Sigma) or anti-HA (3F10, Roche) with protein-G-sepharose (GE Healthcare). Immuno-complexes were isolated, fractionated by SDS-PAGE and identified by immunoblot using anti-Flag, anti-HA, or anti-Gsk3β.
Ubiquitination assay
Cells were transfected with a plasmid encoding a HA-ubiquitin. Twenty-four hours after transfection, the proteasome inhibitor MG132 (10 μM) was added. Six hours later, anti-HA immunoprecipitates were recovered and immuniblotted with anti-Aurora-A antibody.
Analysis of mRNA
Total RNA was purified using TRIzol (Invitrogen) according to the manufacturer’s instructions and then 5 μg of each sample was reverse transcribed using the SuperScript II RNaseH first-strand synthesis system (Invitrogen). PCR was performed with 2 μl of cDNA from each sample using Extaq DNA polymerase (TAKARA). Primers for Flag-tagged Aurora-A were as follows: sense 5′-GACTACAAAGACCATGACGGT-3′ (for Flag) and antisense 5′-TGGTGCATATTCCAGAATTAGG-3′ (Aurora-A 621-642).
Mice and tumor induction
Fbxw7 and Pten single or double heterozygous knockout (KO) mice were generated by crossing Fbxw7+/− heterozygous KO mice (C57BL/6J) with Pten+/− heterozygous KO mice (C57BL/6J) purchased from Jax Laboratories. 5-week old mice of both sexes were exposed to a single dose of 4Gy whole body γ-irradiation and monitored daily until moribund, then killed and autopsied. Mice were bred and treated under UCSF LARC regulations.
Statistical Analysis
The Kaplan-Meier method was used to compare the survival time post-radiation between different genotypic groups of mice using the SPSS statistical package.
Results
Fbxw7 binds to and targets Aurora-A for ubiquitin-mediated degradation
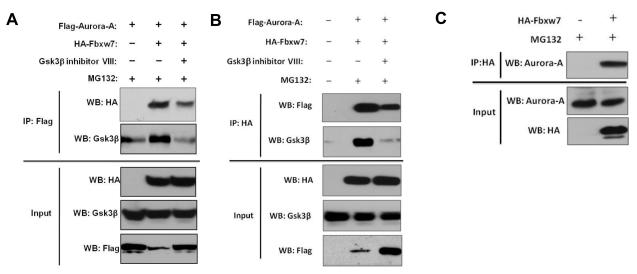
We previously showed that depletion of Fbxw7 resulted in increased Aurora-A expression (17); however, the mechanism by which this regulation occurs is unknown. Previous study suggests that FBXW7 binds Aurora-A in immunoprecipitation assays (16). We hypothesize that FBXW7 may physically interact with Aurora-A to regulate its ubiquitination and turnover by proteasomal degradation. To address this, 293T cells were co-transfected with Flag-tagged Aurora-A and HA-tagged FBXW7. Next, immunoprecipitation and subsequent immunoblot analyses using antibodies directed against the Flag or HA tag were performed and revealed that HA-tagged FBXW7 coimmunoprecipitated with Flag-tagged Aurora-A, indicating a physical interaction between the two proteins (Figure 1A and B). Moreover, we found that HA-tagged FBXW7 also co-immunoprecipitated with endogenous Aurora-A (Figure 1C).
Figure 1.
Aurora-A interacts with FBXW7 and GSK3β. (A and B) Coimmunoprecipitation of Flag-tagged Aurora-A and HA-tagged FBXW7 in transiently transfected 293T cells. Immunoprecipitations were performed with either (A) anti-Flag or (B) anti-HA antibodies. Immunoprecipitates were fractionated by SDS-PAGE and subsequently immunoblotted with (A) anti-HA or (B) anti-Flag antibodies. Coimmunoprecipitation of endogenous GSK3β was also detected using anti-GSK3β antibody. Lower panel shows the input levels of FBXW7, GSK3β and Aurora-A in the lysates. (C) Coimmunoprecipitation of HA-tagged FBXW7 with endogenous Aurora-A. 293T cells were transiently transfected with HA-tagged FBXW7. Immunoprecipitation was performed with anti-HA antibody and subsequently immunoblotted with anti-Aurora-A antibody. Lower panel shows the input levels of FBXW7 and Aurora-A in the lysates. All results are representative of three independent experiments.
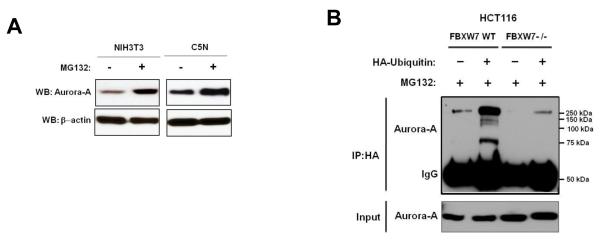
Next, we determined whether the regulation of Aurora-A by Fbxw7 is through the proteasome-dependent degradation pathway. NIH3T3 and C5N cells were treated with the proteasome inhibitor MG132. Proteasome inhibition caused a significant increase in Aurora-A levels in both cell lines (Figure 2A), suggesting that Aurora-A is regulated in a post-translational manner. To examine whether the ubiquitination status of Aurora-A is Fbxw7-dependent, HCT116 FBXW7 wild-type (WT) and FBXW7−/− cells were transfected with a vector containing CMV promoter-driven HA-ubiquitin. Immunoprecipitation of HA followed by immunoblot analysis of Aurora-A showed that Aurora-A ubiquitination was only found in HCT116 FBXW7 WT cells, but not in HCT116 FBXW7−/− cells (Figure 2B), consistent with previous findings (19). In summary, these results suggest that FBXW7 binds to Aurora-A and targets Aurora-A for ubiquitin-mediated degradation through the proteosome.
Figure 2.
Aurora-A ubiquitination is Fbxw7-dependent. (A) NIH3T3 and C5N cells show increased endogenous Aurora-A levels upon treatment with proteasome inhibitor MG132. (B) HCT116 FBXW7 WT and HCT116 FBXW7−/− cells were transfected with HA-ubiquitin. Immunoprecipitation of HA followed by Western blot analysis of Aurora-A showed that ubiquitination of Aurora-A was only seen in the HCT116 FBXW7 WT cells (column 2), and not in HCT116 FBXW7−/− cells (column 4). All results are representative of three independent experiments.
Fbxw7 mediates ubiquitination of Aurora-A in a Gsk3β dependent manner
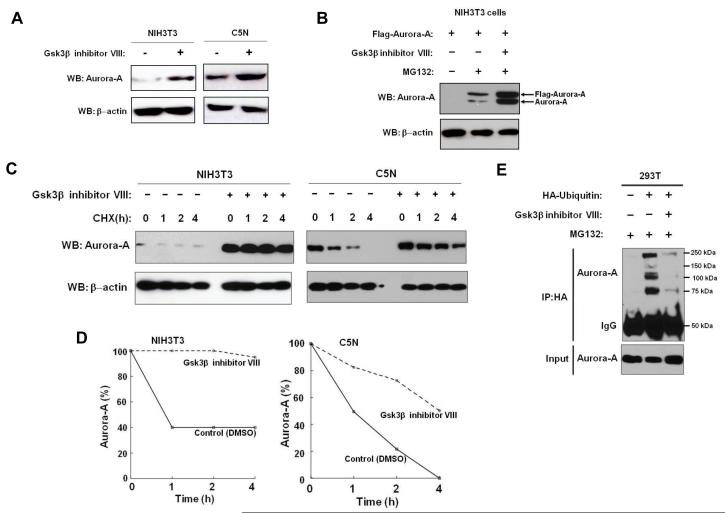
Since Fbxw7-mediated ubiquitination of most substrates (such as Cyclin E, Jun, and Myc) occurs in a Gsk3β-dependent manner, we examined whether Fbxw7 ubiquitination of Aurora-A is also dependent on Gsk3β activity. First, Gsk3β co-immunoprecipitated with both Flag-tagged Aurora-A and HA-tagged Fbxw7 (Figure 1A and 1B), suggesting that Gsk3β may exist in a complex with Aurora-A and Fbxw7. Additionally, in the presence of Gsk3β inhibitor, the binding between Fbxw7 and Aurora-A was dramatically reduced (Figure 1A and 1B, lane 3), indicating that physical interaction between Fbxw7 and Aurora-A is dependent on Gsk3β activity. Moreover, Gsk3β inhibitor treatment of both NIH-3T3 and C5N cell lines resulted in a significant increase in Aurora-A levels (Figure 3A). The protein levels of both endogenous and exogenous Aurora-A increased even further in cells treated with both the proteasome and Gsk3β inhibitors (Figure 3B). Gsk3β inhibitor treatment also dramatically increased the stability and half-life of Aurora-A (Figure 3C and 3D). Finally, we investigated whether Gsk3β inhibitor treatment blocked Aurora-A ubiquitination by Fbxw7. 293T cells were transfected with a vector containing CMV promoter-driven HA-ubiquitin and were then treated with Gsk3β inhibitor. Immunoprecipitation of HA followed by immunoblot analysis of Aurora-A showed that Aurora-A ubiquitination was dramatically reduced in the cells following Gsk3β inhibitor treatment (Figure 3E). Taken together, these results indicate that Gsk3βis able to physically interact with both Fbxw7 and Aurora-A and that Gsk3β is required for Fbxw7-mediated ubiquitination of Aurora-A.
Figure 3.
GSK3β regulates the ubiquitination and degradation of Aurora-A by Fbxw7. (A) NIH3T3 and C5N cells show increased Aurora-A levels upon treatment with Gsk3βinhibitor VIII. (B) NIH3T3 cells transfected with Flag-tagged Aurora-A show increased endogenous and exogenous Aurora-A levels upon treatment with MG132. Further increase in Aurora-A levels is observed when cells were treated with both MG132 and Gsk3β inhibitor VIII. (C) Gsk3β inhibitor VIII treatment stabilizes Aurora-A protein. CHX indicates hours post cycloheximide treatment. (D) Quantification of Aurora-A protein levels shown in panel (C). (E) Gsk3β inhibitor VIII treatment dramatically reduces ubiquitination of Aurora-A. All results are representative of three independent experiments.
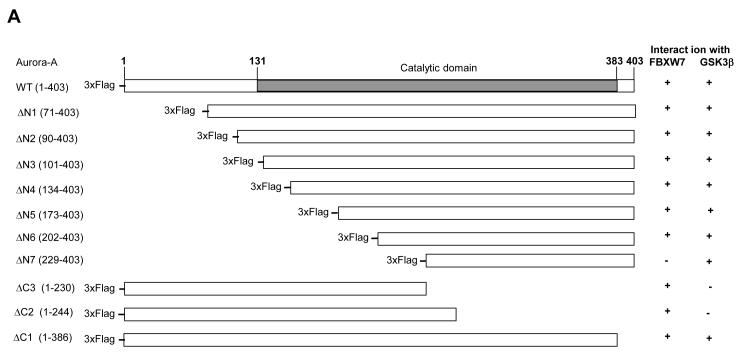
Mapping the FBXW7-binding site within Aurora-A
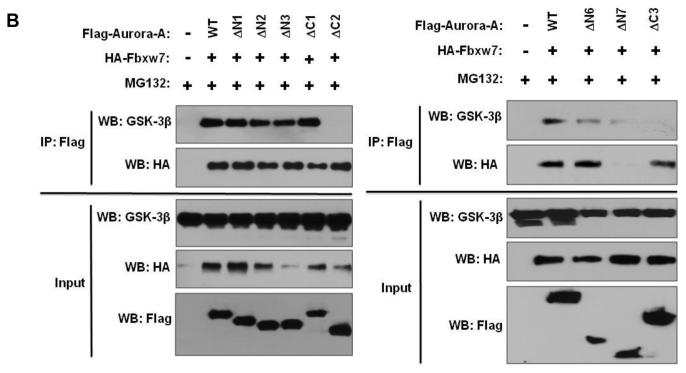
The consensus CDC4 phosphodegron (CPD) sequence I/L-I/L/P-S/T-P-XXXX (where lysine and arginine are unfavorable in the X locations) of FBXW7 was not found in Aurora-A using bioinformatics analysis. In order to identify the region within Aurora-A that is responsible for interaction with FBXW7, we made serial truncations of Aurora-A (Figure 4A), coexpressed them with HA-tagged Fbxw7 in HEK293T cells, and tested the ability of the truncated proteins to bind to FBXW7 by immunoprecipitation. FBXW7 bound to ΔN1-ΔN6 and ΔC1-ΔC3, but not to ΔN7 (Figure 4A and B, Supplementary Figure S1), implicating residues 202-230 of Aurora-A as the critical region necessary for interaction with FBXW7. Closer inspection of this region showed that two potential sites are similar to the FBXW7 CPD (Figure 4C). To investigate the significance of each individual site for binding FBXW7, we generated two mutants: T217A/E221A and S226A/E230A. S226A/E230A retained the ability to bind FBXW7 although this interaction was significantly reduced as compared to wild type Aurora-A (Figure 4D). On the other hand, FBXW7 binding capability was completely abolished in T217A/E221A, indicating that the T217E221 site is largely responsible for the physical interaction between Aurora-A and FBXW7.
Figure 4.
Identification of the FBXW7-binding site within Aurora-A. (A) Schematic representation of full length Aurora-A and its truncated forms that were tested for their ability to bind Fbxw7. Numbers in brackets indicate the amino acids that comprise the various truncated Aurora-A proteins. “+” indicates that the truncated Aurora-A is able to bind to FBXW7 or GSK3β while “−” indicates that the truncated Aurora-A is unable to bind to FBXW7 or GSK3β. (B) Coimmunoprecipitation of the truncated forms of Flag-tagged Aurora-A with either HA-tagged Fbxw7 or GSK3β. HA-tagged FBXW7 and truncated forms of Flag-tagged Aurora-A were expressed in 293T cells, followed by immunoprecipitation with anti-Flag antibodies and immunoblotting with antibodies against HA or GSK3β. Lower panel shows the input levels of GSK3β, FBXW7, and Aurora-A proteins in the lysates. (C) Potential FBXW7-binding sites within Aurora-A. Numbers indicate the amino acid residues that were substituted with alanines to generate the corresponding mutant Aurora-A. (D) Coimmunoprecipitation of HA-tagged Fbxw7 with wild type or mutant forms of Flag-tagged Aurora-A in transiently transfected 293T cells. Immunoprecipitations were performed with anti-Flag antibody and subsequently immunoblotted with anti-HA antibodies. Lower panel shows the input levels of FBXW7 and Aurora-A proteins in the lysates.
Mapping the GSK3β-binding site within Aurora-A
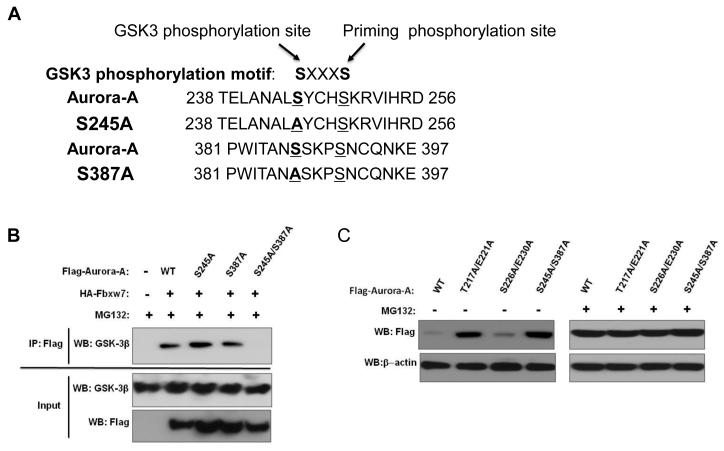
In order to map the GSK3β binding region in Aurora-A, we used the same serial truncated proteins of Aurora-A described above to test their ability to bind to GSK3β. Only ΔC2 and ΔC3 were not able to bind GSK3β, indicating that the 173 C-terminal residues of Aurora-A are required for interaction with GSK3β (Figure 4A and 4B, Supplementary Figure S1). Using the PhosphoMotif Finder software, we found that this C-terminal region of Aurora-A (amino acids 230-403) harbors two potential GSK3β phosphorylation sites (Figure 5A). To investigate the significance of each individual phosphorylation site, we systematically disrupted each potential sites with alanine substitutions (Figure 5A). Although both single site Aurora-A mutants S245A and S387A interacted with GSK3β, the S245A/S387A double mutant completely lost the capacity to bind to GSK3β (Figure 5B). Interestingly, the S245A/S387A double mutant also displayed significantly reduced binding capability to FBXW7 (Figure 4D).
Figure 5.
Identification of GSK3β–binding sites within Aurora-A. (A) Potential GSK3β binding sites within Aurora-A. Numbers indicate the amino acid residues that were substituted with alanines to generate the corresponding mutant Aurora-A. (B) Coimmunoprecipitation of endogenous GSK3β with wild type or mutant forms of Flag-tagged Aurora-A in transiently transfected 293T cells. Immunoprecipitations were performed with anti-Flag antibody and subsequently immunoblotted with anti-GSK3β antibodies. Lower panel shows the input levels of GSK3β and Aurora-A proteins in the lysates. (C) Mutations at the Fbxw7- and GSKβ-binding sites within Aurora-A result in increased levels of Aurora-A. This effect is abolished in the presence of proteasome inhibitor MG132.
FBXW7- and GSK3β-binding sites within Aurora-A are crucial for proteasome-dependent degradation of Aurora-A
Since FBXW7 appears to mediate ubiquitination of Aurora-A in a GSK3β-dependent manner, we investigated whether disruption of the interactions between FBXW7/Aurora-A and GSK3β/Aurora-A would block proteasome-dependent degradation of Aurora-A. First, 293T cells were transfected with either flag-tagged wild type Aurora-A, T217A/E221A, S226A/E230A, or S245A/S387A mutants. Subsequent immunoblot analysis with anti-flag antibody revealed that while wild type and the S226A/E230A mutant exhibited low levels of expression, the T217A/E221A and S245A/S387A mutants displayed significantly greater protein levels (Figure 5C, left panel), suggesting that disruption of either FBXW7- or GSK3β-binding site within Aurora-A substantially stabilizes (or decreases turnover of) Aurora-A. In contrast, when these cells were treated with the proteasome inhibitor MG132, all groups displayed comparably high levels of Aurora-A protein (Figure 5C, right panel). When comparing M132 treated and untreated cells, a significant increase in levels of wild type Aurora-A and the S226A/E230A mutant occurred following M132 treatment, while levels of the T217A/E221A and S245A/S387A mutants did not change. In order to verify that the observed differences in Aurora-A levels resulted from post-translational regulation, transcript levels of the wild type and mutant forms of Aurora-A were measured by semi-quantitative RT-PCR, which showed no differences between any of the Aurora-A variants (Supplementary Figure S2). As a whole, these data suggest that the interactions of Aurora-A with FBXW7 and GSK3β are crucial for proteasome-dependent turnover of Aurora-A.
Loss of Pten stabilizes Aurora-A via the Akt/Gsk3β pathway
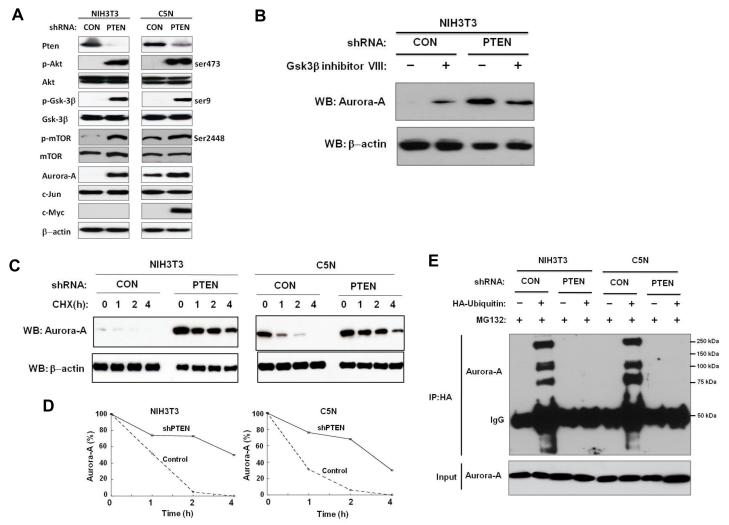
Pten is a negative regulator of the Pi3k/Akt pathway (20). Activation of Pi3k/Akt signaling leads to the phosphorylation (Ser-9) and inactivation of Gsk3β (18). Thus, we investigated whether Pten could regulate Aurora-A via the Akt/Gsk3β pathway. In order to examine the effects of Pten down-regulation on Aurora-A level and stability, we designed a short hairpin RNA (shRNA) against Pten (shPten) and generated stable transfectants in NIH3T3 and C5N cells. The shRNA effectively reduced Pten protein expression in both cell lines (Figure 6A). Down-regulation of Pten led to the increase in Aurora-A levels as compared to controls (Figure 6A). We also analyzed levels of other Fbxw7 substrates that were shown to be ubiquitinated in a Gsk3β-dependent manner. Levels of c-Jun were unaffected in both cell lines (Figure 6A). c-Myc was undetectable in NIH3T3 cells even after downregulation of Pten, while levels of c-Myc increased in C5N cells with shPten (Figure 6A). The expression levels of p-Akt (serine-473) were also significantly increased in shPten transfected cells and presumably were responsible for the inactivation of Gsk3β. Indeed, the level of p-Gsk3β (Ser-9) increased dramatically with down-regulation of Pten. In contrast, the total level of Gsk3β did not change. As expected, the level of p-mTor (Ser2448) increased whereas the level of total mTor did not change (Figure 6A). Furthermore, in NIH3T3 cells with shPten, Gsk3β inhibitor treatment did not cause an increase in Aurora-A levels, indicating that Pten regulates Aurora-A via the Akt/Gsk3β pathway (Figure 6B).
Figure 6.
Pten regulates Aurora-A by attenuating Aurora-A degradation by Fbxw7. All results are representative of three independent experiments. (A) shRNA knockdown of PTEN results in higher levels of Aurora-A in both NIH3T3 and C5N cells. (B) Upon treatment with Gsk3β inhibitor VIII, Aurora-A levels remain the same following shRNA knockdown of PTEN in NIH3T3 cells. (C) shRNA knockdown of PTEN stabilizes Aurora-A. CHX indicates hours post cycloheximide treatment. (D) Quantification of Aurora-A levels shown in panel (C). (E) shRNA knockdown of PTEN abolishes ubiqutination of Aurora-A.
The stability of Aurora-A was also examined in shPten cells after treatment with cycloheximide. Aurora-A was much more stably maintained in shPten cells as compared to the control cells (Figure 6C and D). Furthermore, down-regulation of Pten blocked Aurora-A ubiquitination (Figure 6E). In summary, these findings are similar to those that were observed with Gsk3β inhibitor treatment, further suggesting that loss of Pten attenuates the degradation of Aurora-A by Fbxw7 through the Akt/Gsk3β pathway.
Pten synergizes with Fbxw7 in radiation-induced tumor development
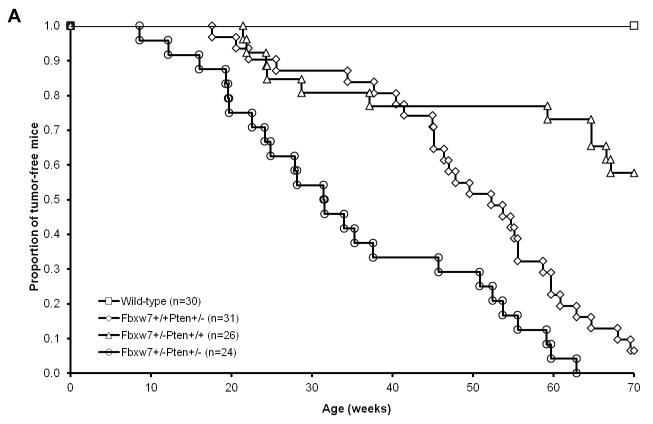
We have previously used a mouse model of radiation-induced lymphoma to demonstrate that haploinsufficiency for P53, Pten or Fbxw7 confers susceptibility to lymphoma development (21-23). Since we have established that Pten and Fbxw7 pathways both impact Aurora-A levels and mTor signaling; therefore, we speculate that simultaneous loss of Pten and Fbxw7 may result in a synergistic effect on tumor development in vivo. In order to investigate this possibility, we crossed Fbxw7+/− mice with Pten+/− mice to generate Pten+/−Fbxw7+/− mice. 24 Pten+/−Fbxw7+/−, 26 Fbxw7+/−, 31 Pten+/−, and 30 wild-type mice were exposed to a single dose of 4Gy whole body γ irradiation at 5 weeks of age. Within 65 weeks, none of wild type mice developed any tumors (Figure 7A). In contrast, a significant portion of Fbxw7+/− (40%) and Pten+/− (95%) mice developed tumors by 65 weeks post-irradiation (p-value <0.0001) (Figure 7). Interestingly, the double heterozygous Fbxw7+/−Pten+/− mice developed tumors much faster than either Fbxw7+/− or Pten+/− mice (p-value < 0.0001) (Figure 7). These data indicate that Pten synergizes with Fbxw7 in suppressing radiation-induced tumorigenesis.
Figure 7.
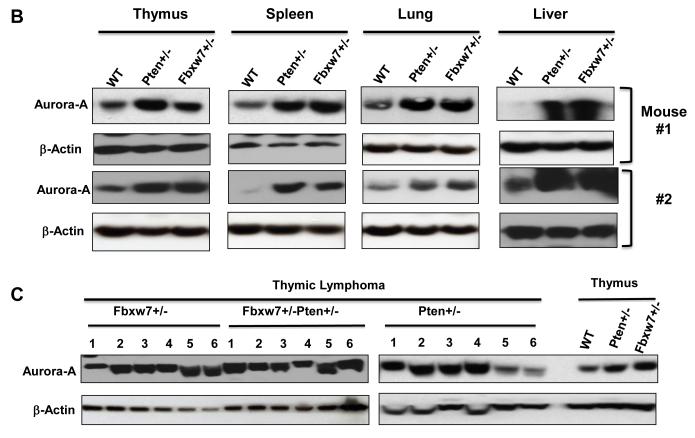
Pten interacts with Fbxw7 in suppressing radiation-induced tumorigenesis. (A) Radiation-induced tumor latency is significantly shortened in Fbxw7±Pten+/− mice as compared to either Fbxw7+/− or Pten+/− mice. Wild-type (open squares; n=30), Fbxw7+/− Pten+/− (open circles; n=24), Fbxw7+/−Pten+/+ (open triangles; n=26), and Fbxw7+/+Pten+/− (open diamonds; n=31) mice were exposed to a single dose of 4 Gy whole body γ irradiation at 5 weeks of age and followed up for 65 weeks post-radiation. (B) Western blot showing increased Aurora-A level when deletion of one copy of either Fbxw7 or Pten in normal thymus, spleen, lung and liver tissue. (C) Detection of Aurora-A protein level in radiation-induced tumors from Fbxw7+/−, Pten+/− and Fbxw7+/−Pten+/− mice by Western blotting.
Next we investigated the effects of loss of either Fbxw7 or Pten on Aurora-A protein levels in the normal and tumor tissues by Western blotting. One consistent observation was that normal tissues from Fbxw7+/− mice had higher protein levels of Aurora-A than the equivalent tissue from wild-type mice (Figure 7B). Similar observations were made with the tissues from Pten+/− mice (Figure 7B). It is also found that tumors retained the higher starting levels of endogenous Aurora-A protein, but few tumors from Pten+/− mice showed the lower Aurora-A level (Figure 7C). These data together with our previous findings (15) suggests a novel interaction between the Fbxw7 and Pten tumor suppressor pathways that controls the levels of the oncoprotein Aurora-A kinase and mTor, but we could not fully exclude the possibility of other oncogenes, such as Myc. Additional studies will be granted.
Discussion
Aurora-A controls chromosome segregation during mitosis, and is amplified and/or over-expressed in a large proportion of human tumors. However, the frequency of Aurora-A overexpression in human cancers is much higher than that of its amplification. For example, in breast cancers, Aurora-A is amplified in 12% of cases whereas it is overexpressed in 98% of cases. The discrepancy between overexpression and amplification suggests that elevated Aurora-A levels are likely mediated through other mechanisms such as post-translational modifications. In this study, we demonstrate that two tumor suppressor genes, Fbxw7 and Pten, regulate Aurora-A levels in a post-translational manner.
Our results reveal that Aurora-A is targeted for ubiquitin-dependent degradation by FBXW7 in a process that is regulated by GSK3β. A recent study showed that overexpression of Aurora-A in AGS and SNU1 gastric cancer cells led to increased phosphorylation and inactivation of Gsk3β (24), suggesting that a positive feedback loop exists between Gsk3β and Aurora-A. This process may be mediated through Akt since it has been shown that Aurora-A can activate Akt through phosphorylation (24, 25). As a result, it is possible that Aurora-A activates Akt followed by Akt inactivation of Gsk3β resulting in the increase of Aurora-A levels. Furthermore, based on our results that Pten negatively regulates Aurora-A through AKT/GSK3β, together with the recent report by Taga et. al. (26) that long-term overexpression of Aurora-A in cells leads to degradation of Pten, it appears that yet another layer of positive feedback may be operating within this complex signaling network (Supplementary Figure S3).
Unlike other FBXW7 targets, the consensus CDC4 phosphodegron motif was not found in Aurora-A. Using a series of Aurora-A truncated proteins, we have now identified residues 202 to 230 of the Aurora-A as the minimal region required for binding to FBXW7, which is located within Aurora-A catalytic domain. Moreover, the residues T217/E221 have been defined critical for binding to FBXW7 as our results showed that mutation of these two residues totally abolished the interaction between Aurora-A and FBXW7. Interestingly, recent studies showed that T217 is an important residue for developing small inhibitors that are highly selective for targeting Aurora-A (27, 28).
In this study, we also found two GSK3β binding sites in Aurora-A. Our results indicate that both sites are equivalently important for the interaction between Aurora-A and GSK3βMutation of either site alone is insufficient to block their interaction. Surprisingly, we found that the GSK3β binding sites in Aurora-A are distinct from the FBXW7 binding site, which differs from what is found in other FBXW7 targets, such as c-Myc and CCNE1. Mutation of the GSK3β binding sites within Aurora-A or pharmacologic inhibition of GSK3β reduces the binding affinity between Aurora-A and FBXW7, suggesting that GSK3β binding to Aurora-A promotes the interaction between Aurora-A and FBXW7. Nevertheless, both GSK3β and FBXW7 binding sites play an equivalent role in degradation of Aurora-A. Mutation of critical residues in either sites substantially stabilizes Aurora-A.
In conclusion, we have identified a novel interaction between the Fbxw7 and Pten tumor suppressor pathways that controls the levels of the oncoprotein Aurora-A kinase. We show that Fbxw7 degrades Aurora-A through physical interaction. Like most of the other known Fbxw7 substrates, ubiquitination of Aurora-A by Fbxw7 is a GSK3β-dependent event. Furthermore, we demonstrate that loss of Pten also increases the stability of Aurora-A through the Akt/GSK3β pathway. Using a genetic mouse model, we report that mice heterozygous for both Fbxw7 and Pten have significantly reduced tumor latency compared to mice heterozygous for either Fbxw7 or Pten, and normal tissues from Fbxw7+/− or Pten+/− mice have higher protein levels of Aurora-A than the equivalent tissue from wild-type mice. Moreover such higher starting levels of endogenous Aurora-A protein sustained in the tumors generated from Fbxw7+/− and Fbxw7+/−Pten+/− mice and in most of tumors from Pten+/− mice. In view of the toxicity of presently available inhibitors of Aurora-A kinase in clinical trials, these findings may lead to the design of alternative approaches to manipulate the levels of Aurora-A kinase protein for cancer therapy.
Supplementary Material
Acknowledgements
We thank B. Vogelstein for providing us with the HCT116 WT and HCT116 FBXW7−/− cell lines; K.I. Nakayama for providing Fbxw7 knockout mice and vectors (HA-FBXW7 and HA-FBXW7ΔF); and O. Tetsu for vector encoding HA-ubiquitin.
Grant Support
JHM was supported by the National Institutes of Health, National Cancer Institute grant R01 CA116481, the Low Dose Scientific Focus Area, Office of Biological & Environmental Research, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231, and Laboratory Directed Research & Development Program (LDRD). AB was supported by the Department of Energy Low Dose Radiation Research Program DESC0003679, and the National Cancer Institute grant U01 CA141455. JPL was partially supported by FEDER, MICINN (PLE2009-119), FIS (PI10/00328) and “Fundación Eugenio Rodríguez-Pascual”.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Fu J, Bian M, Jiang Q, Zhang C. Roles of Aurora kinases in mitosis and tumorigenesis. Mol Cancer Res. 2007;5:1–10. doi: 10.1158/1541-7786.MCR-06-0208. [DOI] [PubMed] [Google Scholar]
- 2.Sen S, Zhou H, Zhang RD, Yoon DS, Vakar-Lopez F, Ito S, et al. Amplification/overexpression of a mitotic kinase gene in human bladder cancer. J Natl Cancer Inst. 2002;94:1320–9. doi: 10.1093/jnci/94.17.1320. [DOI] [PubMed] [Google Scholar]
- 3.Li D, Zhu J, Firozi PF, Abbruzzese JL, Evans DB, Cleary K, et al. Overexpression of oncogenic STK15/BTAK/Aurora A kinase in human pancreatic cancer. Clin Cancer Res. 2003;9:991–7. [PubMed] [Google Scholar]
- 4.Sakakura C, Hagiwara A, Yasuoka R, Fujita Y, Nakanishi M, Masuda K, et al. Tumour-amplified kinase BTAK is amplified and overexpressed in gastric cancers with possible involvement in aneuploid formation. Br J Cancer. 2001;84:824–31. doi: 10.1054/bjoc.2000.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishida N, Nagasaka T, Kashiwagi K, Boland CR, Goel A. High copy amplification of the Aurora-A gene is associated with chromosomal instability phenotype in human colorectal cancers. Cancer Biol Ther. 2007;6:525–33. doi: 10.4161/cbt.6.4.3817. [DOI] [PubMed] [Google Scholar]
- 6.Lassmann S, Shen Y, Jutting U, Wiehle P, Walch A, Gitsch G, et al. Predictive value of Aurora-A/STK15 expression for late stage epithelial ovarian cancer patients treated by adjuvant chemotherapy. Clin Cancer Res. 2007;13:4083–91. doi: 10.1158/1078-0432.CCR-06-2775. [DOI] [PubMed] [Google Scholar]
- 7.Zhou H, Kuang J, Zhong L, Kuo WL, Gray JW, Sahin A, et al. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20:189–93. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]
- 8.Zhang D, Hirota T, Marumoto T, Shimizu M, Kunitoku N, Sasayama T, et al. CreloxP-controlled periodic Aurora-A overexpression induces mitotic abnormalities and hyperplasia in mammary glands of mouse models. Oncogene. 2004;23:8720–30. doi: 10.1038/sj.onc.1208153. [DOI] [PubMed] [Google Scholar]
- 9.Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T, et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004;10:262–7. doi: 10.1038/nm1003. [DOI] [PubMed] [Google Scholar]
- 10.Tan Y, Sangfelt O, Spruck C. The Fbxw7/hCdc4 tumor suppressor in human cancer. Cancer Lett. 2008;271:1–12. doi: 10.1016/j.canlet.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 11.Yada M, Hatakeyama S, Kamura T, Nishiyama M, Tsunematsu R, Imaki H, et al. Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. Embo J. 2004;23:2116–25. doi: 10.1038/sj.emboj.7600217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strohmaier H, Spruck CH, Kaiser P, Won KA, Sangfelt O, Reed SI. Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature. 2001;413:316–22. doi: 10.1038/35095076. [DOI] [PubMed] [Google Scholar]
- 13.Tetzlaff MT, Yu W, Li M, Zhang P, Finegold M, Mahon K, et al. Defective cardiovascular development and elevated cyclin E and Notch proteins in mice lacking the Fbw7 F-box protein. Proc Natl Acad Sci U S A. 2004;101:3338–45. doi: 10.1073/pnas.0307875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei W, Jin J, Schlisio S, Harper JW, Kaelin WG., Jr. The v-Jun point mutation allows c-Jun to escape GSK3-dependent recognition and destruction by the Fbw7 ubiquitin ligase. Cancer Cell. 2005;8:25–33. doi: 10.1016/j.ccr.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Mao JH, Kim IJ, Wu D, Climent J, Kang HC, DelRosario R, et al. FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science. 2008;321:1499–502. doi: 10.1126/science.1162981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otto T, Horn S, Brockmann M, Eilers U, Schuttrumpf L, Popov N, et al. Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer Cell. 2009;15:67–78. doi: 10.1016/j.ccr.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Mao JH, Perez-Losada J, Wu D, Delrosario R, Tsunematsu R, Nakayama KI, et al. Fbxw7/Cdc4 is a p53-dependent, haploinsufficient tumour suppressor gene. Nature. 2004;432:775–9. doi: 10.1038/nature03155. [DOI] [PubMed] [Google Scholar]
- 18.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–9. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 19.Fujii Y, Yada M, Nishiyama M, Kamura T, Takahashi H, Tsunematsu R, et al. Fbxw7 contributes to tumor suppression by targeting multiple proteins for ubiquitin-dependent degradation. Cancer Sci. 2006;97:729–36. doi: 10.1111/j.1349-7006.2006.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–14. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Kemp CJ, Wheldon T, Balmain A. p53-deficient mice are extremely susceptible to radiation-induced tumorigenesis. Nat Genet. 1994;8:66–9. doi: 10.1038/ng0994-66. [DOI] [PubMed] [Google Scholar]
- 22.Mao JH, Wu D, Perez-Losada J, Nagase H, DelRosario R, Balmain A. Genetic interactions between Pten and p53 in radiation-induced lymphoma development. Oncogene. 2003;22:8379–85. doi: 10.1038/sj.onc.1207083. [DOI] [PubMed] [Google Scholar]
- 23.Mao JH, To MD, Perez-Losada J, Wu D, Del Rosario R, Balmain A. Mutually exclusive mutations of the Pten and ras pathways in skin tumor progression. Genes Dev. 2004;18:1800–5. doi: 10.1101/gad.1213804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dar AA, Belkhiri A, El-Rifai W. The aurora kinase A regulates GSK-3beta in gastric cancer cells. Oncogene. 2009;28:866–75. doi: 10.1038/onc.2008.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang H, He L, Kruk P, Nicosia SV, Cheng JQ. Aurora-A induces cell survival and chemoresistance by activation of Akt through a p53-dependent manner in ovarian cancer cells. Int J Cancer. 2006;119:2304–12. doi: 10.1002/ijc.22154. [DOI] [PubMed] [Google Scholar]
- 26.Taga M, Hirooka E, Ouchi T. Essential roles of mTOR/Akt pathway in Aurora-A cell transformation. Int J Biol Sci. 2009;5:444–50. doi: 10.7150/ijbs.5.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sloane DA, Trikic MZ, Chu ML, Lamers MB, Mason CS, Mueller I, et al. Drug-resistant aurora A mutants for cellular target validation of the small molecule kinase inhibitors MLN8054 and MLN8237. ACS Chem Biol. 2010;5:563–76. doi: 10.1021/cb100053q. [DOI] [PubMed] [Google Scholar]
- 28.Dodson CA, Kosmopoulou M, Richards MW, Atrash B, Bavetsias V, Blagg J, et al. Crystal structure of an Aurora-A mutant that mimics Aurora-B bound to MLN8054: insights into selectivity and drug design. Biochem J. 2010;427:19–28. doi: 10.1042/BJ20091530. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.