Abstract
CD4+CD25+ regulatory T cells (Tregs) mediate immune suppression and prevent autoimmune disorders. Recently, Tregs were found to present in atherosclerotic lesions and play an important role in the progression of atherosclerosis. Statins have immunomodulatory properties, and the effect of statins on atherosclerosis depends in part on their immunomodulatory mechanisms. We sought to determine whether statins exhibit an effect on Tregs in atherosclerotic plaques and in peripheral circulation of patients with acute coronary syndrome (ACS). In an in vivo experiment, we induced atherosclerotic plaques in apolipoprotein E–deficient (ApoE−/−) mice. The mice were randomly divided into two groups for 6-wk treatment: simvastatin (50 mg/kg/d) or vehicle (control). Simvastatin significantly increased the number of Tregs and the expression of Treg marker Foxp3 (Forkhead/winged helix transcription factor), transforming growth factor (TGF)-β and interleukin (IL)-10 in atherosclerotic plaques. Moreover, simvastatin played an important role in modulating the balance between antiinflammatory (Tregs and Th2 cells) and proinflammatory (Th17 and Th1 cells) subsets of T cells. In an in vitro experiment, peripheral blood mononuclear cells (PBMCs) were isolated from patients with ACS and incubated with simvastatin. After an incubation for 96 h, simvastatin significantly enhanced the frequency and functional suppressive properties of Tregs. Therefore, statin treatment may influence Tregs in atherosclerotic lesions. Furthermore, statins improved the quantity and suppressive function of Tregs in ACS patients.
INTRODUCTION
The pathogenesis of atherosclerosis is multifactorial. Much attention has been given to the role of the immune system in the development of atherosclerosis. Both innate and adaptive immunity are involved in the progression and destabilization of atherosclerotic plaque (1). Also, accelerated atherosclerosis was found in some autoimmune diseases such as systemic lupus erythematosus, systemic sclerosis and rheumatoid arthritis (2,3).
T cell–mediated pathogenic immune responses contribute to atherosclerotic plaque formation and progression (4). Interferon (IFN)-γ–producing T helper 1 (Th1) cells and interleukin (IL)-17– producing Th17 cells may compose the predominant activated T cells (5). Th1 and Th17 cells played a key role in the progression of atherosclerosis and plaque instability (6). On the contrary, CD4+CD25+ regulatory T cells (Tregs), as a healthy T-cell compartment, were capable of mediating immune suppression, preventing activated T-cell responses and autoimmune disorders (7). Tregs might modulate the balance of other T-cells such as Th1, Th2 and Th17 cells (8–10). Lack or dysfunction of Tregs can destroy immune homeostasis and result in many pathological conditions. Recently, considerable evidence supported that Tregs play a central role in progression of atherosclerosis (11). It has been reported that atherosclerotic lesions were decreased with tail-vein delivery of Tregs, while being significantly increased with injection of Treg-depleting antibody in animal models (11,12). Furthermore, the number of Tregs decreased and their functional properties were compromised in patients with acute coronary syndrome (ACS) (13). Forkhead/winged helix transcription factor (Foxp3), a specific marker for Tregs, is essential for Treg regulatory activity and survival, and lack of Foxp3 may lead to Treg dysfunction (14,15).
Statins (HMG-CoA [hydroxymethyl-glutaryl–coenzyme A] reductase inhibitors) reduce the risk of cardiovascular disease and stroke and have been used widely in primary and secondary prevention of atherosclerosis. These drugs improve endothelial function, reduce inflammation, prevent the progression of atherosclerosis and promote stability in vulnerable atherosclerotic plaque (16). In addition to their potent lipid-lowering capabilities, statins have beneficial pleio tropic effects (17). They possess immunomodulatory properties, including inhibiting interferon (IFN)-γinduced expression of major histocompatibility complex (MHC) class II genes and effector T-cell activation, and their role in atherosclerosis depends in part on their immunomodulatory mechanism (18,19).
However, whether statin treatment is able to influence Treg numbers in atherosclerotic plaques is still unclear. Therefore, we aimed to detect the change in levels of Tregs and their master transcription regulator (Foxp3) in atherosclerotic plaques after simvastatin treatment in apolipoprotein E–deficient (ApoE−/−) mice models. We also investigated the effect of simvastatin treatment on the levels of Tregs and immunosuppressive function in the peripheral circulation of patients with ACS.
MATERIALS AND METHODS
Animals
A total of 40 male ApoE−/− mice on a C57BL/6 background (10 wks old) were obtained from Beijing University Animal Research Center. All mice were housed in our facility and fed a “Western-type” diet (0.25% cholesterol and 15% cocoa butter) and water ad libitum throughout the study. The institutional ethics committee of Shandong University approved the animal use in the study.
Two weeks after the start of the diet, all ApoE−/− mice were anesthetized with intraperitoneal pentobarbital sodium (40 mg/kg). Then a silastic perivascular collar (3 mm long, 0.3 mm internal diameter) was placed around the right common carotid artery under sterile conditions to induce atherosclerotic lesions (20,21). Six weeks after surgery, all mice were randomly assigned to two groups (n = 20 per group): a simvastatin group that received intragastric administration of simvastatin (50 mg/kg/d) in 0.5% methylcellulose and a control group that received methylcellulose alone. The dose of simvastatin was based on the doses used in previous studies (18). After 6 wks, the mice were euthanized and tissues were harvested for further analysis.
Lipid profile
At the end of 14 wks, mice were fasted overnight. Blood samples were collected by cardiac puncture at the time of sacrifice, and serum was separated. The concentrations of total cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) cholesterol were measured.
Immunohistochemistry
In the animals, the right common carotid arteries were removed and consecutive sections (5-μm thickness) were collected from each mouse for immunohistochemical analysis. Frozen sections of right common carotid arteries were performed for immunohistochemical analysis, including natural Tregs (Foxp3, 1:100; eBioscience, San Diego, CA, USA), IL-4 (1:100; Abcam, Cambridge, UK), IL-1β (1:100; Abcam), IL-17 (1:100; Abcam) and IFN-γ (1:100; Abcam).
Image Pro Plus 6.0 (Media Cybernetics, Bethesda, MD, USA) was used to quantify, and each lesion analysis was assessed by an observer blinded to the study.
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was isolated from the right common carotid arteries from the mice with use of Trizol (Invitrogen, Carlsbad, CA, USA). Real-time–polymerase chain reaction (RT-PCR) was performed to determine the gene expression of Foxp3, transforming growth factor (TGF)-β, IL-10, IL-4, IL-1β, IL-17, IFN-γ and glyc-eraldehyde-3-phosphate dehydrogenase (GAPDH). The primer sequences were as follows: Foxp3, sense 5′-CCCAT CCCCA GGAGT CTTG-3′, antisense 5′-ACCAT GACTA GGGGC AC TGTA-3′; TGF-β, sense 5′-CTCCC GTGGC TTCTA GTGC-3′, antisense 5′-GCCTT AGTTT GGACA GGATC TG-3′; IL-10, sense 5′-GCTCT TACTG ACTGG CATGA G-3′, antisense 5′-CGCAG CTCTA GGAGC ATGTG-3′; IL-4, sense 5′-CATCG GCATT TTGAA CGAGG TCA-3′, antisense 5′-GCTAC GGACC TAAGT AGCTA TTC-3′; IL-1β, sense 5′-GCAAC TGTTC CTGAA CTCAA CT-3′, antisense 5′-ATCTT TTGGG GTCCG TCAAC T-3′; IL-17, sense 5′-TCCCT CTGTG ATCTG GGAAG-3′, antisense 5′-CTCGA CCCTG AAAGT GAAGG-3′; IFN-γ, sense 5′-CATTG AAAGC CTAGA AAGTC TG-3′, antisense 5′-CTCAT GAATG CATCC TTTTT CG-3′; and GAPDH, sense 5′-AGGTC GGTGT GAACG GATTT G-3′, antisense 5′-TGTAG ACCAT GTAGT TGAGG TCA-3′. mRNA levels were normalized to that of the housekeeping gene GAPDH. Data are representative of five independent experiments.
Western Blot Analysis
The right common carotid arteries of ApoE−/− mice were isolated and protein was extracted. Proteins samples were separated by 10–12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred to nitrocellulose membranes. The membranes were blocked in 5% nonfat milk for 2 h at room temperature, followed by incubation overnight at 4°C with appropriate primary antibody: anti-β-actin antibody (1:1,000; Cell Signaling Technology, Danvers, MA, USA), anti-Foxp3 antibody (1:500, eBioscience), anti-TGF-β antibody (1:500, Abcam) and anti-IL-10 antibody (1:1,000, Abcam). After an incubation with peroxidase-conjugated secondary antibodies for 2 h at room temperature, the membranes were determined with chemiluminescent substrate (Millipore, Billerica, MA, USA). β-Actin immuno blot analysis was used to verify equal loading of protein.
Enzyme-Linked Immunosorbent Assay
Murine sera were obtained and stored at −80°C. The concentrations of IL-10, IFN-γ and IL-17 in serum were determined by use of an enzyme-linked immunosorbent assay (ELISA) kit (Bender MedSystems, Burlingame, CA, USA). Each sample was assayed in triplicate.
Isolation of Peripheral Blood Mononuclear Cells and Cell Culture
We obtained blood samples from 10 male ACS patients (mean age 54 ± 5 years) with confirmed atherosclerosis by coronary arteriography. Exclusion criteria included the following: (a) ongoing or previous treatment with statins or immunosuppressive drugs, (b) hypertension, (c) diabetes mellitus, (d) hyperlipidemia, (e) smoking, (f) cancer, (g) chronic immune-mediated disorders and (h) liver or kidney disease. Our institution’s ethics committee approved the study, and all patients gave their informed written consent for use of blood samples.
Peripheral blood mononuclear cells (PBMCs) were isolated from blood samples and prepared at 1 × 106 cells/mL in RPMI 1640 medium containing 10% fetal bovine serum and 1% penicillin/ streptomycin without additives (control) or with simvastatin (1 or 10 μmol/L) for 96 h. The dose of simvastatin was on the basis of the doses used in previous studies (22,23).
Flow Cytometry
After 96 h incubation, PBMCs in different cultures were harvested for flow cytometry to detect CD4+CD25+Foxp3+ Tregs with use of the Human Regulatory T Cell Staining Kit (eBioscience). Prepared PBMCs were incubated with fluorescein isothiocyanate (FITC)-labeled anti-human CD4 antibodies and Allo-phycocyanin (APC)-labeled anti-human CD25 antibodies for surface staining. Then, cells were washed, resuspended in a fixation and incubated at 4°C. After washing, fixation and permeabilization, cells were stained with phycoerythrin (PE)-labeled anti-human Foxp3 antibody at 4°C in the dark and then underwent flow cytometry with a fluorescent- activated cell sorter (FACS) (BD Biosciences, San Jose, CA, USA) and analyzed with CellQuest software.
Functional Suppression Assays
Purified Tregs and CD4+CD25− cells were sorted from PBMCs by use of the CD4+CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Purity of the two kinds of cells was assessed by FACS (>95%).
In addition, different ratios of Tregs and CD4+CD25− cells (1:1, 1:2, 1:4, 1:8) were added to 96-well plates with plate-bound anti-CD3 monoclonal antibody (1 μg/well) and cocultured in RPMI 1640 medium containing 10% fetal bovine serum, 1% penicillin/ streptomycin, in the presence of 105 CD4− cells pretreated with mitomycin C (Sigma-Aldrich, St. Louis, MO, USA) for 72 h. A total of 1 μCi [3H]-thymidine was added for the final 16 h, and proliferation was assayed with use of a scintillation counter (β-counter). Percentage inhibition of proliferation was determined by the following formula: 1 – (median [3H]-thymidine uptake of Tregs/CD4+CD25− coculture/ median [3H]-thymidine uptake of CD4+CD25− cells) (24,25). The experiment was repeated in the presence of simvastatin at 1 or 10 μmol/L.
Statistical Analysis
SPSS v13.0 (SPSS, Chicago, IL, USA) was used for data analysis. Data are presented as mean ± SD. Student two-tail t test was used for comparing two groups, and one-way analysis of variance was used for more than two groups. P < 0.05 was considered statistically significant.
RESULTS
Body Weight and Biochemical Parameters
At the time of sacrifice, simvastatin-treated and control mice did not differ in body weight (P > 0.05), and simvastatin did not alter lipid levels (total cholesterol, triglycerides and LDL and HDL cholesterol compared with control group (P > 0.05, Table 1).
Table 1.
Body weight and biochemical parameters in two groups of mice.
| Parameters | Control group | Simvastatin group |
|---|---|---|
| Body weight (g) | 30.74 ± 3.24 | 30.22 ± 3.83 |
| Total cholesterol (mmol/L) | 20.82 ± 1.58 | 19.71 ± 1.86 |
| Triglycerides (mmol/L) | 1.42 ± 0.47 | 1.24 ± 0.21 |
| LDL cholesterol (mmol/L) | 5.68 ± 1.12 | 5.60 ± 1.01 |
| HDL cholesterol (mmol/L) | 1.77 ± 0.31 | 1.84 ± 0.32 |
Simvastatin Upregulated the Expression of Foxp3, IL-10 and TGF-β in Atherosclerotic Plaques
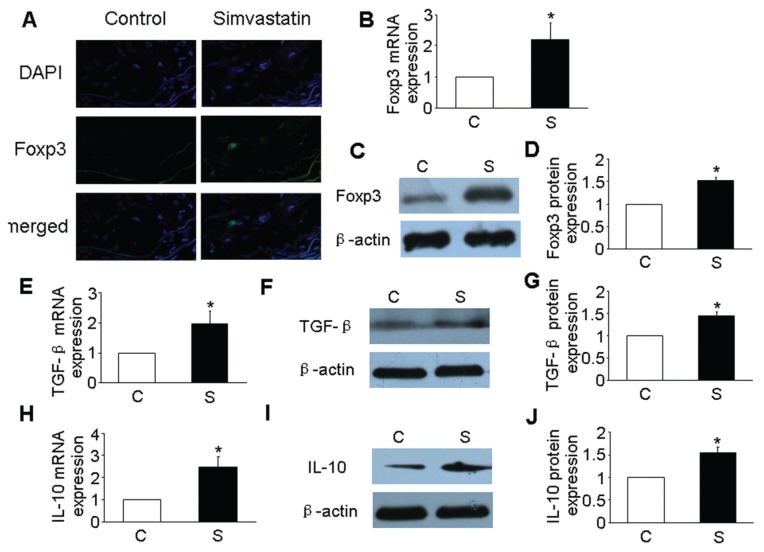
To identify the distribution of Tregs in atherosclerotic plaques, we targeted the Foxp3 transcription factor and measured its levels in atherosclerotic lesions. After 6 wks of simvastatin treatment, immunohistochemical studies showed an increase in the number of Tregs within atherosclerotic plaque (Figure 1A). Furthermore, the mRNA and protein expression of Foxp3 was markedly increased in the simvastatin group versus the control group (P < 0.05, Figures 1B–D). These results confirmed that simvastatin might induce an accumulation of Tregs in atherosclerotic plaques.
Figure 1.
Simvastatin promoted the numbers of Tregs and the expression of Treg markers in atherosclerotic plaques. (A) Tregs were visualized (blue color for 4′,6-diamidino-2-phenylindole [DAPI] staining nuclei and green color depicted Foxp3+ Tregs). (B) mRNA expression of Foxp3 in atherosclerotic plaques of two groups of mice. (C) Protein expression of Foxp3 in atherosclerotic plaques of two groups of mice by Western blot. (D) Quantitative analysis of the results in (C). (E) mRNA expression of TGF-β in atherosclerotic plaques of two groups of mice. (F) Protein expression of TGF-β in atherosclerotic plaques of two groups of mice by Western blot. (G) Quantitative analysis of the results in (F). (H) mRNA expression of IL-10 in atherosclerotic plaques of two groups of mice. (I) Protein expression of IL-10 in atherosclerotic plaques of two groups of mice by Western blot. (J) Quantitative analysis of the results in (I). *P < 0.05 versus control group. C, control group; S, simvastatin group.
Tregs have an ability to produce high levels TGF-β and IL-10. TGF-β and IL-10, Treg-associated cytokines, may have a role in reducing the inflammatory reaction in antiatherosclerosis (15). In line with the change in Foxp3 expression, mRNA and protein expression of TGF-β and IL-10 was significantly increased in the simvastatin group compared with the control group (P < 0.05, Figures 1E–J).
Simvastatin Changed the Cytokine Production in Atherosclerotic Plaques
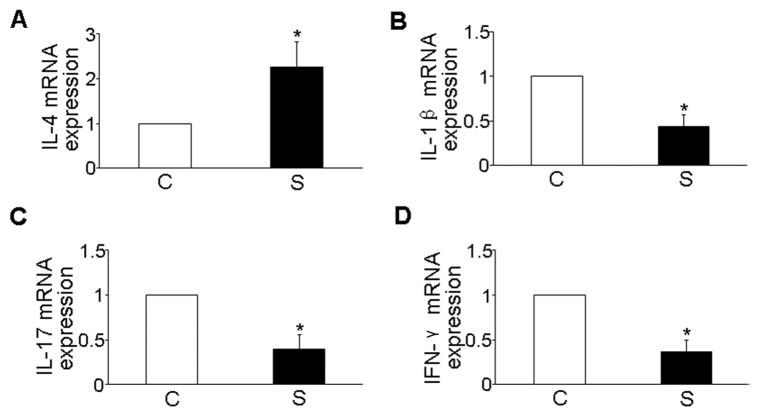
In ApoE−/− mice, we investigated mRNA expression of cytokines (IL-4, IL-1β, IL-17 and IFN-γ) in the carotid plaque using RT-PCR in two groups of mice. Simvastatin treatment led to a significant increase of IL-4 and a decrease of IL-1β, IL-17 and IFN-γ in mRNA expressions (P < 0.05, Figure 2).
Figure 2.
Effects of simvastatin on mRNA expression of cytokines in atherosclerotic plaques. (A) mRNA expression of IL-4 in two groups of mice. (B) mRNA expression of IL-1β in two groups of mice. (C) mRNA expression of IL-17 in two groups of mice. (D) mRNA expression of IFN-γ in two groups of mice. *P < 0.05 versus control group. C, control group; S, simvastatin group.
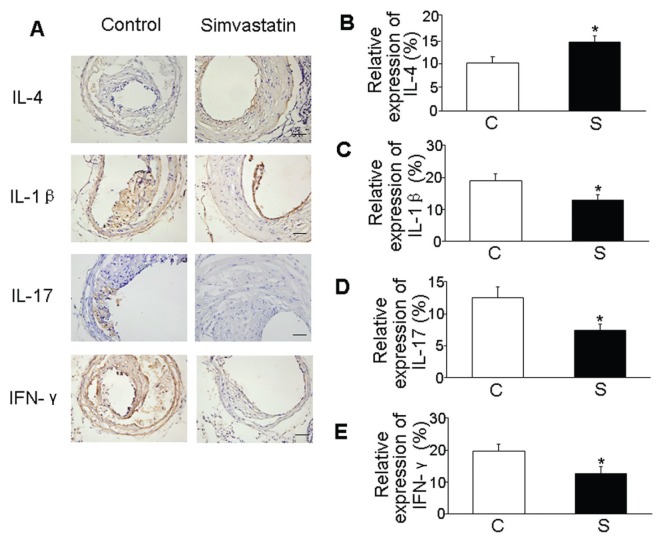
The protein expression level of cytokine in the carotid plaques was also assessed by immunohistochemistry. As compared with the control group, simvastatin significantly upregulated the relative contents of IL-4 and downregulated IL-1β, IL-17 and IFN-γ in the carotid plaque (P < 0.05, Figure 3).
Figure 3.
Effects of simvastatin on protein expression of cytokines in atherosclerotic plaques. (A) Representative immunostaining of IL-4, IL-1β, IL-17 and IFN-γ in two groups of mice. (B) Quantitative analysis of IL-4 protein expression. (C) Quantitative analysis of IL-1β protein expression. (D) Quantitative analysis of IL-17 protein expression. (E) Quantitative analysis of IFN-γ protein expression. *P < 0.05 versus control group. C, control group; S, simvastatin group. Scale bar: 100 μm.
Simvastatin Influenced Circulatory Levels of IL-10, IFN-γ and IL-17
The serum levels of IL-10 were greatly increased in the simvastatin group compared with the control group, but the circulatory levels of IFN-γ and IL-17 were significantly reduced after simvastatin treatment (P < 0.05, Figure 4).
Figure 4.
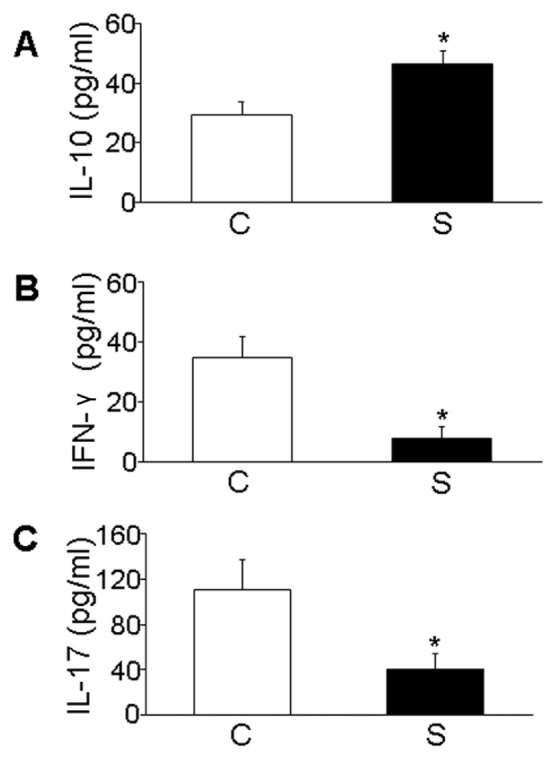
Effects of simvastatin on serum levels of cytokines in the mice. (A) Levels of IL-10 by ELISA. (B) Levels of IL-17 by ELISA. (C) Levels of IFN-γ by ELISA. *P < 0.05 versus control group. C, control group; S, simvastatin group.
Simvastatin Improved the Proportion and Regulatory Function of Tregs in PBMCs from ACS Patients
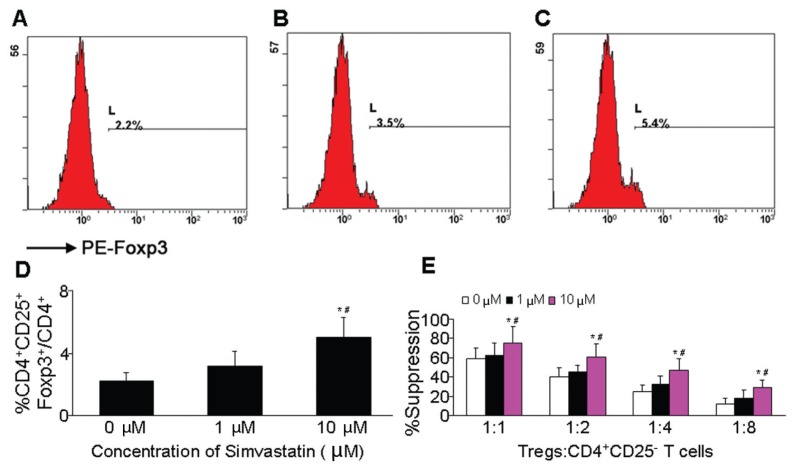
PBMCs were cultured with simvastatin at different concentrations, and the proportion of Tregs in PBMCs was analyzed by CD4+/CD25+/Foxp3+ staining. The proportion of CD4+CD25+Foxp3+ Tregs to total CD4+ cells was 2.18 ± 0.57%, 3.15 ± 0.99% and 5.00 ± 1.27% in the control group and with 1 and 10 μmol/L simvastatin, respectively (Figures 5A–D). Compared with the control group, 10 μmol/L simvastatin increased the proportion of CD4+CD25+Foxp3+ Tregs to total CD4+ cells (P < 0.05). Although 1 μmol/L simvastatin produced a slight increase in the frequency of Tregs relative to the control group, the effect was not significant (P > 0.05). Therefore, simvastatin promoted an increase of Tregs in the peripheral circulation of ACS patients.
Figure 5.
Simvastatin improved the proportion and regulatory function of Tregs in PBMCs from ACS patients. (A) Representative FACS analysis of percentage CD4+CD25+Foxp3+ Tregs to total CD4+ cells in the control group. (B) Representative FACS analysis of percentage CD4+CD25+Foxp3+ Tregs to total CD4+ cells in 1 μmol/L simvastatin group. (C) Representative FACS analysis of percentage CD4+CD25+Foxp3+ Tregs to total CD4+ cells in the 10 μmol/L simvastatin group. (D) FACS analysis results: percentage CD4+CD25+Foxp3+ Tregs to total CD4+ cells in three groups of PBMCs. (E) Purified Tregs and CD4+CD25− cells from PBMCs were incubated at different ratios (Tregs:CD4+CD25− cells = 1:1, 1:2, 1:4, 1:8) without (control) or with simvastatin. [3H]-thymidine was added and proliferation was evaluated. *P < 0.05 versus control group. #P < 0.05 versus 1 μmol/L simvastatin group.
We also analyzed the regulatory function of Tregs. Purified Tregs and CD4+CD25− effector cells were isolated from PBMCs of ACS patients. We examined a possible concentration-dependent effect of simvastatin on the inhibitory function of Tregs for effector T cells. The suppression rate with 10 μmol/L simvastatin was higher than that for the control group at different ratios of Tregs and CD4+CD25− cells (1:1, 1:2, 1:4, 1:8) (P < 0.05). Moreover, 10 μmol/L simvastatin increased the extent of suppression relative to 1 μmol/L simvastatin (P < 0.05) (Figure 5E). Simvastain at the low concentration did not have any effect. Therefore, simvastatin treatment enhanced the suppressive function of Tregs of ACS patients.
DISCUSSION
Recently, Tregs were found to play an important role in the progression of atherosclerosis. In this study, we found that simvastatin has a direct effect on Tregs in atherosclerotic plaques in mice and in peripheral circulation of patients with ACS. Six-week simvastatin treatment significantly increased the number of Tregs and the expression of the Treg marker Foxp3, TGF-β and IL-10 in atherosclerotic plaques in ApoE−/− mice. Furthermore, simvastatin significantly enhanced the frequency and functional suppressive properties of Tregs in ACS patients.
Immune activation is involved in the pathogenesis of atherosclerosis, and the role of the immune system in atherosclerotic process has received considerable attention. The imbalance of regulatory and pathogenic immunity may promote the development of atherosclerosis (1). Statins have been widely used in preventing and treating atherosclerosis. They have pleiotropic effects beyond that of simply lowering plasma lipid levels. Statin therapy contributes to reducing the secretion of inflammatory cytokines, inhibiting atherogenic procession and stabilizing vulnerable plaque (16,26). In recent years, the potent modulatory mechanisms of statins on the immune system have been investigated (18). Statins have a number of immunomodulatory effects and modulate immune system–mediated activities, including inhibiting the production of inflammatory cytokines associated with immunity, T lymphocyte activation, mononuclear cell proliferation and antigen-presenting capacity (24,27). Extensive evidence also indicated that statins reduce the production of inflammatory cytokines and stabilize vulnerable plaques in atherosclerosis, in part because of their immunomodulatory mechanism (18,19).
Tregs are a unique subset of T cells. Although the number of Tregs is small among the total CD4+ T cells, Tregs have an indispensable role in the immune system, including restraining immune responses and maintaining immune tolerance and self-tolerance (15). It has been suggested that Tregs may have an important protective role in the development and progression of atherosclerosis (11). Adoptive transfer of Tregs could lead to a significant attenuation of atherogenesis, whereas atherosclerotic lesions were significantly increased with injection of Treg-depleting antibody in ApoE−/− mice models (11,12).
Tregs are present in atherosclerotic lesions and implicated in the suppression of the chronic inflammatory process in the development of atherosclerotic lesions (14). However, no study has investigated the effect of statins on Tregs in atherosclerotic plaques. As well known, atherosclerotic lesions can develop in ApoE−/− mice throughout the arterial tree and such mice are thought to be a well-established genetic animal model of atherosclerosis, with many morphologic characteristics resembling that in humans (28). Therefore, in this study, we induced atherosclerotic plaque in ApoE−/−mice by placing a silastic perivascular collar around the right common carotid artery and introducing a lipid-rich diet (20,21). Then we administered simvastatin to mice for 6 wks and investigated the effect of statins on Tregs in atherosclerotic plaques. Foxp3 dominates the development and function of Tregs and is considered a reliable marker of Tregs (14). Deletion or mutation of Foxp3 halts the development of Tregs (15). In the present study, immunohistochemistry showed a larger number of accumulation Tregs in atherosclerotic plaques of simvastatin-treated mice compared with controls. Furthermore, Foxp3 mRNA and protein levels in advanced atherosclerotic lesions were significantly increased with simvastatin therapy. Therefore, the result shows that simvastatin promote an accumulation of Tregs within the atherosclerotic plaques. Interestingly, we did not find a significant difference in serum levels of total cholesterol, triglycerides and LDL cholesterol with and without simvastatin in ApoE−/− mice, which was consistent with the previous studies (27,28), indicating that simvastatin influenced the number of Tregs in plaques, independent of its lipid-lowering properties.
Tregs secrete two major cytokines (TGF-β and IL-10), which are associated with their survival and function (30). TGF-β plays an important role in regulating the signaling pathway that promotes Foxp3 expression and Treg differentiation (10). Previous study demonstrated that TGF-β has pleiotropic effects on some immune cells (31). It has been shown that Tregs prevent atherosclerosis via TGF-β signaling and Tregs do not relieve the progression of atherosclerosis and inflammatory response if Tregs fail to respond to TGF-β signaling (15,32). Increased concentration of TGF-β and IL-10 was associated with suppressing pathogenic inflammatory responses, regulating immunity and promoting atherosclerotic plaque stabilization (33). On the contrary, deletion of TGF-β or IL-10 signaling accelerates atherosclerosis and plaque vulnerability (34,35). In the present study, mRNA and protein levels of TGF-β and IL-10 were significantly increased in atherosclerotic plaques in ApoE−/− mice after 6 wks of simvastatin treatment. Therefore, our results showed that the accumulation of Tregs by statins treatment in atherosclerotic plaques correlated with the increase of TGF-β and IL-10.
Cumulative data have shown that T cell–mediated pathogenic immune responses contribute to atherosclerotic formation and progression (4). Solid evidence suggests that a considerable number of activated T cells accumulate in vulnerable plaques, and their activation accelerates atherosclerotic plaque destabilization, which may initiate plaque rupture and the onset of acute cardiovascular events (1,36). T cells were classified as Th1, Th2 and Th17 cells and Tregs by assessing their intracellular cytokine profile. The activation of the immune system led to an inflammatory cascade and initiated the differentiation of T cells to a Th1 and Th17 phenotype (6). Th1 and Th17 cells mediated proinflammatory responses in atherosclerotic plaques and exerted a robust synergistic effect on the initiation of atherosclerosis and plaque instability (37,38). Tregs protect T-cell homeostasis, inhibit T-cell migration from peripheral lymph tissues into plaques and release of antiinflammatory cytokines (TGF-β and IL-10) and prevent the activation and proliferation of effector T cells in atheroma (32). Importantly, Tregs can modulate Th1 and Th17 response in the atherosclerotic plaques (38). Targeting Th1 and Th17 response and recovering the balance between Tregs and effector T cells may be a beneficial strategy in atherosclerosis (38). Whereas Th2 cells were characteristic with IL-4 production and downregulated the inflammatory response (33), Tregs represented an important role in modulation of immune homeostasis, partly by adjusting the Th1/Th2 balance (39). In this study, we determined whether T-cell activation and differentiation was changed in carotid plaques after statin treatment. We found that simvastatin treatment increased the mRNA and protein expression of IL-4 and decreased the levels of IL-1β, IL-17 and IFN-γ in carotid plaques. Therefore, the results showed that simvastatin promoted an accumulation of Tregs, subsequently decreased Th1 and Th17 response and modulated the Th1/Th2 balance toward a Th2 phenotype in the atherosclerotic plaques, and this result may be an additional mechanism of simvastatin in stabilizing vulnerable plaques.
ELISA revealed increased serum level of IL-10 and decreased serum level of IFN-γ and IL-17 in simvastatin-treated mice compared with the control group. Therefore, simvastatin inhibited the secretion of Th1 and Th17 cytokines and promoted the secretion of antiinflammatory cytokines, indicating a shift toward a more antiinflammatory immune response.
We also determined the effect of statins on Tregs in the peripheral circulation of ACS patients. The number of Tregs and Foxp3 expression was conspicuously decreased, and the functional suppressive properties of Tregs were compromised in ACS patients (9,13). In this study, we discovered that simvastatin treatment of PBMCs from patients with ACS increased the number of Tregs. Furthermore, simvastatin greatly enhanced the inhibitory effect of Tregs on the proliferation of effector T cells. Therefore, simvastatin treatment expanded the peripheral Treg pool and repaired their compromised functional inhibitory properties in ACS patients.
CONCLUSION
In conclusion, our study confirmed that simvastatin treatment resulted in increased expression of Foxp3 and accumulation of Tregs in atherosclerotic lesions of ApoE−/− mice. Furthermore, simvastatin increased the number of Tregs and repaired their compromised functional inhibitory properties in patients with ACS. Therefore, statins may benefit atherosclerosis plaque in part through Treg activation. Therefore, further studies are needed to elucidate the underlying mechanisms.
ACKNOWLEDGMENTS
This work was supported by the National 973 Basic Research Program of China (2010CB732605, 2011CB503906), the Program of Introducing Talents of Discipline to Universities (B07035), the State Program of National Natural Science Foundation of China for Innovative Research Group (81021001) and the State Key Program of National Natural Science of China (60831003) and through grants from the National Natural Science Foundation of China (30900607, 81100207, 30873325, 30700301, 30971096, 81000126, 81000127).
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 2.Aprahamian T, et al. Simvastatin treatment ameliorates autoimmune disease associated with accelerated atherosclerosis in a murine lupus model. J Immunol. 2006;177:3028–34. doi: 10.4049/jimmunol.177.5.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riboldi P, Gerosa M, Luzzana C, Catelli L. Cardiac involvement in systemic autoimmune diseases. Clin Rev Allergy Immunol. 2002;23:247–61. doi: 10.1385/CRIAI:23:3:247. [DOI] [PubMed] [Google Scholar]
- 4.Hansson GK, Lippy P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–19. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 5.Smolders J, et al. Vitamin D status is positively correlated with regulatory T cell function in patients with multiple sclerosis. PLoS One. 2009;4:e6635. doi: 10.1371/journal.pone.0006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong AW, Voyles SV, Armstrong EJ, Fuller EN, Rutledge JC. A tale of two plaques: convergent mechanisms of T-cell-mediated inflammation in psoriasis and atherosclerosis. Exp Dermatol. 2011;20:544–9. doi: 10.1111/j.1600-0625.2011.01308.x. [DOI] [PubMed] [Google Scholar]
- 7.O’Garra A, Viwira P. Regulatory Tcells and mechanisms of immune system control. Nat Med. 2004;10:801–5. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- 8.Youssef S, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 9.Han SF, et al. The opposite-direction modulation of CD4+CD25+ Tregs and T helper 1 cells in acute coronary syndromes. Clin Immunol. 2007;124:90–7. doi: 10.1016/j.clim.2007.03.546. [DOI] [PubMed] [Google Scholar]
- 10.Mucida D, et al. Reciprocal TH17 and regulatory Tcell differentiation mediated by retinoic acid. Science. 2007;317:255–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 11.Mor A, et al. Role of naturally occurring CD4+CD25+ regulatory T cells in experimental atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:893–900. doi: 10.1161/01.ATV.0000259365.31469.89. [DOI] [PubMed] [Google Scholar]
- 12.Ait-Oufella H, et al. Naturally regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–80. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 13.Mor A, Luboshits G, Planer D, Keren G, George J. Altered status of CD4(+)CD25(+) regulatory T cells in patients with acute coronary syndromes. Eur Heart J. 2006;27:2530–7. doi: 10.1093/eurheartj/ehl222. [DOI] [PubMed] [Google Scholar]
- 14.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 15.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–7. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura K, et al. Statin prevents plaque disruption in apoE-knockout mouse model through pleiotropic effect on acute inflammation. Atherosclerosis. 2009;206:355–61. doi: 10.1016/j.atherosclerosis.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Umeji K, et al. Comparative effects of pitavastatin and probucol on oxidative stress, Cu/Zn superoxide dismutase, PPAR, and aortic stiffness in hypercholesterolemia. Am J Physiol Heart Circ Physiol. 2006;291:H2522–32. doi: 10.1152/ajpheart.01198.2005. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Jin J, Peng X, Ramgolam VS, Markovic-Plese S. Simvastatin inhibits IL-17 secretion by targeting multiple IL-17-regulatory cytokines and by inhibiting the expression of IL-17 transcription factor RORC in CD4+ lymphocytes. J Immunol. 2008;180:6988–96. doi: 10.4049/jimmunol.180.10.6988. [DOI] [PubMed] [Google Scholar]
- 19.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4:977–87. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 20.Ni M, et al. Atherosclerotic plaque disruption induced by stress and lipopolysaccharide in apolipoprotein E knockout mice. Am J Physiol Heart Circ Physiol. 2009;296:H1598–606. doi: 10.1152/ajpheart.01202.2008. [DOI] [PubMed] [Google Scholar]
- 21.Cheng C, et al. Heme oxygenase 1 determines atherosclerotic lesion progression into a vulnerable plaque. Circulation. 2009;119:3017–27. doi: 10.1161/CIRCULATIONAHA.108.808618. [DOI] [PubMed] [Google Scholar]
- 22.Medina RJ, O’Neill CL, Devine AB, Gardiner TA, Stitt AW. The pleiotropic effects of simvastatin on retinal microvascular endothelium has important implications for ischaemic retinopathies. PLoS One. 2008;3:e2584. doi: 10.1371/journal.pone.0002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsakiri A, Tsiantoulas D, Frederiksen J, Svane IM. Increased immunopotency of monocyte derived dendritic cells from patients with optic neuritis is inhibited in vitro by simvastatin. Exp Neurol. 2010;221:320–8. doi: 10.1016/j.expneurol.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Mausner-Fainberg K, et al. The effect of HMG-CoA reductase inhibitors on naturally occurring CD4+CD25+ T cells. Atherosclerosis. 2008;197:829–39. doi: 10.1016/j.atherosclerosis.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 25.Feng J, et al. Regulatory T cells ameliorate hyperhomocysteinaemia-accelerated atherosclerosis in apoE−/− mice. Cardiovasc Res. 2009;84:155–63. doi: 10.1093/cvr/cvp182. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura K, et al. Statin prevents plaque disruption in apoE-knockout mouse model through pleiotropic effect on acute inflammation. Atherosclerosis. 2009;206:355–61. doi: 10.1016/j.atherosclerosis.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Peng X, et al. Immunomodulatory effects of 3-hydroxy- 3-methylglutaryl coenzyme-A reductase inhibitors, potential therapy for relapsing remitting multiple sclerosis. J Neuroimmunol. 2006;178:130–9. doi: 10.1016/j.jneuroim.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Johnson J, et al. Plaque rupture after short periods of fat feeding in the apolipoprotein E-knockout mouse: model characterization and effects of pravastatin treatment. Circulation. 2005;111:1422–30. doi: 10.1161/01.CIR.0000158435.98035.8D. [DOI] [PubMed] [Google Scholar]
- 29.Sparrow CP, et al. Simvastatin has anti-inflammatory and antiatherosclerotic activities independent of plasma cholesterol lowering. Arterioscler Thromb Vasc Biol. 2001;21:115–21. doi: 10.1161/01.atv.21.1.115. [DOI] [PubMed] [Google Scholar]
- 30.Kim HJ, Hwang SJ, Kim BK, Jung KC, Chung DH. NKT cells play critical roles in the induction of oral tolerance by inducing regulatory T cells producing IL-10 and transforming growth factor beta, and by clonally deleting antigen- specific T cells. Immunology. 2006;118:101–11. doi: 10.1111/j.1365-2567.2006.02346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grainger DJ, Witchell CM, Metcalfe JC. Tamoxifen elevates transforming growth factor-beta and suppresses diet-induced formation of lipid lesions in mouse aorta. Nat Med. 1995;1:1067–73. doi: 10.1038/nm1095-1067. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki N, et al. Oral anti-CD3 antibody treatment induces regulatory T cells and inhibits the development of atherosclerosis in mice. Circulation. 2009;120:1996–2005. doi: 10.1161/CIRCULATIONAHA.109.863431. [DOI] [PubMed] [Google Scholar]
- 33.Mallat Z, et al. Induction of a regulatory T cell type 1 response reduces the development of atherosclerosis in apolipoprotein E-knockout mice. Circulation. 2003;108:1232–7. doi: 10.1161/01.CIR.0000089083.61317.A1. [DOI] [PubMed] [Google Scholar]
- 34.Mallat Z, et al. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ Res. 2001;89:930–4. doi: 10.1161/hh2201.099415. [DOI] [PubMed] [Google Scholar]
- 35.Caligiuri G, et al. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med. 2003;9:10–7. [PMC free article] [PubMed] [Google Scholar]
- 36.Ovchinnikova O, et al. T-cell activation leads to reduced collagen maturation in atherosclerotic plaques of ApoE−/− mice. Am J Pathol. 2009;174:693–700. doi: 10.2353/ajpath.2009.080561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eid RE, et al. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–32. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Cytokine network and T cell immunity in atherosclerosis. Semin Immunopathol. 2009;31:23–33. doi: 10.1007/s00281-009-0143-x. [DOI] [PubMed] [Google Scholar]
- 39.Liu F, et al. CD4+CD25+Foxp3+ regulatory T cells depletion may attenuate the development of silica-induced lung fibrosis in mice. PLoS One. 2010;5:e15404. doi: 10.1371/journal.pone.0015404. [DOI] [PMC free article] [PubMed] [Google Scholar]