Abstract
The importance of the priming of the lung environment by past infections is being increasingly recognized. Exposure to any given antigen can either improve or worsen the outcome of subsequent lung infections, depending on the immunological history of the host. Thus, an ability to impart transient alterations in the lung environment in anticipation of future insult could provide an important novel therapy for emerging infectious diseases. In this study, we show that nasal administration of virus-like particles (VLPs) before, or immediately after, lethal challenge with methicillin-resistant Staphylococcus aureus (MRSA) of mice i) ensures complete recovery from lung infection and near absolute clearance of bacteria within 12 hours of challenge, ii) reduces host response-induced lung tissue damage, iii) promotes recruitment and efficient bacterial clearance by neutrophils and CD11c+ cells, and iv) protects macrophages from MRSA-induced necrosis. VLP-mediated protection against MRSA relied on innate immunity. Complete recovery occurred in VLP-dosed mice with severe combined immunodeficiency, but not in wild-type mice depleted of either Ly6G+ or CD11c+ cells. Early IL-13 production associated with VLP-induced CD11c+ cells was essential for VLP-induced protection. These results indicate that VLP-induced alteration of the lung environment protects the host from lethal MRSA pneumonia by enhancing phagocyte recruitment and killing and by reducing inflammation-induced tissue damage via IL-13–dependent mechanisms.
The immune system is a plastic entity, in which the history of prior exposures modulates responses to future insults. It is well documented that the occurrence and even the exact sequence of infections can skew the immune response to a subsequent threat toward an inflammatory or anti-inflammatory phenotype,1–5 depending on the type of insult,1,5 its location,6 and even the stage of host development.2 The modulatory effect of former insults can be long lasting and can influence the outcome and prognosis of an individual long after the infection has been cleared.2,7 For example, acute influenza infection can decrease immune-mediated lung damage associated with subsequent respiratory syncytial virus infection and inhibit replication of vaccinia virus, but it can also enhance replication of lymphocytic choriomeningitis virus and murine cytomegalovirus.7 Microbial products, such as CpG, lipopolysaccharide, or other bacterial toxins, can induce protection against a variety of subsequent respiratory pathogens.8–10 The homeostatic profile of the lung can also be altered by noninfectious substances. Morphine reduces Toll-like receptor 9–NF-κB signaling, which leads to impaired clearance of Streptococcus pneumoniae.11 Cigarette smoke down-regulates Toll-like receptor 2 expression on alveolar macrophages (AMs)12 and inhibits expression of proinflammatory cytokines,13 which may increase susceptibility to respiratory bacterial infections. Immune imprinting by the nontoxic heat-labile endotoxin (LTK63) of Escherichia coli, delivered as a mucosal adjuvant, induces a transient inflammatory response that reduces inflammation to subsequent type 1 and 2 helper T-cell pathogens (namely, influenza, respiratory syncytial virus, and Cryptococcus neoformans).9 Therefore, the ability to transiently modulate the immune environment in preparation for a future infectious challenge, without the need for the induction of harmful inflammation, would be beneficial as preventative and therapeutic treatments.
We have previously reported that pulmonary delivery of a noninfectious, self-assembled protein cage nanoparticle (PCN) induced low-grade inflammation in the absence of tissue damage, yet it enhanced the protection of mice against subsequent sublethal and lethal infections by unrelated viruses, including influenza, severe acute respiratory syndrome virus, and pneumovirus.14 This protection is associated with the formation of inducible bronchus-associated lymphoid tissue (iBALT) that modulates the local lung environment in the absence of harmful inflammation.14,15
PCN is a hollow, spherical protein cage that self-assembles from 24 identical subunits of small heat shock protein, derived from Methanocaldococcus jannaschii. Although PCNs are not of viral origin, their small size (12-nm diameter), self-assembled particulate structure, immunogenicity, and lack of genetic material characterize them as virus-like particles (VLPs).14,16,17 Known for their potent adjuvant-like properties,18 VLPs can enhance immune responses to parental infectious agents, such as hepatitis C,19 dengue virus,20 Norwalk virus,21,22 or human papillomavirus-induced cervical cancer.23,24 In addition, VLPs have been used as a platform for targeting cells16 and as a delivery vehicle to present the immune system with fused or conjugated heterologous epitopes,25 and even to induce autoantibodies to self molecules involved in chronic diseases.25,26 The work reported by our laboratory adds yet another dimension to the VLP-mediated immune modulation (namely, the ability to induce protective immunity to a variety of unrelated antigens).14
Invasive methicillin-resistant Staphylococcus aureus (MRSA) infections result annually in more deaths (>18,000 cases) than any other single infectious agent in the United States.27 In fact, the mortality associated with MRSA exceeds the mortality resulting from combined, human immunodeficiency, hepatitis, and influenza viruses.28,29 The pulsed-field gel electrophoresis type USA300 is the most prevalent community-associated MRSA strain in United States. USA300 typically causes skin and soft tissue infections; however, it is also capable of causing severe staphylococcal diseases, including necrotizing pneumonia that can transpire in otherwise healthy individuals.30,31
In the current study, PCN administration before, or shortly after, respiratory infection of mice with MRSA minimized morbidity and prevented mortality. Major reduction of bacterial burden was observed as early as 8 hours after infection in PCN-dosed mice. This protection was associated with improved recruitment of neutrophils and enhanced antibacterial activity of both neutrophils and lung CD11c+ cells. The reliance on innate immunity for PCN-mediated protection was indicated by the complete recovery of MRSA-infected mice with severe combined immunodeficiency (SCID) dosed with PCN and the significant loss of protection in PCN-dosed wild-type (WT) mice depleted of either Ly6G+ neutrophils or CD11c+ cells. Interestingly, PCN-mediated protection was not limited to the lung, because bone marrow (BM) cells from mice intranasally dosed with PCN, but not with PBS, efficiently killed MRSA in vitro. Phenotypical analyses revealed that PCN-dosed mice retained a high M1:M2 macrophage ratio throughout the infection; however, the efficient bacterial killing in PCN-dosed mice was dependent on the early presence of IL-13 and IL-4, in that mice deficient in either of these cytokines had reduced PCN-mediated protection against MRSA pneumonia.
Materials and Methods
Mice
Male and female WT BALB/c, C57BL/6 (CD45.2), SCID, C-X-C chemokine receptor (CXCR) 2 knockout (KO), IL-13 KO, and CCR2 KO mice were bred at Montana State University, Bozeman. The breeding pairs of IL-13 KO mice were originally generated by Dr. Andrew N.J. McKenzie (Medical Research Council, Laboratory of Molecular Biology, Cambridge, UK) and were kindly provided by Dr. Andrij Holian (University of Montana, Missoula). The breeding pairs of WT and remaining KO mice were obtained from The Jackson Laboratories (Bar Harbor, MI). All mice were maintained at Montana State University Animal Resources Center in individual ventilated cages under high efficiency particulate arresting-filter barrier conditions and were fed sterile food and water ad libitum. All animal care procedures were in accordance with institutional polices for animal health and well-being; 7- to 8-week-old mice were used in all experiments.
Administration of Nanoparticles
PCNs were prepared as previously described.14,16 Mice were lightly anesthetized with oxygen-delivered isoflurane, USP (Piramal, Bethlehem, PA), and were intranasally dosed with 50 μL of sterile PBS or PCN containing 100 μg of protein. This dosing procedure was repeated five times either daily or every couple of days for 2 weeks, as designated in the figures.
MRSA Infection
The LAC strain of MRSA (pulsed-field type USA300) was a kind gift from Dr. Jovanka Voyich (Montana State University). The USA300 MRSA was used for infections because it has been described as a causative agent of severe community-onset pneumonia in healthy individuals.32 Before MRSA infection, PCN- and PBS-dosed mice were rested for 6 to 7 days to ensure removal of nanoparticles from the body.17 Fresh or frozen bacteria grown to OD600 1.5 in tryptic soy broth were used for in vivo infections and in vitro cultures. Between 5 × 107 and 5 × 108 (where indicated) microorganisms were used for the infection dose. The erythromycin-sensitive MRSA strain (AH1263 USA300 community-associated MRSA ErmS Lac)33 was provided by Dr. Alex Horswill (University of Iowa, Iowa City).
Collection and Preparation of BALF
Mice were sacrificed at the designated time points by i.p. administration of a lethal dose of sodium pentobarbital (90 mg/kg), followed by exsanguination when the mice no longer exhibited a pedal reflex. Bronchoalveolar lavage fluid (BALF) was obtained by washing the lungs with 2 mL of 3 mmol/L EDTA in PBS in two aliquots of 1 mL each. The cellular composition of BALF was determined by hemocytometer cell counts and differential counts of cytospins after staining with Quick-Diff solution (Siemens; Medical Solutions Diagnostics, Tarrytown, NY). After centrifugation, the cell-free BALF supernatant was recovered and stored at −80°C for future use to determine levels of cytokines and lung tissue damage.
Histological Analyses
Lungs used for histological analyses were instilled and fixed in 10% buffered formalin phosphate (Fisher Scientific, Fairlawn, NJ) for 24 hours. Paraffin-embedded lung sections (5 μm thick) were stained with H&E and evaluated under a microscope (Eclipse E800; Nikon Inc., Melville, NY) at ×10 and ×60 objective magnification.
Survival and Determination of Bacterial Load
Mice were monitored bidaily up to 96 hours after infection. Lung bacterial burden was determined based on the counted number of colony-forming units (CFUs) cultured out of 100 μL of serially diluted whole lung homogenates (LHs) on tryptic soy agar (TSA) plates at 37°C in 5% CO2. Plated colonies were counted after an overnight incubation.
In Vivo Depletion Studies
Mice previously dosed with PCN or PBS were i.p. injected with anti-CD11c monoclonal antibody (mAb) [HB224/N418; for depletion of AMs and dendritic cells (DCs)] or anti-Ly6G mAb (1A8; for depletion of neutrophils), both obtained from BioXcell (West Lebanon, NH). The total volume of 500 μL (containing 250 μg of either Ab) was delivered by injection on days −3, −1, and 0 in relation to MRSA infection (day 0). Cell depletion was verified by flow cytometry at sacrifice and was determined to be at least 98% efficient for depletion of Ly6G+ and >97% efficient for depletion of CD11c+ cells in both PCN- and PBS-dosed mice.
Neutrophil Preparation, Adoptive Transfer, and Trafficking into the Lungs
Neutrophils were isolated from the BM of naïve BALB/c mice by Percoll gradient, as previously described.34 Briefly, BM was flushed from dissected tibias and femurs, resuspended in HBSS (Mediatech Inc., Herndon, VA), supplemented with 0.1% bovine serum albumin and 1% glucose, and filtered through a 70-μm nylon cell strainer (BD, Franklin Lakes, NJ) to remove clumps and bone particles. BM cells were centrifuged (600 × g, 10 minutes) and resuspended in 45% Percoll PLUS solution (GE Healthcare Bio-Sciences Corp, Piscataway, NJ). The Percoll gradient was prepared by layering the polypropylene tube with 81%, 62%, 55%, and 50% of Percoll solution in HBSS. The BM cells in 45% Percoll were carefully suspended on a top of the gradient. After centrifugation (1600 × g, 30 minutes), the neutrophil fraction (band between 81% and 62% Percoll gradient) was carefully removed and washed twice with HBSS. The purity of the neutrophil fraction was confirmed by microscopic examination of cells after the Diff-Quick staining procedure and was assessed to be >97% pure. Purified neutrophils were labeled with Vybrant-DiI (Molecular Probes, Eugene, OR) dye, according to the manufacturer's recommendations. Mice were dosed nasally with PCN or PBS for 5 consecutive days. One week after the last dose, mice were infected with MRSA and, 1 hour later, adoptively transferred through tail vein injection with 1 × 107 Vybrant-labeled neutrophils. At 4 hours after adoptive transfer, mice were sacrificed. The number of Vybrant+ cells in BALF was determined by fluorescence-activated cell sorting (FACS).
Cytokine Analyses
Cytokine analyses were performed on cell-free BALF. Levels of interferon-γ, tumor necrosis factor (TNF)-α, IL-6, and monocyte chemoattractant protein (MCP)-1 were determined by Cytometric Bead Array (BD Bioscience, San Jose, CA), according to manufacturer's recommendations, and analyzed by BD LSRII Flow Cytometer (BD Bioscience) using BD Cytometric Bead Array analysis software. Levels of IL-13 and IL-4 were determined by a sandwich enzyme-linked immunosorbent assay kit (Ready-SET-Go; eBioscience, San Diego, CA). BALF samples were tested in triplicate.
Flow Cytometry
A single-cell suspension was obtained by centrifugation of BALF. Red blood cells were lysed with AKC Lysis Buffer (150 mmol/L NH4Cl, 1 mmol/L KHCO3, and 0.1 mmol/L Na2EDTA; pH 7.2 to 7.4) in room temperature for 5 minutes. Cells were washed with PBS twice and blocked with Fc receptor block (2.4G2 hybridoma made in house) for 20 minutes at room temperature. Cells were stained with fluorochrome-labeled antibodies to extracellular markers: CD11c (clone N418; eBioscience), CD11b (clone M1/70; eBioscience), F4/80 (clone BM8; eBioscience), major histocompatibility complex (MHC) II (clone M5/114.15.2; eBioscience), and CD206 (clone MR5D3; AbD Serotec, Raleigh, NC). After a 30-minutes incubation, cells were washed twice in FACS buffer (1% fetal bovine serum/PBS) and stained with streptavidin–allophycocyanin for 30 minutes. After staining for extracellular markers, cells were washed in FACS buffer and fixed in 4% paraformaldehyde for 20 minutes. Fixed cells were washed once in FACS buffer and in 0.2% saponin made in FACS buffer, and they were stained for 30 minutes with phosphatidylethanolamine-conjugated Abs to mouse IL-13 (clone eBio13A; eBioscience) or with rat IgG1 isotype control Ab (clone eBRG1; eBioscience). Samples were acquired on FACSCanto running FACSDiva software (both obtained from BD Bioscience). Approximately 50,000 events were routinely acquired per sample. FlowJo software (Tree Star, Inc., Ashland, OR) was used for analysis.
Cell Selection, Bacterial Killing, and Bacterial Uptake Assays
A single-cell suspension obtained after red blood cell lysis and filtration (30 μm) of LH was run through magnetic-activated cell sorting (MACS)–positive selection columns to obtain pure Ly6G+ and Ly6G− cell populations (>97% and >98% purity, respectively). In separate experiments, LH cells were first selected for CD11c+ cells (positive MACS CD11c selection) and the CD11c− cells were subsequently depleted of Ly6G+ cells by MACS Ly6G depletion columns (>97% purity). The purity of all fractions was confirmed by FACS, according to manufacturers' recommendations.
Bacterial Killing Assay
For these experiments, designated populations of cells were plated in a 1:1 ratio with normal mouse serum (NMS)–opsonized MRSA (50% NMS, 30 minutes, 37°C, 5% CO2) on NMS-coated flat-well culture plates (100% NMS, 30 minutes, 37°C, 5% CO2). The killing assay was conducted at 37°C for 1.5 and 3 hours. After this time, 0.1% saponin was added to each well to release internalized bacteria and total CFUs were determined for each experimental sample by plating the 10-fold dilutions on TSA plates. In the experiments in which two different variants of the LAC strain were used (in vivo, MT1263 erythromycin-sensitive LAC; and in vitro, WT LAC erythromycin resistant), the bacterial burden was evaluated by plating on TSA/erythromycin plates (25 μg/mL).
Bacterial Uptake Assay
This assay was performed as previously described.35 Briefly, cells isolated from BMs of mice dosed with PCN and PBS (>78% polymorphonuclear cells, as determined by differential count) were plated on tissue culture plates previously incubated at 37°C for 30 minutes with 100% NMS or heat-inactivated NMS or incubated without serum. Cells were left to adhere to the wells for 15 minutes at room temperature, followed by a 10-minute incubation on ice to prevent activation. NMS-opsonized, heat-inactivated NMS–opsonized, or not opsonized MRSA was added in a 1:1 ratio to the wells, and plates were synchronized by centrifugation at 8°C, 500 × g for 7 minutes.35 After synchronization, cells were incubated at 37°C for 30 minutes. The uptake of bacteria was stopped after 30 minutes, and 40 μg/mL of gentamicin was added to each well to kill the extracellular bacteria. Subsequently, total supernatants were removed by aspiration and cells were resuspended in 0.1% saponin solution to allow the release of intracellular bacteria. Wells containing MRSA only (but no mammalian cells) were used as a baseline for gentamicin killing of extracellular bacteria.
Statistical Analyses
The differences between treatment groups were analyzed using the Student's t-test or analysis of variance. For the differences in survival, Kaplan-Meier curves were plotted and analyzed using GraphPad Prism software, version 4.0 (GraphPad, La Jolla, CA). Statistical differences of P < 0.05 were considered significant.
Results
Nasal Administration of PCNs Protects from Pulmonary MRSA Infection
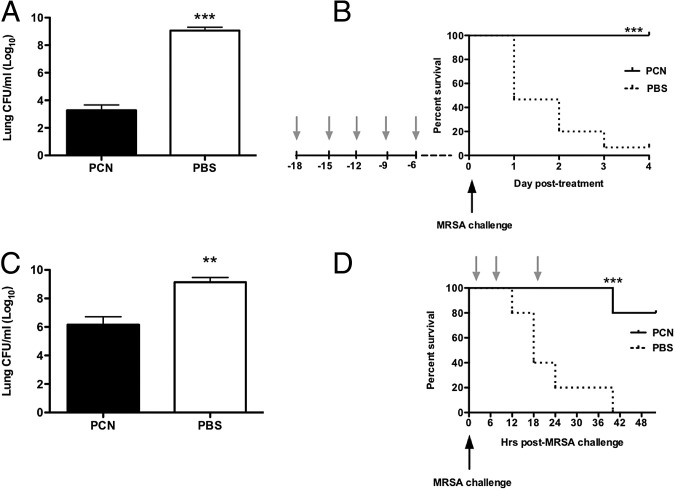
Our previous studies14 indicated that nasal administration of a type of VLP, PCN, prevented body weight loss and significantly reduced lung viral titers in mice infected with murine-adapted influenza or severe acute respiratory syndrome viruses. Herein, we determined whether nasal administration of PCN prevented mortality associated with pulmonary MRSA infection. WT mice dosed five times with PCN and rested for a week before MRSA infection, compared with PBS-dosed mice, showed more than a millionfold reduction in bacterial titers in the lungs (Figure 1A). At 72 hours after infection with 5 × 107 CFUs of MRSA per dose, we isolated 1.9 × 103 CFUs of bacteria from the lungs of PCN-dosed mice and 1.2 × 109 CFUs of bacteria from the lungs of PBS-dosed mice. Significant decreases in bacterial burden in spleen, liver, and kidney were observed in PCN-dosed mice when compared with PBS-dosed mice (see Supplemental Figure S1 at http://ajp.amjpathol.org). Interestingly, the blood of both PCN- and PBS-dosed mice was virtually free of bacteria up to 72 hours after infection and over the range of challenge doses used in multiple experiments (5 × 107 to 5 × 108 CFUs; data not shown). The reduction of lung bacterial burden directly correlated to survival of all PCN-dosed mice (Figure 1B). In contrast to PCN-dosed mice, the mortality of PBS-dosed mice reached >50% at 24 hours after infection, which was directly associated with high bacterial burden in lungs. The mortality rate of PBS-dosed mice was dependent on the infectious dose and varied between approximately 50% survival at 72 hours for PBS-dosed BALB/c mice infected with 5 × 107 CFUs and <20% survival for these mice when infected with 5 × 108 CFUs of MRSA. In contrast to PBS-dosed mice, PCN-dosed BALB/c mice were entirely protected from MRSA-related mortality in all dose regimens tested (5 × 107 to 5 × 108 CFUs).
Figure 1.
Administration of VLPs protects against MRSA pneumonia. A and B: Female BALB/c mice are nasally dosed five times every other day over 2 weeks (arrow) with PCN or PBS. One week after the last dose, mice are challenged with MRSA. C and D: Mice are infected with MRSA and then treated with PCN or PBS three times: 1, 7, and 19 hours after infection. A and C: The bacterial burden in lungs is determined 72 hours after infection. B and D: The survival of mice is monitored daily. Each experiment is repeated three times. The mean ± SD from 9 to 15 mice per group (representing three combined independent experiments) is shown. **P < 0.01, ***P < 0.001 versus PCN-dosed mice.
Nasal Delivery of P22 VLP Reduces Bacterial Burden in Lungs of MRSA-Infected Mice
To determine whether induced protection against MRSA pneumonia was specific to PCN, we investigated whether P22 VLP was also protective. The P22 VLP was derived from the P22 bacteriophage that infected Salmonella.36,37 This VLP consisted of 420 identical copies of the P22 coat protein (each 45.5 kDa); it did not contain nucleic acids and, thus, was not infectious. Nasal administration of P22 VLP before MRSA infection prevented mortality and reduced bacterial burdens in the lungs, similar to PCN administration (see Supplemental Figure S2 at http://ajp.amjpathol.org).
PCN Administration Shortly after MRSA Challenge Resolves Pneumonia and Protects from Mortality
Next, we investigated whether PCN, given after exposure to MRSA infection, also protected mice from lethal MRSA pneumonia. Indeed, intranasal therapy with PCN started 1 hour after MRSA challenge, and repeated again at 7 and 19 hours after infection, significantly reduced bacterial burden (>1000-fold reduction) and prevented mortality (Figure 1, C and D).
The Early Events of the First 12 Hours after MRSA Infection Are Crucial for Disease Resolution in PCN-Dosed Mice
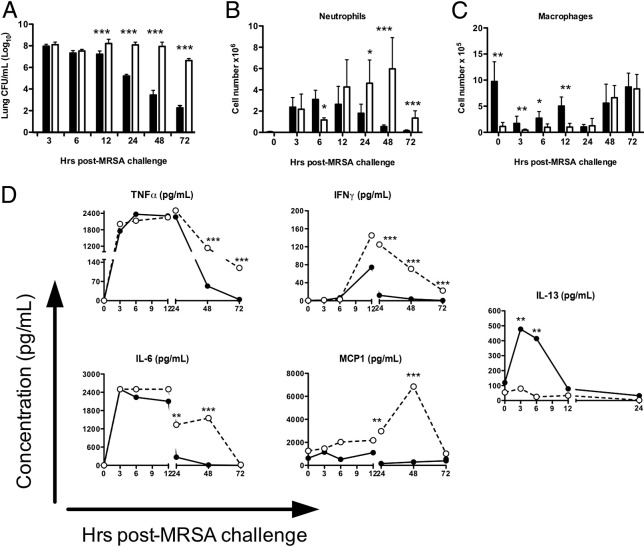
To determine how soon after MRSA infection PCN administration began to exert its protective effects, PCN-dosed mice were sacrificed at designated time points after MRSA (Figure 2). By 12 hours after infection, a 10-fold difference in bacterial burden was present between PCN- and PBS-dosed mice; by 72 hours, the lungs of PCN-dosed mice showed more than a millionfold reduction in bacterial burden when compared with PBS-dosed mice (Figure 2A). This decline of bacterial numbers in PCN-dosed mice was coupled with increased numbers of neutrophils and macrophages in the first 12 hours after MRSA infection (Figure 2, B and C). Neutrophils were absent in the lung lavages of PCN- and PBS-dosed mice at infection (0 hours). Significantly more macrophages were present in the BALF of PCN-dosed mice than in the PBS-dosed mice at infection. At 3 hours after infection, the numbers of macrophages in PCN-dosed mice remained significantly higher when compared with PBS-dosed mice, but were notably reduced when compared with uninfected PCN-dosed mice. This decline was most likely a result of migration of macrophages to the lung parenchyma or more firm adherence to alveolar epithelium, a phenomenon described originally by Sonozaki and Cohen38 as a macrophage disappearance reaction. In PCN-dosed mice, neutrophils and macrophages remained elevated between 3 and 12 hours after infection, but were reduced by 24 hours (Figure 2, B and C). After 24 hours, and in compliance with the return of homeostatic conditions in the lungs of PCN-dosed mice, neutrophil numbers were reduced to trace levels, whereas AMs repopulated the lung. In contrast to PCN-dosed mice, the numbers of neutrophils in PBS-dosed mice increased steadily until after 48 hours of infection. The numbers of macrophages in PBS-dosed mice did not start to increase until 48 hours after infection (Figure 2C). Bacterial burden in the lungs of PBS-dosed mice reflected the delayed and/or inefficient macrophage and neutrophil responses and remained high throughout the experiment (Figure 2A).
Figure 2.
PCN reduces bacterial burden and down-regulates harmful inflammation in the first 24 hours after infection. Mice are dosed five times daily with PCN or PBS a week before MRSA infection, and were sacrificed at the designated time points. A: The LHs are evaluated for bacterial growth. B–D: BALF is collected on sacrifice. Numbers of neutrophils (B) and mononuclear cells (C) are enumerated by differential count. D: Cell-free supernatant is evaluated for production of cytokines TNF-α, interferon (IFN)-γ, IL-13, IL-6, and MCP-1. Experiments are repeated three times. The mean ± SD of five to six mice per group is shown. *P < 0.05, **P < 0.01, and ***P < 0.001 versus PCN-dosed mice. Black bars/dots, PCN; white bars/dots, PBS.
Successful clearance of MRSA required an inflammatory response, but an excess of inflammation is responsible for tissue damage and decreased survival of MRSA-infected mice.39,40 In this regard, the levels of lactate dehydrogenase (LDH) and albumin in BALF of MRSA-infected PCN-dosed mice remained low throughout the disease, whereas in PBS-dosed mice, these levels were increasing until 48 hours after infection (see Supplemental Figure S3, A and B, at http://ajp.amjpathol.org). In addition, starting at 6 hours after MRSA infection, levels of albumin in BALF of PBS-dosed mice were significantly increased when compared with PCN-dosed mice. This was important because, at 6 hours after infection, no differences in lung bacterial burden were seen between PCN- and PBS-dosed mice. Therefore, the lack of increases in albumin in PCN-dosed mice was indicative of reduced host response-mediated tissue damage in PCN-dosed mice. There were no statistical differences in BALF levels of TNF-α, interferon-γ, IL-6, and MCP-1 in the first 6 hours after MRSA infection between PCN- and PBS-dosed mice (Figure 2D). Between 12 and 24 hours after MRSA infection, when the bacterial burdens in PCN-dosed mice were significantly less, the levels of these inflammatory cytokines started to decline. However, in PBS-dosed mice, these cytokines remained high (IL-6 and TNF-α) or continued to increase (MCP-1), which was associated with the lack of infection resolution and consistently high bacterial burden in the lungs of these mice (Figure 2). Interestingly, of the anti-inflammatory cytokines tested, only levels of IL-13 were significantly elevated in the BALF of PCN-dosed mice, up to 8 hours after infection (Figure 2D).
PCN Reduces Lung Tissue Pathology
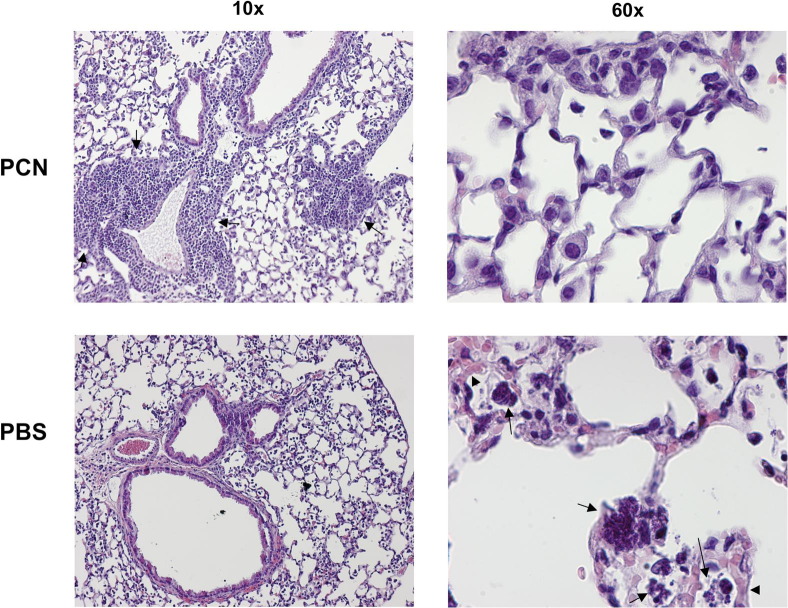
In concurrence with decreased levels of LDH and albumin and more efficient clearance of bacteria by innate cells in PCN-dosed mice, we observed that at 20 hours after MRSA infection, PCN-dosed mice showed greatly reduced signs of tissue damage, as evaluated by staining of lung sections with H&E (Figure 3). In agreement with our previous report,14 iBALT was present in PCN- but not in PBS-dosed mice, in association with airways and blood vessels (Figure 3). Phagocytic cells in the lung parenchyma of PCN-dosed mice were intact; at 20 hours after MRSA infection, these cells consisted predominantly of mononuclear cells that presumably were returning to the lung to re-establish tissue homeostasis (Figure 3). In contrast to PCN-dosed mice, phagocytes in the lungs of PBS-dosed mice were often disrupted and filled with bacteria (Figure 3). The vasculature of these mice was greatly congested and showed signs of interstitial edema (Figure 3).
Figure 3.
PCN protects mice from MRSA-induced lung tissue pathology. C57BL/6 mice are nasally dosed with PCN or PBS five times daily and challenged with 1 × 108 CFUs of MRSA a week later. At 20 hours after MRSA infection, mice are sacrificed and their lungs are fixed in formalin. Embedded sections are then stained with H&E and analyzed by microscopy. Pictures of representative lung sections from PCN- and PBS-dosed mice, taken using ×10 and ×60 magnification objectives, are shown. Arrows, iBALT (×10 PCN picture) and phagocytes filled with bacteria or disrupted phagocytes (×60 PBS picture). Arrowheads, interstitial congestion and edema (×60 PBS picture).
PCN-Mediated Protection Against MRSA Subsides between 3 and 5 Weeks after Treatment
To determine how long PCN-mediated protection persisted, PCN- or PBS-dosed mice were infected with 5 × 107 CFUs of MRSA at 1, 2, 3, or 5 weeks after PCN or PBS treatment. Mice infected with MRSA either 1 or 2 weeks after PCN treatment showed significantly improved bacterial clearance (<4 Log10) when compared with PCN-dosed mice rested for either 3 or 5 weeks before challenge (>5 Log10; see Supplemental Figure S4 at http://ajp.amjpathol.org). No difference in bacterial burden was observed between PCN-dosed mice that were infected with MRSA at either 3 or 5 weeks after treatment, but PCN-dosed mice at all time points showed improved bacterial clearance when compared with their respective PBS-dosed MRSA-infected control mice.
Different Phenotypes of CD11c+ Macrophages and DCs Are Induced in Response to MRSA Infection in the Lungs of PCN-Dosed Mice
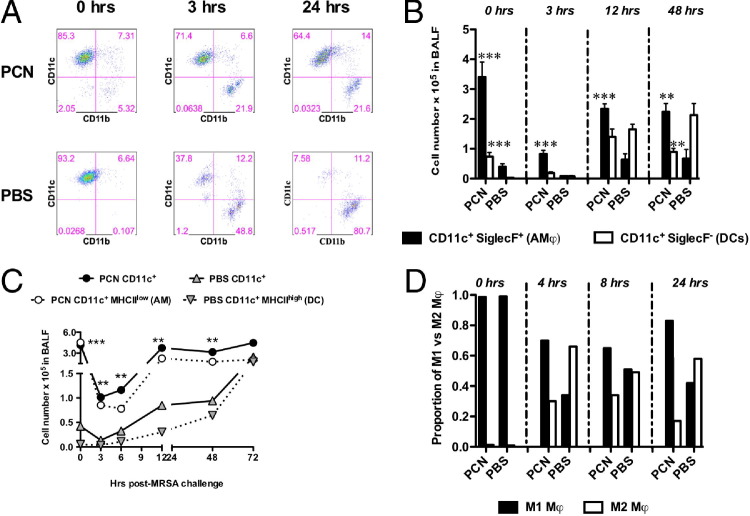
Our results, presented in Figure 2, suggested a role for macrophages in PCN-mediated protection from MRSA. In an effort to define the phenotype and kinetics of macrophages, which in a steady-state lung expressed CD11c,41 FACS analyses were performed on cells isolated from BALF (Figure 4) and LHs (data not shown) of mice infected with 5 × 107 CFUs of MRSA. By gating on the total live cells (confirmed by staining with 7-aminoactinomycin D), we confirmed that at time 0 hours (before MRSA infection), approximately 90% of the cells recovered in the BALF of both PCN- and PBS-dosed mice were CD11c+ (Figure 4A and Table 1). More important, even though >90% of cells in the BALF of PBS-dosed mice were CD11c+, the absolute number of these cells represented just a fraction of CD11c+ cells in the BALF of PCN-dosed mice (Table 1). Early after MRSA infection, the percentage of cells expressing CD11c significantly decreased in the BALF of PBS-dosed mice to approximately 40% at 3 hours after infection and to <10% at 24 hours after infection (Figure 4A). In contrast to PBS-dosed mice, in the BALF of PCN-dosed mice, CD11c+ cells remained at approximately 70% throughout the infection. CD11b+ cells that were virtually absent in PCN- and PBS-dosed mice before infection (Figure 4A: at 0 hours, <6% of CD11b+ cells) increased significantly after infection in PBS-dosed mice (approximately 50% at 3 hours and approximately 80% at 24 hours), but in the BALF of PCN-dosed mice, these cells peaked at approximately 20% at 3 hours after infection and these percentages remained unchanged until 24 hours after infection (Figure 4A). Despite their lower percentage, the numbers of CD11b+ cells were similar between PCN- and PBS-dosed mice (data not shown).
Figure 4.
CD11c+ macrophages and DCs are induced by PCN administration. Mice are dosed five times daily with PCN or PBS a week before MRSA infection. Mice are sacrificed at designated time points after infection, and cells isolated from BALF are stained and analyzed by FACS. The gating strategy is as follows. A: The live cells gate is set on FCS (forward scatter; representing cell volume) versus SSC (side scatter; representing the inner complexity of the particle like granularity of a cell). Staining for CD11c versus CD11b is determined by gating total live cells. Representative FACS plots are shown. B: The expression of SiglecF is evaluated on live CD11c+ F4/80+ or CD11c+ F4/80− cells. C: Live cells are gated for F4/80+ cells and then for CD11c+ versus MHCII+ cells. CD11c+MHCIIlow AMs are shown for PCN-dosed mice, and CD11c+MHCIIhigh DCs are shown for PBS-dosed mice. D: In an independent experiment, the presence of M1 (CD11c+CD206−) and M2 (CD11c+CD206+) cells is determined after gating on live F4/80+ cells. Each panel represents the results from a single experiment (mean ± SD from five mice per group). Each experiment is repeated two to three times. **P < 0.01, ***P < 0.001 versus PBS-dosed mice.
Table 1.
Kinetics of CD11c+ Cells in the BALF of PCN- and PBS-Dosed Mice
| Time after MRSA (hours)⁎ | Treatment† | % of Total F4/80+CD11c+ cells‡ | No. of total F4/80+CD11c+ cells§ | No. of (SiglecF+CD11c+ cells) AMs¶ | % of (SiglecF+CD11c+) AMs | % of (SiglecF−CD11c+) DCs¶ | No. of (SiglecF−CD11c+) DCs |
|---|---|---|---|---|---|---|---|
| 0 | PCN | 85.6 + 3.4 | 4.2 × 105∥ | 3.4 × 105∥ | 80.9 + 4.7 | 19.1 + 4.7⁎⁎ | 0.7 × 105⁎⁎ |
| 0 | PBS | 91.0 + 2.4 | 0.4 × 105 | 0.4 × 105 | 94.2 + 1.6 | 5.8 + 1.6 | 0.03 × 105 |
| 3 | PCN | 42.66 + 15.2†† | 1 × 105⁎⁎ | 0.8 × 105⁎⁎ | 81 + 1.8∥ | 19 + 1.8∥ | 0.2 × 105∥ |
| 3 | PBS | 21.5 + 4.2 | 0.14 × 105 | 0. × 105 | 50.4 + 6.9 | 49.6 + 13.9 | 0.08 × 105 |
| 6 | PCN | 15.6 + 5.9 | 1.2 × 105∥ | 0.9 × 105∥ | 78.1 + 7.0 | 11.9 + 3.7†† | 0.2 × 105∥ |
| 6 | PBS | 11.34 + 3.6 | 0.33 × 105 | 0.3 × 105 | 88.1 + 3.7†† | 21.9 + 7.0 | 0.04 × 105 |
| 12 | PCN | 18.3 + 2.2⁎⁎ | 3.8 × 105∥ | 2.34 × 105∥ | 63.86 + 10.7∥ | 36.1 + 13†† | 1.4 × 105 |
| 12 | PBS | 3.74 + 1.22 | 0.8 × 105 | 0.6 × 105 | 35.32 + 7.5 | 64.68 + 14.6 | 1.6 × 105 |
| 24 | PCN | 30 + 11⁎⁎ | 0.9 × 105†† | 0.8 × 105†† | 72.58 + 4.9∥ | 27.4 + 4.9∥ | 0.3 × 105 |
| 24 | PBS | 4.32 + 1.1 | 0.24 × 105 | 0.3 × 105 | 49.32 + 6.7 | 50.68 + 11.7 | 0.3 × 105 |
| 48 | PCN | 41.6 + 9.4⁎⁎ | 3.2 × 105∥ | 2.2 × 105†† | 69.1 + 5.1⁎⁎ | 30.9 + 5.1⁎⁎ | 1.0 × 105†† |
| 48 | PBS | 6.8 + 2.9 | 0.9 × 105 | 0.7 × 105 | 29.09 + 6.9 | 70.91 + 12.0 | 2.1 × 105 |
| 72 | PCN | 47.9 + 8.9⁎⁎ | 4.5 × 105†† | 2.5 × 105∥ | 71.72 + 7.2⁎⁎ | 28.28 + 7.3⁎⁎ | 1.0 × 105†† |
| 72 | PBS | 15.2 + 3.9 | 2.4 × 105 | 1.0 × 105 | 34.4 + 8.3 | 65.6 + 8.3 | 1.5 × 105 |
A week after the last PCN or PBS treatment, mice were infected with MRSA.
BALB/c mice were intranasally dosed with PCN or PBS and rested for a week.
BALF cells were stained and analyzed by FACS. The gating strategy was as described in the legend to Figure 4.
The absolute numbers of cells were calculated by multiplying the percentage of live cells by the cell number counted under hemocytometer/100%.
SiglecF+ and SiglecF− were determined by gating on CD11c+ cells.
P < 0.01 versus PBS-dosed mice.
P < 0.001 versus PBS-dosed mice.
P < 0.05 versus PBS-dosed mice.
In the lungs, cells that expressed CD11c could be AMs and/or DCs.42 We used multiple staining strategies to distinguish between AMs and DCs, including either differential expression of SiglecF43–45 and F4/8042 (Figure 4B) or various expression levels of MHCII46 (Figure 4C). We confirmed that CD11c+MHCIIlow cells were also SiglecF+ (and, thus, were AMs) and CD11c+MHCIIhigh cells were SiglecF− (and, thus, were DCs; data not shown). When compared with PBS-dosed mice, the numbers of total CD11c+ cells, and CD11c+ F4/80+ SiglecF+ AM and SiglecF− DCs, were significantly higher in PCN-dosed mice before and shortly after MRSA infection (Figure 4, A and B, and Table 1). Even though numbers of AMs (CD11c+SiglecF+ cells in Figure 4B) remained significantly lower in PBS-dosed mice until 48 and 72 hours after infection, the numbers of DCs (CD11c+SiglecF− cells in Figure 4B and CD11c+MHCIIhigh cells in Figure 4C) in these mice increased rapidly at 12 hours after infection and significantly exceeded the numbers of DCs in PCN-dosed mice at later points after infection (Figure 4B and Table 1). M1 macrophages are considered to be the short-term responders to infections, whereas the M2 phenotype is usually associated with wound healing and less damaging chronic inflammatory responses.47,48 We observed that, in PCN-dosed mice at 4 hours after MRSA infection, there was threefold more M1 (CD11c+ CD206−) than M2 (CD11c+ CD206+) macrophages (Figure 4D). At 8 hours after infection in PCN-dosed mice, the dominance of M1 phenotype was smaller, yet still significant (twofold more of M1 macrophages); at 24 hours after infection, when the bacterial burden in the lungs of PCN-dosed mice was further diminishing (Figure 2A), the M1 prevalence over the M2 phenotype was fivefold. Contrary to PCN-dosed mice, PBS-dosed mice had almost threefold as many M2 macrophages as M1 at 4 hours after infection. At 8 hours after infection in these mice, the numbers of M1 and M2 macrophages in BALF were similar, but at 24 hours after infection, the numbers of M2 cells again exceeded the numbers of M1 cells (Figure 4D).
CD11c+ Cells and Neutrophils from PCN-Dosed Mice Are More Efficient in Killing MRSA
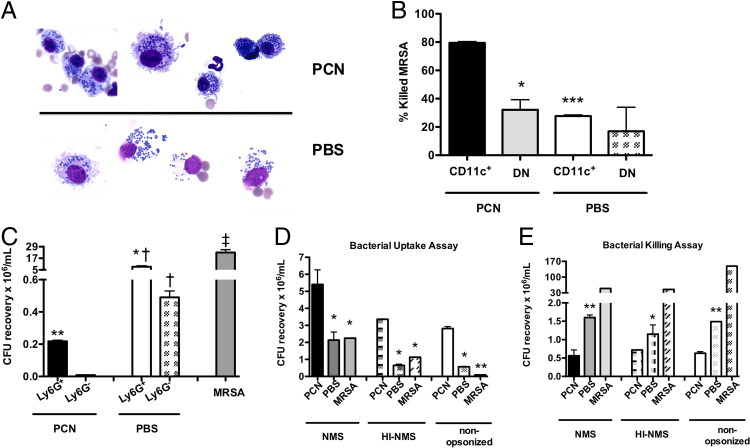
Our results thus far suggested a role for macrophages and neutrophils in PCN-induced protection. Thus, we determined whether these cells in PCN-dosed mice were better in killing MRSA than cells from PBS-dosed mice. Morphological assessment of these cells at 3 hours after MRSA infection indicated that mononuclear cells in the BALF of PCN-dosed mice were, for the most part, intact and appeared to contain bacteria (Figure 5A). At the same time, plasma membranes of mononuclear cells in the BALF of PBS-dosed mice were largely disrupted and, consequently, cell-free portions of the BALF from these mice contained frequent bacteria (Figure 5A). At 3 hours after infection, in contrast to mononuclear cells in the BALF of PCN-dosed mice, the morphological appearance of mononuclear cells in the BALF of PBS-dosed mice suggested that these cells underwent necrosis.
Figure 5.
CD11c+ cells and neutrophils from PCN-dosed mice are more efficient in killing MRSA than the respective cells from PBS-dosed mice. A: Mice are dosed five times daily with PCN or PBS a week before MRSA infection. A micrograph of slide sections containing BALF cells isolated from PCN- or PBS-dosed mice 3 hours after MRSA infection. B: Mice are dosed with PCN or PBS and are sacrificed 1 week after the last dose. LHs are selected for CD11c+ cells and CD11c−Ly6G− (DN) cells by MACS columns. After selection, cells are incubated in a 1:1 ratio with MRSA for 1.5 hours, and CFUs are determined by plating on TSA plates, followed by an overnight incubation. The mean and SD of duplicate samples from three to four mice per group is shown. C: One week after the PCN or PBS dose, mice are infected with erythromycin-sensitive MRSA mutant, and 2.5 hours later, mice are sacrificed. LHs are selected for Ly6G+ and Ly6G− cells that are incubated in a 1:1 ratio with WT MRSA for 3 hours. The CFUs of WT MRSA are determined after overnight incubation on TSA/erythromycin plates. D and E: Total BM cells isolated a week after the last PCN or PBS administration are plated in a 10:1 (D) or 1:1 (E) bacteria:BM cell ratio. The MRSA used for experiments is opsonized with NMS or heat-inactivated NMS or not opsonized. D: For the bacterial uptake assay, after a 30-minute incubation, gentamicin is added to each well to kill extracellular bacteria. The CFUs of bacteria taken up by BM cells are measured by plating. E: Bacterial killing by BM cells is assessed after a 3-hour incubation. *P < 0.05, **P < 0.01, and ***P < 0.001 versus PCN-dosed mice; †P < 0.05 versus the respective Ly6G+ or Ly6G− cells from PCN-dosed mice; ‡P < 0.05 versus MRSA-only control. Experiments are performed at least twice, with a similar result.
Results described in Figure 4D showed that, in contrast to macrophages in PBS-dosed mice, these cells in PCN-dosed mice were predominantly M1. By applying cell isolation techniques, we obtained >97% pure population of CD11c+Ly6G− cells that were F4/80+, as determined by FACS, and >90% pure population of F4/80+ CD11c−Ly6G− double-negative (DN) cells (presumably inflammatory monocytes) from LHs of PCN- or PBS-dosed but not infected mice (Figure 5B). Next, we investigated which of these cell subsets were better in killing MRSA. As expected, PCN-CD11c+ cells were more efficient than DN cells in killing bacteria in vitro (killing efficacy of CD11c+ cells, approximately 80%; DN cells, approximately 35%; Figure 5B). Interestingly, no difference in bacterial killing was observed between CD11c+ and DN cells isolated from not infected PBS-dosed mice, and either of these cells killed only approximately 30% of bacteria. In the following experiment, we isolated Ly6G+ and Ly6G− cells from the lungs of PCN- or PBS-dosed mice that, 2.5 hours earlier, were infected with erythromycin-sensitive MRSA. Similarly, when lung Ly6G+ neutrophils and Ly6G− cells isolated from erythromycin-MRSA–infected PCN- or PBS-dosed mice were examined for their ability to kill WT MRSA, both of these cell subsets from PCN-dosed mice were more effective in killing bacteria than the respective cell subsets from PBS-dosed mice (Figure 5C). Moreover, Ly6G− cells were better in killing MRSA than Ly6G+ cells in both experimental groups.
Within 1 hour after nasal installation, the PCN was found in respiratory lymph nodes (tracheobronchial and cervical) and, in 24 hours, they were nearly completely gone from the body.17 To determine whether PCN-mediated protection from MRSA infection was limited to the lung environment, we isolated BM cells from mice that had been dosed with PCN or PBS a week earlier. The BM cells were then incubated with MRSA to determine the efficiency of uptake and killing of bacteria in vitro. After 30 minutes of incubation, BM cells from PCN-dosed mice took up twofold more bacteria than BM cells from PBS-dosed mice (Figure 5D). The rate of internalization was highest when bacteria were opsonized with NMS, although even unopsonized bacteria and bacteria opsonized with heat-inactivated NMS were also internalized and killed by BM cells from PCN-dosed mice (Figure 5, D and E). When compared with the background MRSA levels (bacteria incubated alone and treated with gentamicin), similar numbers of bacteria were retrieved from wells incubated with BM cells from PBS-dosed mice, indicating that, during the 30 minutes of incubation time, BM cells from PBS-dosed mice failed to efficiently take up MRSA (Figure 5D). Interestingly, after 3 hours of incubation, BM cells from PBS-dosed mice killed MRSA, although not as well as BM cells derived from PCN-dosed mice (Figure 5E). During the 3 hours of in vitro incubation, BM cells from PCN-dosed mice killed, on average, two to three times more bacteria than these cells from PBS-dosed mice, and killing of MRSA by BM cells from PCN-dosed mice was enhanced, although not fully dependent, on opsonization by complement (Figure 5E).
PCN-Mediated Protection Against MRSA Is Independent of Adaptive Immunity
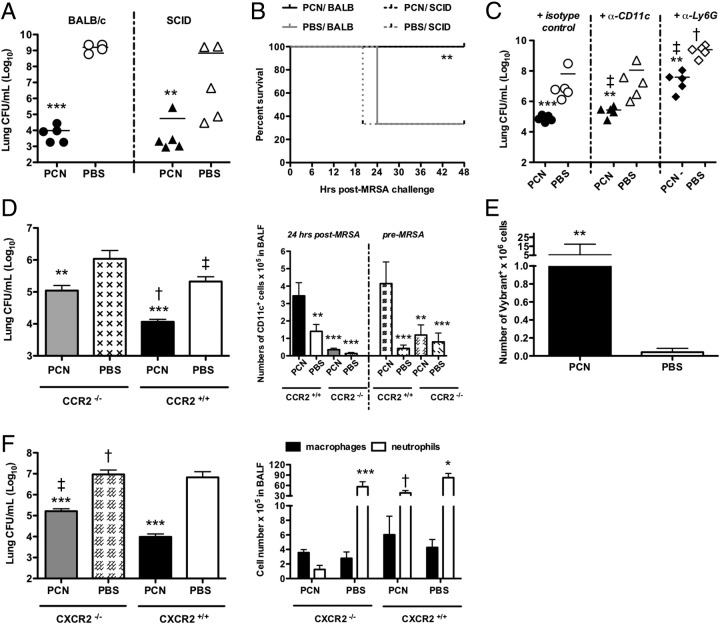
We previously observed that the accelerated protection against viruses that was induced by PCN administration was dependent, in part, on the formation of iBALT, because the PCN administration to mice deficient in adaptive immunity, such as SCID, B-cell–deficient muMT, or WT mice depleted of CD4 T cells, did not prevent influenza infection.14 To discern whether PCN protection against MRSA also required functional adaptive immunity, PCN-dosed SCID mice were challenged with 5 × 107 CFUs of bacteria. SCID and WT mice dosed with PCN before infection showed a similar significant reduction in the lung bacterial burden at 24 and 48 hours after infection, when compared with their PBS-dosed littermates (Figure 6A and data not shown, respectively). In addition, 100% of PCN-dosed SCID and WT mice survived MRSA infection (Figure 6B), whereas only 40% of PBS-dosed SCID and WT mice survived to 24 hours after infection.
Figure 6.
PCN-mediated protection against MRSA depends on innate immune cells. Mice are dosed five times daily with PCN or PBS a week before MRSA infection. A: The bacterial burden is evaluated in murine lungs 24 hours after infection. B: Survival is evaluated during a 2-day period. C: Mice are treated with anti-CD11c mAb or anti-Ly6G mAb on day −3, −1, and 0, in relation to MRSA infection, and lung bacterial burden is evaluated 24 hours after infection. The results represent individual mice from one of two independent experiments. D: Mice are sacrificed 24 hours after infection. Bacterial burden in the lungs was determined (left panel), and numbers of CD11c+ cells in BALF are determined by FACS after staining with fluorochrome-labeled anti-CD11c mAb before and after infection (right panel). The mean ± SD of five mice per group is shown and represents the results of one of two independent experiments. E: At 1 hour after MRSA infection, mice are adoptively transferred with Vybrant-DiI–labeled naïve neutrophils, and 4 hours later, mice were sacrificed. The number of Vybrant+ cells in the BALF is determined by FACS. The mean and SD of eight mice per group (combined two independent experiments) is shown. F: Mice are sacrificed 24 hours after infection. The bacterial burden in the lungs is determined (left panel), and numbers of polymorphonuclear and mononuclear cells in the BALF are determined by differential counts (right panel). The mean ± SD of five mice per group is shown and represents the results of one of two independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 versus PBS-dosed mice; †P < 0.001; ‡P < 0.05 versus PCN-dosed WT mice.
Both Ly6G+ Neutrophils and CD11c+ Cells Are Responsible for PCN-Mediated Protection from MRSA
Although neutrophils are regarded to be the early and predominant cells responsible for fighting bacterial infections, including MRSA,49–51 increasing importance is assigned to CD11c+ cells46 in such killing. To discriminate which groups of innate cells were responsible for PCN-mediated protection against MRSA, we depleted either Ly6G+ (neutrophils) or CD11c+ cells (macrophages and DCs) from PCN-dosed mice on day −3, −1, and 0, before MRSA infection. In mice dosed with PCN, depletion of either neutrophils or CD11c+ cells impaired resistance to infection, because the bacterial burdens in the lungs of depleted mice were significantly greater than the burdens in the lungs of PCN-dosed mice treated with isotype control Abs (Figure 6C, P < 0.05). Moreover, in PCN-dosed mice, neutrophil depletion resulted in much higher bacterial numbers than did CD11c+ cell depletion (P < 0.05), indicating the importance of neutrophil function in PCN-induced protection. Depletion of CD11c+ cells from PBS-dosed mice did not affect the lung CFU counts when compared with nondepleted PBS-dosed mice. However, the lungs of PBS-dosed mice depleted of neutrophils showed increases in CFUs when compared with PBS-dosed mice not depleted of neutrophils (P < 0.001), indicating the importance of neutrophils in controlling MRSA, even in the absence of PCN. More important, regardless of whether the groups of mice were undepleted, depleted of neutrophils, or depleted of CD11c+ cells, the PCN-dosed mice were always more resistant to MRSA pneumonia than the PBS-dosed mice.
Uncompromised Infiltration of Monocytes Is Required for PCN-Mediated Protection Against MRSA
Depletion of CD11c+ cells in PCN-dosed mice resulted in a partial inhibition of PCN-induced protection against MRSA (Figure 6C). Whether the protective CD11c+ cells were recruited monocytes resulting from PCN treatment, PCN-altered resident macrophages, or PCN-altered recruited monocytes remained to be determined. CCR2 is a receptor molecule for MCP-1.52 Consequently, monocytes in CCR2 KO mice are impaired in their ability to infiltrate inflammatory sites and, thus, recruitment of monocytes in response to PCN treatment before MRSA infection should also be compromised. Indeed, numbers of CD11c+ cells were significantly reduced in PCN-dosed CCR2 KO mice both before and 24 hours after MRSA infection when compared with PCN-dosed WT mice (Figure 6D). Also, PCN-dosed CCR2 KO mice were less competent than the PCN-dosed WT mice in clearing MRSA infection (Figure 6D). In this regard, at 24 hours after infection, PCN-dosed CCR2 KO mice showed >10-fold more bacteria in their lungs than PCN-dosed WT mice, indicating that CCR2 signaling played a role in PCN-induced resistance to MRSA. Interestingly, when compared with PBS-dosed WT mice, PBS-dosed CCR2 KO mice showed elevated bacterial burden, but reduced CD11c+ cell numbers, at 24 hours after infection, implicating the role of recruited monocytes in clearance of MRSA infection in these mice as well. All CCR2 KO mice, independent of treatment, survived MRSA infection up to 96 hours, when the study was terminated (see Supplemental Figure S5A at http://ajp.amjpathol.org). This suggested that absence of the CCR2 receptor had no effect on survival of PCN-dosed mice, and even improved the survival of PBS-dosed mice.
PCN Enhances Neutrophil Trafficking into the Lungs in Response to MRSA Infection
To determine whether administration of PCN enhanced neutrophil recruitment into the lungs, mice predosed with either PCN or PBS were infected with MRSA and, 1 hour later, adoptively transferred with Vybrant-DiI–labeled BM neutrophils isolated from naïve littermates. The number of Vybrant+ cells in LHs of PCN-dosed mice 5 hours after MRSA infection was significantly higher when compared with the lungs of PBS-dosed mice (Figure 6E, P < 0.01), indicating that PCN changed the lung environment, resulting in enhanced neutrophil recruitment in response to infection. Consistent with this observation were the findings of both a significant decrease of neutrophil recruitment in PCN-dosed CXCR2 KO mice (Figure 6F, P < 0.001) and lower effectiveness in clearing MRSA infection (P < 0.05) compared with PCN-dosed WT mice. Moreover, the inability of PCN-dosed CXCR2 KO mice to recruit neutrophils to MRSA-infected lungs decreased their survival compared with PCN-dosed WT mice. In this regard, the PCN-dosed KO mice started dying by day 4 after infection; and by 5 days after infection, >80% of PCN-dosed CXCR2 KO mice had succumbed to MRSA infection, whereas all of the PCN-treated WT mice survived through 5 days (see Supplemental Figure S5B at http://ajp.amjpathol.org). More important, when compared with PCN-dosed CXCR2 KO mice, PBS-dosed CXCR2 KO mice were less effective in clearing MRSA infection, despite the CXCR2-independent recruitment of neutrophils to the lungs of PBS-dosed CXCR2 KO mice, but PCN- and PBS-dosed KO mice showed a similar lethality rate. This suggested that CXCR2 signaling was important for PCN-induced protection against lethality from MRSA infection, but not important for the PCN-independent resistance to MRSA pneumonia.
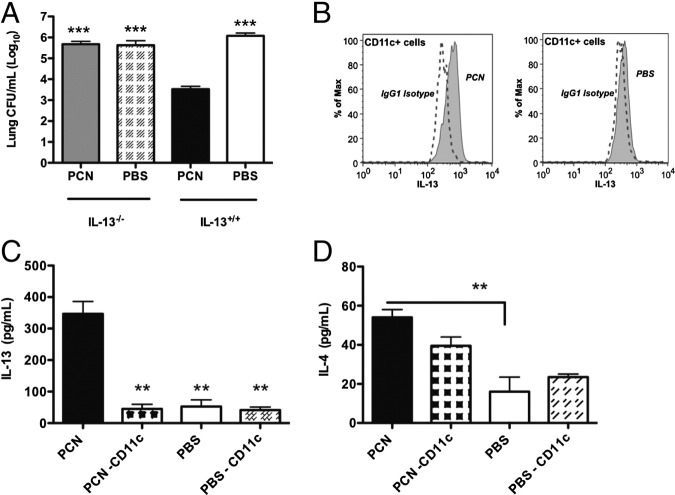
IL-13 Is Required for PCN-Mediated Protection Against MRSA
We found that levels of IL-13 were significantly elevated in the BALF of PCN-dosed mice up to 9 hours after MRSA infection (Figure 2D). To determine whether anti-inflammatory cytokines played an important role in PCN-mediated protection against MRSA, we analyzed bacterial burden in the lungs of PBS- or PCN-dosed IL-13 and IL-4 KO mice 24 hours after infection. Interestingly, no difference in bacterial clearance from the lungs was observed between PCN- and PBS-dosed IL-13 KO and PBS-dosed WT mice, but when those three groups of mice were compared with PCN-dosed WT mice, the three groups of mice each had at least 100-fold more bacteria in their lungs (Figure 7A). Consistent with the lack of bacterial clearance, PCN-dosed IL-13 KO mice reached 50% mortality within the first 24 hours after MRSA infection (see Supplemental Figure S5C at http://ajp.amjpathol.org). PCN-dosed IL-4 KO mice cleared bacteria more efficiently than PCN-dosed IL-13 KO mice, but not nearly as well as PCN-dosed WT mice (data not shown). We also found that CD11c+ cells in PCN-dosed mice produced IL-13 at 2.5 hours after MRSA infection (Figure 7B). This observation was further confirmed by the virtual absence of IL-13 in BALFs of PCN-dosed WT mice that, before infection, were depleted of CD11c+ cells, much like PBS-dosed mice independent of the presence of CD11c+ cells (Figure 7C). When tested, IL-4 levels were significantly elevated in PCN-dosed undepleted mice, when compared with PBS-dosed mice either depleted or not depleted of CD11c+ cells (Figure 7D).
Figure 7.
CD11c+ cell-induced early IL-13 secretion is important for disease resolution by PCN-dosed mice. A: Mice are dosed five times daily with PCN or PBS a week before MRSA infection and sacrificed 24 hours after infection. The bacterial burden in lungs is determined. B: PCN- and PBS-dosed mice are infected with 1 × 108 CFUs of MRSA. FACS staining is performed on cells isolated from the BALF of these mice 2.5 hours after MRSA infection. Representative plots of IL-13+ CD11c+ cells are shown compared with these CD11c+ cells stained with isotype control Abs. C and D: Mice are depleted of CD11c+ cells on day −3, −1, and 0, in relation to MRSA infection, as described in the legend for Figure 6C. Mice are sacrificed 4 hours after infection, and the concentration of IL-13 (C) and IL-4 (D) is determined in their cell-free BALF. Each experiment is performed twice. The mean ± SD of three to four mice per group from a single experiment is shown. **P < 0.01, ***P < 0.001 versus PCN-dosed mice.
Discussion
We show herein that intranasal delivery of VLPs, such as PCN, induced near complete resistance to lethal MRSA pneumonia. Mice dosed with PCN either before or shortly after MRSA infection were protected from mortality and showed up to a millionfold reduction in bacterial burden, when compared with PBS-dosed mice. Remarkably, when mice were treated with a VLP unrelated to PCN, P22, a similar degree of protection and recovery was observed. This suggests that the protective effect induced by either of the VLPs can be attributed to shared physical characteristics, such as repeating protein subunits, and small size, similar to a virus, rather than the origin of the VLP. VLPs, because of their ability to stimulate pathogen-associated molecular pattern recognition receptors on DCs, are effective inducers of innate, and adaptive, immunity.18–20,53 Thus, VLPs have been used predominantly for their adjuvant properties19,21 and their ability to deliver either genetically conjugated or chemically fused epitopes for the induction of specific immune responses.25,54 However, their potential role as self-proprietors of immune modulation has not been extensively investigated, and the present data suggest that they could be used in this regard.
Because of the early resolution of MRSA infection by more efficient neutrophils and macrophages in PCN-dosed mice, decreased signs of cellular damage (measured by extracellular release of LDH) or tissue leaking (measured by serum albumin level) were found in the BALF of PCN-dosed mice. In contrast to PCN-dosed mice, levels of albumin and LDH in the BALF of PBS-dosed mice increased as early as 6 and 12 hours after MRSA infection, respectively. These increases coincided with inefficient bacterial clearance in the lungs of PBS-dosed mice by infiltrating neutrophils and mononuclear cells, which, as early as 2 to 3 hours after infection, showed signs of necrosis and extracellular release of bacteria. At 6 hours after infection, lung bacterial burden was similar in PCN- and PBS-dosed mice; thus, in PCN-dosed mice, the host response to MRSA caused less lung damage, which is similar to what we found in PCN-dosed mice infected with influenza.14 The more efficient bacterial clearance and abridged lung damage coincided with the greatly reduced lung tissue pathological characteristics, apparent by the lack of congestion and interstitial edema at 20 hours after MRSA infection in the lungs of PCN-dosed, but not PBS-dosed, mice.
Up to 12 hours after MRSA infection, most cells in the BALF of PBS-dosed mice could be classified as macrophages, based on their expression of SiglecF and intermediate levels of MHCII. However, in PCN-dosed mice at these time points, SiglecF−/MHCIIhigh DCs accounted for slightly >10% of CD11c+ cells. The role of CD11c+ cells in pulmonary MRSA infections just recently gained attention.46 According to the study by Martin et al,46 clodronate-induced depletion of CD11c+ AMs did not affect bacterial burden in the lungs, but was associated with increased mortality; however, when CD11c+ cells were depleted in CD11c–enhanced green fluorescence protein–diphtheria toxin mice, the bacterial burden in the lungs increased, although no changes in mortality were observed. In this regard, it is plausible that PCN-induced CD11c+ DCs, although not numerous in the BALF, are important for protection from MRSA pneumonia.
The altered phagocytosis of apoptotic cells in the lung has been linked to the overwhelming inflammation that can lead to extensive tissue damage and even autoimmunity.55 Not surprisingly, one of the survival mechanisms exploited by intracellular bacteria includes reprogramming of the phagocytic cells, which allows them to escape intracellular killing. In this regard, Mtb was shown to avoid killing by macrophages via induction of cell necrosis rather than apoptosis.56 Also, MRSA-induced programmed necrosis rapidly killed neutrophils, but not bacteria.57 In our experiments, as early as 2 to 3 hours after MRSA infection, mononuclear cells isolated from PCN-dosed mice were intact and associated with bacteria. At the same time after infection, the plasma membrane of macrophages from PBS-dosed mice was already destroyed, and the remaining cell debris showed signs of organelle swelling, all of which is usually associated with necrosis. Furthermore, in contrast to PCN-dosed mice, extracellular portions of the BALF of PBS-dosed mice contained numerous bacteria, suggesting inefficient killing by cells in PBS-dosed mice. These results implicate that lung macrophages in PCN-dosed mice were resistant to MRSA-induced necrosis and, thus, were better able to kill MRSA.
Enhancement of the primary immune response is responsible for PCN-induced protection against body weight loss and reduction of viral titers in the lungs of mice infected with either murine influenza or severe acute respiratory syndrome viruses.14 Herein, we report that PCN-mediated protection against MRSA infection was entirely dependent on functional innate immunity. In this regard, PCN-dosed SCID mice showed similar recovery rates and nearly identical reduction of bacterial burden as PCN-dosed WT mice, and depletion of CD4 or CD8 T cells even further enhanced PCN-mediated protection (data not shown). Moreover, when depleted of either neutrophils or CD11c+ cells, PCN-dosed mice were less resistant to MRSA pneumonia. However, depletion of neutrophils after PCN administration resulted in the loss of more than two logs of protection and increased mortality of mice, whereas CD11c depletion resulted in the loss of less than a log of protection and had no effect on mouse survival. There is an obvious inconsistency between how efficient CD11c cells from PCN-dosed mice were in killing MRSA in vitro and only a moderate effect that the depletion of these cells had on bacterial burden in PCN-dosed mice in vivo. This discrepancy could be attributed to the overall fewer macrophages in the lungs when compared with neutrophils in PCN-dosed mice. Therefore, although CD11c+ cells appear more efficient in killing MRSA in vitro, neutrophils may have a greater in vivo effect because of their prevalence in the MRSA-infected lung.
CCR2 is a receptor molecule for MCP-1; therefore, the trafficking of monocytes to inflammatory sites is compromised in CCR2 KO mice.52 Even before MRSA infection, the numbers of CD11c+ cells in CCR2 KO mice dosed with PCN were significantly lower than the numbers of these cells in PCN-dosed WT mice, and were similar to the numbers of CD11c+ cells in PBS-dosed WT and CCR2 KO mice. At 24 hours after MRSA infection, the bacterial burden of CCR2-deficient PCN-dosed mice was significantly elevated when compared with PCN-dosed WT mice, which is consistent with the well-established importance of recruited monocytes in fighting pulmonary microbial infections.7,52,58 Therefore, we concluded that many CD11c+ cells in the lungs of PCN-dosed mice at infection are newly recruited resident cells. Despite sharing a similar FACS profile with CD11c+ cells in PBS-dosed mice, these cells were recruited to the lung in response to PCN administration; thus, they represent a diverse cell population, perhaps one that is more efficient in fighting bacterial infection.
Neutrophils are considered the primary line of defense against MRSA infections,49,59 although they are also actively targeted by MRSA to improve its survival within the host.57,60,61 In our experiments, PCN-dosed WT mice showed enhanced neutrophil recruitment, but also a much faster decline in neutrophil numbers in the MRSA-inflamed lung, which presumably prevented extensive tissue damage. However, PCN-dosed CXCR2 KO mice infected with MRSA showed increased bacterial burden at 24 hours after infection, increased mortality, and a deficiency of neutrophil recruitment when compared with PCN-dosed WT mice. In contrast, CXCR2 KO mice that received PBS had nearly identical numbers of neutrophils as PBS-dosed WT mice. This suggests that, in contrast to PBS-dosed mice, MRSA-induced recruitment of neutrophils in PCN-dosed mice was CXCR2 dependent. Because no difference in bacterial burden was observed between PBS-dosed CXCR2 KO and WT mice at 24 hours after infection, we concluded, in agreement with other studies,57,61 that neutrophils alone are not sufficient in clearing MRSA infection.
Among the CD11c+F4/80+ cells isolated from PCN-dosed MRSA-infected mice, the high ratio of M1 (CD11c+CD206−) to M2 (CD11c+CD206+) cells persisted throughout the disease. In contrast to these mice, most macrophages in MRSA-infected, PBS-dosed mice showed characteristics of M2 cells. It is well described that M1 macrophages are more efficient responders to an acute infection, whereas the long-term stage M2 cells, even though they cause less tissue damage, are also less efficient in killing microbes.62 Furthermore, M2 macrophages can be subdivided into three subclasses (a to c), in which M2a cells develop in the presence of IL-13/IL-4 and lipopolysaccharide enhances the M2b phenotype.63 M2a AMs supported M1 cells and promoted lung defense against Pneumocystis murina.64 Interestingly, induction of IL-13 and IL-4 was observed early after MRSA infection of PCN-dosed, but not PBS-dosed, mice, suggesting that M2 cells in PCN- and PBS-dosed mice can differ in their ability to support M1 cells in response to MRSA infection. Despite the presence of IL-13 in the BALF of MRSA-infected PCN-dosed, but not PBS-dosed, mice, the levels of proinflammatory cytokines remained similar between these mice until 12 hours after infection. After 12 hours of infection and likely as a consequence of disease resolution in PCN-dosed mice, a sudden reduction of proinflammatory cytokines occurred in these mice. The importance of IL-13 in PCN-mediated protection from lethal MRSA pneumonia was indicated by the lack of recovery and disease resolution in PCN-dosed IL-13 KO mice, the increased mortality of these mice, and the realization of only partial protection in PCN-dosed IL-4 KO mice (data not shown). IL-13 causes neutrophil activation and recruitment via induction of IL-8–like chemoattractant on airway epithelium.65,66 The role of IL-13 in pulmonary clearance of methicillin-sensitive S. aureus was reported by Olszewski et al.67 In their hands, IL-13 KO mice showed delayed clearance of bacteria, but when mice were reconstituted with exogenous IL-13, normal clearance was restored. We have determined that CD11c+ cells in PCN-dosed, but not in PBS-dosed, mice are accountable for the production of IL-13, whereas the production of IL-4 was only partially dependent on the presence of CD11c+ cells. Which subsets of PCN-induced CD11c+ cells are involved in IL-13 and/or IL-4 production, and the exact mechanism of IL-13–dependent protection in our model, requires further investigation.
In summary, this work describes a novel approach of priming of the lungs via administration of noninfectious VLPs, resulting in enhanced efficacy of the innate immune response, but omits the risk of tissue-damaging inflammation that occurs with pathogen-induced alteration of lung environment. Our results indicate that improved uptake and killing of MRSA by neutrophils and CD11c+ cells, and possibly cell-independent mechanisms, are involved. To our knowledge, this is the first report describing the importance of CD11c+ cells in IL-13–dependent protection from MRSA pneumonia. Our results provide important insights into the mechanisms of host anti-MRSA response in the lung and suggest that VLPs could be a viable means of enhancing these mechanisms.
Acknowledgments
We thank members of the Harmsen and Voyich laboratories (Montana State University) for their assistance in performing the described assays and Amy Servid from the Douglas laboratory (Montana State University) for preparation of P22 VLPs.
Footnotes
Supported by grants from the NIH [NIH/National Institute of Allergy and Infectious Diseases1R56AI089458, NIH/IDeA Networks of Biomedical Research ExcellenceP20 RR16455 (GM123456), NIH/Centers of Biomedical Research ExcellenceP20 RR020185 (GM123456), and NIH/Rocky Mountain Regional Center for ExcellenceU54 AI065357], an equipment grant from M.J. Murdock Charitable Trust, and the Montana State University Agricultural Experimental Station.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at http://dx.doi.org/10.1016/j.ajpath.2012.03.018.
Contributor Information
Agnieszka Rynda-Apple, Email: agnieszka.rynda@montana.edu.
Allen G. Harmsen, Email: aharmsen@montana.edu.
Supplementary data
PCN administration prevents multiorgan failure. Mice are nasally dosed with PCN or PBS five times during the 2-week period. One week after the last dose, mice are challenged with MRSA. A and B: BALB/c mice receive 5 × 107 CFUs of bacteria, and bacterial burden in the liver (A) and kidney (B) is determined 72 hours after infection. C: C57BL/6 mice receive a challenge dose containing 3.6 × 108 CFUs of MRSA, and bacterial burden in the lungs is determined 24 hours after infection. *P < 0.05, ***P < 0.001 versus PBS-dosed mice.
PCN-unrelated VLPs protect against MRSA. BALB/c mice are dosed five times daily with 100 μg of PCN or P22 VLP or with equal volume of PBS. One week after the last dose, mice are infected with MRSA and sacrificed 24 or 48 hours later to determine the bacterial burden in the lungs. ***P < 0.001 versus PBS-dosed mice.
PCN reduces harmful inflammation in the first 24 hours after infection. BALB/c mice are intranasally dosed with PCN or PBS five times within a 2-week period. One week after the last dose, mice are infected with MRSA and sacrificed at the designated time points after infection. Cell-free BALF is tested for the presence of LDH (A) and serum albumin (B). The experiment is repeated two times. The mean and SD of five to six mice per group is shown. Each sample is plated in duplicate. ∞P < 0.05, **P < 0.01, and ***P < 0.001 versus PCN-dosed mice.
PCN-mediated protection against MRSA declines between 3 and 5 weeks after treatment. BALB/c mice are nasally dosed with PCN or PBS daily for 5 days. After the last PCN or PBS administration, mice are rested for the designated time period (1, 2, 3, or 5 weeks) and subsequently challenged with 5 × 107 CFUs of MRSA. Lung bacterial burden is evaluated by plating on TSA plates 24 hours after MRSA infection. The graph shows the average bacterial burden (CFUs) and SD for five mice per group. ***P < 0.001, **P < 0.01 versus PCN-dosed mice; ∞P < 0.001 versus PCN-dosed mice at 1 week.
Survival of PCN-dosed CXCR2 and IL-13 KO, but not CCR2, mice is compromised in response to MRSA infection. Mice are nasally dosed with PCN or PBS daily for 5 days. One week after the last treatment, WT BALB/c and CXCR2 KO mice are infected with 5 × 107 CFUs of MRSA (A) and WT C57BL/6, CCR2 KO, and IL-13 KO mice were infected with 1 × 108 CFUs of MRSA (B and C). All mice are monitored bidaily to determine the survival.
References
- 1.Walzl G., Tafuro S., Moss P., Openshaw P.J., Hussell T. Influenza virus lung infection protects from respiratory syncytial virus-induced immunopathology. J Exp Med. 2000;192:1317–1326. doi: 10.1084/jem.192.9.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Folkerts G., Walzl G., Openshaw P.J. Do common childhood infections “teach” the immune system not to be allergic? Immunol Today. 2000;21:118–120. doi: 10.1016/s0167-5699(00)01582-6. [DOI] [PubMed] [Google Scholar]
- 3.Chen H.D., Fraire A.E., Joris I., Brehm M.A., Welsh R.M., Selin L.K. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat Immunol. 2001;2:1067–1076. doi: 10.1038/ni727. [DOI] [PubMed] [Google Scholar]
- 4.Chen H.D., Fraire A.E., Joris I., Welsh R.M., Selin L.K. Specific history of heterologous virus infections determines anti-viral immunity and immunopathology in the lung. Am J Pathol. 2003;163:1341–1355. doi: 10.1016/S0002-9440(10)63493-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walzl G., Humphreys I.R., Marshall B.G., Edwards L., Openshaw P.J., Shaw R.J., Hussell T. Prior exposure to live Mycobacterium bovis BCG decreases Cryptococcus neoformans-induced lung eosinophilia in a gamma interferon-dependent manner. Infect Immun. 2003;71:3384–3391. doi: 10.1128/IAI.71.6.3384-3391.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams A.E., Edwards L., Hussell T. Colonic bacterial infection abrogates eosinophilic pulmonary disease. J Infect Dis. 2006;193:223–230. doi: 10.1086/498915. [DOI] [PubMed] [Google Scholar]
- 7.Wissinger E., Goulding J., Hussell T. Immune homeostasis in the respiratory tract and its impact on heterologous infection. Semin Immunol. 2009;21:147–155. doi: 10.1016/j.smim.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Edwards L., Williams A.E., Krieg A.M., Rae A.J., Snelgrove R.J., Hussell T. Stimulation via Toll-like receptor 9 reduces Cryptococcus neoformans-induced pulmonary inflammation in an IL-12-dependent manner. Eur J Immunol. 2005;35:273–281. doi: 10.1002/eji.200425640. [DOI] [PubMed] [Google Scholar]
- 9.Williams A.E., Edwards L., Humphreys I.R., Snelgrove R., Rae A., Rappuoli R., Hussell T. Innate imprinting by the modified heat-labile toxin of Escherichia coli (LTK63) provides generic protection against lung infectious disease. J Immunol. 2004;173:7435–7443. doi: 10.4049/jimmunol.173.12.7435. [DOI] [PubMed] [Google Scholar]
- 10.Watkins A.C., Caputo F.J., Badami C., Barlos D., Xu da Z., Lu Q., Feketeova E., Deitch E.A. Mesenteric lymph duct ligation attenuates lung injury and neutrophil activation after intraperitoneal injection of endotoxin in rats. J Trauma. 2008;64:126–130. doi: 10.1097/TA.0b013e3181574a8a. [DOI] [PubMed] [Google Scholar]
- 11.Wang J., Barke R.A., Charboneau R., Schwendener R., Roy S. Morphine induces defects in early response of alveolar macrophages to Streptococcus pneumoniae by modulating TLR9-NF-kappa B signaling. J Immunol. 2008;180:3594–3600. doi: 10.4049/jimmunol.180.5.3594. [DOI] [PubMed] [Google Scholar]
- 12.Droemann D., Goldmann T., Tiedje T., Zabel P., Dalhoff K., Schaaf B. Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respir Res. 2005;6:68. doi: 10.1186/1465-9921-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H., Cowan M.J., Hasday J.D., Vogel S.N., Medvedev A.E. Tobacco smoking inhibits expression of proinflammatory cytokines and activation of IL-1R-associated kinase, p38, and NF-kappaB in alveolar macrophages stimulated with TLR2 and TLR4 agonists. J Immunol. 2007;179:6097–6106. doi: 10.4049/jimmunol.179.9.6097. [DOI] [PubMed] [Google Scholar]
- 14.Wiley J.A., Richert L.E., Swain S.D., Harmsen A., Barnard D.L., Randall T.D., Jutila M., Douglas T., Broomell C., Young M., Harmsen A.G. Inducible bronchus-associated lymphoid tissue elicited by a protein cage nanoparticle enhances protection in mice against diverse respiratory viruses. PLoS One. 2009;4:e7142. doi: 10.1371/journal.pone.0007142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rangel-Moreno J., Hartson L., Navarro C., Gaxiola M., Selman M., Randall T.D. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest. 2006;116:3183–3194. doi: 10.1172/JCI28756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flenniken M.L., Willits D.A., Harmsen A.L., Liepold L.O., Harmsen A.G., Young M.J., Douglas T. Melanoma and lymphocyte cell-specific targeting incorporated into a heat shock protein cage architecture. Chem Biol. 2006;13:161–170. doi: 10.1016/j.chembiol.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Kaiser C.R., Flenniken M.L., Gillitzer E., Harmsen A.L., Harmsen A.G., Jutila M.A., Douglas T., Young M.J. Biodistribution studies of protein cage nanoparticles demonstrate broad tissue distribution and rapid clearance in vivo. Int J Nanomedicine. 2007;2:715–733. [PMC free article] [PubMed] [Google Scholar]
- 18.Jennings G.T., Bachmann M.F. The coming of age of virus-like particle vaccines. Biol Chem. 2008;389:521–536. doi: 10.1515/bc.2008.064. [DOI] [PubMed] [Google Scholar]
- 19.Lechmann M., Murata K., Satoi J., Vergalla J., Baumert T.F., Liang T.J. Hepatitis C virus-like particles induce virus-specific humoral and cellular immune responses in mice. Hepatology. 2001;34:417–423. doi: 10.1053/jhep.2001.26523. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S., Liang M., Gu W., Li C., Miao F., Wang X., Jin C., Zhang L., Zhang F., Zhang Q., Jiang L., Li M., Li D. Vaccination with dengue virus-like particles induces humoral and cellular immune responses in mice. Virol J. 2011;8:333. doi: 10.1186/1743-422X-8-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velasquez L.S., Shira S., Berta A.N., Kilbourne J., Medi B.M., Tizard I., Ni Y., Arntzen C.J., Herbst-Kralovetz M.M. Intranasal delivery of Norwalk virus-like particles formulated in an in situ gelling, dry powder vaccine. Vaccine. 2011;29:5221–5231. doi: 10.1016/j.vaccine.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herbst-Kralovetz M., Mason H.S., Chen Q. Norwalk virus-like particles as vaccines. Expert Rev Vaccines. 2010;9:299–307. doi: 10.1586/erv.09.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barr E., Sings H.L. Prophylactic HPV vaccines: new interventions for cancer control. Vaccine. 2008;26:6244–6257. doi: 10.1016/j.vaccine.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 24.Harper D.M. Currently approved prophylactic HPV vaccines. Expert Rev Vaccines. 2009;8:1663–1679. doi: 10.1586/erv.09.123. [DOI] [PubMed] [Google Scholar]
- 25.Chackerian B., Rangel M., Hunter Z., Peabody D.S. Virus and virus-like particle-based immunogens for Alzheimer's disease induce antibody responses against amyloid-beta without concomitant T cell responses. Vaccine. 2006;24:6321–6331. doi: 10.1016/j.vaccine.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 26.Fulurija A., Lutz T.A., Sladko K., Osto M., Wielinga P.Y., Bachmann M.F., Saudan P. Vaccination against GIP for the treatment of obesity. PLoS One. 2008;3:e3163. doi: 10.1371/journal.pone.0003163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klevens R.M., Morrison M.A., Nadle J., Petit S., Gershman K., Ray S., Harrison L.H., Lynfield R., Dumyati G., Townes J.M., Craig A.S., Zell E.R., Fosheim G.E., McDougal L.K., Carey R.B., Fridkin S.K. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 28.Boucher H.W., Corey G.R. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46(Suppl 5):S344–S349. doi: 10.1086/533590. [DOI] [PubMed] [Google Scholar]
- 29.Miller L.S., Cho J.S. Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol. 2011;11:505–518. doi: 10.1038/nri3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graves S.F., Kobayashi S.D., DeLeo F.R. Community-associated methicillin-resistant Staphylococcus aureus immune evasion and virulence. J Mol Med (Berl) 2010;88:109–114. doi: 10.1007/s00109-009-0573-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deleo F.R., Otto M., Kreiswirth B.N., Chambers H.F. Community-associated methicillin-resistant Staphylococcus aureus. Lancet. 2010;375:1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Francis J.S., Doherty M.C., Lopatin U., Johnston C.P., Sinha G., Ross T., Cai M., Hansel N.N., Perl T., Ticehurst J.R., Carroll K., Thomas D.L., Nuermberger E., Bartlett J.G. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005;40:100–107. doi: 10.1086/427148. [DOI] [PubMed] [Google Scholar]
- 33.Boles B.R., Thoendel M., Roth A.J., Horswill A.R. Identification of genes involved in polysaccharide-independent Staphylococcus aureus biofilm formation. PLoS One. 2010;5:e10146. doi: 10.1371/journal.pone.0010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siemen D.W., Schepetkin I.A., Kirpotina L.N., Lei B., Quinn M.T. Neutrophil Isolation from Nonhuman Species. Methods in Molecular Biology. 2007;412:21–34. doi: 10.1007/978-1-59745-467-4_3. [DOI] [PubMed] [Google Scholar]
- 35.Voyich J.M., Braughton K.R., Sturdevant D.E., Whitney A.R., Said-Salim B., Porcella S.F., Long R.D., Dorward D.W., Gardner D.J., Kreiswirth B.N., Musser J.M., DeLeo F.R. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunol. 2005;175:3907–3919. doi: 10.4049/jimmunol.175.6.3907. [DOI] [PubMed] [Google Scholar]
- 36.Prevelige P.E.J. The Bacteriophages. In: Calendar R., editor. Oxford University Press; New York: 2005. pp. 457–468. [Google Scholar]
- 37.Steinmetz N.F., Manchester M. Overview of the manifold VNPs used in nantechnology. In: Manchester M., editor. ed 1. vol 1. Pan Stanford Publishing Pte. Ltd.; Singapore: 2011. pp. 13–48. (Viral Nonoparticles: Tools for Material Science and Biomedicine). ch 2. [Google Scholar]
- 38.Sonozaki H., Cohen S. The macrophage disappearance reaction: mediation by a soluble lymphocyte-derived factor. Cell Immunol. 1971;2:341–352. doi: 10.1016/0008-8749(71)90069-4. [DOI] [PubMed] [Google Scholar]
- 39.Gu J., Zuo J., Lei L., Zhao H., Sun C., Feng X., Du C., Li X., Yang Y., Han W. LysGH15 reduces the inflammation caused by lethal methicillin-resistant Staphylococcus aureus infection in mice. Bioeng Bugs. 2011;2:96–99. doi: 10.4161/bbug.2.2.14883. [DOI] [PubMed] [Google Scholar]
- 40.Watkins R.L., Pallister K.B., Voyich J.M. The SaeR/S gene regulatory system induces a pro-inflammatory cytokine response during Staphylococcus aureus infection. PLoS One. 2011;6:e19939. doi: 10.1371/journal.pone.0019939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goulding J., Snelgrove R., Saldana J., Didierlaurent A., Cavanagh M., Gwyer E., Wales J., Wissinger E.L., Hussell T. Respiratory infections: do we ever recover? Proc Am Thorac Soc. 2007;4:618–625. doi: 10.1513/pats.200706-066TH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kugathasan K., Roediger E.K., Small C.L., McCormick S., Yang P., Xing Z. CD11c+ antigen presenting cells from the alveolar space, lung parenchyma and spleen differ in their phenotype and capabilities to activate naive and antigen-primed T cells. BMC Immunol. 2008;9:48. doi: 10.1186/1471-2172-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fei M., Bhatia S., Oriss T.B., Yarlagadda M., Khare A., Akira S., Saijo S., Iwakura Y., Fallert Junecko B.A., Reinhart T.A., Foreman O., Ray P., Kolls J., Ray A. TNF-alpha from inflammatory dendritic cells (DCs) regulates lung IL-17A/IL-5 levels and neutrophilia versus eosinophilia during persistent fungal infection. Proc Natl Acad Sci U S A. 2011;108:5360–5365. doi: 10.1073/pnas.1015476108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sung S.S., Fu S.M., Rose C.E., Jr, Gaskin F., Ju S.T., Beaty S.R. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol. 2006;176:2161–2172. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- 45.Kim T.S., Braciale T.J. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS One. 2009;4:e4204. doi: 10.1371/journal.pone.0004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin F.J., Parker D., Harfenist B.S., Soong G., Prince A. Participation of CD11c+ leukocytes in methicillin-resistant Staphylococcus aureus clearance from the lung. Infect Immun. 2011;79:1898–1904. doi: 10.1128/IAI.01299-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wojcik A.J., Skaflen M.D., Srinivasan S., Hedrick C.C. A critical role for ABCG1 in macrophage inflammation and lung homeostasis. J Immunol. 2008;180:4273–4282. doi: 10.4049/jimmunol.180.6.4273. [DOI] [PubMed] [Google Scholar]
- 49.Robertson C.M., Perrone E.E., McConnell K.W., Dunne W.M., Boody B., Brahmbhatt T., Diacovo M.J., Van Rooijen N., Hogue L.A., Cannon C.L., Buchman T.G., Hotchkiss R.S., Coopersmith C.M. Neutrophil depletion causes a fatal defect in murine pulmonary Staphylococcus aureus clearance. J Surg Res. 2008;150:278–285. doi: 10.1016/j.jss.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Senavsky C., Craft N., Miller L.S. Bacterial Infections. In: Gaspary A.A., Tyring S.K., editors. Springer; London, UK: 2008. pp. 335–363. [Google Scholar]
- 51.Heyworth P.G., Cross A.R., Curnutte J.T. Chronic granulomatous disease. Curr Opin Immunol. 2003;15:578–584. doi: 10.1016/s0952-7915(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 52.Boring L., Gosling J., Chensue S.W., Kunkel S.L., Farese R.V., Jr, Broxmeyer H.E., Charo I.F. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lenz P., Day P.M., Pang Y.Y., Frye S.A., Jensen P.N., Lowy D.R., Schiller J.T. Papillomavirus-like particles induce acute activation of dendritic cells. J Immunol. 2001;166:5346–5355. doi: 10.4049/jimmunol.166.9.5346. [DOI] [PubMed] [Google Scholar]
- 54.Lacasse P., Denis J., Lapointe R., Leclerc D., Lamarre A. Novel plant virus-based vaccine induces protective cytotoxic T-lymphocyte-mediated antiviral immunity through dendritic cell maturation. J Virol. 2008;82:785–794. doi: 10.1128/JVI.01811-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jennings J.H., Linderman D.J., Hu B., Sonstein J., Curtis J.L. Monocytes recruited to the lungs of mice during immune inflammation ingest apoptotic cells poorly. Am J Respir Cell Mol Biol. 2005;32:108–117. doi: 10.1165/rcmb.2004-0108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Behar S.M., Martin C.J., Booty M.G., Nishimura T., Zhao X., Gan H.X., Divangahi M., Remold H.G. Apoptosis is an innate defense function of macrophages against Mycobacterium tuberculosis. Mucosal Immunol. 2011;4:279–287. doi: 10.1038/mi.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kobayashi S.D., Braughton K.R., Palazzolo-Ballance A.M., Kennedy A.D., Sampaio E., Kristosturyan E., Whitney A.R., Sturdevant D.E., Dorward D.W., Holland S.M., Kreiswirth B.N., Musser J.M., DeLeo F.R. Rapid neutrophil destruction following phagocytosis of Staphylococcus aureus. J Innate Immun. 2010;2:560–575. doi: 10.1159/000317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skold M., Behar S.M. Tuberculosis triggers a tissue-dependent program of differentiation and acquisition of effector functions by circulating monocytes. J Immunol. 2008;181:6349–6360. doi: 10.4049/jimmunol.181.9.6349. [DOI] [PubMed] [Google Scholar]
- 59.Cheung G.Y., Rigby K., Wang R., Queck S.Y., Braughton K.R., Whitney A.R., Teintze M., DeLeo F.R., Otto M. Staphylococcus epidermidis strategies to avoid killing by human neutrophils. PLoS Pathog. 2010;6:e1001133. doi: 10.1371/journal.ppat.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diep B.A., Chan L., Tattevin P., Kajikawa O., Martin T.R., Basuino L., Mai T.T., Marbach H., Braughton K.R., Whitney A.R., Gardner D.J., Fan X., Tseng C.W., Liu G.Y., Badiou C., Etienne J., Lina G., Matthay M.A., DeLeo F.R., Chambers H.F. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc Natl Acad Sci U S A. 2010;107:5587–5592. doi: 10.1073/pnas.0912403107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gresham H.D., Lowrance J.H., Caver T.E., Wilson B.S., Cheung A.L., Lindberg F.P. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J Immunol. 2000;164:3713–3722. doi: 10.4049/jimmunol.164.7.3713. [DOI] [PubMed] [Google Scholar]
- 62.Ghassabeh G.H., De Baetselier P., Brys L., Noel W., Van Ginderachter J.A., Meerschaut S., Beschin A., Brombacher F., Raes G. Identification of a common gene signature for type II cytokine-associated myeloid cells elicited in vivo in different pathologic conditions. Blood. 2006;108:575–583. doi: 10.1182/blood-2005-04-1485. [DOI] [PubMed] [Google Scholar]
- 63.Martinez F.O., Sica A., Mantovani A., Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 64.Nelson M.P., Christmann B.S., Werner J.L., Metz A.E., Trevor J.L., Lowell C.A., Steele C. IL-33 and M2a alveolar macrophages promote lung defense against the atypical fungal pathogen Pneumocystis murina. J Immunol. 2011;186:2372–2381. doi: 10.4049/jimmunol.1002558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Girard D., Paquin R., Naccache P.H., Beaulieu A.D. Effects of interleukin-13 on human neutrophil functions. J Leukoc Biol. 1996;59:412–419. doi: 10.1002/jlb.59.3.412. [DOI] [PubMed] [Google Scholar]
- 66.Shim J.J., Dabbagh K., Ueki I.F., Dao-Pick T., Burgel P.R., Takeyama K., Tam D.C., Nadel J.A. IL-13 induces mucin production by stimulating epidermal growth factor receptors and by activating neutrophils. Am J Physiol Lung Cell Mol Physiol. 2001;280:L134–L140. doi: 10.1152/ajplung.2001.280.1.L134. [DOI] [PubMed] [Google Scholar]
- 67.Olszewski M.A., Falkowski N.R., Surana R., Sonstein J., Hartman A., Moore B.B., Huffnagle G.B., Toews G.B. Effect of laparotomy on clearance and cytokine induction in Staphylococcus aureus infected lungs. Am J Respir Crit Care Med. 2007;176:921–929. doi: 10.1164/rccm.200606-763OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PCN administration prevents multiorgan failure. Mice are nasally dosed with PCN or PBS five times during the 2-week period. One week after the last dose, mice are challenged with MRSA. A and B: BALB/c mice receive 5 × 107 CFUs of bacteria, and bacterial burden in the liver (A) and kidney (B) is determined 72 hours after infection. C: C57BL/6 mice receive a challenge dose containing 3.6 × 108 CFUs of MRSA, and bacterial burden in the lungs is determined 24 hours after infection. *P < 0.05, ***P < 0.001 versus PBS-dosed mice.
PCN-unrelated VLPs protect against MRSA. BALB/c mice are dosed five times daily with 100 μg of PCN or P22 VLP or with equal volume of PBS. One week after the last dose, mice are infected with MRSA and sacrificed 24 or 48 hours later to determine the bacterial burden in the lungs. ***P < 0.001 versus PBS-dosed mice.
PCN reduces harmful inflammation in the first 24 hours after infection. BALB/c mice are intranasally dosed with PCN or PBS five times within a 2-week period. One week after the last dose, mice are infected with MRSA and sacrificed at the designated time points after infection. Cell-free BALF is tested for the presence of LDH (A) and serum albumin (B). The experiment is repeated two times. The mean and SD of five to six mice per group is shown. Each sample is plated in duplicate. ∞P < 0.05, **P < 0.01, and ***P < 0.001 versus PCN-dosed mice.
PCN-mediated protection against MRSA declines between 3 and 5 weeks after treatment. BALB/c mice are nasally dosed with PCN or PBS daily for 5 days. After the last PCN or PBS administration, mice are rested for the designated time period (1, 2, 3, or 5 weeks) and subsequently challenged with 5 × 107 CFUs of MRSA. Lung bacterial burden is evaluated by plating on TSA plates 24 hours after MRSA infection. The graph shows the average bacterial burden (CFUs) and SD for five mice per group. ***P < 0.001, **P < 0.01 versus PCN-dosed mice; ∞P < 0.001 versus PCN-dosed mice at 1 week.
Survival of PCN-dosed CXCR2 and IL-13 KO, but not CCR2, mice is compromised in response to MRSA infection. Mice are nasally dosed with PCN or PBS daily for 5 days. One week after the last treatment, WT BALB/c and CXCR2 KO mice are infected with 5 × 107 CFUs of MRSA (A) and WT C57BL/6, CCR2 KO, and IL-13 KO mice were infected with 1 × 108 CFUs of MRSA (B and C). All mice are monitored bidaily to determine the survival.