Abstract
Wound repair on the cellular and multicellular levels is essential to the survival of complex organisms. In order to avoid further damage, prevent infection, and restore normal function, cells and tissues must rapidly seal and remodel the wounded area. The cytoskeleton is an important component of wound repair in that it is needed for actomyosin contraction, recruitment of repair machineries, and cell migration. Recent use of model systems and high-resolution microscopy has provided new insight into molecular aspects of the cytoskeletal response during wound repair. Here we discuss the role of the cytoskeleton in single-cell, embryonic, and adult repair, as well as the striking resemblance of these processes to normal developmental events and many diseases.
Keywords: Wound repair, Single cells, Multicellular, Cytoskeleton, Actin, Myosin, Purse string, Adherens junctions
Introduction
Living organisms of all shapes and sizes have developed robust processes for healing wounds in order to navigate the continuous physical and chemical stresses of their environment. For a single cell, a rapid wound-repair response is absolutely necessary for survival. If damage to the cell membrane is not quickly repaired, the cell will die due to the loss of cytoplasm and the influx of extracellular molecules. For tissues and organs, wound repair is necessary to maintain homeostasis, avoid infection, and maintain tissue function. Failure to properly repair injuries can result in the death of an organism. Despite the morphological differences, the wound-repair responses in single cells and tissues share similar components and mechanisms: upon injury, the wound is rapidly sealed, the wound area is reconstituted, and the damaged area is then remodeled in order to restore normal function.
A large component of both cellular and tissue-repair responses is the involvement of the cytoskeleton. From the assembly of a contractile actomyosin array to the formation of protrusions from the cells at the leading edge of wounds, the cell cytoskeleton is required to precisely orchestrate the dynamic process of wound repair. Similar to other biological processes requiring cell-shape changes and rearrangements, wound repair is powered by the actin, myosin, and microtubule networks. The actin and myosin networks provide the driving force for the repair process and microtubules serve to traffic membrane and other components to the wound. Rho family GTPases regulate many of the cytoskeleton changes that underpin the repair process. The recent addition of new model systems to the wound repair field, together with advances in imaging, as well as molecular, biochemical, and genetic tools, is providing fresh insights on how single cells and tissues repair their injuries, and is helping to identify new essential players and molecular/signaling pathways regulating these processes. In this review, we highlight the differences and similarities between the cytoskeletal responses in a single cell and in a tissue during wound repair, and correlate these responses with basic developmental processes and diseases.
Single cell wound repair
Cells in mechanically active tissues such as skeletal or cardiac muscle cells [1], as well as cells exposed to constant chemical or physical insults such as cells in the gut epithelium or the skin epidermis [2, 3], show a higher frequency of being wounded in vivo. Damage to individual cells leads to a disruption in their plasma membrane, which requires rapid repair to ensure cell survival. Significantly, cells exposed to bacterial toxins or associated with genetic diseases including muscular dystrophies, skin-blistering conditions, or diabetes can be easily damaged (reviewed in [4]), highlighting the fundamental importance of efficient cell wound repair to maintaining tissue integrity and function.
The immediate response to a cellular wound is to plug the plasma membrane disruption, thereby avoiding an influx of extracellular molecules and preventing the loss of cytoplasm. Membrane breaches are rapidly closed by the recruitment and fusion of internal vesicular membrane with the plasma membrane, a process triggered by Ca2+ influx and other signaling cues (reviewed in [4]). Once the hole in the membrane has been plugged, the cell must remodel the plasma membrane and the underlying cortical cytoskeleton at the wound site to fully restore function (reviewed in [5]) (Fig. 1). Complete restoration of the cellular surface typically occurs within minutes of suffering the damage [5].
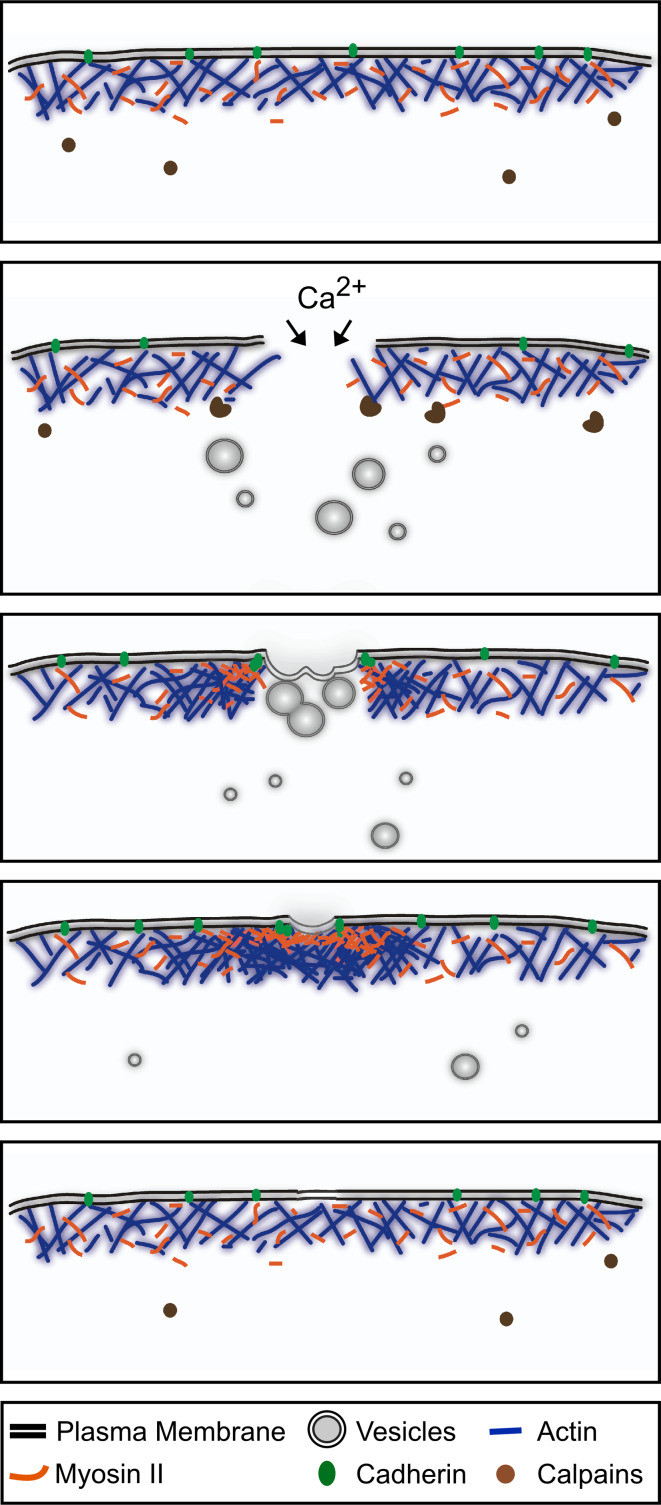
Fig. 1.
Single cell wound repair. Schematic diagram of the single-cell wound-repair process depicting the plasma membrane and cytoskeleton components. Upon plasma membrane disruption, Ca2+ influx and other signals trigger the temporal disassembly of the cortical cytoskeleton. Simultaneously, internal vesicles fuse with each other to form a membrane patch that will directionally migrate to the wound site and fuse with the plasma membrane. Once the membrane has been resealed, the plasma membrane and cortical cytoskeleton are remodeled. This process involves the assembly of an actomyosin ring, the replacement of the membrane patch, and the reconstitution of the cortical cytoskeleton
Sealing the membrane breach
The establishment of a membrane patch at the site of a wound requires a highly dynamic and exact response from the cytoskeleton, including the immediate Ca2+-regulated exocytosis of vesicles, the generation, recruitment, and transport of new vesicles, and the reconstitution of the plasma membrane. Upon wounding, the actin filament network associated with the damaged area is locally and transiently disassembled to allow plugging of the hole: stabilization of F-actin severely decreases plasma membrane resealing [6], whereas drug-induced F-actin depolymerization enhances resealing [7]. Cortical actin disassembly is thought to be signaled by Ca2+ influx, and requires the activity of calpain proteases. In fibroblasts, calpain mediates the rapid fragmentation of talin and vimentin after wounding, as a first step towards the removal of damaged cytoskeleton [8]. Calpain has also been shown to rapidly cleave caldesmon, a calmodulin-dependent actin-linked protein involved in actin filament stabilization and actomyosin contraction, suggesting an alternative mechanism facilitating the rapid disassembly and clearance of the local cytoskeleton at the wound site [9].
The cytoskeleton mediates the localized transport of vesicles to the wound site. The motor activities of kinesin and myosin are required to move the vesicles directionally to the site of the disruption and to promote the exocytic events required for resealing [10, 11]. An intact microtubule network is also critical for proper cell repair (reviewed in [12]). Disruption of the microtubule network impairs the recruitment of vesicles to the wound site, and severely slows the repair process in Drosophila embryos [13]. Wounding also triggers the recruitment of the microtubule-associated protein EB1, which in turn stimulates microtubule elongation and facilitates the transport of Golgi-derived lipids to the wound area [14]. Thus, resealing a plasma membrane disruption in a wounded cell involves the rapid degradation of the cortical cytoskeleton, and the equally fast delivery of vesicular membrane to the site of the wound.
Membrane and cytoskeleton remodeling
Once a membrane plug is in place, a wounded cell must restore the cortical cytoskeleton and repopulate the wounded area with the lipids and proteins normally found in the plasma membrane. Currently, two mechanisms have been put forward to explain how plasma membrane remodeling is achieved. The first model proposes that the plug becomes integrated into the plasma membrane through lipid and protein diffusion. In support of this model, in tissue culture cells, lipids normally found in the Golgi body were observed within the remodeled plasma membrane [14]. A second model proposes that a new plasma membrane forms underneath the membrane plug, with the plug subsequently discarded as a scab. Consistent with this, wound sites in sea urchin eggs have a concave crater-like appearance. It has been suggested that the vesicle patch fuses at specific points or “vertices” around the wound perimeter and is subsequently excluded as a scab after membrane resealing [15]. It is likely that both mechanisms are used in a context- and/or organism-specific manner.
Regardless of which scenario of plasma membrane reconstitution is employed, it is accompanied by cortical cytoskeleton remodeling and the two processes appear to rely on one another. Cytoskeleton remodeling has been studied in tissue culture cells [16], Xenopus oocytes [17–19], and more recently in the early Drosophila embryo [13]. In all models, actin and myosin II are recruited to the wound edge within seconds of the injury, and assemble as contractile arrays surrounding the damaged area. This actomyosin ring then contracts continuously throughout the repair process until the damaged area is closed [13, 17, 18] (Fig. 2a–d). Interestingly, while both actin and myosin II accumulate at the wound edge, their respective areas of accumulation do not completely overlap. In Xenopus oocytes, myosin II concentrates in the interior of the array and overlaps on its outer periphery with stable actin, followed by a zone of dynamic actin [17, 18] (Fig. 2a, b).
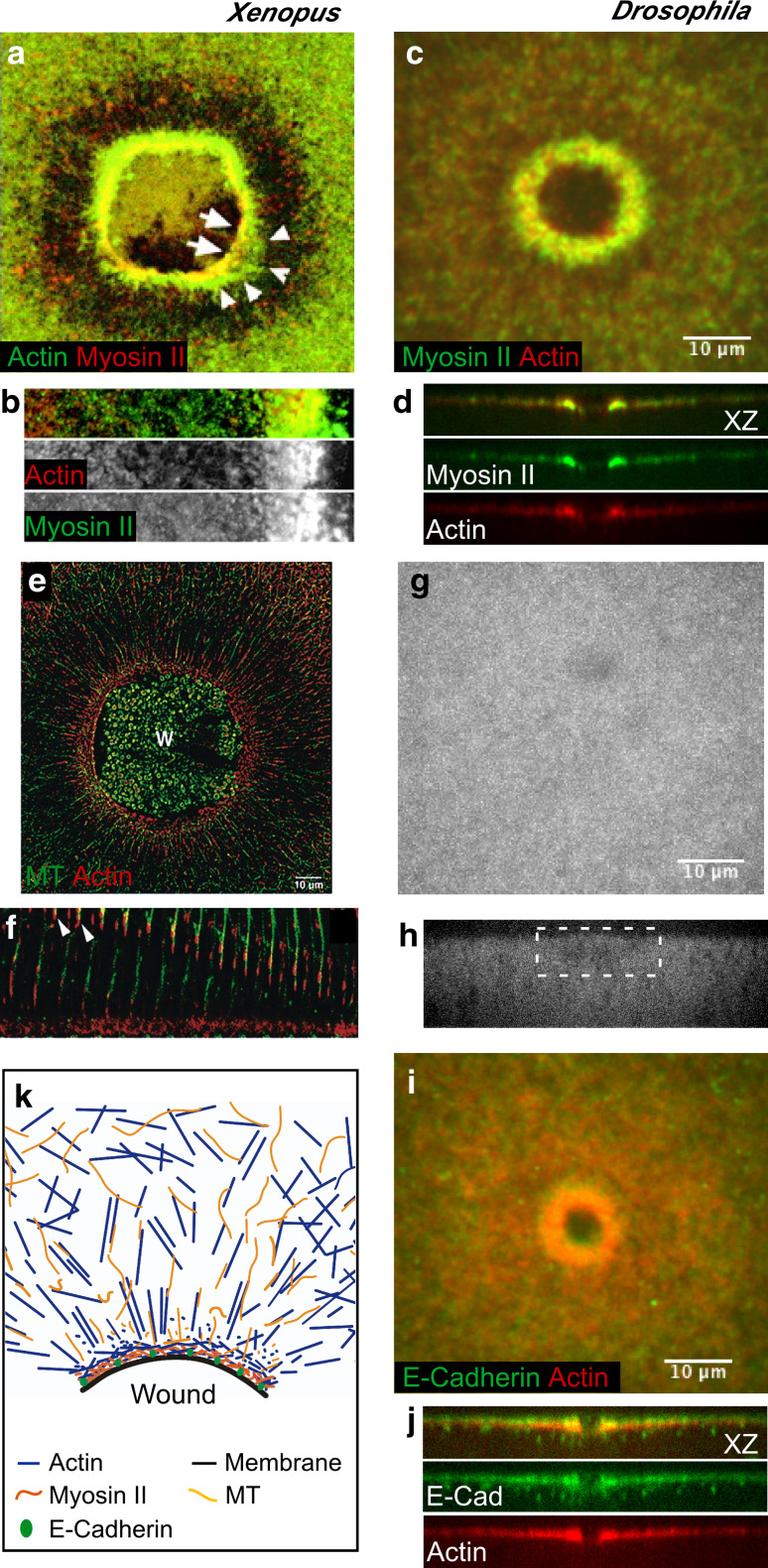
Fig. 2.
Cytoskeleton components of the single cell wound repair response. Fluorescent micrographs of single cell wound repair in Xenopus oocytes (a, b, e, f) and the Drosophila embryo (c, d, g–j), showing the dynamic response of different cytoskeletal components upon wounding. a–d An actomyosin contractile array is assembled at the wound edge a few seconds post-wounding (30–45 s). Actin and/or myosin-enriched zones can be distinguished: myosin II is enriched in the leading edge of the wound followed by an overlapping actin-myosin-enriched zone then a broad array of actin (b, d) (a, b adapted from [18], originally published in Journal of Cell Biology. doi:10.1083/jcb.200103105) (c, d adapted from [13], originally published in Journal of Cell Biology. doi:10.1083/jcb.201011018). e–f Cell wounding triggers the formation of a radial arrangement of microtubules in Xenopus oocytes. Microtubules are transported to the wound in association with actin (arrowheads in f), and modulate the assembly of the actomyosin array (adapted from [19], and reproduced with permission from Elsevier). g, h In the Drosophila embryo, no radial rearrangement of the microtubule network is observed upon wounding (wound site is indicated by white box) (adapted from [13], originally published in Journal of Cell Biology. doi:10.1083/jcb.201011018). i, j E-Cadherin co-localizes with the actomyosin array in single cell wounds in Drosophila, and tethers the ring to the plasma membrane (adapted from [13], originally published in Journal of Cell Biology. doi:10.1083/jcb.201011018). k Cartoon depicting the response of the different cortical cytoskeleton components to cell wounding (adapted from [12])
In Drosophila, actin and myosin also assemble a contractile ring at the wound border (Fig. 2c, d), with an initial enrichment of myosin II at the leading edge of the wound, surrounded by a broader actin array [13]. As healing progresses, three zones can be distinguished within the actomyosin ring: an apical zone of actin, an overlapping actomyosin zone, and a basal area of myosin II enrichment (Fig. 2d). The dynamic changes of the actin and myosin cytoskeleton are necessary for proper single-cell wound repair. Drug-induced depolymerization of filamentous actin prevents healing, as well as the recruitment of plasma membrane and myosin II to the wound site [13, 18]. Similarly, pharmacological or genetic disruption of myosin II results in a disorganized actin array at the wound edge [13, 18].
The assembly of the actomyosin ring is accompanied by the rearrangement of microtubules into a radial array around the wound in Xenopus oocytes [19] (Fig. 2e, f). These microtubules are assembled both locally at the wound edge and away from the wound and then transported towards the wound by associating with cortically flowing actin, and are ultimately cross-linked with the actin filaments [19]. Microtubules also play a role in actin polymerization at the wound edge. In Xenopus oocytes, stabilization of microtubules by taxol treatment induces non-overlapping actin and myosin arrays at the wound edge [19]. In contrast to Xenopus oocytes, microtubule rearrangement is not observed in the Drosophila cell wound repair model (Fig. 2g, h) [13]. Despite this, disruption of the microtubule network severely impairs actin ring formation and plasma membrane recruitment: not only is the actin ring broader and less organized, but a reduced number of vesicles are also observed beneath the wound [13].
The precise and specific recruitment of membrane, actin, and myosin II is dependent on Ca2+ signaling, and modulated by the Rho and Cdc42 small GTPases [20]. Upon wounding in Xenopus oocytes, Rho accumulates as a ring that overlaps with myosin II, while Cdc42 overlaps with the actin ring. This leads to the formation of concentric GTPase zones around the wound edge. Recently, Abr, a protein with GEF and GAP activity, was identified in a candidate screen for potential GTPase regulators of cell wound repair in Xenopus [21]. Abr is recruited from the cytoplasm and concentrated into the Rho zone where its GAP activity is required to locally suppress Cdc42 activity, thereby segregating Rho and Cdc42 into their respective zones.
Recent studies have shown that as the actomyosin ring is closed it pulls the plasma membrane inwards through its association with adhesion molecules. Early studies in Xenopus oocytes suggest that the actomyosin purse string is tethered to the membrane at intervals along the wound edge by an unknown mechanism [18]. In the Drosophila embryo, E-cadherin, a component of adherens junction, acts as the intermediary between the plasma membrane and the actin cytoskeleton: it co-localizes with the actin ring at the wound edge and is excluded from the membrane plug (Fig. 2i, j) [13]. Embryos deficient for E-cadherin exhibit wound overexpansion and improper actin cable formation, indicating a role for E-cadherin in tethering the plasma membrane to the actomyosin ring at the wound border and transferring the force generated by actomyosin cable contraction to the plasma membrane. However, E-cadherin mutant embryos do manage to heal wounds, suggesting that other proteins are likely involved in tethering the membrane to the underlying actomyosin cable and compensating for the lack of E-cadherin. It will be interesting to know which proteins mediate the interaction between E-cadherin and the cortical cytoskeleton, and how other proteins involved in membrane–cytoskeleton interactions, such as ankyrin, which is also involved in cytokinesis [22], spectrin, or ezrin-radixin-moesin (ERM) proteins, affect cell wound healing [23]. Thus, combining the strengths of the different single-cell wound models, as well as the qualitative differences between them, is providing a new list of players and mechanisms required for the cell to repair injuries.
Single cell repair in a multicellular tissue context
Most of the research in single-cell wound repair has utilized stand-alone, single-cell models or in vitro tissue culture. However, in higher-level organisms, single cell repair occurs in the context of other cells and surrounding tissue. Therefore, single cell repair must also be considered in the context of a tissue. As such, wounded single cells not only need to be repaired but are also a signaling source for their neighbors, in order to initiate the tissue-repair response. In the event that single cell repair fails, the surrounding tissue must know when and how to repair itself to maintain tissue homeostasis.
The cellularizing Xenopus embryo provides an excellent model for examining the interplay between the single cell wound response and its multicellular environment. The large cells of this model facilitate the visualization and analysis in vivo of the cell-wound repair processes. Wounding a single cell in the two-cell Xenopus blastomere leads to recruitment of active Rho to the wound edge [24], as previously observed in single cell models. The activation of Rho causes actin and myosin to form a contractile ring that closes the wound (Fig. 3) [24]. The distance between the wound and the nearest cell–cell contact dictates the role that the surrounding tissue will play in the repair process. Wounds near cell–cell contacts trigger the accumulation of actin, myosin, and active GTPases in the neighboring unwounded cell, resulting in an intercellular, hybrid purse string through which both cells work together to close the wound (Fig. 3b, c) [24]. In contrast, wounds made further away from the cell–cell contact fail to trigger actin and myosin accumulation in the neighboring unwounded cell. As these wounds close, the adjoining cells are pulled apart, indicating that the hybrid purse string works to transfer tension from the cell being repaired to its surrounding environment (Fig. 3a). Ca2+ influx appears to be the signal for hybrid purse string formation. Ca2+ rapidly accumulates in the wounded cell and in the adjacent cells that participate in hybrid purse string formation. Similarly, in four-cell sea urchin eggs, wounding of a single cell leads to spikes in the concentration of intracellular calcium in neighboring cells [25].
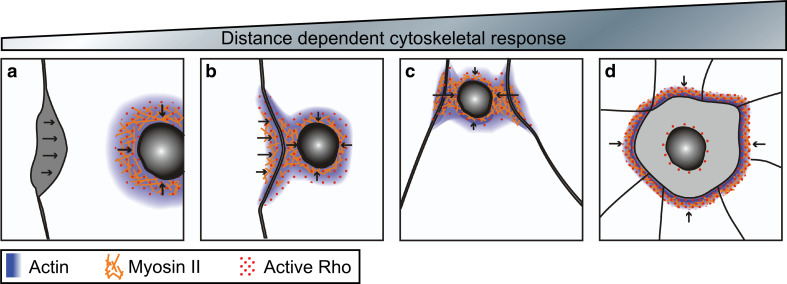
Fig. 3.
Distance-dependent cytoskeletal responses triggered by a damaged single cell within an epithelia. Arrows indicate direction of repair forces. a–d Cartoon depicting the response of neighboring cells upon wounding and the formation of a hybrid purse string in Xenopus oocytes. Wounds distal from neighboring cells repair via formation of an actomyosin purse string, but detach from neighbors as the wound closes (a), while wounds made near to neighboring cells form a hybrid purse string where the actomyosin contractile array forms at the wound as well as at nearby cell contacts (b), as the wound closes the cell contacts ingress (c). Severely damaged cells are extruded from the epithelia by the formation of an actomyosin contractile array between the surrounding neighbor cells (d)
In the event that a larger wound causes irreparable damage to a single cell, the adjoining cells must be able to sense this and initiate tissue repair around the damaged single cell. Interestingly, damaging a single cell in a late Xenopus blastomere to the extent that it cannot survive, elicits a more dramatic response in the cells surrounding the wounded cell than in the wounded cell itself (Figs. 3d, 4a–a″) [24]. The edges of neighboring cells accumulate active Rho and assemble a supracellular actomyosin purse string to remove the damaged cell from the tissue. It is unknown if adherens junctions between the damaged cell and surrounding cells are maintained, or if such a wound causes a breach in the tissue through which harmful compounds or infectious agents could pass. This repair process is reminiscent of the extrusion of apoptotic cells by actin and myosin in epithelial sheets [26]. Apoptotic cells, like a wounded single cell, are not a true hole in the epithelial layer, but a potential breach in sheet integrity. As apoptotic cells are extruded, E-cadherin accumulates at the cell–cell junctions of neighboring cells, along with an actomyosin contractile ring (Fig. 4b–b″). In addition, E-cadherin and occludin, a tight junction protein, are expressed highly at the interfaces between the surrounding cells and the cell being extruded, suggesting that epithelial sheet integrity is not compromised during this process (Fig. 4c, d) [26]. Cell junction proteins have not been investigated closely in the case of an irreparably wounded cell, but it is possible that the accumulation of active Rho along the intracellular actomyosin purse string also recruits these proteins. Cell junction proteins may also keep neighboring cells from losing contact with each other as the wound left by the dying cell is repaired.
Fig. 4.
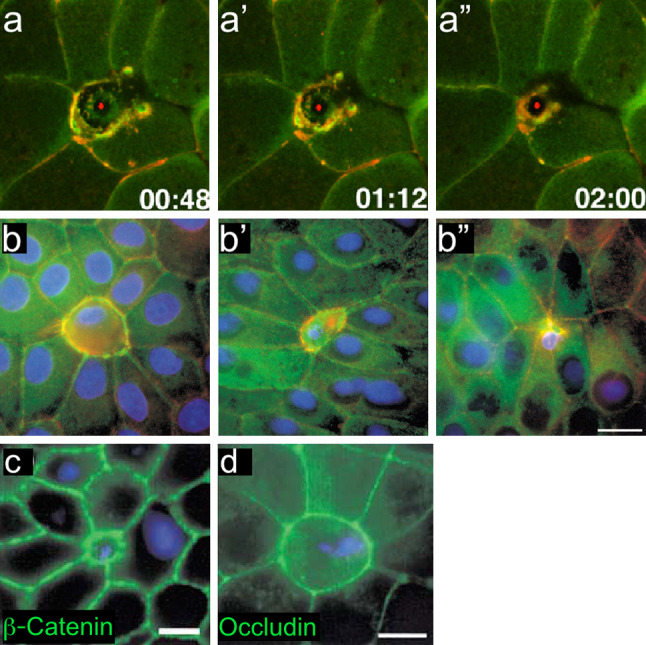
Cells unable to undergo repair in a multicellular context resemble cell extrusion during apoptosis. a–a″ Time-lapse images of a single cell being removed from the Xenopus embryo epithelia. The apoptotic cell is extruded by an actomyosin ring assembling between neighboring cells (adapted from [24], and reproduced with permission from Elsevier). b–b″ Time series showing the interface between an apoptotic cell and its neighbors at early (b), middle (b′), and late (b″) stages of apoptotic cell extrusion. Staining for myosin II (green), actin (red), and DNA (blue) shows that actin and myosin II co-localize around the apoptotic cell. c, d β-catenin (c) and occludin (d) staining shows that adherens and tight junctions are maintained between the apoptotic cell and surrounding tissue, indicating that tissue integrity is maintained during the extrusion process (adapted from [26], and reproduced with permission from Elsevier)
Multicellular repair in embryos
The embryo epidermis is a simple bilayered tissue capable of healing wounds efficiently and without leaving a scar. Epithelial wound repair mechanisms fall in two different categories based on epithelial cell mobility: wound repair can be achieved by the assembly of an actomyosin purse string at the leading edge of the wound linked by adherens junctions; and by the formation of cellular protrusions that allow the cells to crawl forward to close the wound gap (Fig. 5a–b′″). Factors such as wound size and tissue composition may determine the mechanism of repair chosen by the cells [27, 28].
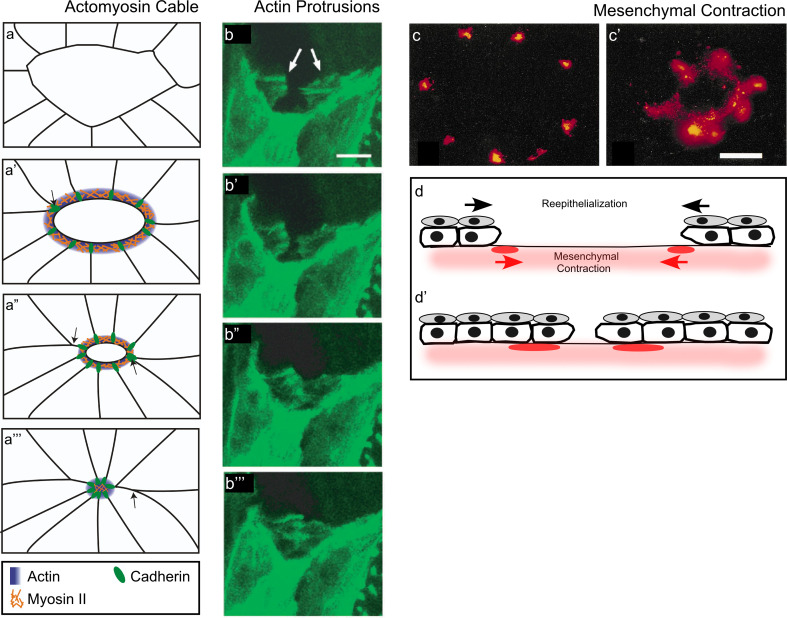
Fig. 5.
Mechanisms of embryo epithelial repair. a–a′″ Cartoon depicting the cell rearrangements and the assembly of the actomyosin purse string in epithelial wound repair. The actomyosin purse string is intercellularly linked through E-cadherin-based adherens junctions. Arrows indicate the cells that will be removed from the leading edge and moved back during repair. b–b′″ Confocal series showing actin protrusions at the wound leading edge. These protrusions use each other as tethers to close the wound (adapted from [31], and reproduced with permission from Nature Publishing Group). c–c′ In mouse embryonic epithelia, the mesenchyme is exposed upon wounding, and its contraction mediates wound closure. Mesenchyme contraction was followed with DiI for 0 h (c) and 12 h (c′) post-wounding (adapted from [91], and reproduced with permission from Springer). d–d′ Cartoon depicting the components contributing to epithelial wound repair: apical layer cell migration and contraction of the underlying mesenchyme (adapted from [91])
The actomyosin purse string
A striking feature of embryonic repair is the formation of a supracellular actomyosin purse string at the leading edge of the wound (Fig. 5a–a′″). This purse string was first described as a mechanism for wound repair in the embryonic chick wing bud, but has since been observed in mouse, frog, and fly embryos [24, 29–31]. Blocking the assembly of new filamentous actin with cytochalasin D in the mouse embryo, results in a failure to re-epithelialize the wound such that they remain open long after control wounds have healed [30]. Notably, the actin cable is under circumferential tension. This tension not only rounds the wound but also cinches the wound shut (reviewed [28]). Non-muscle myosin II is also recruited to the wound edge and co-localizes with the actin cable, and in all likelihood drives the contraction of the supracellular cable [31, 32]. Interestingly, in chick embryos, actin is recruited to the leading edge a few minutes before myosin II, suggesting that myosin II localization is dependent on actin cable assembly [32]. Similar results have been observed in vitro, where actin recruitment is followed by myosin II localization to the wound edge [27].
Another key component of embryonic wound repair machinery is the adherens junction, which links the actomyosin purse string between cells, forming a continuous, intercellular actin cable [32, 33]. Cadherin has been shown to accumulate at cell junctions along the leading edge as the actomyosin purse string is assembled in both the chick wing bud and Drosophila embryo [31, 32]. In addition to stably linking segments of the intracellular cable, junction proteins must dynamically remodel during the repair process to allow some cells to fall back from the leading edge of the wound to reduce crowding and decrease the wound circumference [32].
The Rho GTPase cytoskeletal regulator is essential for the formation of the actomyosin cable and embryos in which Rho is perturbed fail to form a functional purse string [31, 32]. Intriguingly, Rho functioning through its downstream effectors has different biochemical properties that affect all aspects of purse string biology. One possibility is that Rho inhibition directly affects the linear nucleation of actin filaments, which comprise the purse string through its interaction with proteins such as formins (reviewed by [34]). Another possible role of Rho in purse string formation may be activating Rho-kinase that, in turn, phosphorylates the myosin light chain, and may be essential for inducing the contraction of the purse string [35]. Finally, Rho may be necessary for mediating the cadherin-based intracellular linking of the actin cable through its association with α-catenin and p120catenin [36].
Cellular protrusions
Actin-based cellular protrusions from cells at the wound periphery are also a commonly observed feature of wound repair and provide an alternate means to close wounds. In the Drosophila embryo, dynamic actin-rich protrusions at the leading edge of the wound are observed throughout the repair process (Fig. 5b–b′″) [31]. Initially, these protrusions appear as filopodia, which may act to sense the substratum ahead of the leading edge, however, lamellipodia are also observed later in the repair process and work to pull the cell forward to reduce the damaged area [31]. In the final stages of wound closure, the filopodia function to mechanically close the wound by tethering to other filopodia in neighboring cells and ‘zippering’ the leading edge shut. As wound size is reduced, the frequency of contra-lateral protrusions contacting one another increases, suggesting a role for protrusions in accelerating wound closure towards the end of the repair process [31]. This increase in protrusions at the leading edge leads to the formation of ‘zippering fronts’ that eventually facilitate wound closure without the use of an actin cable [31]. The expression of a dominant negative form of Cdc42 in the epithelia of the Drosophila embryo abolishes the formation of actin-based protrusions. As a consequence, the cells at the leading edge of the wound are unable to undergo the final step of knitting, and a small hole remains in the epithelial layer [31].
Beta-Heavy Spectrin (βH-Spectrin) and its binding partner, α-Spectrin, form the apical Spectrin-based membrane skeleton (SBMS), which links the actin cytoskeleton to the membrane thus stabilizing the membrane and helping maintain cell shape. Besides linking the cytoskeleton to the membrane, the SBMS has also been shown to maintain Zonula adherens, act as a scaffold for protein interactions, and function in contractile actin-ring-based and apical constrictions. Disrupting the apical SBMS in Drosophila embryos perturbs both the actin cable and protrusions. βH-Spectrin co-localizes with actin along the leading edge of wounds in Drosophila embryos. Wounded βH-Spectrin mutant (karst d11138) embryos are unable to form a robust actin cable or cellular protrusions, and remain open long after their wild-type counterparts have closed [33].
Different wound-repair models tend to use one or the other mechanism of closure: lamellipodial crawling is often observed for large wounds, whereas actin purse string contraction is observed with smaller wounds. However, these mechanisms need not be mutually exclusive. While protrusions can compensate for the lack of the actomyosin purse string, the purse string alone is insufficient to completely compensate for a lack of protrusions, especially during the final stages of the wound closure. Thus, the relationship between the actin cable and cellular protrusions is complex and may be context-dependent.
Mesenchymal tissue contraction
In mouse and chick embryos, actin-rich protrusions have not been shown to contribute to the wound repair process. Instead, the contraction of the mesenchymal tissue underlying the epithelia plays a pivotal role in the repair response (Fig. 5c–d′). Mouse and chick embryonic epithelia are organized into an outer stratified epidermis composed of the periderm, a layer of squamous cells, and a basal layer of cuboidal cells, which in turn lie atop a bed of mesenchymal connective tissue. The mesenchyme is made up of extracellular matrix (ECM) and mesenchymal stem cells that can differentiate into all types of connective tissue (bone, cartilage, adipose, and fibroblast). Evidence for active mesenchymal contraction comes from studies in the mouse hind limb where the mesenchyme exposed by the wound was dyed post-wounding and contracts to approximately half its original area during the process of wound repair (Fig. 5c–c′) [30]. Additionally, McCluskey and Martin [30] selectively blocked the formation of the actin purse string with cytochalasin D without impairing mesenchymal tissue. While these embryos are extremely delayed in the repair process, they significantly reduce the wound area through mesenchymal contraction [32].
The mechanisms underlying mesenchymal contraction are not yet known. Staining wounded embryonic tissue in mice and sheep has shown that the mesenchymal cells underlying wounds do not express alpha smooth muscle actin (αSMA) [37], a hallmark of myofibroblasts, which contribute most of the contractile force in adult wounds (reviewed in [38]). However, in vimentin knockdown mouse embryos, fibroblasts do not undergo stereotypic contractions and, as such, wound repair is significantly impaired [39]. Vimentin-deficient fibroblasts in knockdown mice display impaired function of other cytoskeletal elements such as actin and myosin, and vimentin-deficient cultured fibroblasts appear to have altered actin filament morphology [39]. Vimentin filaments often terminate in focal adhesions, and could therefore play a role in cell adhesion to the ECM thereby affecting the ability of mesenchymal cells to participate in wound repair.
Multicellular repair in adults
Adult wound repair differs drastically from embryonic repair: it requires clot formation, an inflammatory response is elicited, and wound contraction is typically driven by fibroblasts rather than an intracellular actin cable (reviewed in [40]). Because of its clinical and day-to-day relevance to people, adult wound repair has been studied for centuries. For the most part, studies have focused on gross changes in tissue morphology, the immune response, and signaling activity at the wound site. However, recent technical advances in genetics, molecular biology, and microscopy have shifted the focus of these studies to the role of the cytoskeleton.
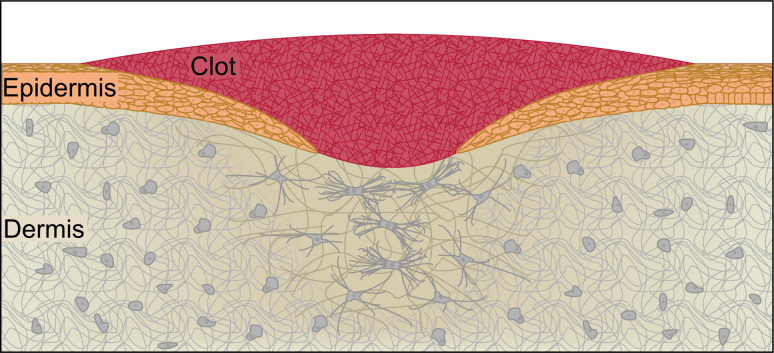
Adult wounds vary greatly from muscle tears and skin lacerations to chemical damage in the intestinal epithelium. In this review, we will focus on the repair of mammalian skin, which is made of two layers: the outer epidermis, which is composed of multiple layers of keratinocytes, and the dermis, an underlying layer of connective tissue, which is composed of a fibrous protein meshwork imbedded with fibroblasts, capillaries, hair follicles, sweat glands, and nerve endings (Fig. 6). Adult skin primarily serves as a barrier against the external environment, and wound repair must quickly reestablish this barrier function and effectively coordinate the restoration of secondary tissue functions. The adult wound-repair response occurs in four sequential but partially overlapping phases: clot formation, inflammation, tissue formation, and tissue remodeling.
Fig. 6.
Adult epithelial repair. Three to 10 days after the initial wound, new tissue is being formed and remodeled in the wound bed. The initial clot is slowly sloughed off as a scab, while the underlying keratinocytes migrate to re-epithelialize the wound. As the keratinocytes migrate, they deposit a new basement membrane and undergo rapid cycles of cell division in order to completely restore the epidermis. At this stage, fibroblasts and new capillaries have populated the underlying dermis. The fibroblasts have begun to differentiate into proto-myofibroblasts and eventually myofibroblasts, which will continuously replace the fibers that make up the ECM and apply powerful contractile forces to the wound bed
After the initial injury, keratinocytes from the wound periphery and from hair follicle sheaths [41], as well as fibroblasts beside and beneath the wound, are called to repopulate the wound site (reviewed in [40]). Depending on the size of the wound, keratinocytes take hours to days to migrate through the clot, proliferate, and cover the entire wound surface. In healthy skin, keratinocytes are attached to one another through desmosomes and anchored to the underlying basement membrane through hemidesmosomes (Fig. 7). Dissolution of these structures is necessary to allow keratinocyte migration (reviewed in [42]). Migrating keratinocytes increase the expression of integrins that bind extracellular matrix fibers found in the wound bed such as fibronectin, vitronectin, laminin, and tenascin (reviewed in [43, 44]). However, shifts in integrin expression must be coupled with changes in integrin regulation in order to establish a migratory phenotype. For example, α6β4 integrin, the principal hemidesmosome adhesion molecule, also plays an important role in migration by binding to laminin 332 fibers deposited into the wound bed (reviewed in [45, 46]).
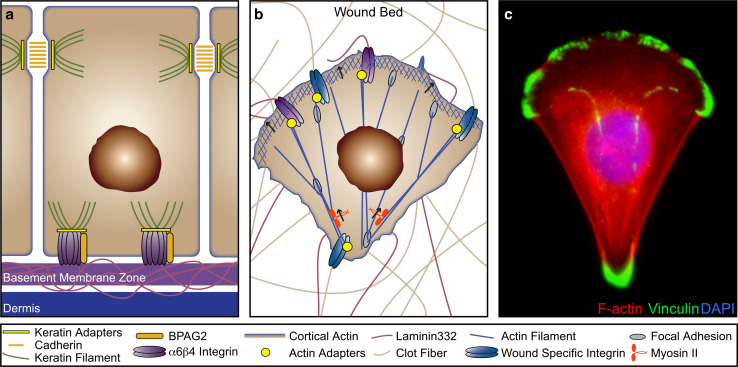
Fig. 7.
Keratinocytes in wound repair. a, b During wound repair, keratinocytes shift from a stationary barrier into highly motile invasive cells requiring dramatic cytoskeletal reconfiguration. Cartoon depicting a stationary keratinocyte in healthy epithelia (a) and migrating through a wound bed (b). c Spreading human primary keratinocyte stained for the focal adhesion protein vinculin (green), F-actin (red) and the nucleus (blue) (adapted from The Cell: An Image Library, CIL:12655, contributed by Julien Gautrot)
Migration relies on the coordination of forward protrusion and rearward retraction forces; all of which are mediated by the actin and microtubule cytoskeleton. Cells in a 3D matrix, like those migrating through the wound bed, typically migrate through a combination of amoeboid (membrane blebbing-based) and mesenchymal (stress fiber and adhesion-based) mechanisms (reviewed in [47]). Both of these mechanisms largely rely on the actin cytoskeleton for force generation and cell-shape changes. During wound repair, adhesive complexes of migrating cells must be stable enough to maintain points of contact, yet plastic enough to form at the leading edge and disassemble at the trailing edge. Migrating keratinocytes form structures known as focal adhesions, which are more dynamic than desmosomes or hemidesmosomes. Recent studies have uncovered flightless I as a key regulator of hemidesmosome and focal adhesion stability [48]. Flightless I was shown to delay wound repair by inhibiting paxillin phosphorylation, which stabilizes adhesion complexes and prevents the essential process of integrin turnover in migrating keratinocytes [48]. Once formed, focal adhesions are closely associated with myosin II and the Arp2/3 complex, cytoskeletal components responsible for force generation and actin filament nucleation [49, 50]. In the lamella, focal adhesions act as a platform for the elongating actin filaments that drive cellular protrusions forward at the leading edge of the cell (reviewed in [51]). Like hemidesmosomes, focal adhesions are integrin-based. However, instead of linking to keratin filaments, they link to actin filaments through various adapters, including plectin, paxillin, vinculin, talin, and actinin [52].
As the wound bed is repopulated, fibroblasts begin remodeling and contracting the wounded dermis until the ECM is returned to its original composition. For smaller wounds, the remodeling phase may only last a few days or weeks, whereas the process can take months or years to complete for larger wounds. Adult skin repair often fails at this tissue remodeling stage. If fibroblasts remodel too little ECM or do so too slowly, the damaged tissue remains fragile and a chronic wound can develop. On the other hand, myofibroblasts can perpetually contract the wound bed, a condition known as fibrosis, resulting in a hardened and often immobile region of scar tissue (reviewed in [40, 53]).
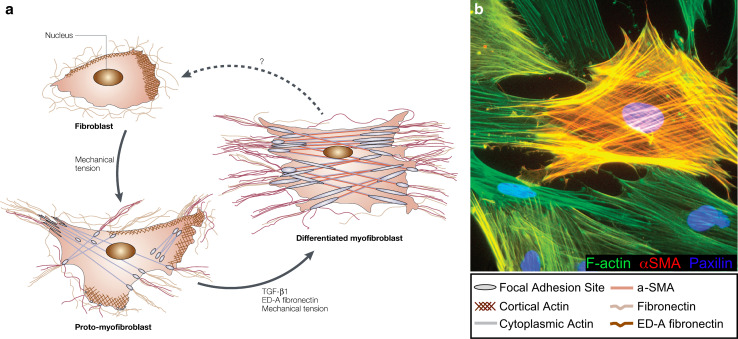
Fibroblasts must differentiate into myofibroblasts in order to apply enough force to effectively contract the wound bed (Fig. 8) (reviewed by [38, 54]). Myosin-II based contraction along stress fibers is responsible for myofibroblast force generation, a process that is largely regulated by Rho, myosin light chain kinase, and myosin light chain phosphatase [55]. The forces generated by myofibroblasts are transferred to the ECM through a specialized focal adhesion structure known as a fibronexus (actin filaments bound to integrin receptors), which in turn bind to fibronectin fibrils (reviewed by [38]). The amount of tensile forces exerted by myofibroblasts is directly linked to their level of αSMA expression [56, 57]. Decreasing αSMA levels makes myofibroblasts more motile [58], whereas introducing an amino terminal peptide of αSMA reduces contraction of myofibroblasts [59]. However, the exact mechanism by which αSMA increases the maximum amount of force a myofibroblast can apply is unknown. It also remains to be seen how the myofibroblast senses which direction to apply the force, how much force to apply, and when to stop applying force.
Fig. 8.
Fibroblast differentiation and activity in the wound bed. Fibroblasts differentiate into proto-myofibroblasts and eventually into myofibroblasts as they contract the wound bed and remodel the ECM. a Cartoon depicting the transformations of the cell’s cytoskeleton and the surrounding ECM as the fibroblast differentiates into a myofibroblast (adapted from [38], with permission from Nature Publishing Group). b Rat subcutaneous fibroblasts were differentiated into myofibroblasts in vitro. Cells were immunostained for αSMA (red) to visualize contractile stress fibers, F-actin (green), and nuclei (blue) (adapted from [92], with permission from Nature Publishing Group)
Intriguingly, certain wounds in adult tissue utilize an intracellular actomyosin cable instead of myofibroblasts to contract the wound [60–62], indicating that the cytoskeletal response may be tissue-specific and not solely dependent on developmental stage. Between the second and third trimester, fetal skin rapidly transitions from embryonic to adult-like repair. One study has found that paxillin, a focal adhesion adapter protein, is associated with the actin cable in embryonic wounds while gelsolin, an actin-severing protein, associates with the actin fibers of adult-like wounds [63]. Flightless I, which contains multiple gelsolin-like domains, is upregulated and colocalizes with actin in the leading edge cells of adult-like wounds but not embryonic wounds [64]. These data indicate that changes in the expression and localization of cytoskeletal modulators may help determine the exact mechanisms used during wound repair. Intermediate filaments present another exciting development for the wound repair field as they appear to affect keratinocyte migration and could be used to modulate repair [32, 39, 65–68].
Although most studies in adult repair have been done in mice, the Drosophila larva has emerged as a powerful genetic system for studying wound repair in post-embryonic tissue [69]. In larvae, repair follows the same steps as mammalian models. However, genetic tools and a much simpler immune response simplify the task of dissecting the cytoskeletal and signaling mechanisms underlying repair. Non-muscle myosin II was found to play a crucial role in larval wound repair. Its localization towards the trailing side of the cells in the migrating sheet is necessary for successful re-epithelialization, indicating a role for myosin in retracting the tail of migrating cells [70]. In addition, an RNAi screen was conducted in larvae, which revealed the JNK signaling pathway, as well as multiple regulators of the cytoskeleton (e.g., the Rac and Cdc42 GTPases, SCAR and Arp2/3 complex members, paxillin), as significant players in adult tissue repair [71].
Relationship of wound repair to normal morphogenetic processes and future perspectives
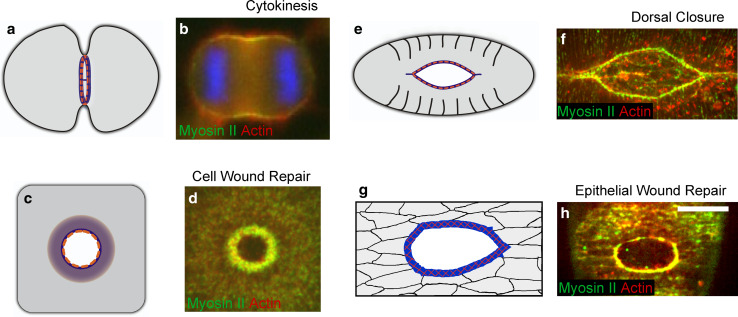
A core cytoskeletal component of wound repair is the actin/non-muscle myosin II contractile apparatus. Whether it takes the form of a ring around the wound edge or linear arrays within the wound bed, actomyosin contraction provides a major driving force for wound closure. Contractile actomyosin arrays are also key components in many normal biological processes (Fig. 9). Notably, an actomyosin ring is the driving force for cytokinesis in eukaryotic cells [72, 73]. Despite some differences in architecture and assembly, cytokinesis requires the recruitment of actin filaments and myosin II to the equator of the cell to form a contractile actomyosin ring that mediates cell cleavage from fission yeast to mammalian cells (reviewed in [74]). Similarly, studies in Drosophila and Caenorhabditis elegans have revealed the importance of supracellular actomyosin purse strings in closing tissue gaps during normal morphogenesis. In these cases, the actomyosin arrays span multiple cells and are linked by adherens junctions, creating a continuous contractile cable that contracts to bring the opposing epithelial sheets together [75–77], suggesting that contractile supracellular actomyosin purse strings are a conserved driving force in morphogenesis. However, a complete mechanism for controlling the size and shape of the actomyosin contractile apparatus has yet to be determined.
Fig. 9.
Transient actomyosin arrays are the driving force in multiple biological processes. a Schematic diagram of the actomyosin contractile ring that assembles during cytokinesis (in all diagrams actin is blue, and myosin II is orange). b Actomyosin array in a dividing HeLa cell. During cytokinesis, F-actin (red) and myosin II (green) assemble a contractile ring at the cell equator region mediating cell division (adapted from [81], reproduced with permission from Springer). c Diagram of the actomyosin contractile array in single cell wound repair. d An actomyosin contractile array is assembled at the leading edge of single cell wounds. Confocal image showing actin (red) and myosin II (green) recruitment at the wound site in the early Drosophila embryo (adapted from [13], originally published in Journal of Cell Biology. doi:10.1083/jcb.201011018). e Cartoon of dorsal closure in the Drosophila embryo depicting the actomyosin purse string. f A continuous supracellular actomyosin purse string contributes to dorsal closure in the Drosophila embryo, actin (red) and myosin II (green) are enriched at the leading edge of the dorsal hole and drive epithelialization. g Cartoon of tissue wound repair where a multicellular actomyosin purse string is assembled at the wound leading edge. h The actomyosin (actin red and myosin II green) purse string is observed at the leading edge of multicellular wounds in late Drosophila embryos few minutes after the tissue damage (adapted from [93], originally published in Bioarchitecture. doi:10.4161/bioa.1.3.17091)
The similarities between wound repair and normal morphogenetic processes extend to the regulators of the cytoskeleton response. Rho family small GTPases are well-known regulators of the cytoskeleton and particularly of transient contractile arrays such as observed in cytokinesis [78], single cell wound repair [20], dorsal closure [79], and tissue repair [31]. Similar to the Rho and Cdc42 active zones observed in single cell wound repair [20], a Rho active zone develops at the site where the cytokinetic apparatus is assembled in echinoderm and Xenopus embryos [80]. Recent studies suggest that both GTPases are also active around the cell equator during cytokinesis in culture cells [81–83]. Similarly, genetic evidence from Drosophila indicates that Rho, Rac, and Cdc42 are required in cytokinesis [84, 85]. However, little is known about how cells choose anchor points for contractile machinery, determine its length and thickness, or build a curved rather than a linear actomyosin apparatus.
Actomyosin contraction cannot aid in the repair process without a physical link to the wound edge. During multicellular repair, E-cadherin transmits the forces of intracellular contraction to neighboring cells, forming an intracellular actomyosin purse string around the wound. While stable adherens junctions are needed to transmit contractile forces, they also display a level of plasticity that is not well understood. For example, epithelial cells are able to ‘slide’ back from the leading edge of the wound without disrupting the actomyosin cable, even when they form an essential part of the cable. This requires cells to break and reform their cell–cell contacts or move apical junctions laterally through the cell membrane during contact rearrangement. Either way, cells at the leading edge of a wound must balance the need for stable, force-transmitting junctions against the necessary reorganization of cell contacts during repair. Surprisingly, E-cadherin also transmits the contractile forces of the actomyosin ring during single cell repair, where adjacent cells are often unavailable for the formation of apical junctions [13]. This suggests that cadherins have the potential to link actin to the membrane precluding the need for it to form intercellular junctions.
Cadherin is not the only protein known that can transfer actomyosin contractile forces to the wound edge: Spectrin, as well as ERM family proteins, have been shown to physically link actin to the plasma membrane [86, 87]. Loss of Spectrin during embryonic wound repair results in both an impaired actin cable and a reduction of cellular protrusions [33]. Spectrin is actively transcribed in adult keratinocytes [88], but its role in repair has not been investigated. In addition, Spectrin, along with ankyrin and protein 4.1, are all calpain substrates and may be cleaved during the calpain-dependent removal of damaged cortical cytoskeleton during the initial stages of single cell repair [89]. It has been shown that early Drosophila embryos lacking E-cadherin can still form a contractile ring and close a wound [13], a process that may be mediated by Spectrin or the ERM family of proteins.
At the morphological level, another striking similarity between wound repair and normal morphogenetic processes is the formation of actin-rich protrusions. In both, multicellular wound repair and dorsal closure, actin-rich protrusions are observed in the leading edge cells where they sense the environment, facilitate cell migration, and mediate the zippering between opposing epithelial sheets [18, 28, 31, 90]. In all of these cases, the actin-rich protrusions contact each other and mediate the final steps of closure to form a permanent and neat seam.
Overall, the emerging picture is one of striking conservation among wound-repair models and types of repair. Similar cytoskeletal elements are utilized from single-cell to adult tissue repair, and in basic processes such as cytokinesis and morphogenesis, reflecting the evolutionary conservation of basic cytoskeleton machineries and the capacity of the cell and organism to adapt these machineries for multiple biological functions. However, these same machineries can work against the organism. Many of the cytoskeletal responses to wounds are also utilized by metastasizing cancer cells. Therefore, understanding the cellular mechanisms governing wound repair will inform a broad range of clinical circumstances in addition to advancing our knowledge of wound repair and cytoskeleton.
Acknowledgments
We thank Parkhurst lab members for their interest, advice, and comments on the manuscript. This work was supported by NIH Grant GM092731 to SMP.
Abbreviations
- ERM
Ezrin-radixin-moesin
- SBMS
Spectrin-based membrane skeleton
- ECM
Extracellular matrix
- αSMA
Alpha smooth muscle actin
References
- 1.Clarke MS, Caldwell RW, Chiao H, Miyake K, McNeil PL. Contraction-induced cell wounding and release of fibroblast growth factor in heart. Circ Res. 1995;76(6):927–934. doi: 10.1161/01.RES.76.6.927. [DOI] [PubMed] [Google Scholar]
- 2.McNeil PL, Ito S. Gastrointestinal cell plasma membrane wounding and resealing in vivo. Gastroenterology. 1989;96(5 Pt 1):1238–1248. doi: 10.1016/s0016-5085(89)80010-1. [DOI] [PubMed] [Google Scholar]
- 3.McNeil PL, Ito S. Molecular traffic through plasma membrane disruptions of cells in vivo. J Cell Sci. 1990;96(Pt 3):549–556. doi: 10.1242/jcs.96.3.549. [DOI] [PubMed] [Google Scholar]
- 4.McNeil PL, Steinhardt RA. Plasma membrane disruption: repair, prevention, adaptation. Annu Rev Cell Dev Biol. 2003;19:697–731. doi: 10.1146/annurev.cellbio.19.111301.140101. [DOI] [PubMed] [Google Scholar]
- 5.Bement WM, Yu HY, Burkel BM, Vaughan EM, Clark AG. Rehabilitation and the single cell. Curr Opin Cell Biol. 2007;19(1):95–100. doi: 10.1016/j.ceb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Togo T, Krasieva TB, Steinhardt RA. A decrease in membrane tension precedes successful cell-membrane repair. Mol Biol Cell. 2000;11(12):4339–4346. doi: 10.1091/mbc.11.12.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyake K, McNeil PL, Suzuki K, Tsunoda R, Sugai N. An actin barrier to resealing. J Cell Sci. 2001;114(Pt 19):3487–3494. doi: 10.1242/jcs.114.19.3487. [DOI] [PubMed] [Google Scholar]
- 8.Mellgren RL, Zhang W, Miyake K, McNeil PL. Calpain is required for the rapid, calcium-dependent repair of wounded plasma membrane. J Biol Chem. 2007;282(4):2567–2575. doi: 10.1074/jbc.M604560200. [DOI] [PubMed] [Google Scholar]
- 9.Mellgren RL. A plasma membrane wound proteome: reversible externalization of intracellular proteins following reparable mechanical damage. J Biol Chem. 2010;285(47):36597–36607. doi: 10.1074/jbc.M110.110015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bi GQ, Morris RL, Liao G, Alderton JM, Scholey JM, Steinhardt RA. Kinesin- and myosin-driven steps of vesicle recruitment for Ca2+ -regulated exocytosis. J Cell Biol. 1997;138(5):999–1008. doi: 10.1083/jcb.138.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinhardt RA, Bi G, Alderton JM. Cell membrane resealing by a vesicular mechanism similar to neurotransmitter release. Science. 1994;263(5145):390–393. doi: 10.1126/science.7904084. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez OC, Schaefer AW, Mandato CA, Forscher P, Bement WM, Waterman-Storer CM. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat Cell Biol. 2003;5(7):599–609. doi: 10.1038/ncb0703-599. [DOI] [PubMed] [Google Scholar]
- 13.Abreu-Blanco MT, Verboon JM, Parkhurst SM. Cell wound repair in Drosophila occurs through three distinct phases of membrane and cytoskeletal remodeling. J Cell Biol. 2011;193(3):455–464. doi: 10.1083/jcb.201011018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Togo T. Disruption of the plasma membrane stimulates rearrangement of microtubules and lipid traffic toward the wound site. J Cell Sci. 2006;119(Pt 13):2780–2786. doi: 10.1242/jcs.03006. [DOI] [PubMed] [Google Scholar]
- 15.McNeil PL, Vogel SS, Miyake K, Terasaki M. Patching plasma membrane disruptions with cytoplasmic membrane. J Cell Sci. 2000;113(Pt 11):1891–1902. doi: 10.1242/jcs.113.11.1891. [DOI] [PubMed] [Google Scholar]
- 16.Godin LM, Vergen J, Prakash YS, Pagano RE, Hubmayr RD. Spatiotemporal dynamics of actin remodeling and endomembrane trafficking in alveolar epithelial type I cell wound healing. Am J Physiol Lung Cell Mol Physiol. 2011;300(4):L615–L623. doi: 10.1152/ajplung.00265.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bement WM, Mandato CA, Kirsch MN. Wound-induced assembly and closure of an actomyosin purse string in Xenopus oocytes. Curr Biol. 1999;9(11):579–587. doi: 10.1016/S0960-9822(99)80261-9. [DOI] [PubMed] [Google Scholar]
- 18.Mandato CA, Bement WM. Contraction and polymerization cooperate to assemble and close actomyosin rings around Xenopus oocyte wounds. J Cell Biol. 2001;154(4):785–797. doi: 10.1083/jcb.200103105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandato CA, Bement WM. Actomyosin transports microtubules and microtubules control actomyosin recruitment during Xenopus oocyte wound healing. Curr Biol. 2003;13(13):1096–1105. doi: 10.1016/S0960-9822(03)00420-2. [DOI] [PubMed] [Google Scholar]
- 20.Benink HA, Bement WM. Concentric zones of active RhoA and Cdc42 around single cell wounds. J Cell Biol. 2005;168(3):429–439. doi: 10.1083/jcb.200411109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaughan EM, Miller AL, Yu HY, Bement WM. Control of local Rho GTPase crosstalk by Abr. Curr Biol. 2011;21(4):270–277. doi: 10.1016/j.cub.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett V. The molecular basis for membrane—cytoskeleton association in human erythrocytes. J Cell Biochem. 1982;18(1):49–65. doi: 10.1002/jcb.1982.240180106. [DOI] [PubMed] [Google Scholar]
- 23.Fievet B, Louvard D, Arpin M. ERM proteins in epithelial cell organization and functions. Biochim Biophys Acta. 2007;1773(5):653–660. doi: 10.1016/j.bbamcr.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Clark AG, Miller AL, Vaughan E, Yu HY, Penkert R, Bement WM. Integration of single and multicellular wound responses. Curr Biol. 2009;19(16):1389–1395. doi: 10.1016/j.cub.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Covian-Nares JF, Koushik SV, Puhl HL, 3rd, Vogel SS. Membrane wounding triggers ATP release and dysferlin-mediated intercellular calcium signaling. J Cell Sci. 2010;123(Pt 11):1884–1893. doi: 10.1242/jcs.066084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenblatt J, Raff MC, Cramer LP. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol. 2001;11(23):1847–1857. doi: 10.1016/S0960-9822(01)00587-5. [DOI] [PubMed] [Google Scholar]
- 27.Bement WM, Forscher P, Mooseker MS. A novel cytoskeletal structure involved in purse string wound closure and cell polarity maintenance. J Cell Biol. 1993;121(3):565–578. doi: 10.1083/jcb.121.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacinto A, Martinez-Arias A, Martin P. Mechanisms of epithelial fusion and repair. Nat Cell Biol. 2001;3(5):E117–E123. doi: 10.1038/35074643. [DOI] [PubMed] [Google Scholar]
- 29.Martin P, Lewis J. Actin cables and epidermal movement in embryonic wound healing. Nature. 1992;360(6400):179–183. doi: 10.1038/360179a0. [DOI] [PubMed] [Google Scholar]
- 30.McCluskey J, Martin P. Analysis of the tissue movements of embryonic wound healing—DiI studies in the limb bud stage mouse embryo. Dev Biol. 1995;170(1):102–114. doi: 10.1006/dbio.1995.1199. [DOI] [PubMed] [Google Scholar]
- 31.Wood W, Jacinto A, Grose R, Woolner S, Gale J, Wilson C, Martin P. Wound healing recapitulates morphogenesis in Drosophila embryos. Nat Cell Biol. 2002;4(11):907–912. doi: 10.1038/ncb875. [DOI] [PubMed] [Google Scholar]
- 32.Brock J, McCluskey J, Baribault H, Martin P. Perfect wound healing in the keratin 8 deficient mouse embryo. Cell Motil Cytoskelet. 1996;35(4):358–366. doi: 10.1002/(SICI)1097-0169(1996)35:4<358::AID-CM7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 33.Campos I, Geiger JA, Santos AC, Carlos V, Jacinto A. Genetic screen in Drosophila melanogaster uncovers a novel set of genes required for embryonic epithelial repair. Genetics. 2010;184(1):129–140. doi: 10.1534/genetics.109.110288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu R, Linardopoulou EV, Osborn GE, Parkhurst SM. Formins in development: orchestrating body plan origami. Biochim Biophys Acta 1803. 2010;2:207–225. doi: 10.1016/j.bbamcr.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271(34):20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 36.Magie CR, Pinto-Santini D, Parkhurst SM. Rho1 interacts with p120ctn and alpha-catenin, and regulates cadherin-based adherens junction components in Drosophila. Development. 2002;129(16):3771–3782. doi: 10.1242/dev.129.16.3771. [DOI] [PubMed] [Google Scholar]
- 37.Estes JM, Vande Berg JS, Adzick NS, MacGillivray TE, Desmouliere A, Gabbiani G. Phenotypic and functional features of myofibroblasts in sheep fetal wounds. Differentiation. 1994;56(3):173–181. doi: 10.1046/j.1432-0436.1994.5630173.x. [DOI] [PubMed] [Google Scholar]
- 38.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Natl Rev Mol Cell Biol. 2002;3(5):349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 39.Eckes B, Colucci-Guyon E, Smola H, Nodder S, Babinet C, Krieg T, Martin P. Impaired wound healing in embryonic and adult mice lacking vimentin. J Cell Sci. 2000;113(Pt 13):2455–2462. doi: 10.1242/jcs.113.13.2455. [DOI] [PubMed] [Google Scholar]
- 40.Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276(5309):75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 41.Jahoda CA, Reynolds AJ. Hair follicle dermal sheath cells: unsung participants in wound healing. Lancet. 2001;358(9291):1445–1448. doi: 10.1016/S0140-6736(01)06532-1. [DOI] [PubMed] [Google Scholar]
- 42.Margadant C, Frijns E, Wilhelmsen K, Sonnenberg A. Regulation of hemidesmosome disassembly by growth factor receptors. Curr Opin Cell Biol. 2008;20(5):589–596. doi: 10.1016/j.ceb.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Juhasz I, Murphy GF, Yan HC, Herlyn M, Albelda SM. Regulation of extracellular matrix proteins and integrin cell substratum adhesion receptors on epithelium during cutaneous human wound healing in vivo. Am J Pathol. 1993;143(5):1458–1469. [PMC free article] [PubMed] [Google Scholar]
- 44.Raja SivamaniK, Garcia MS, Isseroff RR. Wound re-epithelialization: modulating keratinocyte migration in wound healing. Front Biosci. 2007;12:2849–2868. doi: 10.2741/2277. [DOI] [PubMed] [Google Scholar]
- 45.Litjens SH, de Pereda JM, Sonnenberg A. Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol. 2006;16(7):376–383. doi: 10.1016/j.tcb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Margadant C, Raymond K, Kreft M, Sachs N, Janssen H, Sonnenberg A. Integrin alpha3beta1 inhibits directional migration and wound re-epithelialization in the skin. J Cell Sci. 2009;122(Pt 2):278–288. doi: 10.1242/jcs.029108. [DOI] [PubMed] [Google Scholar]
- 47.Grinnell F, Petroll WM. Cell motility and mechanics in three-dimensional collagen matrices. Annu Rev Cell Dev Biol. 2010;26:335–361. doi: 10.1146/annurev.cellbio.042308.113318. [DOI] [PubMed] [Google Scholar]
- 48.Kopecki Z, Arkell R, Powell BC, Cowin AJ. Flightless I regulates hemidesmosome formation and integrin-mediated cellular adhesion and migration during wound repair. J Invest Dermatol. 2009;129(8):2031–2045. doi: 10.1038/jid.2008.461. [DOI] [PubMed] [Google Scholar]
- 49.Serrels B, Serrels A, Brunton VG, Holt M, McLean GW, Gray CH, Jones GE, Frame MC. Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat Cell Biol. 2007;9(9):1046–1056. doi: 10.1038/ncb1626. [DOI] [PubMed] [Google Scholar]
- 50.Tang DD, Turner CE, Gunst SJ. Expression of non-phosphorylatable paxillin mutants in canine tracheal smooth muscle inhibits tension development. J Physiol. 2003;553(Pt 1):21–35. doi: 10.1113/jphysiol.2003.045047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Small JV, Resch GP. The comings and goings of actin: coupling protrusion and retraction in cell motility. Curr Opin Cell Biol. 2005;17(5):517–523. doi: 10.1016/j.ceb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 52.Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9(8):858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Satish L, Kathju S. Cellular and molecular characteristics of scarless versus fibrotic wound healing. Dermatol Res Pract. 2010;2010:790234. doi: 10.1155/2010/790234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127(3):526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 55.Parizi M, Howard EW, Tomasek JJ. Regulation of LPA-promoted myofibroblast contraction: role of Rho, myosin light chain kinase, and myosin light chain phosphatase. Exp Cell Res. 2000;254(2):210–220. doi: 10.1006/excr.1999.4754. [DOI] [PubMed] [Google Scholar]
- 56.Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990;63(1):21–29. [PubMed] [Google Scholar]
- 57.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12(9):2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ronnov-Jessen L, Petersen OW. A function for filamentous alpha-smooth muscle actin: retardation of motility in fibroblasts. J Cell Biol. 1996;134(1):67–80. doi: 10.1083/jcb.134.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hinz B, Gabbiani G, Chaponnier C. The NH2-terminal peptide of alpha-smooth muscle actin inhibits force generation by the myofibroblast in vitro and in vivo. J Cell Biol. 2002;157(4):657–663. doi: 10.1083/jcb.200201049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Danjo Y, Gipson IK. Actin ‘purse string’ filaments are anchored by E-cadherin-mediated adherens junctions at the leading edge of the epithelial wound, providing coordinated cell movement. J Cell Sci. 1998;111(Pt 22):3323–3332. doi: 10.1242/jcs.111.22.3323. [DOI] [PubMed] [Google Scholar]
- 61.Grootjans J, Thuijls G, Derikx JP, van Dam RM, Dejong CH, Buurman WA. Rapid lamina propria retraction and zipper-like constriction of the epithelium preserves the epithelial lining in human small intestine exposed to ischaemia-reperfusion. J Pathol. 2011;224(3):411–419. doi: 10.1002/path.2882. [DOI] [PubMed] [Google Scholar]
- 62.Russo JM, Florian P, Shen L, Graham WV, Tretiakova MS, Gitter AH, Mrsny RJ, Turner JR. Distinct temporal-spatial roles for rho kinase and myosin light chain kinase in epithelial purse-string wound closure. Gastroenterology. 2005;128(4):987–1001. doi: 10.1053/j.gastro.2005.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cowin AJ, Hatzirodos N, Teusner JT, Belford DA. Differential effect of wounding on actin and its associated proteins, paxillin and gelsolin, in fetal skin explants. J Invest Dermatol. 2003;120(6):1118–1129. doi: 10.1046/j.1523-1747.2003.12231.x. [DOI] [PubMed] [Google Scholar]
- 64.Lin CH, Waters JM, Powell BC, Arkell RM, Cowin AJ. Decreased expression of Flightless I, a gelsolin family member and developmental regulator, in early-gestation fetal wounds improves healing. Mamm Genome. 2011;22(5–6):341–352. doi: 10.1007/s00335-011-9320-z. [DOI] [PubMed] [Google Scholar]
- 65.Jang SI, Kalinin A, Takahashi K, Marekov LN, Steinert PM. Characterization of human epiplakin: RNAi-mediated epiplakin depletion leads to the disruption of keratin and vimentin IF networks. J Cell Sci. 2005;118(Pt 4):781–793. doi: 10.1242/jcs.01647. [DOI] [PubMed] [Google Scholar]
- 66.Wong P, Coulombe PA. Loss of keratin 6 (K6) proteins reveals a function for intermediate filaments during wound repair. J Cell Biol. 2003;163(2):327–337. doi: 10.1083/jcb.200305032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woolley K, Martin P. Conserved mechanisms of repair: from damaged single cells to wounds in multicellular tissues. Bioessays. 2000;22(10):911–919. doi: 10.1002/1521-1878(200010)22:10<911::AID-BIES6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 68.Wu X, Kodama A, Fuchs E. ACF7 regulates cytoskeletal-focal adhesion dynamics and migration and has ATPase activity. Cell. 2008;135(1):137–148. doi: 10.1016/j.cell.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Galko MJ, Krasnow MA. Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol. 2004;2(8):E239. doi: 10.1371/journal.pbio.0020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kwon YC, Baek SH, Lee H, Choe KM. Nonmuscle myosin II localization is regulated by JNK during Drosophila larval wound healing. Biochem Biophys Res Commun. 2010;393(4):656–661. doi: 10.1016/j.bbrc.2010.02.047. [DOI] [PubMed] [Google Scholar]
- 71.Lesch C, Jo J, Wu Y, Fish GS, Galko MJ. A targeted UAS-RNAi screen in Drosophila larvae identifies wound closure genes regulating distinct cellular processes. Genetics. 2010 doi: 10.1534/genetics.110.121822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Field C, Li R, Oegema K. Cytokinesis in eukaryotes: a mechanistic comparison. Curr Opin Cell Biol. 1999;11(1):68–80. doi: 10.1016/S0955-0674(99)80009-X. [DOI] [PubMed] [Google Scholar]
- 73.Pollard TD. Mechanics of cytokinesis in eukaryotes. Curr Opin Cell Biol. 2010;22(1):50–56. doi: 10.1016/j.ceb.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pollard TD, Wu JQ. Understanding cytokinesis: lessons from fission yeast. Natl Rev Mol Cell Biol. 2010;11(2):149–155. doi: 10.1038/nrm2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hutson MS, Tokutake Y, Chang MS, Bloor JW, Venakides S, Kiehart DP, Edwards GS. Forces for morphogenesis investigated with laser microsurgery and quantitative modeling. Science. 2003;300(5616):145–149. doi: 10.1126/science.1079552. [DOI] [PubMed] [Google Scholar]
- 76.Kiehart DP, Galbraith CG, Edwards KA, Rickoll WL, Montague RA. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J Cell Biol. 2000;149(2):471–490. doi: 10.1083/jcb.149.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams-Masson EM, Malik AN, Hardin J. An actin-mediated two-step mechanism is required for ventral enclosure of the C. elegans hypodermis. Development. 1997;124(15):2889–2901. doi: 10.1242/dev.124.15.2889. [DOI] [PubMed] [Google Scholar]
- 78.Kishi K, Sasaki T, Kuroda S, Itoh T, Takai Y. Regulation of cytoplasmic division of Xenopus embryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) J Cell Biol. 1993;120(5):1187–1195. doi: 10.1083/jcb.120.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Woolner S, Jacinto A, Martin P. The small GTPase Rac plays multiple roles in epithelial sheet fusion–dynamic studies of Drosophila dorsal closure. Dev Biol. 2005;282(1):163–173. doi: 10.1016/j.ydbio.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 80.Bement WM, Benink HA, von Dassow G. A microtubule-dependent zone of active RhoA during cleavage plane specification. J Cell Biol. 2005;170(1):91–101. doi: 10.1083/jcb.200501131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kamijo K, Ohara N, Abe M, Uchimura T, Hosoya H, Lee JS, Miki T. Dissecting the role of Rho-mediated signaling in contractile ring formation. Mol Biol Cell. 2006;17(1):43–55. doi: 10.1091/mbc.E05-06-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoshizaki H, Ohba Y, Kurokawa K, Itoh RE, Nakamura T, Mochizuki N, Nagashima K, Matsuda M. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. J Cell Biol. 2003;162(2):223–232. doi: 10.1083/jcb.200212049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yuce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J Cell Biol. 2005;170(4):571–582. doi: 10.1083/jcb.200501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Crawford JM, Harden N, Leung T, Lim L, Kiehart DP. Cellularization in Drosophila melanogaster is disrupted by the inhibition of rho activity and the activation of Cdc42 function. Dev Biol. 1998;204(1):151–164. doi: 10.1006/dbio.1998.9061. [DOI] [PubMed] [Google Scholar]
- 85.D’Avino PP, Savoian MS, Glover DM. Mutations in sticky lead to defective organization of the contractile ring during cytokinesis and are enhanced by Rho and suppressed by Rac. J Cell Biol. 2004;166(1):61–71. doi: 10.1083/jcb.200402157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Neisch AL, Fehon RG. Ezrin, Radixin and Moesin: key regulators of membrane-cortex interactions and signaling. Curr Opin Cell Biol. 2011;23(4):377–382. doi: 10.1016/j.ceb.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thomas GH. Spectrin: the ghost in the machine. Bioessays. 2001;23(2):152–160. doi: 10.1002/1521-1878(200102)23:2<152::AID-BIES1022>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 88.Mutha S, Langston A, Bonifas JM, Epstein EH., Jr Biochemical identification of alpha-fodrin and protein 4.1 in human keratinocytes. J Invest Dermatol. 1991;97(3):383–388. doi: 10.1111/1523-1747.ep12480948. [DOI] [PubMed] [Google Scholar]
- 89.Baines AJ. The spectrin-ankyrin-4.1-adducin membrane skeleton: adapting eukaryotic cells to the demands of animal life. Protoplasma. 2010;244(1–4):99–131. doi: 10.1007/s00709-010-0181-1. [DOI] [PubMed] [Google Scholar]
- 90.Jacinto A, Wood W, Woolner S, Hiley C, Turner L, Wilson C, Martinez-Arias A, Martin P. Dynamic analysis of actin cable function during Drosophila dorsal closure. Curr Biol. 2002;12(14):1245–1250. doi: 10.1016/S0960-9822(02)00955-7. [DOI] [PubMed] [Google Scholar]
- 91.Nodder S, Martin P. Wound healing in embryos: a review. Anat Embryol (Berl) 1997;195(3):215–228. doi: 10.1007/s004290050041. [DOI] [PubMed] [Google Scholar]
- 92.Modarressi A, Pietramaggiori G, Godbout C, Vigato E, Pittet B, Hinz B. Hypoxia impairs skin myofibroblast differentiation and function. J Invest Dermatol. 2010;130(12):2818–2827. doi: 10.1038/jid.2010.224. [DOI] [PubMed] [Google Scholar]
- 93.Abreu-Blanco MT, Verboon JM, Parkhurst SM. Single cell wound repair: dealing with life’s little traumas. Bioarchitecture. 2011;1(3):114–121. doi: 10.4161/bioa.1.3.17091. [DOI] [PMC free article] [PubMed] [Google Scholar]