Abstract
Cutaneous wound healing is a complex process involving blood clotting, inflammation, migration of keratinocytes, angiogenesis, and, ultimately, tissue remodeling and wound closure. Many of these processes involve transforming growth factor-β (TGF-β) signaling, and mice lacking components of the TGF-β signaling pathway are defective in wound healing. We show herein that CLIC4, an integral component of the TGF-β pathway, is highly up-regulated in skin wounds. We genetically deleted murine CLIC4 and generated a colony on a C57Bl/6 background. CLIC4NULL mice were viable and fertile but had smaller litters than did wild-type mice. After 6 months of age, up to 40% of null mice developed spontaneous skin erosions. Reepithelialization of induced full-thickness skin wounds and superficial corneal wounds was delayed in CLIC4NULL mice, resolution of inflammation was delayed, and expression of β4 integrin and p21 was reduced in lysates of constitutive and wounded CLIC4NULL skin. The induced level of phosphorylated Smad2 in response to TGF-β was reduced in cultured CLIC4NULL keratinocytes relative to in wild-type cells, and CLIC4NULL keratinocytes migrated slower than did wild-type keratinocytes and did not increase migration in response to TGF-β. CLIC4NULL keratinocytes were also less adherent on plates coated with matrix secreted by wild-type keratinocytes. These results indicate that CLIC4 participates in skin healing and corneal wound reepithelialization through enhancement of epithelial migration by a mechanism that may involve a compromised TGF-β pathway.
CLIC4 belongs to the family of chloride intracellular channel proteins, which is composed of seven family members: p64, CLIC1 to CLIC5, and parchorin.1 Although the nature of participation of the membrane-associated CLIC4 in chloride transport is unsettled,2 the soluble protein is multifunctional and resides in multiple cellular compartments, including the cytoplasm. In the CLIC4 protein sequence is a C-terminal nuclear localization signal that promotes its translocation to the nucleus on several types of cellular stress.3,4 CLIC4 is a direct downstream response gene for p53- and c-Myc–mediated transcription and is essential for p53- and c-Myc–mediated apoptosis.5,6
CLIC proteins are highly conserved throughout evolution, and a CLIC homologue, EXC-4, is essential for excretory canal tube formation in Caenorhabditis elegans.7 CLIC4 has a similar function in vascular tubulogenesis in vitro, where its expression is induced in endothelial cells exposed to vascular endothelial growth factor-A, and its silencing decreases capillary network formation and sprouting and lumen formation in vitro.8,9 Recently, defective angiogenesis was the primary phenotype reported in a CLIC4NULL mouse model.10
A function for CLIC4 in transforming growth factor-β (TGF-β) signaling was first suggested subsequent to an mRNA analysis of genes modified during the TGF-β–mediated conversion of fibroblasts to myofibroblasts.11 This connection and the mechanism involved was solidified by the discovery that nuclear CLIC4 binds to phospho-Smads (p-Smads) 2 and 3 and inhibits dephosphorylation by its selective phosphatase PPM1a, thus prolonging the intracellular TGF-β signal and enhancing downstream responses.12
To study the broader consequences of CLIC4 depletion in vivo, we generated mice lacking its expression. These results confirm the participation of CLIC4 in TGF-β signaling and reveal an important role for CLIC4 in skin and corneal wound healing. The experimental results suggest that these two findings are related.
Materials and Methods
Targeting Strategy of CLIC4 Locus and CLIC4NULL Mouse Generation
Recombineering technology was used for generation of the targeting vector.13 Briefly, bacterial artificial chromosome RP23-57P18 (BSP001) containing a 184-kb DNA sequence spanning 134,699,828 and 134,884,152 of mouse chromosome 4 containing the CLIC4 locus was purchased from Invitrogen (Carlsbad, CA). pBluescript light vector (Agilent Technologies Inc., Santa Clara, CA) containing the thymidine kinase gene (TK1) was used as a backbone for the targeting vector. The region of interest, including 4.2 kb upstream and 3.3 kb downstream of the second exon of the CLIC4 locus, was recombined into the targeting vector (see Supplemental Figure S1A at http://ajp.amjpathol.org). One loxP site was inserted 1.1 kb upstream, and the second loxP site was introduced 643 bp downstream of CLIC4 exon 2. The final targeting vector also contained a neomycin cassette flanked by FLP recognition target sites for positive selection and a TK gene for negative selection of embryonic stem cell clones. Generation of chimeric CLIC4 conditional knockout mice was obtained by standard technology. Briefly, the targeting vector (BSP031) was linearized by NotI digestion and was electroporated into mouse embryonic stem cells v6.4 (hybrid 129Sv/C57BL/6). Embryonic stem cell clones were subsequently selected with G418 and ganciclovir. Correctly targeted clones were identified by Southern blot analysis using probes specific to regions external to the 5′ and 3′ homology arms (see Supplemental Figure S1B at http://ajp.amjpathol.org). The 5′ probe was amplified using oligonucleotides 5′-GGGATTGGTCAGTTTGAAGACA-3′ (OSP048) and 5′-AGTGAGAGACTAGGAGCTAGAC-3′ (OSP049), and the 3′ probe was amplified using oligonucleotides 5′-CATGAAGAATCTTACTTGCACT-3′ (OSP050) and 5′-TGAGACATAAAACTGAAAAC-3′ (OSP051). Correctly targeted clones were injected into blastocysts, and germline transmission was achieved from mating an 85% chimera to a C57BL/6 wild-type (WT) female.
Genotyping
PCR was routinely used for genotyping. WT and CLIC4FLOX mice were identified by genotyping with OSP147 and OSP190 oligonucleotides, which gives rise to a 604-bp WT band and a 446-bp flox band. Two sets of oligonucleotides were used for genotyping WT and CLIC4NULL mice. Oligonucleotides OSP147 and OSP190 produce a 496-bp WT band, and oligonucleotides OSP146 and OSP148 give rise to a 372-bp CLIC4NULL band. The sequences of the oligonucleotides used for genotyping are as follows: 5′-TCCCCATCTCCCTTTGAATCTTG-3′ (OSP146), 5′-CATGTTATTTCATGGAGCAAGAA-3′ (OSP147), 5′-CACGGTTTAGCCAGGCTGACTGG-3′ (OSP148), and 5′-AAGTAAACAAGCAGGGGACTTC-3′ (OSP190).
Skin Wounding
Full-thickness and epidermal abrasion wounds were performed on mice that were approximately 50 days old during the resting phase of the hair cycle. An 8-mm-diameter, circular, full-thickness wound was made on the back, and the mice were observed for 12 days. Pictures were taken intermittently, and samples were collected for immunohistochemical (IHC) analysis at required time points. Epidermal abrasion was performed using a small felt wheel attached to a handheld electric tool (Dremel tool). A 2 cm2 region on the lower back was abraded. Wound depth was controlled by applying the same rotation speed and pressure. Abraded skin was clearly identified by the pink color of the deepidermalized area, and mice that bled during the procedure were discarded from the study and immediately euthanized. Skin samples were collected for IHC analysis 8 days after wounding. All the animal experiments were performed in accordance with the guidelines and approval of the National Cancer Institute Animal Care and Use Committee.
Corneal Wounding
Corneal epithelium debridement was performed on 24 WT and 27 CLIC4NULL mice to study the effect of loss of CLIC4 on corneal wound healing.14 After anesthesia with ketamine and xylazine, proparacaine ophthalamic ointment eye drops were used to eliminate blink eye reflex. After demarcating a 1.5-mm wound area at the center of the cornea using a trephine, the epithelium was removed using a dulled blade as described previously15; wound closure was evaluated 18 hours after wounding. The area of the remaining wound was determined using ImageJ software version 1.44p (NIH, Bethesda, MD).
Cell Culture, Migration, and Attachment Assays
Skin keratinocytes were isolated from newborn WT and CLIC4NULL mice as described previously.16 Keratinocytes were cultured in minimum essential medium (Gibco S-MEM; Invitrogen) containing 8% serum and a low calcium level (0.05 mmol/L). The methods for the migration assay have been reported previously.17 In brief, keratinocytes were seeded on 24-well plates and were allowed to grow for 2 days in low-calcium 8% serum containing media before imaging using an Olympus IX81 research microscope (Olympus America Inc., Center Valley, PA) equipped with a ProScan motorized stage (Prior Scientific Inc., Rockland, MA) and a temperature- and CO2-controlled chamber (LiveCell incubation system; Neue Biosciences, Camp Hill, PA). Using relief contrast optics, 10× images were taken per well every 10 minutes for 16 hours 40 minutes (100 images). For each variable, triplicate wells were tracked and images managed using Metamorph image analysis software (Molecular Devices Corp., Chicago, IL), where velocities of 15 cells per well were calculated using the track cell module. From each cell tracked, average velocity, net displacement, and total displacement were determined. For TGF-β neutralizing antibody studies, 1 μg/mL of neutralizing antibody (AB-101-NA; R&D Systems, Minneapolis, MN) or control IgY (AB-101-Cl; R&D Systems) was added to serum containing media at day 2, and cells were tracked between days 2 and 3. Results of two independent experiments are reported. For experiments involving TGF-β treatment, TGF-β (No. 101-B1-001; R&D Systems) was dissolved in 4 mmol/L HCl containing 0.1% serum albumin at concentrations indicated on the figures. Cell attachment assays were performed on plastic dishes coated with matrix previously secreted by WT keratinocytes and extracted with ammonium hydroxide.18 WT and CLIC4NULL keratinocytes cultured for 5 days were trypsinized, and 300,000 cells were plated onto the matrix. After 30 minutes of attachment, the medium was removed and the dishes were rinsed twice with low-calcium medium, and attached cell number was assessed by MTT assay as described (No. G4102; Promega Corp., Madison, WI).
Immunoblotting
Protein lysate was prepared from whole skin or cultured cells in lysis buffer (No. 9803; Cell Signaling Technology Inc., Beverly, MA). EDTA-free Halt protease inhibitor (No. 78437; Thermo Scientific, Waltham, MA) and phosphatase inhibitor (No. 78420; Pierce Biotechnology, Rockford, IL) were used during lysis buffer preparation. Protein concentration was measured using Bradford reagent (No. 500–0006; Bio-Rad Laboratories, Hercules, CA), and 10 to 50 μg of protein lysate was loaded in premade 12.5% Tris-HCl protein gels (Nos. 345–0014 and 345–0016; Bio-Rad Laboratories). The protein was transferred to either a polyvinylidene difluoride (No. 162–0177; Bio-Rad Laboratories) or a nitrocellulose (No. RPN303E; GE Healthcare, Piscataway, NJ) membrane and was probed with appropriate antibodies. CLIC4 and CLIC1 monoclonal antibodies were obtained from BD Biosciences, San Jose, CA. The polyclonal antibodies against β4 integrin, α3 integrin, and β1 integrin that were used for immunoblots were described previously.19 P-Smad2 (ser465/467) (No. 3101; Cell Signaling Technology Inc.), p-Smad3 (a gift from Ed Leof, Mayo Clinic), E-cadherin (No. sc-7870; Santa Cruz Biotechnologies, Santa Cruz, CA), and p21 (No. sc-6246; Santa Cruz Biotechnologies) antibodies were used. Blots were developed using SuperSignal West Dura (No. 34076) and SuperSignal West Pico (No. 34078) chemiluminescent substrates from Thermo Scientific.
Histologic and IHC Analyses
Skin samples were fixed in 10% neutral buffered formalin overnight and then were embedded in paraffin. The Pathology/Histotechnology Laboratory of the National Cancer Institute, Frederick, MD, performed some of the IHC staining. The sections were stained with the following antibodies: BrdU (No. A21301MP; Invitrogen), Ki-67 (No. ab16667; Abcam Inc., Cambridge, MA), CD45 (No. 550539; BD Biosciences), CD31 (No. sc-1506; Santa Cruz Biotechnologies), myeloperoxidase (No. A0398; Dako, Carpinteria, CA), and F4/80 (No. 14–4801; eBioscience, San Diego, CA). CLIC4, p-Smad2, and keratin 6 staining used previously published methods.20 Slides were scanned and analyzed using a ScanScope XT scanner and ImageScope viewing software version 11.0.2.725 (Aperio Technologies Inc., Vista, CA) or were photographed using an Eclipse E800 microscope (Nikon Instruments, Melville, NY) or an Axioplan microscope (Carl Zeiss MicroImaging GmbH, Jena, Germany).
High-Resolution Confocal Microscopy
Corneal tissues were processed for confocal imaging as described elsewhere.21 After sacrifice, the eyes were removed; the corneas were fixed; the retina, lens, and iris were discarded; and four incisions were made in the cornea to permit flattening. After staining with the indicated antibodies, DAPI or propidium iodide was used to visualize the nuclei, and the corneas were placed with the epithelial side up in Fluoromount G mounting media (No. 17984–25; EMS, Hatfield, PA) and coverslipped. Images were acquired using the Zeiss LSM 710 Axio Examiner upright microscope and LSM 710 confocal system (Carl Zeiss MicroImaging GmbH) with 34 spectral detection channels and two transmitted light photomultipliers, five laser lines (458, 488, 514, 561, and 633 nm), control electronics, ZEN 2009 software, and x/y/z motorized scanning stage. Eighteen to 20 optical sections were acquired sequentially (z = 1 μm) using a 63× objective lens (numerical aperture = 1.4). Three-dimensional images were obtained using Volocity image analysis software version 5.0 (PerkinElmer, Waltham, MA). Images captured using confocal microscopy were merged and presented en face and/or rotated at 90° for cross-sectional views. For assessment of corneal thickness, the number of 1-μm z sections required to image the entire corneal thickness and the epithelium alone were determined at three locations for five corneas for each genotype.
Statistical Analyses
The attachment assays were repeated at least three times. Statistical analysis was performed using unpaired two-tailed t-tests, and data with P < 0.05 were considered significant. The statistical analysis for the migration assay was performed by one-way analysis of variance, and a minimum of 45 cells were evaluated for each genotype and condition in each well. The experiment was repeated twice.
Results
Generation of CLIC4NULL Mice
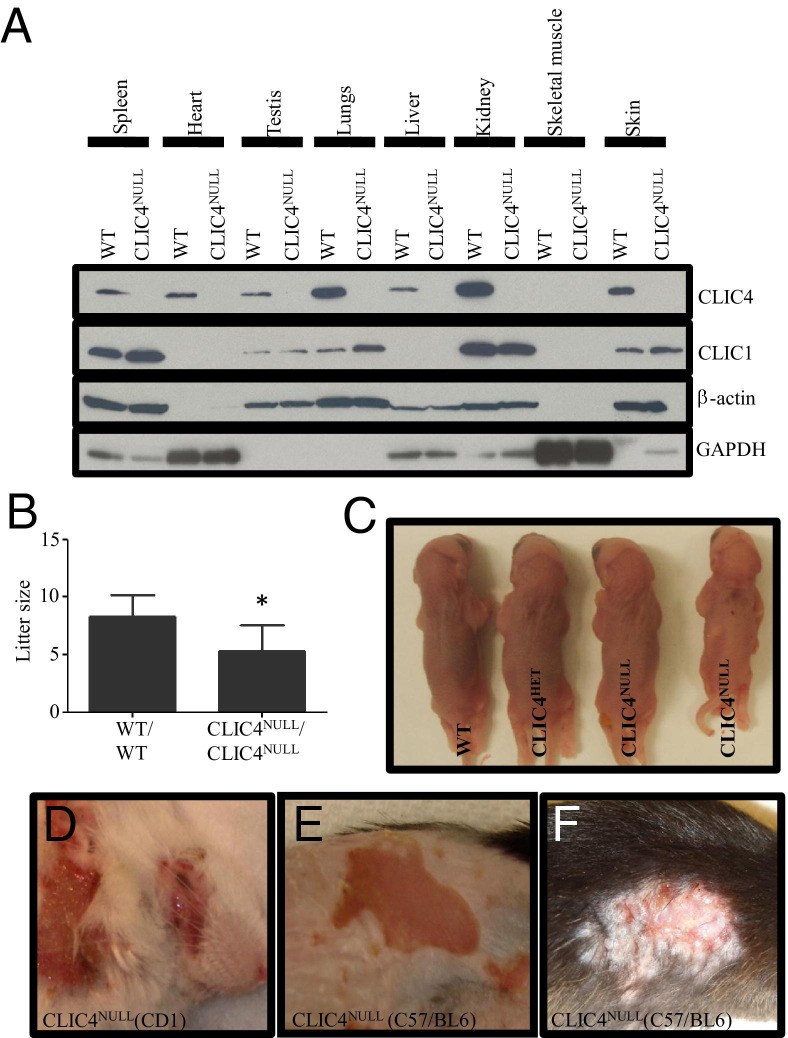
To study the in vivo function of CLIC4, we generated conditional CLIC4NULL mice. Mouse chromosomal locus 4D harbors the CLIC4 gene; it consists of six exons that code for a 253–amino acid protein. Sequence analysis of the CLIC4 gene revealed that removal of the second exon would be the most appropriate strategy, resulting in the loss of functional CLIC4 protein. Therefore, we designed the target vector to insert loxP sites surrounding the second exon of the CLIC4 gene (see Supplemental Figure S1A at http://ajp.amjpathol.org). Loss of the second exon would lead to a truncated form of CLIC4 consisting of the first 24 amino acids lacking any recognizable domain. Coincidently, a CLIC4 conventional knockout mouse being generated simultaneously in another laboratory targeted the identical location.10 Conditional CLIC4NULL animals were mated with C57BL/6 transgenic mice expressing Cre recombinase under the control of EIIa promoter22 to abrogate the expression of CLIC4 in the entire mouse. Immunoblot analysis of CLIC4 in spleen, heart, testis, lungs, liver, kidney, skeletal muscle, and skin confirmed the ubiquitous loss of CLIC4. Expression of CLIC1 was modestly increased in spleen, lungs, and skin in CLIC4NULL mice, suggesting a potential compensation (Figure 1A). Even the loss of one allele of CLIC4 led to considerable reduction in its expression (see Supplemental Figure S1C at http://ajp.amjpathol.org). Statistical analysis of littermates from heterozygous parents showed 22% of the live births to be of the genotype CLIC4NULL, not significantly different from the expected 25% ratio (data not shown). Analysis of the litter size from 32 WT parents and 28 CLIC4NULL parents revealed a statistically significant difference in average litter size (8.3 and 5.3, respectively) (Figure 1B). Ten percent of CLIC4NULL mice weighed visibly less than their WT littermates (Figure 1C).
Figure 1.
CLIC4NULL mice develop spontaneous skin erosions. A: Protein lysates were isolated from spleen, heart, testis, lungs, liver, kidney, skeletal muscle, and skin of WT and CLIC4NULL mice and were probed for CLIC4, CLIC1, β-actin, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression. B: Mean ± SEM litter size of WT and CLIC4NULL matings. (WT: 8.31 ± 0.33, n = 32; CLIC4NULL: 5.32 ± 0.42, n = 28; *P ≤ 0.0001). C: A comparison of the overall size of WT, CLIC4HET, and CLIC4NULL pups from one litter; approximately 10% of CLIC4NULL pups are visibly smaller. D–F: Skin erosions in CLIC4NULL mice in CD1 background (D) and C57/BL6 background (E and F).
Spontaneous Skin Erosions in CLIC4NULL Mice
Because previous studies have indicated important functions for CLIC4 in cultured skin keratinocytes,1 we first focused our attention to determine whether a skin phenotype would emerge from deletion of CLIC4 in vivo. CLIC4NULL mice are viable and not readily distinguishable from their WT littermates when full size. On standard H&E staining, WT and CLIC4NULL skin were identical (see Supplemental Figure S2, A and B, at http://ajp.amjpathol.org), and Ki-67 and BrdU staining revealed no statistically significant difference in proliferation of epidermal keratinocytes between WT and CLIC4NULL skin (see Supplemental Figure S2, C–F, at http://ajp.amjpathol.org). The absence of cutaneous CLIC4 did not evoke an inflammatory infiltrate as measured by CD45 staining (see Supplemental Figure S2, G and H, at http://ajp.amjpathol.org). However, some CLIC4NULL mice developed skin erosions around their face and neck and across the entire body. These erosions occur in approximately 25% of mice in CD1 background developed in a coauthor's laboratory (J.E.) (Figure 1D) and in 40% of mice in C57Bl/6 background that are older than 6 months monitored for 4 months at the National Cancer Institute mouse facility (Figure 1, E and F). During this time, 1 of 22 WT animals also developed a milder dermatitis-like skin abnormality. Histologic analysis of the skin erosions revealed hyperplasia as evidenced by keratin 6 and Ki-67 staining and neutrophilic infiltration as detected by myeloperoxidase staining (see Supplemental Figure S3 at http://ajp.amjpathol.org).
Wound-Healing Defect in CLIC4NULL Mice
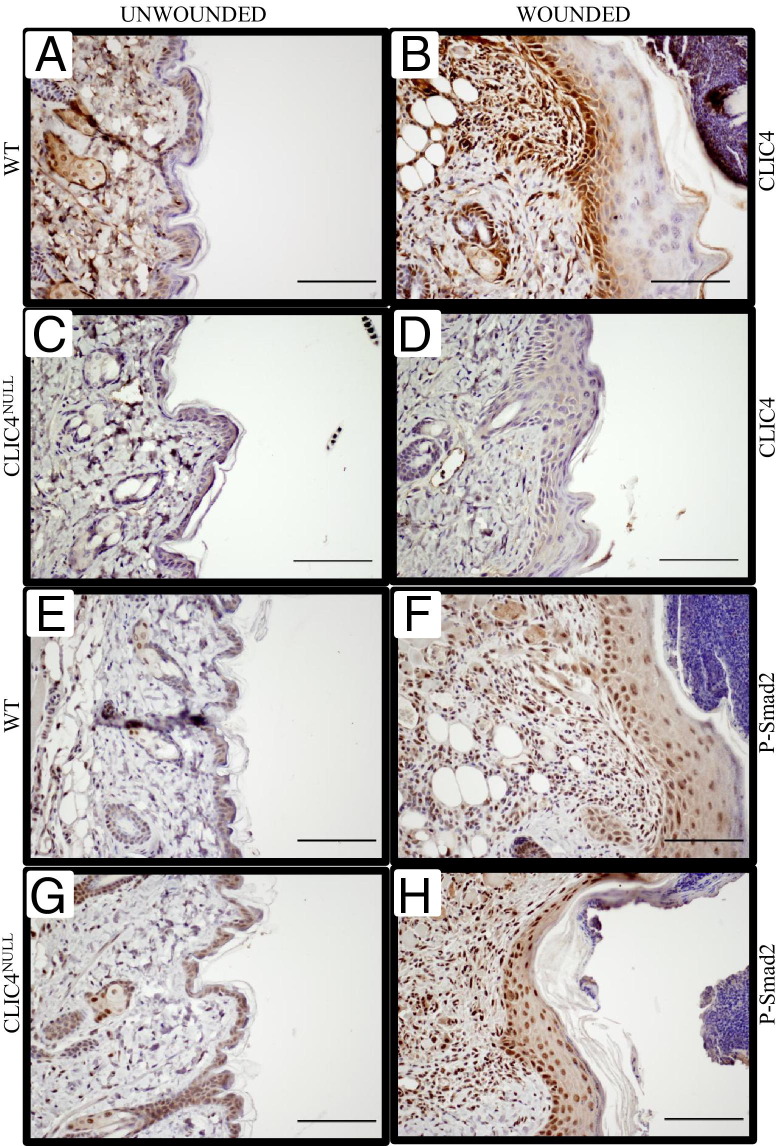
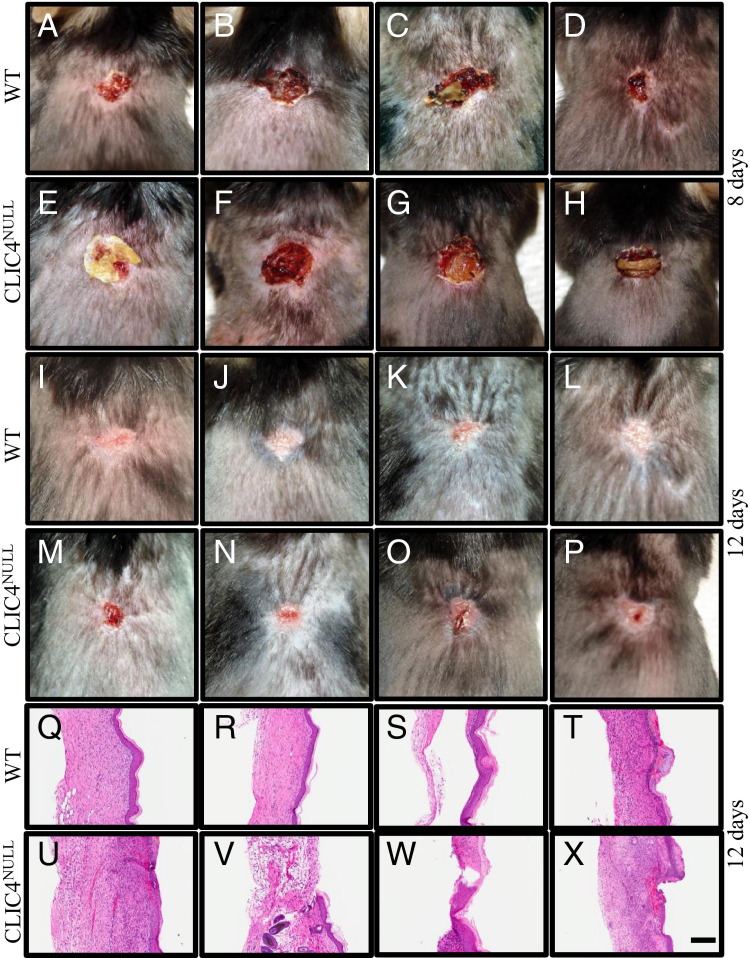
We investigated whether the spontaneous skin erosions in CLIC4NULL mice occur because of defective healing of wounds that develop in caged housing. We first studied the expression of CLIC4 in wounded skin. In normal resting WT skin, CLIC4 is localized to a distinct population of nuclei along the basal layer of the epidermis and in hair follicles (Figure 2A). Specific expression of CLIC4 is highly up-regulated in the epidermis and in the dermal compartment in experimentally induced skin wounds (Figure 2B). In contrast, CLIC4 is absent from resting and wounded areas of CLIC4NULL skin (Figure 2, C and D). CLIC4 is an integral component of TGF-β signaling, and TGF-β is involved in wound healing. However, neither constitutive nor wound-induced nuclear p-Smad2 was impaired by the absence of CLIC4 in mouse skin (Figure 2, E–H). In both genotypes, p-Smad2 was detected in nuclei of basal and suprabasal epidermis at the wound margins and in infiltrating cells in the dermis 4 days after wounding. To study the wound-healing process, we made 8-mm full-thickness wounds on a cohort of eight WT and eight CLIC4NULL mice and observed them for 12 days. Wound closure was delayed in CLIC4NULL mice after 8 days compared with in WT mice (Figure 3, A–H). WT wound closure was complete after 12 days, but the CLIC4NULL wounds remained open at this time point (Figure 3, I–P). We confirmed the complete reepithelialization of WT wounds histologically after 12 days, but areas remained without closure in all wounds on CLIC4NULL mice 12 days after wounding (Figure 3, Q–X).
Figure 2.
CLIC4 is nuclear in basal epidermis and is up-regulated in wounded epidermis and dermis. Skin from WT (A and B) and CLIC4NULL (C and D) mice was immunostained with CLIC4 monoclonal antibody 4 days after full-thickness wounding. A and C show the unwounded area, and B and D show the wound region. Skin sections from mice in the same experiment were immunostained for P-Smad2. Nuclear staining in the epidermis and hair follicles is detected in WT (E) and CLIC4NULL (G) resting skin, and nuclear p-Smad2 is increased in epidermis and dermis of wounded WT (F) and CLIC4NULL (H) skin. Scale bars: 100 μm.
Figure 3.
The loss of CLIC4 delays wound healing. An 8-mm, circular, full-thickness wound was made on the backs of eight WT and eight CLIC4NULL mice, and the mice were subsequently monitored for 12 days. After 8 days, four independent WT mice (A–D) and four independent CLIC4NULL mice (E–H) were photographed. I–P: The same mice were photographed after 12 days to monitor the extent of wound closure. Skin from the back wound site of four independent WT (Q–T) and CLIC4NULL (U–X) mice was collected after 12 days, fixed in 10% neutral buffered formalin, and H&E stained. Sections show the epidermis and dermis at the wound site. Scale bar = 200 μm (Q–X).
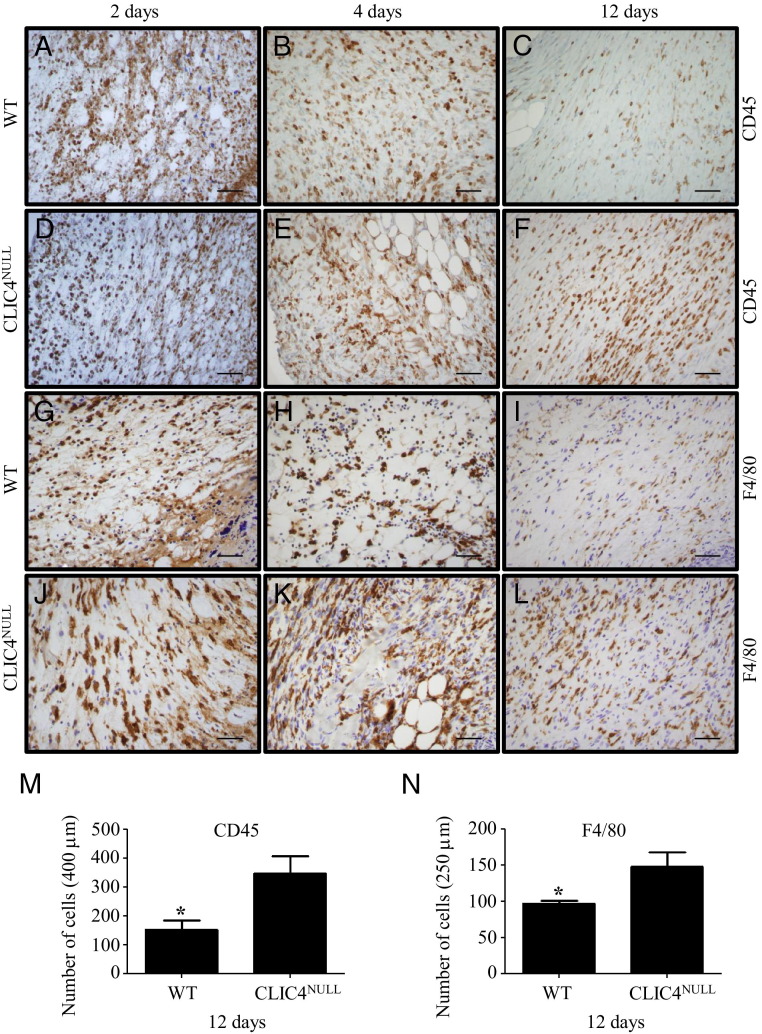
Epidermal abrasion wounding involving a group of six mice for each genotype also revealed moderately delayed wound resolution in CLIC4NULL mice compared with their WT counterparts (see Supplemental Figure S4 at http://ajp.amjpathol.org). One aspect of wound healing is resolution of the dermal inflammatory infiltrate invariably associated with induced wounds. IHC staining of full-thickness wounds from early healing (2 and 4 days) and near resolution (12 days) indicated similar early recruitment of CD45+ and F4/80+ macrophages into the wound site but sustained a statistically significant presence of these inflammatory cells in 12-day-old CLIC4NULL wounds (Figure 4).
Figure 4.
Delayed resolution of immune cells in wounds in CLIC4NULL mice. IHC staining was performed on 2-, 4-, and 12-day-old full-thickness wounds using antibodies to detect inflammatory infiltrates (CD45; A–F) and macrophages (F4/80; G–L). Sections show the dermis of the wound site on a WT mouse stained for CD45 (A–C) and F4/80 (G–I) and on a CLIC4NULL mouse stained for CD45 (D–F) and F4/80 (J–L). Scale bars: 50 μm. M: Mean ± SEM CD45-positive cells in 400 μm of the wound area were counted by Aperio Imagescope software version 11.0.2.725: WT (n = 4), 152 ± 65; CLIC4NULL (n = 3), 346 ± 107. N: Mean ± SEM F4/80-positive cells in 250 μm of wound area: WT (n = 4), 97 ± 4; CLIC4NULL (n = 3), 147 ± 20. *P = 0.03.
Corneal Wounds Heal Slower in CLIC4NULL Mice
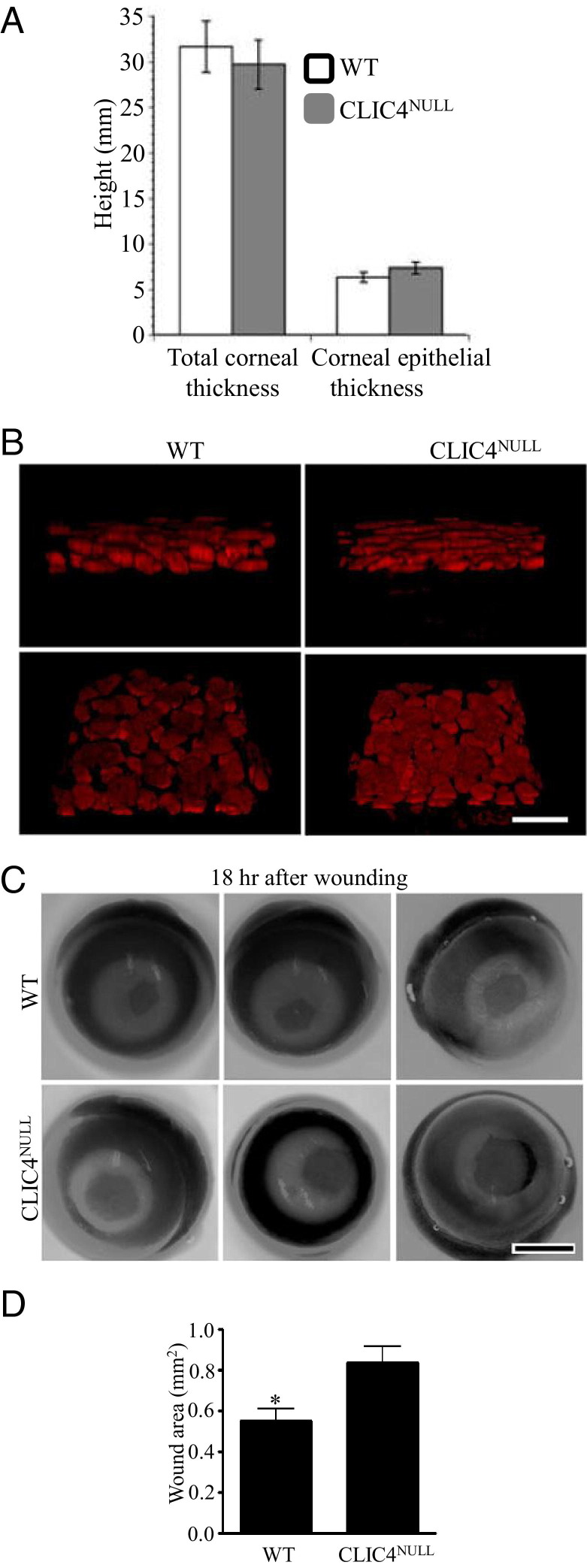
Skin wound healing involves reepithelialization and angiogenesis, and CLIC4 is known to influence angiogenesis in vitro and in vivo.8–10 Corneal debridement wounds remove the corneal epithelium only and reepithelialize without angiogenesis. To sort out the underlying cause of the healing delay in the CLIC4NULL skin, we used corneal wound healing as a model of rapid and highly quantitative reepithelialization in response to superficial debridement.23–25 The corneas of CLIC4NULL mice appeared normal and were clear on gross inspection. Total corneal and epithelial thickness in CLIC4NULL corneas was the same as in WT corneas (Figure 5A). However, when high-resolution three-dimensional confocal images were obtained of propidium iodide–stained corneal epithelium of WT and CLIC4NULL mice, the number of epithelial cell layers was greater in the unwounded epithelium of the CLIC4NULL corneas (Figure 5B). Corneal epithelial debridement was performed in 24 WT and 27 CLIC4NULL corneas in C57BL/6 background. The extent of wound closure was measured 18 hours after the debridement. The study revealed a statistically significant decrease in wound closure in CLIC4NULL corneas (Figure 5, C and D). The average wound area remaining in CLIC4NULL mice after 18 hours was 0.83 mm2, as opposed to 0.54 mm2 in WT corneas (P = 0.0079). When unwounded and wounded corneas were subjected to high-resolution three-dimensional confocal imaging, differences in the localization of β4 integrin, E-cadherin, and ZO-1 were seen in unwounded epithelia and during reepithelialization in CLIC4NULL corneas (see Supplemental Figure S5 at http://ajp.amjpathol.org). These data confirm that delayed reepithelialization contributes to the in vivo healing defects observed in these mice. They also suggest that differences in epithelial cell stratification and expression of cell-cell and cell-substrate adhesion proteins contribute to delayed healing in CLIC4NULL mice.
Figure 5.
Corneal wound healing is impaired in CLIC4NULL mice. A: For assessment of mean ± SEM corneal thickness, using ×40 magnification, the number of 1-μm z sections required to image the entire corneal thickness and the entire corneal epithelium were assessed after propidium iodide staining of flat-mounted corneas at three different locations at the centers of five corneas for each genotype. B: Representative high-resolution (×63) three-dimensional confocal images obtained as described showing the propidium iodide–stained nuclei of the corneal epithelium of WT and CLIC4NULL mice in cross section and after rotation to demonstrate their organization. C: Epithelial debridement was performed by removing 1.5 mm of the central corneal epithelium of 24 WT and 27 CLIC4NULL mice. After 18 hours, mice were sacrificed, eyes were enucleated and stained as described in Materials and Methods, and images were obtained. Three representative WT and CLIC4NULL cornea are shown. D: The mean ± SEM area of the remaining open wound was determined using ImageJ software: WT, 0.54 ± 0.05; CLIC4NULL, 0.83 ± 0.08. *P < 0.01. Scale bars: 6 μm (B); 1 mm (C).
Some Aspects of TGF-β Signaling Are Reduced in Cultured CLIC4NULL Mouse Keratinocytes
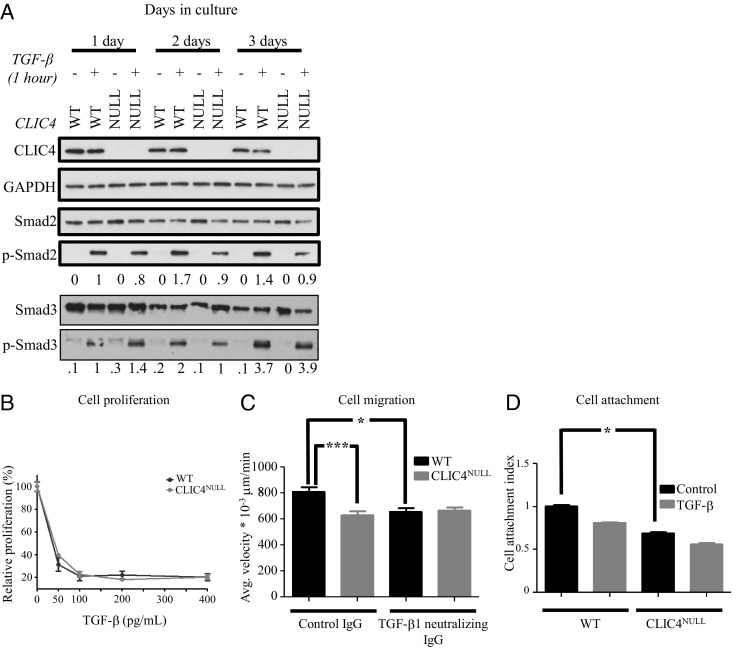
Previous studies on cultured keratinocytes have revealed that CLIC4 enhances TGF-β signaling by preventing dephosphorylation of p-Smad2/3 in the nucleus.12 Consistent with this result, the induction of p-Smad2 by TGF-β is lower in cultured CLIC4NULL keratinocytes than in WT keratinocytes (Figure 6A), whereas Smad2 levels are constant. In contrast, the induction of p-Smad3 is not reproducibly different among the genotypes. This may be related to the absence of a discernible difference in TGF-β–induced growth arrest in the genotypes as measured by thymidine incorporation (Figure 6B).12 However, single-cell velocity measurements indicate that CLIC4NULL keratinocytes migrate significantly slower than do WT keratinocytes, and their migration is not changed by removal of TGF-β from the culture medium, whereas the more rapid migration of WT keratinocytes is diminished in the absence of TGF-β (Figure 6C). Keratinocyte attachment to a keratinocyte-secreted cell matrix is also significantly lower in CLIC4NULL cells compared with in WT cells, and in this case, neither cell type seems to respond to TGF-β by significant changes in attachment (Figure 6D). These results suggest that for some parameters, such as cell migration and p-Smad induction, TGF-β responses are reduced in keratinocytes of CLIC4NULL mice.
Figure 6.
TGF-β signaling is impaired in CLIC4NULL keratinocytes. A: 1-, 2-, and 3-day-old cultures of WT and CLIC4NULL keratinocytes in low-serum medium (0.1%) were treated for 1 hour with TGF-β1 (50 pg/mL). Protein lysates were subsequently probed for p-Smad2, Smad2, p-Smad3, Smad3, CLIC4, β-actin, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). B: Keratinocytes were treated with 0, 50, 100, 200, and 400 pg/mL of TGF-β overnight. Cells were subsequently incubated with 2 μCi of 3H-thymidine for 4 hours. Relative proliferation is presented as a percentage of 3H-thymidine incorporation relative to cells not treated with TGF-β. Data are given as mean ± SEM. C: Keratinocyte migration was measured as described in Materials and Methods. Mean ± SEM migration of cells recorded: WT (control IgG), 813 ± 34 μm/min × 10−3 (n = 105); WT (TGF-β1 neutralizing IgG), 629 ± 30 μm/min × 10−3 (n = 105); CLIC4NULL (control IgG), 658 ± 27 μm/min × 10−3 (n = 135); and CLIC4NULL (TGF-β1 neutralizing IgG), 664 ± 27 μm/min × 10−3 (n = 135). D: Keratinocytes from each genotype were grown in complete medium for 5 days and trypsinized, and 300,000 cells were challenged to attach on keratinocyte matrix for 30 minutes. Viable attached cells were quantified via MTT assay; y axis is the optical density at 570 nm subtracted for background at 670 nm. *P < 0.05, ***P < 0.001.
Wound-Related Proteins Are Altered in CLIC4NULL Skin
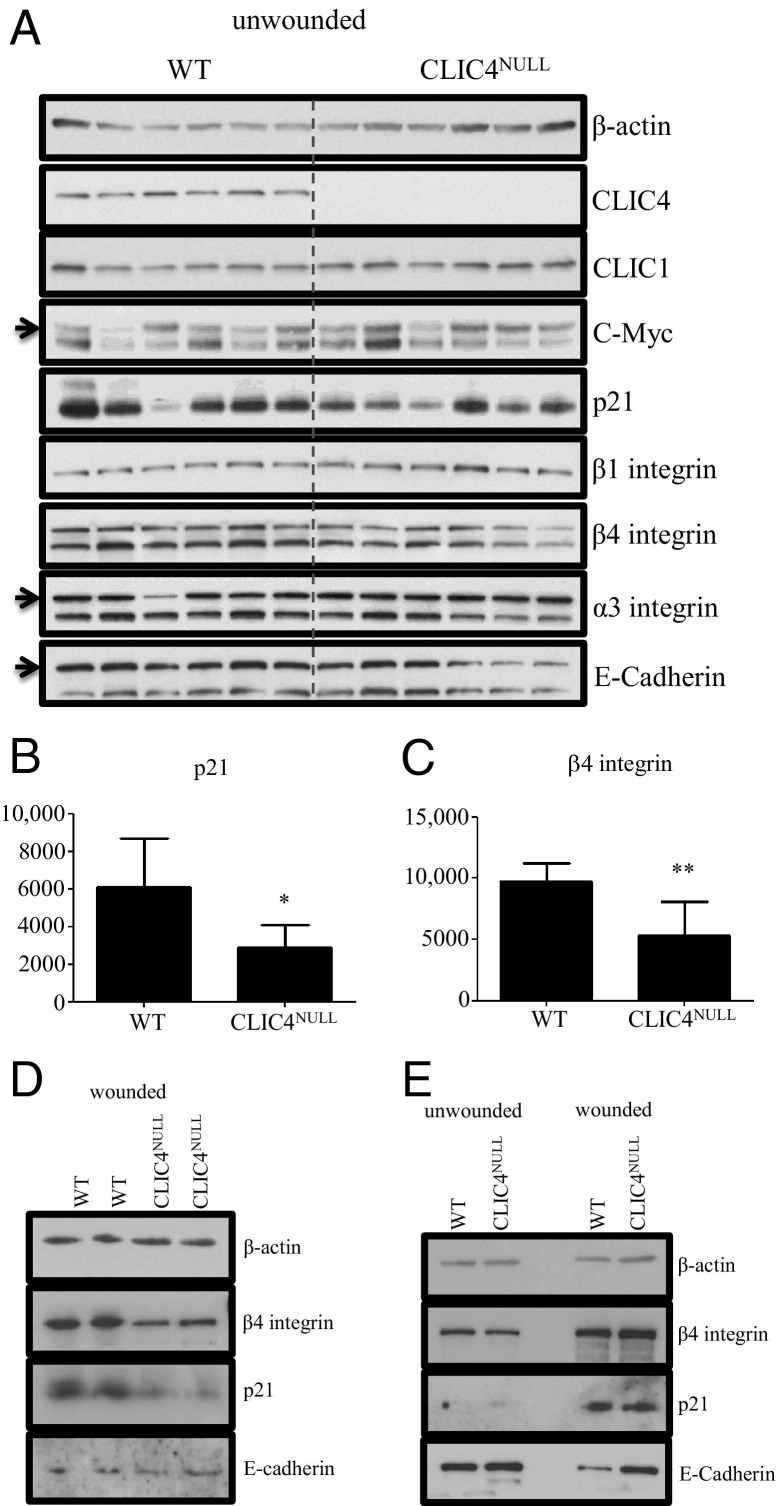
To determine the in vivo consequences of ablation of CLIC4 on proteins involved in skin wound healing, we performed immunoblotting on lysates isolated from untreated, resting skin samples of six independent WT mice and six independent CLIC4NULL mice of the same age and hair cycle (Figure 7A). Densitometric analysis of the immunoblots on resting skin revealed statistically significant decreased expression of p21 (Figure 7B) and β4 integrin (Figure 7C) in CLIC4NULL skin lysates. Based on these results, we performed additional experiments on skin lysates from two WT and two CLIC4NULL wounded mice. These proteins were also reduced in skin lysates 4 days after wounding (Figure 7D). p21 is a downstream effector of TGF-β–mediated growth inhibition,26–28 whereas α6β4 integrin is involved in keratinocyte attachment and migration.29–31 Changes in these proteins were not observed in lysates of resting or wounded corneas from either genotype, but E-cadherin expression was reduced at the edge of wounded WT corneal samples but not in wounded CLIC4NULL corneas (Figure 7E), in agreement with immunofluorescence analysis of E-cadherin in wounded cornea (see Supplemental Figure S5, J–L, at http://ajp.amjpathol.org). These changes in resting and wounded skin and cornea could modify wound healing, as documented by the loss of CLIC4.
Figure 7.
Attachment and cell cycle proteins differ in WT and CLIC4NULL skin. A: Protein samples were isolated from untreated resting skin of six independent WT and six CLIC4NULL mice. Samples were immunoblotted for β-actin, CLIC4, CLIC1, c-Myc, p21, β1 integrin, β4 integrin, α3 integrin, and E-cadherin. B and C: Densitometry on X-ray films was performed using ImageJ software to quantify levels of p21 (B) and β4 integrin (C). Expression levels are normalized to their respective β-actin controls and are presented as the mean ± SEM of six values: p21: WT, 6070 ± 1066; CLIC4NULL, 2891 ± 490; β4 integrin: WT, 9753 ± 594; CLIC4NULL, 5318 ± 1123. *P = 0.022, **P = 0.0058. D: Protein isolates from wound sites 4 days after full-thickness wounds from two independent WT and CLIC4NULL mice were used for analysis of β-actin, β4 integrin, p21, and E-cadherin E: Protein isolates from unwounded and wounded corneal samples taken 18 hours after superficial debridement wounds were used for immunoblotting to detect β-actin, β4 integrin, p21, and E-cadherin.
Discussion
Increasing interest in CLIC proteins has revealed a variety of pathways in which they participate and several cellular functions for which they are essential, such as vascular tubulogenesis in mammals and formation of a gut lumen in C. elegans. CLIC family proteins are highly conserved in vertebrates and invertebrates, indicating that they perform functions that are essential for living organisms.32 The high sequence homology among CLIC family members also suggests that they have overlapping functions, which may account for the survival of hosts in which one family member is genetically ablated. In this report, we demonstrate a new function for a key member of the family, CLIC4, as a participant in healing of wounds in skin and cornea. These data suggest that a key function for CLIC4 in that process is to enhance the ability of keratinocytes to reepithelialize the wound site. Wound healing is a complex process that requires a coordinated action of multiple cellular and tissue responses, such as inflammation, proliferation and migration of keratinocytes, angiogenesis, and extracellular matrix deposition. Platelet-derived growth factor, fibroblast growth factor, epidermal growth factor, vascular endothelial growth factor, and TGF-β signaling pathways have been widely implicated in these diverse processes, which take place during wound healing. Among these pathways, TGF-β signaling plays a dominant role in all the previously mentioned vital events.33–37
In the present study, genetic ablation of CLIC4 in mice did not cause an embryonic or postnatal phenotype or infertility, although it did reduce litter size, suggesting that a subtle change in fecundity or embryonic lethality may exist. Certain organs displayed a higher level of CLIC1 expression, which may indicate compensation since CLIC1 is the closest homologue to CLIC4 in sequence. The major phenotype observed was a high frequency of spontaneous skin erosions as mice aged past 3 months, with an acceleration of spontaneous wounds occurring in 40% of mice older than 6 months on a C57BL/6 background. We cannot discern whether these wounds result from grooming or scratching behavior that does not heal or from some constitutive weakness leading to breakdown of the epidermis. However, we have shown that induced wounds in the skin are associated with a marked up-regulation of CLIC4 expression in the epidermis and dermis and that wounds in skin or cornea heal more slowly in CLIC4NULL mice. In addition keratinocytes from CLIC4NULL mice have alterations in pathways important for reepithelialization, such as migration and cell attachment to matrix.
The importance of CLIC4 in TGF-β signaling is now well established,12 and the present data indicate that migration of WT keratinocytes is enhanced by the presence of TGF-β in their environment. In contrast, CLIC4NULL keratinocytes migrate slower, and their migration rate is not influenced by TGF-β. Likewise CLIC4NULL keratinocytes are less adhesive to matrix and have reduced β4 integrin expression, and corneal epithelial cells express a large amount of E-cadherin at the leading edge of corneal wounds. Together, these properties would all contribute to poor wound reepithelialization and could be TGF-β dependent. In support of this possibility is the reduced induction of p-Smad2 in cultured CLIC4NULL keratinocytes treated with TGF-β compared with in WT keratinocytes. However, we cannot definitively ascribe a role for defective TGF-β signaling in the poor wound repair associated with CLIC4 ablation since the level of nuclear p-Smad2 in the wound site increases irrespective of genotype, and isolated CLIC4NULL keratinocytes are as sensitive to TGF-β–mediated growth arrest as are WT keratinocytes. Thus, the precise signaling pathway(s) through which CLIC4 exerts its wound-healing benefits remains an open question.
Two studies detailing other consequences of CLIC4 loss in mice have recently been published. In the first study, CLIC4 was shown to be essential for the acidification of vacuoles during the tubulogenesis process that accompanies blood vessel formation.10 Although CD31 staining revealed an increased presence of endothelial cells in CLIC4NULL wounds (data not shown), further analysis of the wound bed by more specific methods is necessary to study the vascular integrity in these wound sites. Nevertheless, the absence of a requirement for angiogenesis to heal corneal wounds suggests that changes in vascularity are unlikely to underlie the wound-healing defect in the skin. CLIC4 has also been shown to be a regulator of Toll-like receptor signaling in macrophages and essential for clearance of Listeria infection, thus suggesting that CLIC4 participates in immune functions.38 Macrophages recruited to the wound site secrete several proteins that play essential functions during wound healing.39 Although these prohealing functions of macrophages have not yet been studied in the absence of CLIC4, the prolonged presence of macrophages observed in unresolved CLIC4NULL wounds could have an influence other than just as a marker of delayed wound closure.
CLIC4NULL keratinocytes attach less efficiently on matrix secreted by WT keratinocytes consistent with the migration defect. We could not detect any differences in the electrophoretic pattern of matrix proteins elaborated by WT and CLIC4NULL keratinocytes when examined by silver stain (data not shown). Thus, the difference in attachment may not be related to matrix composition but rather to the expression of integrins or other adhesion proteins. β4 integrin is reduced in resting and wounded CLIC4NULL skin. Total loss of β4 integrin causes epidermal blistering40 and anti–β4 integrin antibodies reduce wound healing in bioengineered human skin equivalent.41 Thus, the reduction of β4 integrin in keratinocytes lacking CLIC4 is a likely component underlying the reduced attachment of these cells to matrix.
Since superficial corneal wound closure largely depends on the rate of reepithelialization independent of stromal effects, it seems that the major action of CLIC4 is on the corneal epithelial cells in the corneal wound. It is known that TGF-β contributes to this process. Endogenously released TGF-β by injured epithelial cells enhances corneal reepithelialization in vivo in the mouse by activating canonical SMAD-dependent and noncanonical p38–mitogen-activated protein kinase–dependent pathways42 that lead to the enhanced expression of epithelial mesenchymal transformation–inducible zinc finger transcription factors, including Snail and Slug.43,44 These transcription factors down-regulate epithelial-specific proteins, including E-cadherin and β-catenin, in a gradient from the leading edge back away from the wound margin. Wounds that do not heal in dogs were found to express reduced levels of Slug that were reversed by topical treatment with tetracycline, which enhanced TGF-β activity, increased Slug expression, and improved healing.44 The present data showing reduced reepithelialization rates in the CLIC4 corneas after superficial debridement wounds and retention of E-cadherin and ZO-1 at the leading edge support a role for CLIC4 in TGF-β signaling during corneal reepithelialization.
Acknowledgment
We thank Jennifer E. Dwyer and Shelley Hoover for help with the Aperio ScanScope scanner. We also thank Susana Walters for animal care and support and Christina Lee and Mario Anazano for genotyping.
Footnotes
Supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, and by NIH grants EY08512 (S.P.-G. and M.A.S.) and 5R01HL092131 (J.C.E.).
Supplemental material for this article can be found at http://ajp.amjpathol.org or at http://dx.doi.org/10.1016/j.ajpath.2012.03.025.
Current address of V.C.P., Ohio State University Medical Center, Columbus, Ohio.
Supplementary data
Creation of a CLIC4NULL mouse by loxP-Cre genetic recombination. A: The target vector design used for generation of the mouse using recombination technology. FRT, FLP recognition target. B: DNA was extracted from embryonic stem cells and probed for the presence of the two loxP sites via Southern blot analysis. C: Protein lysate was isolated from keratinocytes from the skin of WT, heterozygous, and CLIC4NULL neonate progeny, and immunoblotting was performed for β-actin and CLIC4.
IHC analysis of CLIC4NULL skin. Skin samples from the back of one representative WT and one CLIC4NULL mouse of the same age were used for staining to detect proliferation (BrdU and Ki-67) and inflammation (CD45). A, C, E, and G: The epidermis and dermis of the WT mouse stained for H&E, BrdU, Ki-67, and CD45, respectively. B, D, F, and H: The epidermis and dermis of the CLIC4NULL mouse stained equivalently. Scale bars: 50 μm.
IHC analysis of skin erosions in WT and CLIC4NULL CD1 mice. Skin sample from a CLIC4NULL mouse with a healing skin erosion on its snout and a corresponding normal skin sample from a WT mouse were fixed in 10% neutral buffered formalin. Staining was performed using antibodies to detect hyperplasia [keratin 6 (K6)], proliferation (Ki-67), and neutrophil infiltration [myeloperoxidase (MPO)]. A, C, E, and G: The epidermis and dermis of the WT mouse stained for H&E, K6, Ki-67, and MPO, respectively. B, D, F, and H: The epidermis and dermis of the CLIC4NULL mouse stained equivalently. Scale bars: 100 μm.
Delayed wound healing in CLIC4NULL mice after epidermal abrasion. A 2-cm2 region on the lower back of six independent WT and six CLIC4NULL mice were abraded with a small felt wheel attached to a handheld Dremel tool. Skin samples were collected after 8 days, fixed in 10% neutral buffered formalin, and stained by H&E. Sections show the epidermis and dermis of the wound site for each independent mouse from WT (left panel) and CLIC4NULL mice (right panel). Scale bars: 250 μm. Wound closure, contraction, and remodeling are delayed in null mouse skin, whereas inflammation is sustained.
High-resolution three-dimensional confocal imaging of WT and CLIC4NULL corneas reveals differences in β4 integrin, E-cadherin, and ZO-1 localization before and after wounding. WT (A–C,G–I, and M–O) and CLIC4NULL (D–F,J–L, and P–R) corneas were stained for β4 integrin (A–F), E-cadherin (G–L), and ZO-1 (M–R). Shown are unwounded corneas (A, D, G, J, M, and P), wounded corneas behind the leading edge (B, E, H, K,N, and Q), and wounded corneas at the leading edge (C, F, I, L, O, and R). The results shown are representative of those obtained from three different corneas for each variable shown. Scale bar = 6 μm.
References
- 1.Shukla A., Yuspa S.H. CLIC4 and Schnurri-2: a dynamic duo in TGF-β signaling with broader implications in cellular homeostasis and disease. Nucleus. 2010;1:144–149. doi: 10.4161/nucl.1.2.10920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Littler D.R., Harrop S.J., Goodchild S.C., Phang J.M., Mynott A.V., Jiang L., Valenzuela S.M., Mazzanti M., Brown L.J., Breit S.N., Curmi P.M. The enigma of the CLIC proteins: ion channels, redox proteins, enzymes, scaffolding proteins? FEBS Lett. 2010;584:2093–2101. doi: 10.1016/j.febslet.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Suh K.S., Mutoh M., Nagashima K., Fernandez-Salas E., Edwards L.E., Hayes D.D., Crutchley J.M., Marin K.G., Dumont R.A., Levy J.M., Cheng C., Garfield S., Yuspa S.H. The organellular chloride channel protein CLIC4/mtCLIC translocates to the nucleus in response to cellular stress and accelerates apoptosis. J Biol Chem. 2004;279:4632–4641. doi: 10.1074/jbc.M311632200. [DOI] [PubMed] [Google Scholar]
- 4.Suh K.S., Malik M., Shukla A., Yuspa S.H. CLIC4, skin homeostasis and cutaneous cancer: surprising connections. Mol Carcinog. 2007;46:599–604. doi: 10.1002/mc.20324. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Salas E., Sagar M., Cheng C., Yuspa S.H., Weinberg W.C. p53 and tumor necrosis factor α regulate the expression of a mitochondrial chloride channel protein. J Biol Chem. 1999;274:36488–36497. doi: 10.1074/jbc.274.51.36488. [DOI] [PubMed] [Google Scholar]
- 6.Shiio Y., Suh K.S., Lee H., Yuspa S.H., Eisenman R.N., Aebersold R. Quantitative proteomic analysis of myc-induced apoptosis: a direct role for Myc induction of the mitochondrial chloride ion channel, mtCLIC/CLIC4. J Biol Chem. 2006;281:2750–2756. doi: 10.1074/jbc.M509349200. [DOI] [PubMed] [Google Scholar]
- 7.Berry K.L., Bulow H.E., Hall D.H., Hobert O. A C. elegans CLIC-like protein required for intracellular tube formation and maintenance. Science. 2003;302:2134–2137. doi: 10.1126/science.1087667. [DOI] [PubMed] [Google Scholar]
- 8.Bohman S., Matsumoto T., Suh K., Dimberg A., Jakobsson L., Yuspa S., Claesson-Welsh L. Proteomic analysis of vascular endothelial growth factor-induced endothelial cell differentiation reveals a role for chloride intracellular channel 4 (CLIC4) in tubular morphogenesis. J Biol Chem. 2005;280:42397–42404. doi: 10.1074/jbc.M506724200. [DOI] [PubMed] [Google Scholar]
- 9.Tung J.J., Hobert O., Berryman M., Kitajewski J. Chloride intracellular channel 4 is involved in endothelial proliferation and morphogenesis in vitro. Angiogenesis. 2009;12:209–220. doi: 10.1007/s10456-009-9139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulmasov B., Bruno J., Gordon N., Hartnett M.E., Edwards J.C. Chloride intracellular channel protein-4 functions in angiogenesis by supporting acidification of vacuoles along the intracellular tubulogenic pathway. Am J Pathol. 2009;174:1084–1096. doi: 10.2353/ajpath.2009.080625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ronnov-Jessen L., Villadsen R., Edwards J.C., Petersen O.W. Differential expression of a chloride intracellular channel gene, CLIC4, in transforming growth factor-β1-mediated conversion of fibroblasts to myofibroblasts. Am J Pathol. 2002;161:471–480. doi: 10.1016/s0002-9440(10)64203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shukla A., Malik M., Cataisson C., Ho Y., Friesen T., Suh K.S., Yuspa S.H. TGF-β signalling is regulated by Schnurri-2-dependent nuclear translocation of CLIC4 and consequent stabilization of phospho-Smad2 and 3. Nat Cell Biol. 2009;11:777–784. doi: 10.1038/ncb1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tessarollo L., Palko M.E., Akagi K., Coppola V. Gene Targeting in Mouse Embryonic Stem Cells. In: Wolfgang Wurst R.K., editor. Humana Press, a part of Springer Science+Business Media LLC; New York: 2009. [Google Scholar]
- 14.Pal-Ghosh S., Pajoohesh-Ganji A., Brown M., Stepp M.A. A mouse model for the study of recurrent corneal epithelial erosions: α9β1 integrin implicated in progression of the disease. Invest Ophthalmol Vis Sci. 2004;45:1775–1788. doi: 10.1167/iovs.03-1194. [DOI] [PubMed] [Google Scholar]
- 15.Pal-Ghosh S., Tadvalkar G., Jurjus R.A., Zieske J.D., Stepp M.A. BALB/c and C57BL6 mouse strains vary in their ability to heal corneal epithelial debridement wounds. Exp Eye Res. 2008;87:478–486. doi: 10.1016/j.exer.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lichti U., Anders J., Yuspa S.H. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc. 2008;3:799–810. doi: 10.1038/nprot.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stepp M.A., Liu Y., Pal-Ghosh S., Jurjus R.A., Tadvalkar G., Sekaran A., Losicco K., Jiang L., Larsen M., Li L., Yuspa S.H. Reduced migration, altered matrix and enhanced TGFβ1 signaling are signatures of mouse keratinocytes lacking Sdc1. J Cell Sci. 2007;120:2851–2863. doi: 10.1242/jcs.03480. [DOI] [PubMed] [Google Scholar]
- 18.Dlugosz A.A., Glick A.B., Tennenbaum T., Weinberg W.C., Yuspa S.H. Isolation and Utilization of epidermal keratinocytes for oncogene research. Method Enzymol. 1995;254:3–20. doi: 10.1016/0076-6879(95)54003-2. [DOI] [PubMed] [Google Scholar]
- 19.Sta Iglesia D.D., Gala P.H., Qiu T., Stepp M.A. Integrin expression during epithelial migration and restratification in the tenascin-C-deficient mouse cornea. J Histochem Cytochem. 2000;48:363–376. doi: 10.1177/002215540004800306. [DOI] [PubMed] [Google Scholar]
- 20.Grondahl-Hansen J., Christensen I.J., Briand P., Pappot H., Mouridsen H.T., Blichert-Toft M., Dano K., Brunner N. Plasminogen activator inhibitor type 1 in cytosolic tumor extracts predicts prognosis in low-risk breast cancer patients. Clin Cancer Res. 1997;3:233–239. [PubMed] [Google Scholar]
- 21.Pajoohesh-Ganji A., Pal-Ghosh S., Simmens S.J., Stepp M.A. Integrins in slow-cycling corneal epithelial cells at the limbus in the mouse. Stem Cells. 2006;24:1075–1086. doi: 10.1634/stemcells.2005-0382. [DOI] [PubMed] [Google Scholar]
- 22.Lakso M., Pichel J.G., Gorman J.R., Sauer B., Okamoto Y., Lee E., Alt F.W., Westphal H. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci U S A. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buck R.C. Cell migration in repair of mouse corneal epithelium. Invest Ophthalmol Vis Sci. 1979;18:767–784. [PubMed] [Google Scholar]
- 24.Sta Iglesia D.D., Stepp M.A. Disruption of the basement membrane after corneal debridement. Invest Ophthalmol Vis Sci. 2000;41:1045–1053. [PubMed] [Google Scholar]
- 25.Stepp M.A., Gibson H.E., Gala P.H., Iglesia D.D., Pajoohesh-Ganji A., Pal-Ghosh S., Brown M., Aquino C., Schwartz A.M., Goldberger O., Hinkes M.T., Bernfield M. Defects in keratinocyte activation during wound healing in the syndecan-1-deficient mouse. J Cell Sci. 2002;115:4517–4531. doi: 10.1242/jcs.00128. [DOI] [PubMed] [Google Scholar]
- 26.Elbendary A., Berchuck A., Davis P., Havrilesky L., Bast R.C., JR, Iglehart J.D., Marks J.R. Transforming growth factor β 1 can induce CIP1/WAF1 expression independent of the p53 pathway in ovarian cancer cells. Cell Growth Differ. 1994;5:1301–1307. [PubMed] [Google Scholar]
- 27.Datto M.B., Li Y., Panus J.F., Howe D.J., Xiong Y., Wang X.F. Transforming growth factor β induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci U S A. 1995;92:5545–5549. doi: 10.1073/pnas.92.12.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C.Y., Suardet L., Little J.B. Potential role of WAF1/Cip1/p21 as a mediator of TGF-β cytoinhibitory effect. J Biol Chem. 1995;270:4971–4974. doi: 10.1074/jbc.270.10.4971. [DOI] [PubMed] [Google Scholar]
- 29.Stepp M.A., Spurr-Michaud S., Tisdale A., Elwell J., Gipson I.K. α 6 β 4 integrin heterodimer is a component of hemidesmosomes. Proc Natl Acad Sci U S A. 1990;87:8970–8974. doi: 10.1073/pnas.87.22.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones J.C., Kurpakus M.A., Cooper H.M., Quaranta V. A function for the integrin α6 β4 in the hemidesmosome. Cell Regul. 1991;2:427–438. doi: 10.1091/mbc.2.6.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sehgal B.U., DeBiase P.J., Matzno S., Chew T.L., Claiborne J.N., Hopkinson S.B., Russell A., Marinkovich M.P., Jones J.C. Integrin β4 regulates migratory behavior of keratinocytes by determining laminin-332 organization. J Biol Chem. 2006;281:35487–35498. doi: 10.1074/jbc.M606317200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Littler D.R., Harrop S.J., Brown L.J., Pankhurst G.J., Mynott A.V., Luciani P., Mandyam R.A., Mazzanti M., Tanda S., Berryman M.A., Breit S.N., Curmi P.M. Comparison of vertebrate and invertebrate CLIC proteins: the crystal structures of Caenorhabditis elegans EXC-4 and Drosophila melanogaster DmCLIC. Proteins. 2008;71:364–378. doi: 10.1002/prot.21704. [DOI] [PubMed] [Google Scholar]
- 33.Werner S., Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 34.Kulkarni A.B., Huh C.G., Becker D., Geiser A., Lyght M., Flanders K.C., Roberts A.B., Sporn M.B., Ward J.M., Karlsson S. Transforming growth factor β 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shull M.M., Ormsby I., Kier A.B., Pawlowski S., Diebold R.J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D., Annunziata N., Doetschman T. Targeted disruption of the mouse transforming growth factor-β 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crowe M.J., Doetschman T., Greenhalgh D.G. Delayed wound healing in immunodeficient TGF-β 1 knockout mice. J Invest Dermatol. 2000;115:3–11. doi: 10.1046/j.1523-1747.2000.00010.x. [DOI] [PubMed] [Google Scholar]
- 37.Owens P., Engelking E., Han G., Haeger S.M., Wang X.J. Epidermal Smad4 deletion results in aberrant wound healing. Am J Pathol. 2010;176:122–133. doi: 10.2353/ajpath.2010.090081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He G., Ma Y., Chou S.Y., Li H., Yang C., Chuang J.Z., Sung C.H., Ding A. Role of CLIC4 in the host innate responses to bacterial lipopolysaccharide. Eur J Immunol. 2011;41:1221–1230. doi: 10.1002/eji.201041266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DiPietro L.A. Wound healing: the role of the macrophage and other immune cells. Shock. 1995;4:233–240. [PubMed] [Google Scholar]
- 40.van der Neut R., Krimpenfort P., Calafat J., Niessen C.M., Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin β 4 null mice. Nat Genet. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- 41.Egles C., Huet H.A., Dogan F., Cho S., Dong S., Smith A., Knight E.B., McLachlan K.R., Garlick J.A. Integrin-blocking antibodies delay keratinocyte re-epithelialization in a human three-dimensional wound healing model. PLoS One. 2010;5:e10528. doi: 10.1371/journal.pone.0010528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saika S., Okada Y., Miyamoto T., Yamanaka O., Ohnishi Y., Ooshima A., Liu C.Y., Weng D., Kao W.W. Role of p38 MAP kinase in regulation of cell migration and proliferation in healing corneal epithelium. Invest Ophthalmol Vis Sci. 2004;45:100–109. doi: 10.1167/iovs.03-0700. [DOI] [PubMed] [Google Scholar]
- 43.Aomatsu K., Arao T., Sugioka K., Matsumoto K., Tamura D., Kudo K., Kaneda H., Tanaka K., Fujita Y., Shimomura Y., Nishio K. TGF-β induces sustained upregulation of SNAI1 and SNAI2 through Smad and non-Smad pathways in a human corneal epithelial cell line. Invest Ophthalmol Vis Sci. 2011;52:2437–2443. doi: 10.1167/iovs.10-5635. [DOI] [PubMed] [Google Scholar]
- 44.Chandler H.L., Colitz C.M., Lu P., Saville W.J., Kusewitt D.F. The role of the slug transcription factor in cell migration during corneal re-epithelialization in the dog. Exp Eye Res. 2007;84:400–411. doi: 10.1016/j.exer.2006.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Creation of a CLIC4NULL mouse by loxP-Cre genetic recombination. A: The target vector design used for generation of the mouse using recombination technology. FRT, FLP recognition target. B: DNA was extracted from embryonic stem cells and probed for the presence of the two loxP sites via Southern blot analysis. C: Protein lysate was isolated from keratinocytes from the skin of WT, heterozygous, and CLIC4NULL neonate progeny, and immunoblotting was performed for β-actin and CLIC4.
IHC analysis of CLIC4NULL skin. Skin samples from the back of one representative WT and one CLIC4NULL mouse of the same age were used for staining to detect proliferation (BrdU and Ki-67) and inflammation (CD45). A, C, E, and G: The epidermis and dermis of the WT mouse stained for H&E, BrdU, Ki-67, and CD45, respectively. B, D, F, and H: The epidermis and dermis of the CLIC4NULL mouse stained equivalently. Scale bars: 50 μm.
IHC analysis of skin erosions in WT and CLIC4NULL CD1 mice. Skin sample from a CLIC4NULL mouse with a healing skin erosion on its snout and a corresponding normal skin sample from a WT mouse were fixed in 10% neutral buffered formalin. Staining was performed using antibodies to detect hyperplasia [keratin 6 (K6)], proliferation (Ki-67), and neutrophil infiltration [myeloperoxidase (MPO)]. A, C, E, and G: The epidermis and dermis of the WT mouse stained for H&E, K6, Ki-67, and MPO, respectively. B, D, F, and H: The epidermis and dermis of the CLIC4NULL mouse stained equivalently. Scale bars: 100 μm.
Delayed wound healing in CLIC4NULL mice after epidermal abrasion. A 2-cm2 region on the lower back of six independent WT and six CLIC4NULL mice were abraded with a small felt wheel attached to a handheld Dremel tool. Skin samples were collected after 8 days, fixed in 10% neutral buffered formalin, and stained by H&E. Sections show the epidermis and dermis of the wound site for each independent mouse from WT (left panel) and CLIC4NULL mice (right panel). Scale bars: 250 μm. Wound closure, contraction, and remodeling are delayed in null mouse skin, whereas inflammation is sustained.
High-resolution three-dimensional confocal imaging of WT and CLIC4NULL corneas reveals differences in β4 integrin, E-cadherin, and ZO-1 localization before and after wounding. WT (A–C,G–I, and M–O) and CLIC4NULL (D–F,J–L, and P–R) corneas were stained for β4 integrin (A–F), E-cadherin (G–L), and ZO-1 (M–R). Shown are unwounded corneas (A, D, G, J, M, and P), wounded corneas behind the leading edge (B, E, H, K,N, and Q), and wounded corneas at the leading edge (C, F, I, L, O, and R). The results shown are representative of those obtained from three different corneas for each variable shown. Scale bar = 6 μm.