Abstract
Purpose
We have previously shown that the anti-malarial agent chloroquine can abrogate the lethal cellular effects of low dose-rate (LDR) radiation in vitro, most likely by activating the ataxia-telangiectasia mutated (ATM) protein. Here, we demonstrate that chloroquine treatment also protects against lethal doses of LDR radiation in vivo.
Methods and Materials
C57BL/6 mice were irradiated with total of 12.8 Gy delivered at 9.4 cGy/hr. ATM null mice from the same background were used to determine the influence of ATM. Chloroquine was administered by two intraperitoneal injections of 59.4 μg per 17 g of body weight, 24 hrs and 4 hrs before irradiation. Bone marrow cells isolated from tibia, fibula and vertebral bones were transplanted into lethally irradiated CD45 congenic recipient mice by retro orbital injection. Chimerism was assessed by flow cytometry. In vitro methyl cellulose colony forming assay of whole bone marrow cells as well as FACS analysis of lineage depleted cells was used to assess the effect of chloroquine on progenitor cells.
Results
Mice pretreated with chloroquine prior to radiation exhibited a significantly higher survival rate compared to mice treated with radiation alone (80 vs.31 percent, p=0.0026). Chloroquine administration prior to radiation did not impact the survival of ATM null mice (p=0.86). Chloroquine also had a significant effect on the early engraftment of bone marrow cells from the irradiated donor mice 6 weeks after the transplantation (4.2 percent vs. 0.4 percent, p=0.015).
Conclusion
Chloroquine administration prior to radiation had a significant effect on the survival of normal but not ATM null mice strongly suggesting that the in vivo effect like the in vitro effect is also ATM dependent. Chloroquine improved the early engraftment of bone marrow cells from LDR irradiated mice, presumably by protecting the progenitor cells from radiation injury. Chloroquine thus could serve as a very useful drug for protection against the harmful effects of LDR radiation.
Keywords: Low dose rate radiation, Chloroquine, Hematopoietic progenitor cells, Ataxia telangiectasia mutated, ATM activation
INTRODUCTION
The biologic effects of ionizing radiation have been studied for over a century, but it was not until deployment of the atomic bomb in 1945 that the clinical manifestations of total body irradiation (TBI) were fully realized. Following acute radiation exposure, hematopoietic defects in particular are observed and are an important cause of death (1–2). Lethal radiation exposure, either through accidents or possible acts of terrorism, remains a threat with the potential to impact large populations. Rather than acute radiation exposure, these exposures are likely to occur over protracted time periods, and the rate of radiation exposure is an important factor in resulting cellular toxicity. Biological responses to lower dose rates vary depending on the cell type as well as dose rate. Previous studies have found that the delivery of radiation at LDR may result in greater or lesser amounts of cell killing in vitro compared to equivalent doses delivered at higher dose-rates (3–4). These differences in cell survival are thought to result from alterations in the cell cycle and/or repair in radiation-induced injury (5–6). ATM is one of the key proteins involved in the response of mammalian cells to radiation-induced injury and is activated by autophosphorylation following DNA damage (7) .
Once activated, ATM subsequently phosphorylates other key proteins involved in the repair of DNA damage (8) . The anti-malarial agent, chloroquine, has been shown to activate ATM without inducing DNA injury presumably by altering chromatin structure (7, 9) . Intriguingly, ATM is not fully activated by LDR radiation, but the addition of chloroquine to cancer cells growing in vitro prior to LDR radiation exposure activates ATM and subsequently reduces cell death from LDR (10) . Here, we examined whether chloroquine could similarly act as a radio-protective agent in vivo and treated mice with chloroquine prior to LDR radiation exposure. We found that chloroquine improved survival in normal but not ATM null mice. We also show that chloroquine enhanced recovery of hematopoietic progenitors responsible for early engraftment. These data expand our knowledge regarding the role of ATM in protection from radiation injury in mammals and highlight the possibility that drugs like chloroquine could be very useful as modulators of LDR radiation-induced injury.
METHODS AND MATERIALS
Cell proliferation assay
Human fibroblast cells obtained from an ATM −/− patient were immortalized using hTERT (GM05823-hTERT ATM−/−). Immortalized wild-type human fibroblast cells (HFF-hTERT ATM+/+) were used as control. Cells were cultured in DMEM with 10% FBS and treated with 48 μg/ml chloroquine for 4 hrs, then washed with PBS and cultured in 10 ml of fresh medium. Flasks were gassed with 5% CO2, sealed and irradiated in an incubated, low dose-rate irradiator with a 137Cs source which can be attenuated to produce various low dose rates (3) for 42.5 hrs at 37°C for a total radiation exposure of 4 Gy at a rate of 9.4 cGy/hr. Cell proliferation was assessed using Cell titer Blue (Promega, Madison, WI).
Mice
Male C57BL/6-CD45.2 mice (Harlan Laboratories, Indianapolis, IN) were used as bone marrow donors and female C57BL/6-CD45.1 mice (National Cancer Institute) as transplant recipients. Male C57BL/6 ATM null mice (St. Jude Children’s Research Hospital, Memphis, TN) were used in experiments to determine the influence of ATM in total body LDR radiation-induced death. ATM status was confirmed by PCR of mouse genomic DNA. All the mice were used at 4–6 weeks of age, and housed under specific pathogen-free conditions in an accredited facility at the XXXXX. All experiments were conducted using protocols approved by the XXXX Institutional Animal Care and Use Committee (IACUC).
Radiation exposure and chloroquine administration
C57BL/6 donor mice were exposed in a low dose-rate irradiator with a 137Cs source attenuated to produce a dose rate of 9.4 cGy/hr in a custom built insulated chamber approved for small animal exposure. Wild type mice were treated for 136 hrs for TBI of 12.8 Gy. ATM null mice were treated for 96 hrs for a TBI of 9 Gy, given their greater radiosensitivity (11). Bone marrow recipient mice were conditioned with 10 Gy given as two 5 Gy fractions at 30 Gy/hr, 4 hrs apart prior to bone marrow transplantation. Chloroquine (Sigma-Aldrich, St. Louis, MO) was dissolved in PBS, filter-sterilized and administered by two intraperitoneal injections of 3.5 mg/kg of body weight, 24 and 4 hrs prior to LDR radiation exposure. The dose of chloroquine administered to the mice was previously determined in dose response experiments (13).
Bone marrow transplantation
Donor mice were sacrificed by cervical dislocation immediately after LDR radiation exposure. Bone marrow cell suspensions were prepared by crushing tibia, fibula and vertebral bones with a mortar and pestle in sterile PBS and then passed through a 70-μm filter. Bone marrow cellularity was determined using a Coulter counter. Whole bone marrow cell suspensions in PBS (300 μl total volume) were injected via the retro-orbital venous sinus into lethally irradiated recipients.
Complete peripheral blood cell count
Peripheral blood (50 μl) was collected from retroocular vessels using heparinized capillary tubes and complete blood cell counts were obtained using a Hemavet950 Hematology system (Drew Scientific, Oxford, CT).
In vitro methylcellulose colony forming assay
Whole bone marrow cell suspensions were cultured in semi-solid methylcellulose medium (M3434, Stem Cell Technologies, Vancouver, Canada) supplemented with recombinant murine SCF (50 ng/ml), IL-3 (10 ng/ml), IL-6 (10 ng/ml), GM-CSF (10 ng/ml) and EPO (3 U/ml). Cells were incubated at 37°C with 5% CO2, and total number of colonies was counted after 10 days using an inverted microscope.
In vivo progenitor assay
1 X 107 whole bone marrow cells were harvested from LDR radiated mice, mixed with 2 X 105 congenic CD45.1 un-irradiated bone marrow cells and transplanted into lethally irradiated C57BL/6 CD45.1 recipients. To assess donor engraftment, peripheral blood (50 μl) was drawn via retro-orbital bleeding into heparinized capillary tubes and stained with anti-mouse CD45.1-FITC and CD45.2-PE antibodies (eBioscience, San Diego, CA), followed by flow cytometry analysis.
Lineage depletion and FACS analysis
Whole bone marrow cell suspensions were stained with a mixture of purified biotin conjugated monoclonal antibodies recognizing mouse Ter-119, CD3e, B220 and Gr-1 (eBioscience, San Diego, CA). Cells positive for lineage markers were partially removed by magnetic bead depletion on a LD column with Anti-Biotin MicroBeads, mouse IgG1 isotype (Miltenyi Biotech, Auburn, CA). The remaining lineage depleted cells were collected in the flow through from the magnetic columns and treated with 35 μg/ml of chloroquine (Sigma) for 2.5 hrs at 37°C and 5% CO2. To detect phosphor-ATM expression, the lineage depleted cells were washed with PBS, fixed in 2% formaldehyde for 10 minutes at room temperature, permeabilized with a mixture of cold 50% methanol and 50% acetone and blocked with 2% FBS in PBS overnight. Cells were then stained with anti-pATM-PE (clone 10H11.E12, Millipore, Billerica, MA), which recognizes phosphorylated Ser-1981, and analyzed using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) and FlowJoTM software (TreeStar, Ashland, OR).
Western blots
Whole bone marrow cells from chloroquine treated or untreated mice were analyzed by western blot according to standard protocols. Briefly total cell lysates were prepared from about 5 × 107 whole bone marrow cells. About 200 μg of the total cell lysate was loaded per lane. Total p53 was detected by using anti p53 (EMD Chemicals, Gibbstown, NJ) and phosphorylated p53 was detected by using anti mouse phospho-p53 (S18) antibody (R&D SYSTEM, Minneapolis, MN) at final concentration of 2 μg/ml.
Statistical analysis
Survival was calculated using Kaplan Meier analysis and a log rank test. Comparisons between groups were performed using a 2-tailed, un-paired Student t-test. p-values of <0.05 were considered statistically significant.
RESULTS
The in vitro protective effect of chloroquine on noncancerous cells from LDR radiation induced death is dependent on ATM
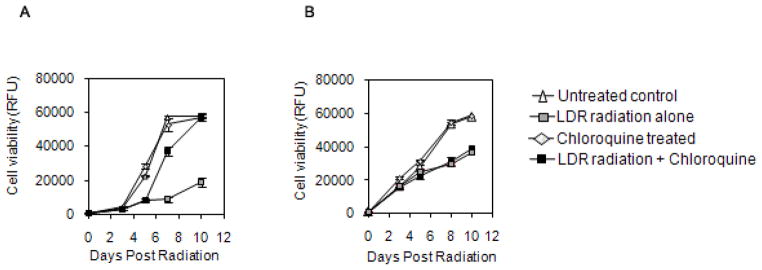
Chloroquine has long been utilized as an anti-malarial agent. It is also known to induce the activation of ATM and has been shown to abrogate the enhanced cytotoxicity of cancer cells exposed to LDR radiation in vitro (10) . To examine whether a similar LDR protective effect of chloroquine in noncancerous human cells is also dependent on ATM, we analyzed the effect of chloroquine on hTERT immortalized human fibroblasts derived from normal donors or patients with ataxia-telangiectasia that lack ATM activity. Treatment of these fibroblasts with chloroquine prior to LDR radiation improved cell viability (Fig. 1A), similar to our previous findings in cancer cells (10) . In contrast, chloroquine failed to protect ATM deficient fibroblasts from LDR irradiation (Fig. 1B).
Fig. 1.
Effect of chloroquine on the proliferation of wild type ATM+/+ human fibroblast cell line (A) or ATM deficient fibroblast cells(B) following 4Gy of ionizing radiation at LDR radiation exposure.
Chloroquine protects mice from LDR radiation induced death
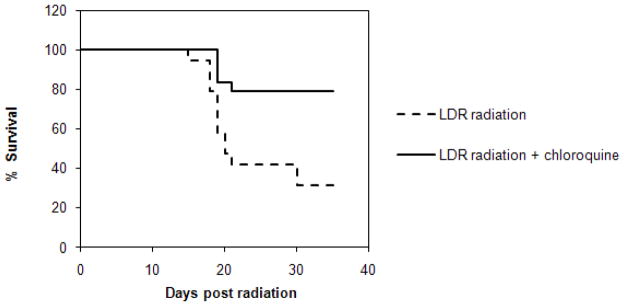
We were similarly interested in understanding the effects of chloroquine in animals treated with the drug prior to total body exposure to LDR. To examine whether similar radio-protective effects can be induced in vivo, we treated normal mice with chloroquine prior to LDR irradiation. Compared to untreated animals (n=19), mice pre-treated with two doses of chloroquine (n=24) demonstrated significantly improved survival rates (Fig. 2A, 31% vs. 80%, p=0.0026).
Fig. 2.
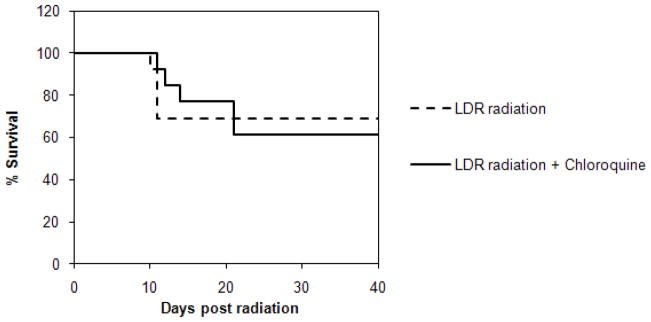
Survival of mice exposed to LDR radiation: Kaplan-Meier survival analysis of normal mice (A) or ATM null mice (B), untreated (dashed lines) or treated with chloroquine (solid line).
The in vivo protective effect of chloroquine on mice from LDR radiation induced death is also dependent on ATM
We then examined whether ATM was specifically required for chloroquine-mediated protection from LDR radiation in vivo and treated ATM null transgenic mice with or without chloroquine prior to LDR radiation exposure. In contrast to wild-type mice (Fig. 2A), we detected no significant differences in survival between chloroquine treated (n=13) and untreated (n=13) ATM null mice (Fig. 2B, 61.5% vs. 69.2%, p=0.86). Therefore, both the in vitro and in vivo radio-protective effects of chloroquine are, at least in part, ATM dependent.
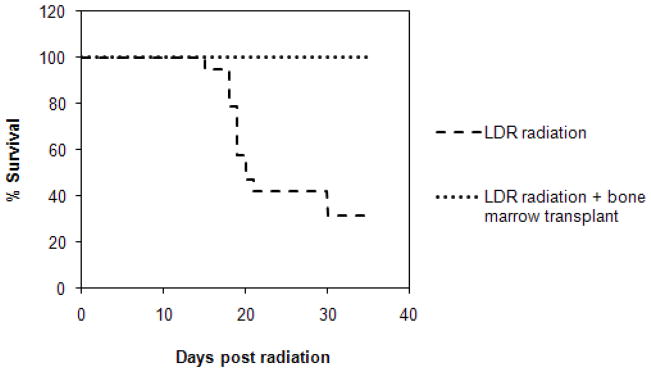
Bone marrow transplantation rescues mice from lethal LDR radiation
Acute exposures to acutely delivered total body radiation at certain doses are known to cause bone marrow failure in mammals. We examined whether hematopoietic failure was also responsible for death of mice following lethal LDR irradiation by carrying out bone marrow transplantation studies. All mice (n=10) receiving healthy whole bone marrow (1 X 106 cells) immediately after LDR radiation survived the exposure. Full necropsies following treatment with LDR radiation alone failed to demonstrate significant damage to the lungs or gastrointestinal tract (data not shown); strongly suggesting that impaired hematopoiesis is the primary cause of death following exposure to LDR radiation in these animals.
Chloroquine protects hematopoietic progenitors from LDR radiation
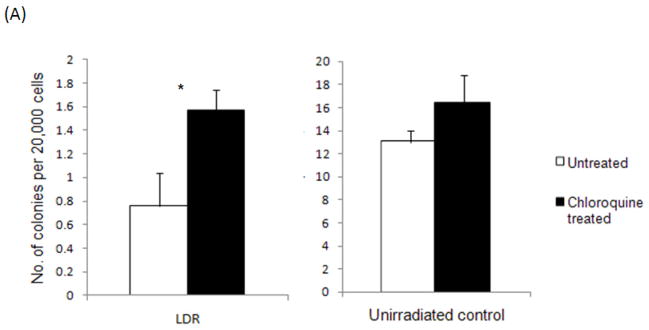
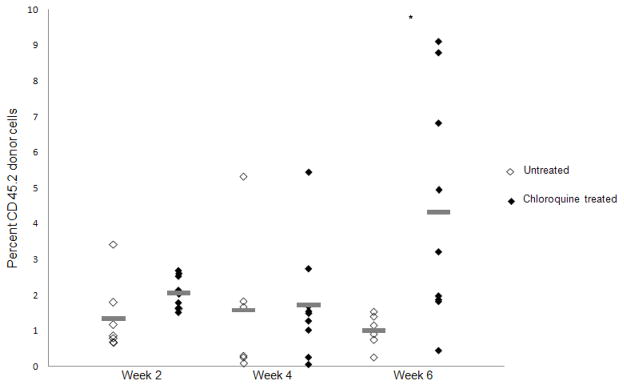
Bone marrow rescue following acute lethal radiation is primarily mediated by committed myeloid progenitors (12) . We therefore examined the effects of chloroquine treatment on myeloid progenitor cell function by plating whole bone marrow cells from LDR irradiated mice in methylcellulose and quantifying colony-formation. Compared to untreated mice, LDR radiation resulted in significantly less colony formation (Fig. 3A, 0.76 vs. 13 colonies per 20,000 cells, p<0.001). Chloroquine treatment significantly improved the recovery of total myeloid CFC following radiation (Fig. 3A, 0.76 vs. 1.57 colonies per 20,000 cells, p=0.02). No significant differences in myeloid CFC were observed in un-irradiated mice treated with or without chloroquine (Fig. 3A, 13 vs.15 colonies per 20,000 cells, p=0.30). We also examined the effects of chloroquine on the in vivo recovery of myeloid progenitors and transplanted 1 X 107 whole bone marrow cells from LDR irradiated C56BL/6 CD45.2 donor mice into congenic C57BL/6 CD45.1 recipient mice. Since myeloid progenitors are responsible for early engraftment, we quantified the peripheral blood chimerism by flow cytometry starting 2 weeks following transplantation. In mice receiving bone marrow from LDR irradiated mice pretreated with chloroquine, the frequency of peripheral blood CD45.2 donor cells was significantly increased compared to untreated mice by 6 weeks post transplant (Fig. 3B, 4.33% vs. 1.00%; p=0.015). In contrast, chloroquine treatment had no significant effect on hematopoietic stem cell mediated long-term engraftment (> 12 weeks) of LDR radiated bone marrow (Supplementary Fig. 1). Consistent with these observations, chloroquine treatment had a significant effect on the recovery of complete peripheral blood cell counts (CBCs) 16 days after bone marrow transplantation (Supplementary Fig. 2). Taken together these results suggest that enhanced survival of LDR irradiated mice treated with chloroquine is primarily mediated through the protection of hematopoietic progenitors.
Fig. 3.
Chloroquine protects hematopoietic progenitors from LDR radiation in vitro and in vivo. Chloroquine pre-treated or untreated whole bone marrow cells from LDR irradiated or non-irradiated mice (A) were assessed for colony formation after 10 days in methylcellulose (*p<0.05; n=3). Short term in vivo engraftment analysis of mice transplanted with 1× 107 whole bone marrow cells following LDR irradiation with or without chloroquine treatment (B), peripheral blood was stained with CD45.1 and CD45.2 antibodies to assess chimerism, each diamond represents one mouse, grey lines represent average engraftment percentages.
Chloroquine activates ATM in mouse hematopoietic progenitor cells
To examine the ability of chloroquine to activate ATM in myeloid progenitors, we treated lineage-depleted hematopoietic stem cells and progenitors isolated from mouse bone marrow cells with chloroquine (35 μg/ml for 2 hours) then examined ATM activation by flow cytometry. Compared to untreated cells, chloroquine significantly increased the level of phosphorylated ATM by approximately 2.5 fold (Figs. 4A and 4B, p <0.05). We also examined the expression of phosphorylated ATM following in vivo treatment with chloroquine; however, we did not detect significant ATM activation in lineage negative bone marrow cells likely due to the cellular processing required to isolate hematopoietic progenitors. We could not detect phosphorylated ATM by standard western blotting techniques of whole bone marrow obtained from treated mice. It is known that it is difficult to detect phosphorylated ATM in mouse tissue (13). Previous studies have used the activation status of p53, a downstream target of activated ATM, as a surrogate for in vivo ATM phosphorylation and have shown increased p53 phosphorylation in tissues from chloroquine treated mice (13).Therefore, we examined the level and activation status of p53 in bone marrow cells obtained from mice treated with chloroquine. Chloroquine treatment increased the levels of both total and phosphorylated p53 (Ser 18) as detected via western blot analysis (Fig. 4C) consistent with ATM activation. Taken together these results support in vivo activation of ATM by chloroquine.
Fig. 4.
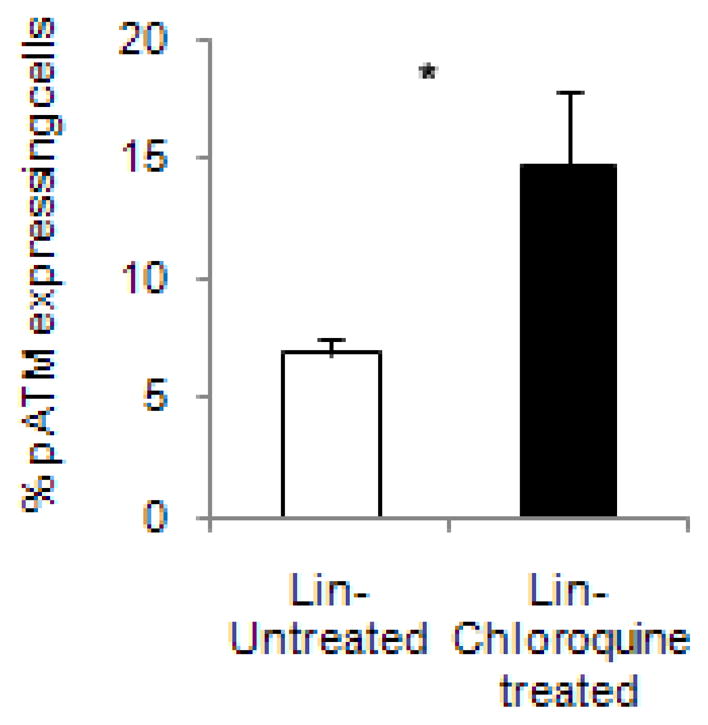
Chloroquine activates ATM in mouse hematopoietic cells. Lineage depleted mouse bone marrow cells were treated with chloroquine and analyzed by flow cytometry for pATM expression, Quantitation of phosphorylated ATM expression(A, *p <0.05) and representative dot plots gated on forward and side scatter followed by pATM expression (B). Untreated cells were stained as control; Western blot of p53 expression in mouse bone marrow cells used as surrogate for ATM activation(C). IR treated mouse used as positive control for p53 phosphorylation received 5 Gy of acute TBI. Graph of fold change of total p53 and p53-p normalized to beta-actin is included (C).
DISCUSSION
Protracted LDR radiation exposures arising from nuclear accidents and terrorism events pose a serious threat to public health and few protective agents are currently available(14) . Here we report a substantial increase in survival of mice pretreated with chloroquine prior to exposure to lethal doses of LDR radiation mediated by the activation of ATM. The precise molecular mechanisms by which chloroquine protects cells from LDR radiation are unknown, but some radioprotective agents, such as aminothiol compounds, are thought to structurally stabilize DNA and decrease the rate of DNA replication (15) . Similar to aminothiols, chloroquine binds to DNA and its intercalation between DNA bases results in the structural modification of chromatin (16). This alteration in chromatin structure is associated with the activation of ATM (7) and DNA damage and repair pathways that otherwise fail to be induced by LDR radiation(10). Activation of ATM by chloroquine alone can lead to the phosphorylation of p53 protein one of the first downstream targets of ATM identified (17). However, other downstream targets of ATM such as H2AX are not phosphorylated by chloroquine-activated ATM and require the presence of double strand breaks for phosphorylation (7). ATM also regulates the cellular response to oxidative stress and reactive oxygen species (ROS), and it is possible that ATM activation enhances cytoprotective mechanisms within progenitors that abrogate the damaging effects of increased ROS levels following exposure to LDR radiation. Recently, ATM has also been found to play a role in regulating cellular metabolism and autophagy in response to increased ROS levels by activating TSC2 and the LKB1/AMPK pathway to repress mTORC1 signaling (18). The loss of Lkb1 severely impairs hematopoiesis in mice primarily by decreasing mitochondrial function (19). Therefore, it is also possible that chloroquine-induced activation of ATM and subsequent LKB1/AMPK signaling may improve the survival of hematopoietic progenitors through the expression of anti-apoptotic factors or modulating autophagy or energy metabolism. It is likely that chloroquine acts by multiple mechanisms in vivo, and it is possible that additional protective functions including those mediated by the bone marrow microenvironment also contribute to its activity. It is also conceivable that the survival benefit associated with chloroquine may be associated with an increased accumulation of genetic alterations and mutations. Therefore, further investigation of chloroquine’s action that include both ATM-mediated and independent processes is warranted. Another potential side effect of chloroquine in combination with radiation may be cutaneous desquamation as reported by Rustogi (20). We did not observe skin reactions in the mice treated with chloroquine and low dose-rate radiation. Nevertheless, given its tolerability and low cost, chloroquine may serve as a readily accessible agent drug for protection from LDR radiation injury.
Supplementary Material
Long term in vivo engraftment analysis of mice transplanted with 1× 107 whole bone marrow cells following LDR irradiation with or without chloroquine treatment. Peripheral blood was stained with CD45.1 and CD45.2 antibodies to assess chimerism. Each diamond represents one mouse; grey lines represent average engraftment percentages.
Chloroquine enhances hematopoietic recovery. Complete blood count analysis of peripheral blood of mice exposed to LDR radiation treated with and without chloroquine (times shown are days post bone marrow transplantation, *p<0.05).
Chloroquine can abrogate the lethal cellular effects of low dose-rate (LDR) radiation in vitro by activating the ataxia-telangiectasia mutated (ATM) protein. Chloroquine administration prior to radiation has a significant effect on early engraftment of bone marrow cells and on the survival of normal but not ATM null mice strongly suggesting that the in vivo effect like the in vitro effect is ATM dependent. Chloroquine may serve as a useful drug for protection against the harmful effects of LDR radiation.
Acknowledgments
This work is supported by the American Society of Hematology Scholar Award, American Society for Clinical Oncology Young Investigator Award, American Association for Cancer Research-Astellas USA Fellowship for Basic Cancer Research to A.A.M. The National Institutes of Health (R01CA127574, P01CA015396, K23CA107040), the Gabrielle’s Angel Foundation for Cancer Research, the Sidney Kimmel Foundation for Cancer Research, and the Goodwin Foundation to W.M., The National Institutes of Health (R01CA71387 and P30CA21765), and the American-Lebanese-Syrian Associated Charities of St. Jude Children’s Research Hospital to M.B.K. and NIH/NCI P50 CA58236 Prostate Cancer SPORE to T.L.D.
Footnotes
CONFLICT OF INTEREST
There are no relevant conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFRENCES
- 1.Meijne EI, van der Winden-van Groenewegen RJ, Ploemacher RE, et al. The effects of x-irradiation on hematopoietic stem cell compartments in the mouse. Exp Hematol. 1991;19:617–623. [PubMed] [Google Scholar]
- 2.Le RG. Hematology of atomic bomb casualties. AMA Arch Intern Med. 1950;86:691–710. doi: 10.1001/archinte.1950.00230170044005. [DOI] [PubMed] [Google Scholar]
- 3.DeWeese TL, Shipman JM, Dillehay LE, et al. Sensitivity of human prostatic carcinoma cell lines to low dose rate radiation exposure. J Urol. 1998;159:591–598. doi: 10.1016/s0022-5347(01)63990-9. [DOI] [PubMed] [Google Scholar]
- 4.Marin LA, Smith CE, Langston MY, et al. Response of glioblastoma cell lines to low dose rate irradiation. Int J Radiat Oncol Biol Phys. 1991;21:397–402. doi: 10.1016/0360-3016(91)90788-6. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell CR, Folkard M, Joiner MC. Effects of exposure to low-dose-rate Co-60 gamma rays on human tumor cells in vitro. Radiation Research. 2002;158:311–318. doi: 10.1667/0033-7587(2002)158[0311:eoetld]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Furre T, Furre IE, Koritzinsky M, et al. Lack of inverse dose-rate effect and binding of the retinoblastoma gene product in the nucleus of human cancer T-47D cells arrested in G2 by ionizing radiation. International Journal of Radiation Biology. 2003;79:413–422. doi: 10.1080/0955300031000140784. [DOI] [PubMed] [Google Scholar]
- 7.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 8.Kastan MB, Lim DS. The many substrates and functions of ATM. Nat Rev Mol Cell Biol. 2000;1:179–186. doi: 10.1038/35043058. [DOI] [PubMed] [Google Scholar]
- 9.Kitagawa R, Bakkenist CJ, McKinnon PJ, et al. Phosphorylation of SMC1 is a critical downstream event in the ATM-NBS1-BRCA1 pathway. Genes Dev. 2004;18:1423–1438. doi: 10.1101/gad.1200304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collis SJ, Schwaninger JM, Ntambi AJ, et al. Evasion of early cellular response mechanisms following low level radiation-induced DNA damage. J Biol Chem. 2004;279:49624–49632. doi: 10.1074/jbc.M409600200. [DOI] [PubMed] [Google Scholar]
- 11.Barlow C, Hirotsune S, Paylor R, et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 12.Na Nakorn T, Traver D, Weissman IL, et al. Myeloerythroid-restricted progenitors are sufficient to confer radioprotection and provide the majority of day 8 CFU-S. J Clin Invest. 2002;109:1579–1585. doi: 10.1172/JCI15272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider JG, Finck BN, Ren J, et al. ATM-dependent suppression of stress signaling reduces vascular disease in metabolic syndrome. Cell Metab. 2006;4:377–389. doi: 10.1016/j.cmet.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Coleman CN, Stone HB, Moulder JE, et al. Medicine. Modulation of radiation injury. Science. 2004;304:693–694. doi: 10.1126/science.1095956. [DOI] [PubMed] [Google Scholar]
- 15.Brown PE. Mechanism of action of aminothiol radioprotectors. Nature. 1967;213:363–364. doi: 10.1038/213363a0. [DOI] [PubMed] [Google Scholar]
- 16.Krajewski WA. Alterations in the internucleosomal DNA helical twist in chromatin of human erythroleukemia cells in vivo influences the chromatin higher-order folding. FEBS Lett. 1995;361:149–152. doi: 10.1016/0014-5793(95)00144-x. [DOI] [PubMed] [Google Scholar]
- 17.Loehberg CR, Thompson T, Kastan MB, et al. Ataxia telangiectasia-mutated and p53 are potential mediators of chloroquine-induced resistance to mammary carcinogenesis. Cancer Research. 2007;67:12026–12033. doi: 10.1158/0008-5472.CAN-07-3058. [DOI] [PubMed] [Google Scholar]
- 18.Alexander A, Cai SL, Kim J, et al. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc Natl Acad Sci U S A. 2010;107:4153–4158. doi: 10.1073/pnas.0913860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gan B, Hu J, Jiang S, et al. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature. 2010;468:701–704. doi: 10.1038/nature09595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rustogi A, Munshi A, Jalali R. Unexpected skin reaction induced by radiotherapy after chloroquine use. Lancet Oncology. 2006;7:608–609. doi: 10.1016/S1470-2045(06)70763-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Long term in vivo engraftment analysis of mice transplanted with 1× 107 whole bone marrow cells following LDR irradiation with or without chloroquine treatment. Peripheral blood was stained with CD45.1 and CD45.2 antibodies to assess chimerism. Each diamond represents one mouse; grey lines represent average engraftment percentages.
Chloroquine enhances hematopoietic recovery. Complete blood count analysis of peripheral blood of mice exposed to LDR radiation treated with and without chloroquine (times shown are days post bone marrow transplantation, *p<0.05).