Abstract
Pain, itch, heat, cold, and touch represent different percepts arising from somatosensory input. How stimuli give rise to these percepts has been debated for over a century. Recent work supports the view that primary afferents are highly specialized to transduce and encode specific stimulus modalities. However, cross-modal interactions (e.g. inhibition or exacerbation of pain by touch) support convergence rather than specificity in central circuits. We outline how peripheral specialization together with central convergence could enable spinal microcircuits to combine inputs from distinctly specialized, co-activated afferents and to modulate the output signals thus formed through computations like normalization. These issues will be discussed alongside recent advances in our understanding of microcircuitry in the superficial dorsal horn.
Introduction
Pain normally serves to alert us to danger. This is exemplified by people with congenital insensitivity to pain, many of whom succumb to minor injuries or disease because they fail to notice health problems normally evidenced by pain [1]. Pain without overt injury is, however, far more common. Such pain can often be traced back to damage to or dysfunction of the nervous system and is termed “neuropathic” [2]. Unlike nociceptive pain in which noxious stimulation is appropriately perceived as painful, neuropathic pain is associated with mechanical allodynia (pain caused by innocuous touch) and, paradoxically, with hypoesthesia (reduced touch sensation) [3]. Such perceptual anomalies provide valuable insight into how sensory information is processed and what impact that processing has on perception.
Other somatosensory percepts include touch, itch, heat and cold. Many believe that each percept is evoked by stimuli representing distinct (sub)modalities, but the neural signals elicited by different modalities often interact [4–9]. Interactions can be unmasked by careful experimentation (e.g. innocuous cooling can elicit pain, but that pain is typically inhibited by touch [9]) and become more obvious under pathological conditions (e.g. mechanical allodynia). These cross-modal interactions suggest that somatosensory percepts are synthesized from the combination of neural signals representing multiple modalities rather than on the basis of signals representing any one modality.
Comparison with other sensory systems is revealing: We see an entire rainbow of colors based on the relative activation of three types of cone photoreceptors (trichromacy) [10], and we tend to smell odorant combinations (configural odor perception) rather than the component odorants (elemental odor perception) despite exquisitely specialized olfactory receptor cells [11]. In both cases, as in somatosensation, primary receptor cells transduce specific features of the physical stimulus but we tend to perceive something more synthetic because of subsequent neural processing. Despite this, cross-modal interactions in the somatosensory system are often considered a design fault (i.e. cross-talk between labeled lines) rather than a potentially important design feature. However, processing enabled by cross-modal interactions could, for instance, help disambiguate stimulus quality and intensity, the same way that comparing the relative activation of cones with different spectral sensitivities disambiguates the color and intensity of light [10].
Before delving into spinal microcircuits, we will follow a top-down approach to establish the importance of central pain processing. Then, following a bottom-up approach, we will consider how spinal microcircuits could implement that processing. Given that spinal microcircuits have been the focus of several recent reviews [12**,13**,14–18], we have emphasized theoretical aspects of pain processing and their relation to microcircuit function in the hope of providing a different perspective on this topic.
Pain theories
Several physiological theories of pain have been developed and can be divided into three groups [for detailed history, see 19,20,21]. According to intensity theory, pain occurs when non-specific cells are activated very strongly. This theory denies peripheral specialization (and, for that reason, has been ruled out) but emphasizes the importance of convergence onto and summation by spinal neurons [22], and thus shares some similarities with pattern theory (see below).
According to specificity theory, pain is subserved by cells activated uniquely by noxious stimulation, i.e. nociceptive-specific (NS) cells. For specificity to be maintained throughout the neuraxis, postsynaptic cells in the “pain pathway” receive input exclusively from presynaptic NS cells and are de facto NS. The neural signal conveyed via this labeled line evokes pain upon arrival at some decoder. Other somatosensory percepts are evoked via separate labeled lines. A critical prediction of this theory is that the specificity that exists peripherally (i.e. in primary afferents) is maintained centrally (i.e. in spinal neurons).
According to pattern theory, perception depends on the relative activation of different types of primary afferents – a spatial pattern at the population level [23] or, as we propose to refer to it, a combinatorial code. A combinatorial rate code is distinct from temporally patterned spiking at the single cell level [e.g. 24], but the term “pattern” has caused confusion in this regard. Nevertheless, spatiotemporally patterned input to spinal circuits is likely to be important, especially given differential conduction velocities among primary afferents. Sensory interaction theory [25] and gate control theory [4*], both of which constitute pattern theories, as well as more recent work [5,26,27*,28**], have all stressed interactions between co-activated inputs. Describing labeled lines as interacting [e.g. 29*,30], despite the inherent self-contradiction, reflects an ongoing effort to reconcile seemingly discrepant observations.
From primary afferent activation to pain – the case for central pain processing
There is unequivocal evidence that primary afferents are specialized to detect certain stimuli [31], e.g. nociceptors detect noxious input. This does not mean that afferents are specialized to evoke certain percepts. Noxious stimulation activates nociceptors and it evokes pain, but pain is not necessarily evoked via (and only via) nociceptor activation. Nociceptor activation and pain are correlated because they share a common cause – noxious stimulation. This suggests but does not prove causation although most everyone, including us, would concede that nociceptor activation normally evokes pain. Causation is supported by microstimulation studies in humans, which showed that activating single afferents evokes somatosensory percepts consistent with the receptive properties of the afferent [32]. The important points, explained below, are (1) that nociceptor activation does not always evoke pain, and (2) that pain can be evoked independent of nociceptor activation.
With regard to the first point, consider that a given stimulus does not always evoke the same percept. For example, capsaicin can evoke pain or itch depending on how it is applied – punctate application evokes itch [33]. This has been suggested to occur because the peripheral endings of “itch” neurons reach more superficial layers of the skin than other neurons [30], the idea being that “itch” neurons achieve their specificity through a combination of transducer phenotype and anatomy (and that itch is suppressed by pain if/when deeper “pain” fibers are activated). An alternative explanation, consistent with the anomalous percepts elicited by punctate thermal stimulation [27*,34], is that cutaneous stimuli are normally distributed (i.e. not punctate) and thus activate multiple afferents, the exact combination of which dictates the evoked sensation. Indeed, hair follicles are each innervated by multiple types of low-threshold mechanoreceptors (LTMRs), which means mechanical stimulation invariably co-activates more than one type of afferent [28**]. Also, the fact that temperature sensation can be qualitatively altered by differentially blocking conduction in Aδ-cold fibers (relative to thermosensitive C fibers) [7,35] supports the link between afferent co-activation and perception.
With regard to the second point, consider that noxious stimulation, although crucial for normal (i.e. nociceptive) pain, is neither necessary nor sufficient to evoke pain. “Pain signals” can originate centrally, as in central neuropathic pain [36], and peripherally generated “pain signals” can be blocked centrally, as in episodic analgesia [37]. This raises an important point: There is nothing innate to primary afferent nociceptors that endows them, and only them, with the capacity to evoke pain. In apparent contradiction to this, it has been shown in mice that ablating C fibers expressing the G protein-coupled receptor Mrgprd reduces sensitivity to noxious mechanical stimulation without affecting thermal sensitivity, whereas pharmacologically ablating a non-overlapping set of fibers expressing TRPV1 reduces sensitivity to noxious heat without affecting mechanical sensitivity [38]. Those data could be taken to suggest that the former cell type is necessary for mechanical pain whereas the latter is necessary for thermal pain; however, that necessity is true only under certain conditions. For instance, myelinated afferents [39,40,41*] and possibly C-LTMRs [42, but see 43] (neither of which express Mrgprd) contribute to mechanical pain under neuropathic conditions [see also 44] and innocuous temperatures can evoke burning pain without activating TRPV1-expressing neurons (see above). Inability of the primary afferents involved in mechanical allodynia and anomalous temperature sensation to normally evoke pain exemplifies how other factors, like downstream microcircuit function and co-activated inputs, are important for perception.
In brief, somatosensory afferents are specialized to encode certain modalities and intensities of stimulation. There is, however, no one-to-one relationship between afferent activation and perception, contrary to what labeled lines predict. Instead, numerous observations speak to the importance of central processing and suggest that co-activated inputs converge and interact within spinal circuits. We will focus on circuits in the superficial dorsal horn (laminae I and II) because of their established importance in pain processing and because they are better understood than those in deeper laminae.
Dorsal horn circuitry
Input
Sensory information is conveyed to the spinal cord dorsal horn via primary afferents. Different types of primary afferents terminate in different laminae (Fig. 1) [45]. Large myelinated fibers also send collaterals into the dorsal columns. Apart from sensory input, the spinal dorsal horn also receives descending modulation including serotonergic fibers from the nucleus raphe magnus, noradrenergic fibers from the locus coeruleus, and GABAergic fibers from the rostral ventromedial medulla [for review see 12].
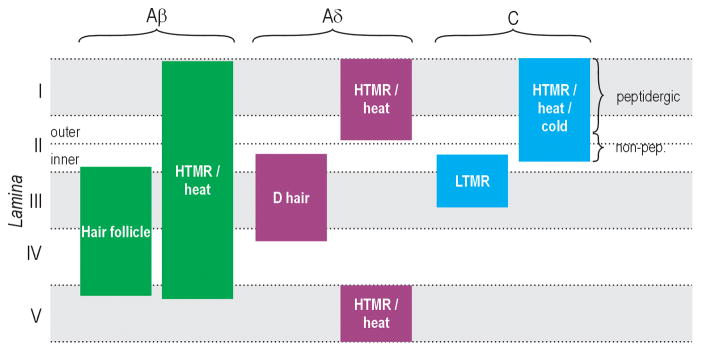
Figure 1. Afferent termination patterns in the spinal dorsal horn.
Primary afferents are routinely categorized as Aβ (thickly myelinated), Aδ (thinly myelinated), and C (unmyelinated) fibers based on conduction velocity, and can be further divided according to their responsiveness to different modalities and intensities of stimulation. The spinal dorsal horn is divided into laminae (indicated along left margin). Different types of afferents terminate in different laminae. HTMR, high-threshold mechanoreceptor. LTMR, low-threshold mechanoreceptor. Modified from [45].
Intrinsic components
Projection neurons (see Output) comprise as few as 5% of all neurons in lamina I of rat lumbar segments [46] and lamina II contains no projection neurons, meaning local interneurons predominate. About one third are GABAergic based on immunocytochemistry [47], and a subset of those co-express glycine, but the majority are excitatory, consistent with paired recordings [48, but see 49]. The neuronal population is, to say the least, heterogeneous [50–54] (Fig. 2). This heterogeneity reflects the distinct ways in which different spinal neurons process information [55,56,57*]. Inclusion within the same circuit of single-spiking neurons that behave as near-optimal coincidence detectors (comparable to those in the auditory midbrain [e.g. 58]) and tonic-spiking neurons that behave as diametrically opposite integrators is intriguing, but the implications for somatosensory processing await further investigation. Below, we highlight our current knowledge of spinal circuitry. For comprehensive reviews, see [12**,13**,14–18].
Figure 2. Classification of neurons in the superficial dorsal horn.
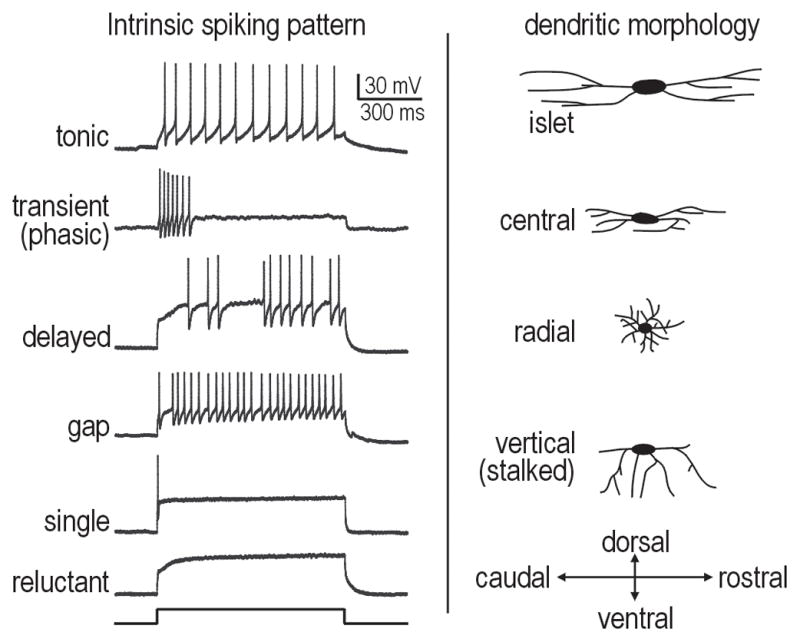
Left column shows sample spiking patterns elicited by current injection into the cell body. Right column shows cartoon representation of differences in dendritic morphology.
Using transgenic mice that express GFP selectively in (subsets of) GABAergic neurons, intrinsic spiking patterns of inhibitory neurons have been identified [59–62,63*] and include tonic-, transient- (phasic-) and single-spiking [e.g. 62]. The same genetic tools have not been used to target excitatory interneurons but immunocytochemical studies provide interesting correlations; for instance, Kv4 channels (responsible for the A-type potassium current) are not co-expressed with GABA [64,65] and are thus present in excitatory interneurons and some projection neurons [66]. The A-type current is associated with several spiking patterns including delayed-, gap-, single- and reluctant-spiking [50,52–54,67]. Delayed-spiking can be accounted for by the inactivation kinetics of the A-type potassium current, whereas single-spiking is accounted for by a separate, noninactivating low-threshold potassium current [57]; gap- and reluctant-spiking arise from interactions between those two currents (unpublished observations; SR and SAP). However, tonic- and transient-spiking patterns have also been described in excitatory interneurons [48] which, given the patterns observed in inhibitory interneurons (see above), suggests there is no simple electrophysiological way of delineating excitatory neurons from inhibitory neurons. Notably, Kv4.2 has been shown to play an important role in pain plasticity [68]. Protein kinase C γ, which is expressed in a subset of excitatory interneurons [69], also plays an important role in neuropathic pain [70].
By combining immunocytochemistry and electrophysiology, Todd and colleagues have shown that inhibitory neurons comprise mostly islet, central and vertical morphologies with mostly tonic spiking, whereas excitatory neurons comprise mostly radial and vertical morphologies with various spiking patterns associated with A-type current (see above) plus central morphology associated with transient spiking [52*]. This is generally consistent with paired recording work by Perl and colleagues (Fig. 3). Tonic-spiking islet cells inhibit transient-spiking central cells [49]. Transient-spiking central cells excite delayed-spiking vertical cells, which in turn excite lamina I projection neurons [71]. Tonic-spiking central cells form reciprocal inhibitory connections with tonic-spiking islet cells and also inhibit vertical and possibly transient-spiking central cells [63*]. In general, excitatory connections tend to depress whereas inhibitory connections facilitate [72]. The two types of inhibitory interneurons receive different C fiber input [63*] and are differentially modulated by serotonin [73]. Opposite effects of noradrenaline on inhibitory interneurons have been reported [73,74]. Yoshimura and colleagues have shown that islet and central cells (both putative inhibitory interneurons) receive monosynaptic excitation uniquely via C fibers (although the former also receive polysynaptic Aδ) and GABAergic inhibition, whereas radial and vertical cells (both putative excitatory interneurons) receive monosynaptic C and Aδ input and mixed GABA/glycinergic inhibition [75*]. Excitatory neurons selectively receive input from TRPA1-expressing C fibers [76]. Notably, all cell types except islet cells receive monosynaptic input from Mrgprd-expressing C fibers [77*], which are purportedly critical for mechanical nociception (see From primary afferent activation to pain – the case for central pain processing). A subset of inhibitory neurons also receive Aβ input [78]. Overall, it appears that most dorsal horn cell types receive convergent input from multiple types of primary afferents.
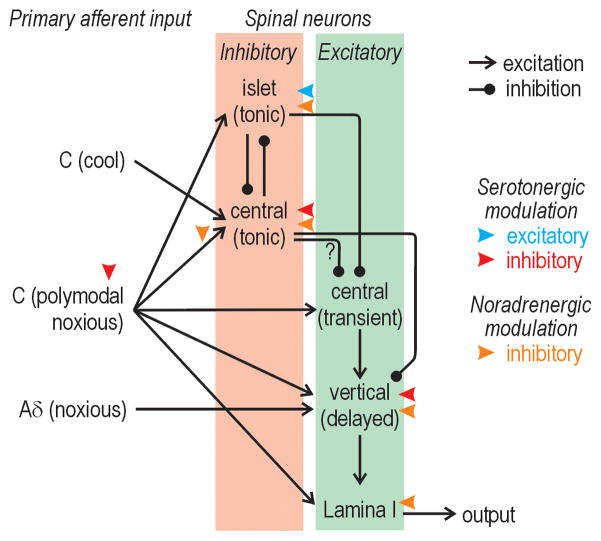
Figure 3. Circuitry in the superficial dorsal horn.
Summary of what is currently known about synaptic input to and connectivity between different types of spinal neurons based primarily on paired recording data [49,63,71,73]. Notably, ref. 63 reports that tonic central cells receive input from TRPM8-expressing C fibers (which have fast conduction velocities relative to other C fibers) whereas ref 49 reports that islet cells receive fast C fiber input (relative to transient central cells); this figure depicts the former results, which are arguably more definitive. Also, this figure does not depict input from Mrgprd-expressing C fibers to all cell types other than islet cells [77], input from TRPA1-expressing C fibers to excitatory interneurons [76], or input from Aβ fibers onto some inhibitory interneurons [78], not to mention the full extent of polsynaptic connections.
Output
A subset of neurons in all laminae except lamina ll send projections to supraspinal targets including the thalamus and brainstem [79,80], with some neurons innervating more than one projection site [for review see 12]. Lamina I projection neurons constitute a large fraction of the spinothalamic tract, conveying information related to pain, itch and temperature. This cell population includes NS neurons (responsive to pinch and/or noxious heat) [81] as well as COLD neurons (responsive to innocuous cooling and inhibited by warming) and HPC neurons (responsive to heat, pinch and cold) [82]. Itch-specific neurons (i.e. selectively activated by histamine) have also been described [83, but see 84]. Notably, however, dorsal horn neurons have inhibitory receptive fields (mapped by innocuous peripheral stimulation) that are larger than their excitatory receptive fields ([85–87]. Moreover, the excitatory receptive field can expand and contract rapidly [88], suggesting that it is dynamically regulated by microcircuit function rather than being hard-wired [see also 89]. Furthermore, lamina I NS cells become responsive to innocuous stimulation after nerve injury, and this can be acutely reproduced by experimental manipulations [90*]. These data demonstrate that pre-existing polysynaptic pathways carrying low-threshold input to “NS” lamina I neurons can be unmasked [see also 91,92,93]. In short, the dynamic multi-modal tuning of spinal projection neurons demonstrates the importance of microcircuit function and argues against labeled lines. Please note that evidence against labeled lines does not equate with evidence against peripheral specialization – this misappropriation has caused too much confusion not to be mentioned here.
Microcircuits and putative computations
The emerging picture is one of spinal circuits composed of numerous excitatory and inhibitory interneurons that (i) have diverse intrinsic coding properties, (ii) receive different patterns of primary afferent input, (iii) connect preferentially to other spinal cell types, and (iv) are differentially modulated. Despite these advances, we are still a long way from understanding exactly how spinal circuits contribute to pain processing. Here, in the hopes of stimulating more research in this direction, we speculate on how microcircuits could implement some simple but potentially important computations.
Figure 4 contrasts the input-output transformation that could be implemented by different circuit motifs. Pure labeled lines, represented by motif 1, do not interact, meaning input a gives output A and input b gives output B regardless of whether inputs a and b are co-activated. By comparison, co-activation of multiple inputs is important in motifs 2–5. Motif 2 shows lateral inhibition between the top and bottom pathways; in this scenario, only the optimally activated pathway will relay output if weak (non-optimal) excitation is overpowered by inhibition [94]. Inclusion of reciprocal inhibition (i.e. between inhibitory interneurons; not shown) could improve categorization [95]. In motif 3, co-activated inputs converge onto a common inhibitory interneuron whose inhibition is therefore proportional to the net input; in this scenario, each output is normalized by total input [96]. In effect, motifs 2 and 3 allow ratios of different inputs to be calculated. Notably, inhibition may not be evident except through its modulation of concurrent excitation, meaning interactions between pathways are likely to be overlooked unless multiple inputs are co-activated. In experiments, co-activation is typically avoided by using stimuli designed to isolate individual inputs. This reductionist tendency may seriously limit our understanding given that realistic stimuli are likely to be complex, and thus conducive to co-activating multiple inputs which could then interact.
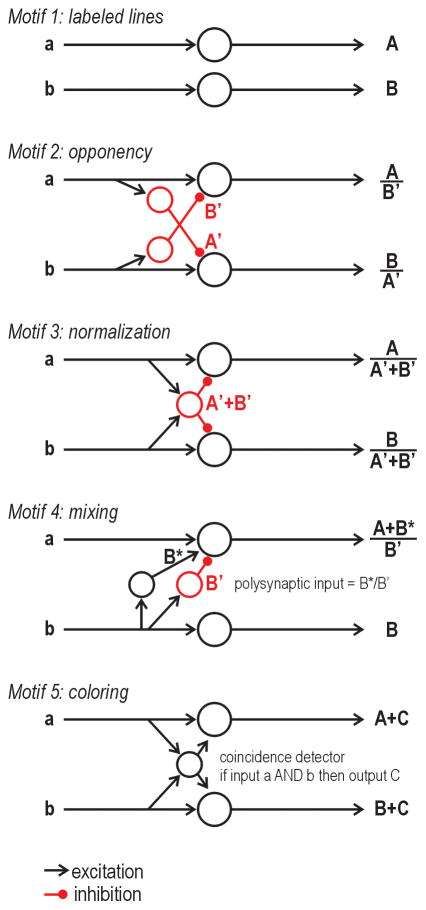
Figure 4. Putative microcircuits and their computational role.
Motif 1: Pure labeled lines do not interact, meaning input a gives output A in the top pathway and input b gives output B in the bottom pathway even when inputs a and b are co-activated. Motif 2: Opponency is implemented by lateral inhibition, meaning output A is modulated by inhibition B′ and output B is modulated by inhibition A′ if inputs a and b are co-activated. Motif 3: Normalization is implemented when inputs a and b converge (perhaps via an excitatory interneuron; not shown) on an inhibitory interneuron whose output A′+B′ modulates outputs A and B. Motif 4: Mixing is implemented by an excitatory interneuron relaying excitation B* to the top circuit, which, if combined with polysynaptic inhibition B′, would give output (A+B*)/B′. This is the first example in which two letters occur in the numerator of the output, which is to say that the labeled line has been corrupted. Motif 5: Coloring is implemented when an excitatory interneuron’s output C indicates that input a and b have occurred together. Output C thus gives context to, or “colors”, outputs A and B. In this example, the excitatory interneuron must behave as a coincidence detector. See text for additional discussion.
In motifs 2 and 3, output in each pathway is scaled by activity in the neighboring pathway but it is not qualitatively altered – one could argue that the pathways still qualify as labeled lines. Motif 4 shows the subtle way in which labeling can be compromised by cross-excitation. Input b (without a) will give output B in the bottom pathway and could excite the top pathway polysynaptically, but polysynaptic excitation will not be evident if B*/B′ is subliminal. Under pathological conditions, B*/B′ may become supraliminal, in which case the top pathway will respond to input b. Even under normal conditions, co-activation of inputs a and b may render input B*/B′ supraliminal; thus, cross-modal interaction would only be (normally) evident when co-activating inputs a and b. It is not clear how such mixing could be useful, but it does seem to occur, as evidenced by mechanical allodynia induced by disinhibition. Motif 5 shows convergent input onto an excitatory interneuron that behaves as a coincidence detector (e.g. single-spiking neuron). Because such a neuron requires simultaneous input from both pathways, it can multiply its input firing rates [97] or implement a logical AND operation, thus enabling its output (which represents the conjunction of inputs) to “color” the regular output of each pathway. This last example highlights the importance of intrinsic cell properties. To continue that theme, consider that normalization is best implemented by inhibitory interneurons that behave as integrators (e.g. tonic-spiking neurons). Our postulated connection between spiking pattern and cell types (i.e. inhibitory vs. excitatory interneuron) is consistent with available data (see Dorsal horn circuitry).
Conclusions
Like in other sensory systems, primary somatosensory afferents are specialized to encode elemental stimulus features. According to specificity theory, the initial neural representation should remain unchanged as the signal passes to postsynaptic neurons along a labeled line, implying a one-to-one relationship between stimulation, primary afferent activation, and perception – evidence does not support this. The same peripheral specialization that is cited as supporting specificity theory (without due regard for exclusive synaptic connectivity to central neurons) is also required for the combinatorial coding proposed here – spinal microcircuits could not calculate the relative activation of different types of primary afferents if the primary afferent population was homogeneous. Such computations require an array of variably specialized afferents and some degree of central convergence. Both requirements seem to be fulfilled.
For now, we can only speculate that spinal microcircuits carry out computations that are known to be important in other sensory systems but which, to date, have attracted little attention in the context of pain processing. Identifying those computations and determining how spinal microcircuits implement them will require much more work, but we are moving closer. What is clear is that information processing by spinal microcircuits is important for how we perceive somatosensory stimuli and that improper processing can produce debilitating perceptual anomalies like mechanical allodynia.
Highlights.
Primary somatosensory afferents are specialized to detect different modalities and intensities of stimulation.
Signals carried by different types of primary afferents converge in the dorsal horn of the spinal cord.
Central convergence compromises central specificity but enables cross-modal interactions.
Spinal circuits may exploit convergence to transform input signals (representing elemental stimulus features) into output signals that are more closely related to perception.
Acknowledgments
SAP is a Rita Allen Foundation Scholar in Pain and the 53rd Mallinckrodt Scholar, and is also funded by an Early Career Grant from the International Association for the Study of Pain and ScanDesign Foundation, the Competitive Medical Research Fund at the University of Pittsburgh, and the National Institute of Neurological Disorders and Stroke (R01 NS076706 and R21 NS074146). The funding sources had no involvement in the preparation of this article. We thank Yves De Koninck, Sergei Karnup, and Rick Koerber for feedback on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baxter DW, Olszewski J. Congenital universal insensitivity to pain. Brain. 1960;83:381–393. doi: 10.1093/brain/83.3.381. [DOI] [PubMed] [Google Scholar]
- 2.Merskey H, Bogduk N. Classification of Chronic Pain. Seattle: IASP Press; 1994. [Google Scholar]
- 3.Maier C, Baron R, Tolle TR, Binder A, Birbaumer N, Birklein F, Gierthmuhlen J, Flor H, Geber C, Huge V, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150:439–450. doi: 10.1016/j.pain.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 5.Green BG. Temperature perception and nociception. J Neurobiol. 2004;61:13–29. doi: 10.1002/neu.20081. [DOI] [PubMed] [Google Scholar]
- 6.Bini G, Cruccu G, Hagbarth KE, Schady W, Torebjork E. Analgesic effect of vibration and cooling on pain induced by intraneural electrical stimulation. Pain. 1984;18:239–248. doi: 10.1016/0304-3959(84)90819-4. [DOI] [PubMed] [Google Scholar]
- 7.Yarnitsky D, Ochoa JL. Release of cold-induced burning pain by block of cold-specific afferent input. Brain. 1990;113 ( Pt 4):893–902. doi: 10.1093/brain/113.4.893. [DOI] [PubMed] [Google Scholar]
- 8.Craig AD, Bushnell MC. The thermal grill illusion: unmasking the burn of cold pain. Science. 1994;265:252–255. doi: 10.1126/science.8023144. [DOI] [PubMed] [Google Scholar]
- 9.Green BG, Pope JV. Innocuous cooling can produce nociceptive sensations that are inhibited during dynamic mechanical contact. Exp Brain Res. 2003;148:290–299. doi: 10.1007/s00221-002-1280-9. [DOI] [PubMed] [Google Scholar]
- 10.Solomon SG, Lennie P. The machinery of colour vision. Nat Rev Neurosci. 2007;8:276–286. doi: 10.1038/nrn2094. [DOI] [PubMed] [Google Scholar]
- 11.Gottfried JA. Central mechanisms of odour object perception. Nat Rev Neurosci. 2010;11:628–641. doi: 10.1038/nrn2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11:823–836. doi: 10.1038/nrn2947. This article provides a thorough and up-to-date review of the spinal dorsal horn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13**.Ribeiro-da-Silva A, De Koninck Y. Morphological and neurochemical organization of the spinal dorsal horn. In: Basbaum AI, Bushnell MC, editors. Science of Pain. Elsevier; 2008. This chapter provides an even more in-depth review than [12], delving into such topics as species differences and synaptic ultrastructure. [Google Scholar]
- 14.Takazawa T, MacDermott AB. Synaptic pathways and inhibitory gates in the spinal cord dorsal horn. Ann N Y Acad Sci. 2010;1198:153–158. doi: 10.1111/j.1749-6632.2010.05501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu SX, Wang W, Li H, Wang YY, Feng YP, Li YQ. The synaptic connectivity that underlies the noxious transmission and modulation within the superficial dorsal horn of the spinal cord. Prog Neurobiol. 2010;91:38–54. doi: 10.1016/j.pneurobio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Graham BA, Brichta AM, Callister RJ. Moving from an averaged to specific view of spinal cord pain processing circuits. J Neurophysiol. 2007;98:1057–1063. doi: 10.1152/jn.00581.2007. [DOI] [PubMed] [Google Scholar]
- 17.Dolique T, Landry M, Nagy F. Spinal cord: dorsal horn. In: Shepherd GM, Grillner S, editors. Handbook of Brain Microcircuits. Oxford University Press; 2010. pp. 237–248. [Google Scholar]
- 18.Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33:550–558. doi: 10.1016/j.tins.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perl ER. Ideas about pain, a historical view. Nat Rev Neurosci. 2007;8:71–80. doi: 10.1038/nrn2042. [DOI] [PubMed] [Google Scholar]
- 20.Perl ER. Pain mechanisms: a commentary on concepts and issues. Prog Neurobiol. 2011;94:20–38. doi: 10.1016/j.pneurobio.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonica JJ. History of pain concepts and therapies. In: Bonica JJ, editor. The Management of Pain. Lea & Febiger; 1990. pp. 2–17. [Google Scholar]
- 22.Livingston WK. Pain Mechanisms. New York: Macmillan; 1943. [Google Scholar]
- 23.Nafe JP. The pressure, pain, and temperature senses. In: Murchison C, editor. Handbook of General Experimental Psychology. Clarck University Press; 1934. pp. 1037–1087. [Google Scholar]
- 24.Iggo A. Cutaneous thermoreceptors in primates and sub-primates. J Physiol (Lond) 1969;200:403–430. doi: 10.1113/jphysiol.1969.sp008701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noordenbos W. Pain. Amsterdam: Elsevier Press; 1959. [Google Scholar]
- 26.McMahon SB, Koltzenburg M. Itching for an explanation. Trends Neurosci. 1992;15:497–501. doi: 10.1016/0166-2236(92)90102-e. [DOI] [PubMed] [Google Scholar]
- 27*.Campero M, Baumann TK, Bostock H, Ochoa JL. Human cutaneous C fibres activated by cooling, heating and menthol. J Physiol. 2009;587:5633–5652. doi: 10.1113/jphysiol.2009.176040. This study describes microneurographic recordings from human cutaneous C fibers. The authors identify a set of C fibers that respond to innocuous cooling, as well as to noxious cold and hot. Based on interaction with other fibers with different temperature-sensitivities, the authors suggest how these C fibers might account for the burning-hot sensation evoked by temperatures at either extreme. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28**.Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147:1615–1627. doi: 10.1016/j.cell.2011.11.027. This study shows that each type of hair follicle is innervated by a unique and invariant combination of mechanoreceptors, and that the central projections of fibers innervating nearby follicles form columns in the spinal dorsal horn. The authors propose a model in which complex tactile stimuli differentially activate unique combinations of mechanoreceptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Ma Q. Labeled lines meet and talk: population coding of somatic sensations. J Clin Invest. 2010;120:3773–3778. doi: 10.1172/JCI43426. This review raises several interesting points about cross-modal interactions related to thermosensation and itch. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross SE. Pain and itch: insights into the neural circuits of aversive somatosensation in health and disease. Curr Opin Neurobiol. 2011;21:880–887. doi: 10.1016/j.conb.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ochoa J, Torebjork E. Sensations evoked by intraneural microstimulation of single mechanoreceptor units innervating the human hand. J Physiol. 1983;342:633–654. doi: 10.1113/jphysiol.1983.sp014873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sikand P, Shimada SG, Green BG, LaMotte RH. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66–75. doi: 10.1016/j.pain.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boring EG. Sensation and Perception in the History of Experimental Psychology. New York: Appleton-Century; 1942. [Google Scholar]
- 35.Fruhstorfer H. Thermal sensibility changes during ischemic nerve block. Pain. 1984;20:355–361. doi: 10.1016/0304-3959(84)90112-X. [DOI] [PubMed] [Google Scholar]
- 36.Henry JL, Panju A, Yashpal K, editors. Central Neuropathic Pain: Focus on Poststroke Pain. Seattle: IASP Press; 2007. [Google Scholar]
- 37.Melzack R, Wall PD, Ty TC. Acute pain in an emergency clinic: latency of onset and descriptor patterns related to different injuries. Pain. 1982;14:33–43. doi: 10.1016/0304-3959(82)90078-1. [DOI] [PubMed] [Google Scholar]
- 38.Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell JN, Raja SN, Meyer RA, Mackinnon SE. Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain. 1988;32:89–94. doi: 10.1016/0304-3959(88)90027-9. [DOI] [PubMed] [Google Scholar]
- 40.Koltzenburg M, Torebjork HE, Wahren LK. Nociceptor modulated central sensitization causes mechanical hyperalgesia in acute chemogenic and chronic neuropathic pain. Brain. 1994;117:579–591. doi: 10.1093/brain/117.3.579. [DOI] [PubMed] [Google Scholar]
- 41*.King T, Qu C, Okun A, Mercado R, Ren J, Brion T, Lai J, Porreca F. Contribution of afferent pathways to nerve injury-induced spontaneous pain and evoked hypersensitivity. Pain. 2011;152:1997–2005. doi: 10.1016/j.pain.2011.04.020. This study is notable for its use of conditioned place preference to assess spontaneous pain for comparison with more typically used reflexive measures of stimulus-evoked pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462:651–655. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gold M. Dissent to evaluation of: Seal RP et al. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009 Dec 3;462(7273):651–5. doi: 10.1038/nature08505. Faculty of 1000, 19 Apr 2010. F1000.com/1285975. Edited by; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321:702–705. doi: 10.1126/science.1156916. [DOI] [PubMed] [Google Scholar]
- 45.Todd AJ, Koerber HR. Neuroanatomical substrates of spinal nociception. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. Elsevier; 2006. [Google Scholar]
- 46.Spike RC, Puskar Z, Andrew D, Todd AJ. A quantitative and morphological study of projection neurons in lamina I of the rat lumbar spinal cord. Eur J Neurosci. 2003;18:2433–2448. doi: 10.1046/j.1460-9568.2003.02981.x. [DOI] [PubMed] [Google Scholar]
- 47.Polgar E, Hughes DI, Riddell JS, Maxwell DJ, Puskar Z, Todd AJ. Selective loss of spinal GABAergic or glycinergic neurons is not necessary for development of thermal hyperalgesia in the chronic constriction injury model of neuropathic pain. Pain. 2003;104:229–239. doi: 10.1016/s0304-3959(03)00011-3. [DOI] [PubMed] [Google Scholar]
- 48.Santos SF, Rebelo S, Derkach VA, Safronov BV. Excitatory interneurons dominate sensory processing in the spinal substantia gelatinosa of rat. J Physiol. 2007;581:241–254. doi: 10.1113/jphysiol.2006.126912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu Y, Perl ER. A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fiber input. J Neurosci. 2003;23:8752–8758. doi: 10.1523/JNEUROSCI.23-25-08752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prescott SA, De Koninck Y. Four cell types with distinctive membrane properties and morphologies in lamina I of the spinal dorsal horn of the adult rat. J Physiol. 2002;539:817–836. doi: 10.1113/jphysiol.2001.013437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santos SF, Melnick IV, Safronov BV. Selective postsynaptic inhibition of tonic-firing neurons in substantia gelatinosa by mu-opioid agonist. Anesthesiology. 2004;101:1177–1183. doi: 10.1097/00000542-200411000-00018. [DOI] [PubMed] [Google Scholar]
- 52*.Yasaka T, Tiong SY, Hughes DI, Riddell JS, Todd AJ. Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain. 2010;151:475–488. doi: 10.1016/j.pain.2010.08.008. This tour-de-force study characterizes an array of different properties in lamina II neurons. The authors show how various properties are interrelated and thereby delineate populations of excitatory and inhibitory interneurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruscheweyh R, Sandkuhler J. Lamina-specific membrane and discharge properties of rat spinal dorsal horn neurones in vitro. J Physiol. 2002;541:231–244. doi: 10.1113/jphysiol.2002.017756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grudt TJ, Perl ER. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J Physiol. 2002;540:189–207. doi: 10.1113/jphysiol.2001.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prescott SA, De Koninck Y. Integration time in a subset of spinal lamina I neurons is lengthened by sodium and calcium currents acting synergistically to prolong subthreshold depolarization. J Neurosci. 2005;25:4743–4754. doi: 10.1523/JNEUROSCI.0356-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Graham BA, Brichta AM, Callister RJ. Pinch-current injection defines two discharge profiles in mouse superficial dorsal horn neurones, in vitro. J Physiol. 2007;578:787–798. doi: 10.1113/jphysiol.2006.123349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57*.Prescott SA, De Koninck Y, Sejnowski TJ. Biophysical basis for three distinct dynamical mechanisms of action potential initiation. PLoS Comput Biol. 2008;4:e1000198. doi: 10.1371/journal.pcbi.1000198. This study uses nonlinear dynamical analysis, simulations, and experiments to explain the different ways in which neurons produce action potentials and how that impacts encoding. The findings highlight the distinct coding properties of different types of spinal neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mathews PJ, Jercog PE, Rinzel J, Scott LL, Golding NL. Control of submillisecond synaptic timing in binaural coincidence detectors by K(v)1 channels. Nat Neurosci. 2010;13:601–609. doi: 10.1038/nn.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hantman AW, van den Pol AN, Perl ER. Morphological and physiological features of a set of spinal substantia gelatinosa neurons defined by green fluorescent protein expression. J Neurosci. 2004;24:836–842. doi: 10.1523/JNEUROSCI.4221-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heinke B, Ruscheweyh R, Forsthuber L, Wunderbaldinger G, Sandkuhler J. Physiological, neurochemical and morphological properties of a subgroup of GABAergic spinal lamina II neurones identified by expression of green fluorescent protein in mice. J Physiol. 2004;560:249–266. doi: 10.1113/jphysiol.2004.070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeilhofer HU, Studler B, Arabadzisz D, Schweizer C, Ahmadi S, Layh B, Bosl MR, Fritschy JM. Glycinergic neurons expressing enhanced green fluorescent protein in bacterial artificial chromosome transgenic mice. J Comp Neurol. 2005;482:123–141. doi: 10.1002/cne.20349. [DOI] [PubMed] [Google Scholar]
- 62.Dougherty KJ, Sawchuk MA, Hochman S. Properties of mouse spinal lamina I GABAergic interneurons. J Neurophysiol. 2005;94:3221–3227. doi: 10.1152/jn.00184.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63*.Zheng J, Lu Y, Perl ER. Inhibitory neurones of the spinal substantia gelatinosa mediate interaction of signals from primary afferents. J Physiol. 2010;588:2065–2075. doi: 10.1113/jphysiol.2010.188052. This study expands upon the previous paired recording work to provide an increasing detailed account of what type of afferent input is received by different spinal cell types, and how those spinal cell types are synaptically interconnected. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu HJ, Gereau RWt. Metabotropic glutamate receptor 5 regulates excitability and Kv4.2-containing K channels primarily in excitatory neurons of the spinal dorsal horn. J Neurophysiol. 2011;105:3010–3021. doi: 10.1152/jn.01050.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang HY, Cheng JK, Shih YH, Chen PH, Wang CL, Tsaur ML. Expression of A-type K channel alpha subunits Kv 4.2 and Kv 4.3 in rat spinal lamina II excitatory interneurons and colocalization with pain-modulating molecules. Eur J Neurosci. 2005;22:1149–1157. doi: 10.1111/j.1460-9568.2005.04283.x. [DOI] [PubMed] [Google Scholar]
- 66.Ruscheweyh R, Ikeda H, Heinke B, Sandkuhler J. Distinctive membrane and discharge properties of rat spinal lamina I projection neurones in vitro. J Physiol. 2004;555:527–543. doi: 10.1113/jphysiol.2003.054049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Graham BA, Brichta AM, Callister RJ. Recording temperature affects the excitability of mouse superficial dorsal horn neurons, in vitro. J Neurophysiol. 2008;99:2048–2059. doi: 10.1152/jn.01176.2007. [DOI] [PubMed] [Google Scholar]
- 68.Hu HJ, Carrasquillo Y, Karim F, Jung WE, Nerbonne JM, Schwarz TL, Gereau RW. The kv4.2 potassium channel subunit is required for pain plasticity. Neuron. 2006;50:89–100. doi: 10.1016/j.neuron.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 69.Polgar E, Fowler JH, McGill MM, Todd AJ. The types of neuron which contain protein kinase C gamma in rat spinal cord. Brain Res. 1999;833:71–80. doi: 10.1016/s0006-8993(99)01500-0. [DOI] [PubMed] [Google Scholar]
- 70.Malmberg AB, Chen C, Tonegawa S, Basbaum AI. Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science. 1997;278:279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- 71.Lu Y, Perl ER. Modular organization of excitatory circuits between neurons of the spinal superficial dorsal horn (laminae I and II) J Neurosci. 2005;25:3900–3907. doi: 10.1523/JNEUROSCI.0102-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang W, Schneider SP. Short-term modulation at synapses between neurons in laminae II-V of the rodent spinal dorsal horn. J Neurophysiol. 2011;105:2920–2930. doi: 10.1152/jn.00684.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu Y, Perl ER. Selective action of noradrenaline and serotonin on neurones of the spinal superficial dorsal horn in the rat. J Physiol. 2007;582:127–136. doi: 10.1113/jphysiol.2007.131565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gassner M, Ruscheweyh R, Sandkuhler J. Direct excitation of spinal GABAergic interneurons by noradrenaline. Pain. 2009;145:204–210. doi: 10.1016/j.pain.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 75*.Yasaka T, Kato G, Furue H, Rashid MH, Sonohata M, Tamae A, Murata Y, Masuko S, Yoshimura M. Cell-type-specific excitatory and inhibitory circuits involving primary afferents in the substantia gelatinosa of the rat spinal dorsal horn in vitro. J Physiol. 2007;581:603–618. doi: 10.1113/jphysiol.2006.123919. This study thoroughly characterizes the distinct patterns of excitatory and inhibitory synaptic inputs to different types of spinal neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Uta D, Furue H, Pickering AE, Rashid MH, Mizuguchi-Takase H, Katafuchi T, Imoto K, Yoshimura M. TRPA1-expressing primary afferents synapse with a morphologically identified subclass of substantia gelatinosa neurons in the adult rat spinal cord. Eur J Neurosci. 2010;31:1960–1973. doi: 10.1111/j.1460-9568.2010.07255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77*.Wang H, Zylka MJ. Mrgprd-expressing polymodal nociceptive neurons innervate most known classes of substantia gelatinosa neurons. J Neurosci. 2009;29:13202–13209. doi: 10.1523/JNEUROSCI.3248-09.2009. This study is notable for its use of channelrhodopsin, which the authors selectively expressed in a subset of primary afferents, to map the connectivity between those afferents and dorsal horn neurons using photostimulation in a spinal slice preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Daniele CA, MacDermott AB. Low-threshold primary afferent drive onto GABAergic interneurons in the superficial dorsal horn of the mouse. J Neurosci. 2009;29:686–695. doi: 10.1523/JNEUROSCI.5120-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dostrovsky JO, Craig AD, McMahon SB, Koltzenburg M. Wall and Melzack’s Textbook of Pain. Elsevier; 2006. Ascending projection systems; pp. 187–203. [Google Scholar]
- 80.Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci. 2003;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- 81.Christensen BN, Perl ER. Spinal neurons specifically excited by noxious or thermal stimuli: marginal zone of the dorsal horn. J Neurophysiol. 1970;33:293–307. doi: 10.1152/jn.1970.33.2.293. [DOI] [PubMed] [Google Scholar]
- 82.Han ZS, Zhang ET, Craig AD. Nociceptive and thermoreceptive lamina I neurons are anatomically distinct. Nat Neurosci. 1998;1:218–225. doi: 10.1038/665. [DOI] [PubMed] [Google Scholar]
- 83.Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci. 2001;4:72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- 84.Simone DA, Zhang X, Li J, Zhang JM, Honda CN, LaMotte RH, Giesler GJ., Jr Comparison of responses of primate spinothalamic tract neurons to pruritic and algogenic stimuli. J Neurophysiol. 2004;91:213–222. doi: 10.1152/jn.00527.2003. [DOI] [PubMed] [Google Scholar]
- 85.Hillman P, Wall PD. Inhibitory and excitatory factors influencing the receptive fields of lamina 5 spinal cord cells. Exp Brain Res. 1969;9:284–306. doi: 10.1007/BF00235240. [DOI] [PubMed] [Google Scholar]
- 86.Steedman WM, Molony V, Iggo A. Nociceptive neurones in the superficial dorsal horn of cat lumbar spinal cord and their primary afferent inputs. Exp Brain Res. 1985;58:171–182. doi: 10.1007/BF00238965. [DOI] [PubMed] [Google Scholar]
- 87.Kato G, Kosugi M, Mizuno M, Strassman AM. Separate inhibitory and excitatory components underlying receptive field organization in superficial medullary dorsal horn neurons. J Neurosci. 2011;31:17300–17305. doi: 10.1523/JNEUROSCI.4474-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dubuisson D, Fitzgerald M, Wall PD. Ameboid receptive fields of cells in laminae 1, 2 and 3. Brain Res. 1979;177:376–378. doi: 10.1016/0006-8993(79)90789-3. [DOI] [PubMed] [Google Scholar]
- 89.Brown PB, Gladfelter WE, Culberson JC, Covalt-Dunning D, Sonty RV, Pubols LM, Millecchia RJ. Somatotopic organization of single primary afferent axon projections to cat spinal cord dorsal horn. J Neurosci. 1991;11:298–309. doi: 10.1523/JNEUROSCI.11-01-00298.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90*.Keller AF, Beggs S, Salter MW, De Koninck Y. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Mol Pain. 2007;3:27. doi: 10.1186/1744-8069-3-27. This study uses a variety of manipulations in vivo to experimentally reproduce the disinhibition caused by nerve injury. The authors show that normally subliminal low-threshold input to lamina I projections neurons can be acutely unmasked, thus illustrating that sensitivity to different stimulus modalities is dependent on microcircuit function rather than being hard wired. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Torsney C, MacDermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci. 2006;26:1833–1843. doi: 10.1523/JNEUROSCI.4584-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baba H, Ji RR, Kohno T, Moore KA, Ataka T, Wakai A, Okamoto M, Woolf CJ. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol Cell Neurosci. 2003;24:818–830. doi: 10.1016/s1044-7431(03)00236-7. [DOI] [PubMed] [Google Scholar]
- 93.Miraucourt LS, Dallel R, Voisin DL. Glycine inhibitory dysfunction turns touch into pain through PKCgamma interneurons. PLoS ONE. 2007;2:e1116. doi: 10.1371/journal.pone.0001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Priebe NJ, Ferster D. Inhibition, spike threshold, and stimulus selectivity in primary visual cortex. Neuron. 2008;57:482–497. doi: 10.1016/j.neuron.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 95.Mysore SP, Knudsen EI. Reciprocal inhibition of inhibition: a circuit motif for flexible categorization in stimulus selection. Neuron. 2012;73:193–205. doi: 10.1016/j.neuron.2011.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carandini M, Heeger DJ. Normalization as a canonical neural computation. Nat Rev Neurosci. 2011;13:51–62. doi: 10.1038/nrn3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Srinivasan MV, Bernard GD. A proposed mechanism for multiplication of neural signals. Biol Cybern. 1976;21:227–236. doi: 10.1007/BF00344168. [DOI] [PubMed] [Google Scholar]