Abstract
Islet amyloid polypeptide (IAPP, Amylin) is responsible for amyloid formation in type 2 diabetes and in islet cell transplants. The only known natural mutation found in mature human IAPP is a Ser-20 to Gly mis-sense mutation, found with small frequency in Chinese and Japanese populations. The mutation appears to be associated with increased risk of early onset type 2 diabetes. Early measurements in the presence of organic co-solvents showed that S20G-IAPP formed amyloid more quickly than the wild type. We confirm that the mutant accelerates amyloid formation under a range of conditions including in the absence of co-solvents. Ser-20 adopts a normal backbone geometry and the side chain makes no steric clashes in models of IAPP amyloid fibers, suggesting that the increase rate of amyloid formation by the mutant does not result from the relief of steric incompatibility in the fiber state. Transmission electronic microscopy, circular dichroism and seeding studies were used to probe the structure of the resulting fibers. The S20G-IAPP peptide is toxic to cultured rat INS-1 β-cells. The sensitivity of amyloid formation to the identity of residue-20 was exploited to design a variant which is much slower to aggregate and which inhibits amyloid formation by wild type IAPP. A S20K mutant forms amyloid with an 18-fold longer lag phase. Thioflavin-T binding assays, together with experiments using a p-cyanophenylalanine variant of human IAPP, show that the designed S20K mutant inhibits amyloid formation by human IAPP. The experiments illustrate how p-cyanophenylalanine can be exploited to monitor amyloid formation even in the presence of other amyloidogenic proteins.
Keywords: Islet amyloid polypeptide, Amylin, Type 2 diabetes, Amyloid-inhibitor, Missense mutation
Introduction
Amyloid formation plays a key role in a wide range of diseases including Parkinson’s disease, Alzheimer’s disease, and type 2 diabetes.1–4 Human islet amyloid polypeptide (IAPP, also known as amylin) is the major component of the pancreatic islet amyloid associated with type 2 diabetes. IAPP is a member of the calcitonin-like family of peptides and is one of the most amyloidogenic polypeptides known (Fig. 1).5–13 The polypeptide is processed with insulin in the pancreatic β-cells, stored in the insulin secretory granule and secreted together with insulin. IAPP normally functions as a partner to insulin,10,14–15 but aggregates in type 2 diabetes to form islet amyloid. Aggregates of the human polypeptide are toxic to cultured β-cells, suggesting that IAPP amyloid fibers or the process of amyloid formation by IAPP contributes to islet β-cell death in type 2 diabetes.8–9,16–22 Increasing evidence argues that IAPP amyloid formation also contributes to the failure of islet cell grafts in islet transplantation.23–26
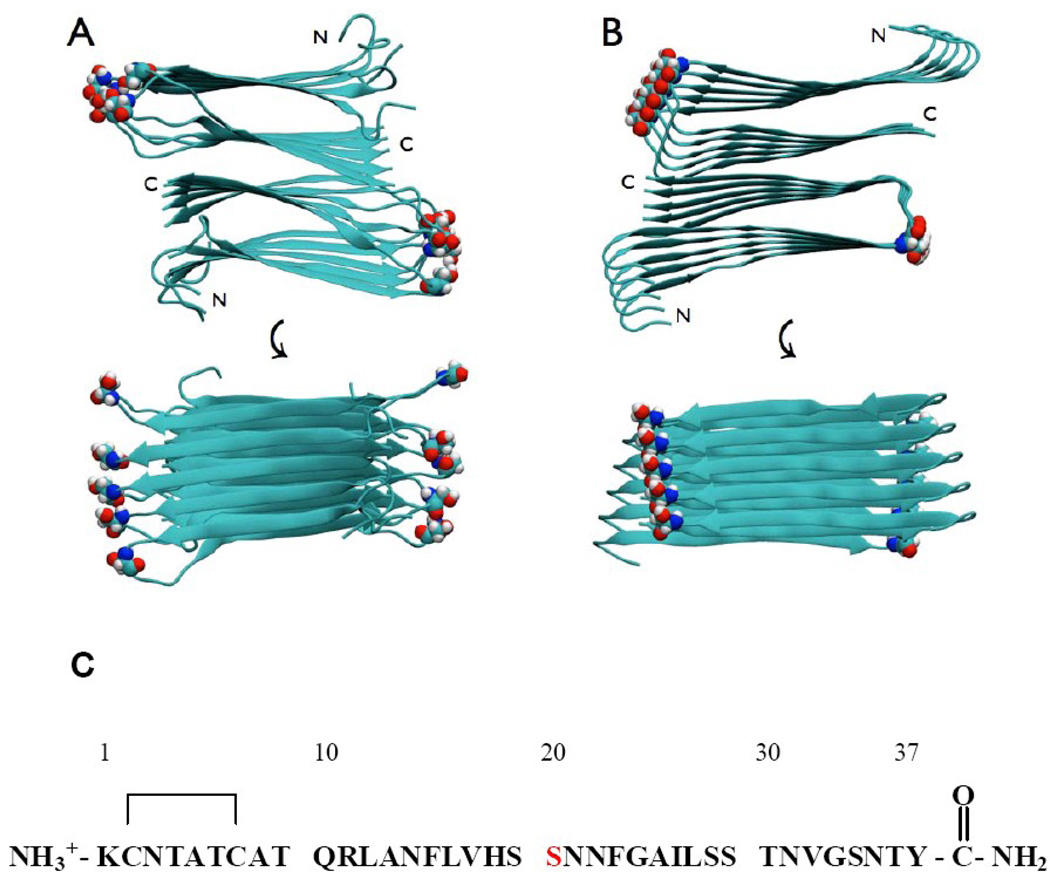
Figure 1.
Structural models of the wild type IAPP amyloid fiber showing the location of Ser-20. Two views are shown: a top down view and an image rotated by 90 degrees. Ser-20 is shown in space-filling representation. (A) The model developed by the NIH group. (B) The model developed by the UCLA group. (C) The primary sequence of human IAPP, residue Ser-20 is colored in red. The wild type peptide contains a disulfide bridge between Cys-2 and Cys-7 and has an amidated C-terminus.
Mutations within a number of amyloidogenic proteins have been directly linked to the early onset or severity of the associated diseases,27–29 but this is generally not the case for IAPP. Only one naturally occurring mutation has been identified within mature human IAPP, a Ser-20 to Gly (S20G-IAPP) mis-sense mutant that is found in a small population of Japanese and Chinese.30–33 There is suggestive evidence that the mutation is linked to an increased risk of early onset type 2 diabetes in the Japanese population.30–31,33 Important early studies indicated that the mutant aggregated more rapidly in a mixed buffer/DMSO system and at low pH, indicating that it is more amyloidogenic under these conditions.33–34
Here we analyze the stereochemical environment of Ser-20 in models of the IAPP amyloid protofiber, examine the kinetics of amyloid assembly of the S20G mutant under a range of conditions, including in the absence of co-solvents at physiological pH, and compare the toxicity of S20G-IAPP to cultured rat INS-1 β-cells relative to wild type IAPP. We demonstrate that the sensitivity of amyloid formation to the identity of postion-20 can be rationally exploited to design slower aggregating variants of IAPP that inhibit amyloid formation by wild type IAPP.
Results and Discussions
Analysis of the environment of Ser-20 in the IAPP amyloid protofiber
There are two atomic level models of the IAPP amyloid protofiber available. One, from the NIH group, is based upon solid state NMR data and scanning transmission electron microscopy mass per unit length data.35 The second was developed by the UCLA group and is based, in part, on x-ray structures of small seven-residue peptide fragments of IAPP.36 The two models are very similar; each is made up of two C2-symmetrically related stacks of IAPP monomers (Fig. 1). The IAPP monomers within each stack adopt a U-shaped structure with the main chain β-sheet hydrogen bonds between adjacent IAPP molecules. Each stack contains two in-register parallel β-sheets; one encompassing residues 8 to 17 and the other residues 28 to 37. Some of the polar residues in IAPP form stacks of sidechain to sidechain hydrogen bonds, but this is not the case for Ser-20. In the two models, the sidechain of a Ser-20 in one layer does not form hydrogen bonds with Ser-20 sidechains in adjacent stacks or layers and the measured O to H distance is on the order of 4.85 Å in the UCLA model. Ser-20 is located in the partially ordered loop that links the two β-sheets in both of the models and does not participate in the ordered back bone hydrogen bonded structure. Two dimensional isotope edited IR (2D-IR) experiments which measure the exciton coupling between labeled 13C18O sites have confirmed that Ser-20 does not participate in well ordered β-sheet structure.37
The S20G mutation accelerates amyloid formation
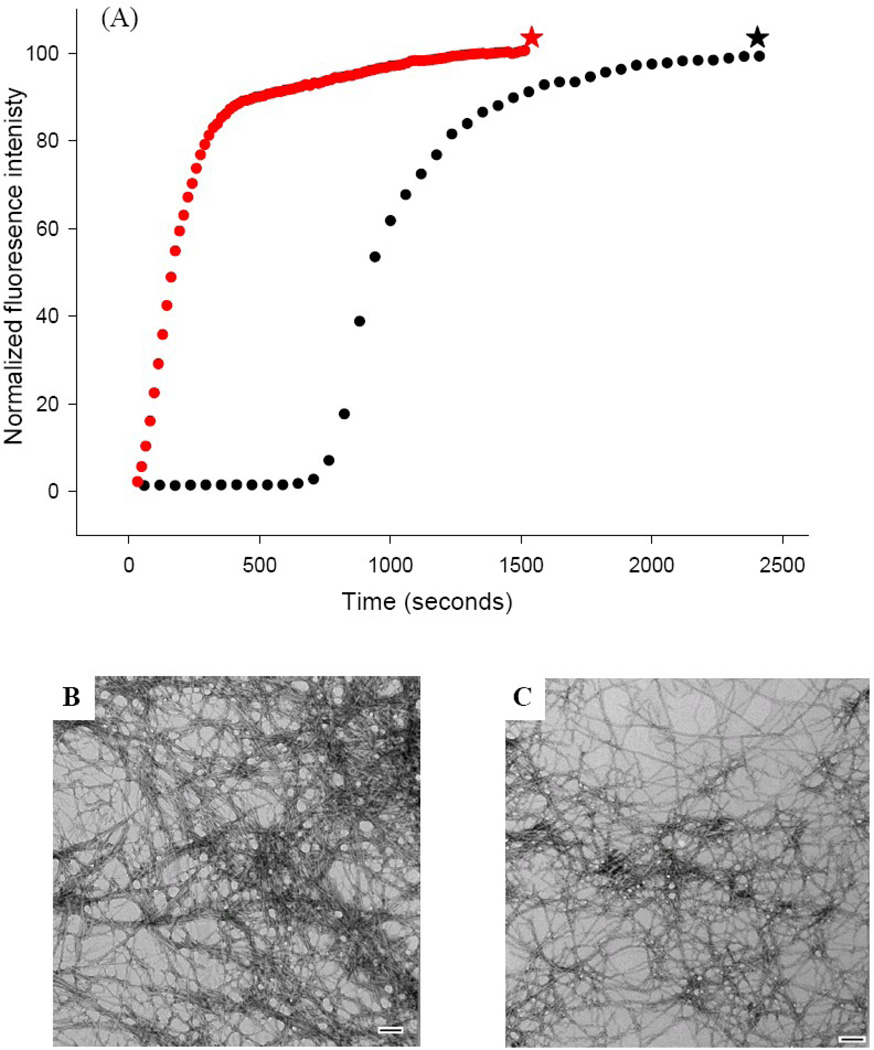
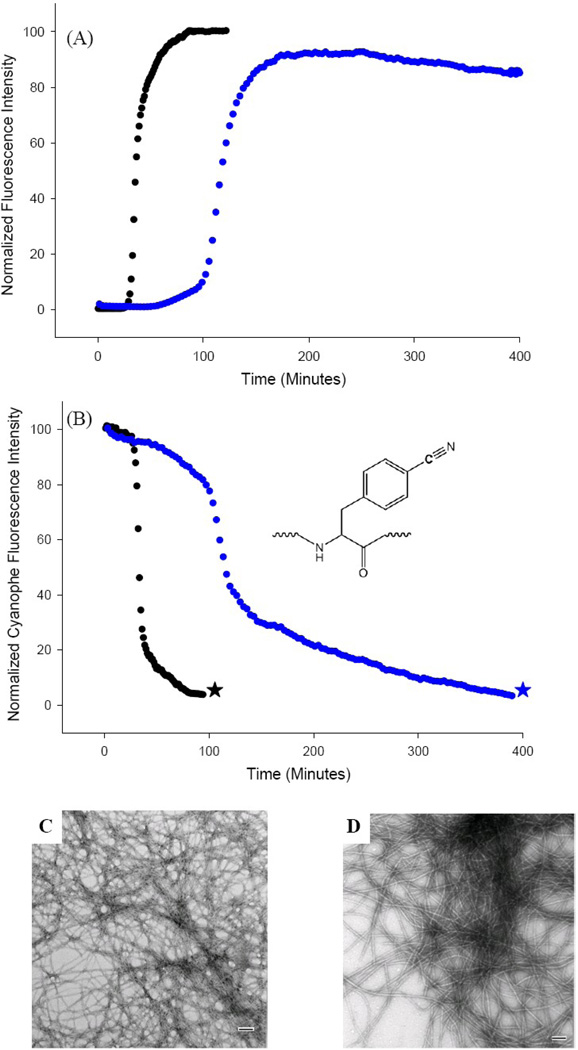
We examined the kinetic consequences of the S20G mutant by conducting thioflavin-T fluorescence studies. Thioflavin-T is a small dye which undergoes a significant increase in quantum yield when it binds to amyloid fibers.38 Initial experiments were conducted at pH 7.4 in the presence of 2% v/v hexafluoroisopropyl alcohol. Additional experiments were conducted in buffer without adding hexafluoroisopropyl alcohol. These conditions have been widely used for biophysical studies of IAPP. The kinetic curve observed for wild type IAPP is very similar to that previously reported for these conditions, with a distinct lag phase followed by a growth phase (Fig. 2A, black curve). TEM images collected at the end of the reaction reveal a dense mat of amyloid fibers (Fig. 2B). The S20G-IAPP peptide behaves very differently. There is no apparent lag phase; instead a monotonic increase in thioflavin-T fluorescence is observed leading to a final plateau region (Fig. 2A, red curve). TEM images were collected from this sample at the end of the reaction and reveal large amounts of amyloid fibers which are similar to wild type fibers (Fig. 2C). CD spectroscopy shows that the wild type and S20G-IAPP fibers are rich in β-sheet (Supplementary Material).
Figure 2.
The S20G mutant of IAPP accelerates amyloid formation. (A) Thioflavin-T fluorescence experiments: wild type IAPP (black), S20G-IAPP (red). (B) TEM image of the amyloid fibers formed by wild type IAPP (black star). (C) TEM image of the amyloid fibers formed by S20G-IAPP (red star). Experiments were conducted at 25°C, 20 mM Tris-HCl, pH 7.4, 2% HFIP (v/v), 16 µM IAPP, with constant stirring. Scale bars in the TEM images represent 100 nm.
Amyloid fibers formed by the S20G mutant can seed amyloid formation by wild type IAPP
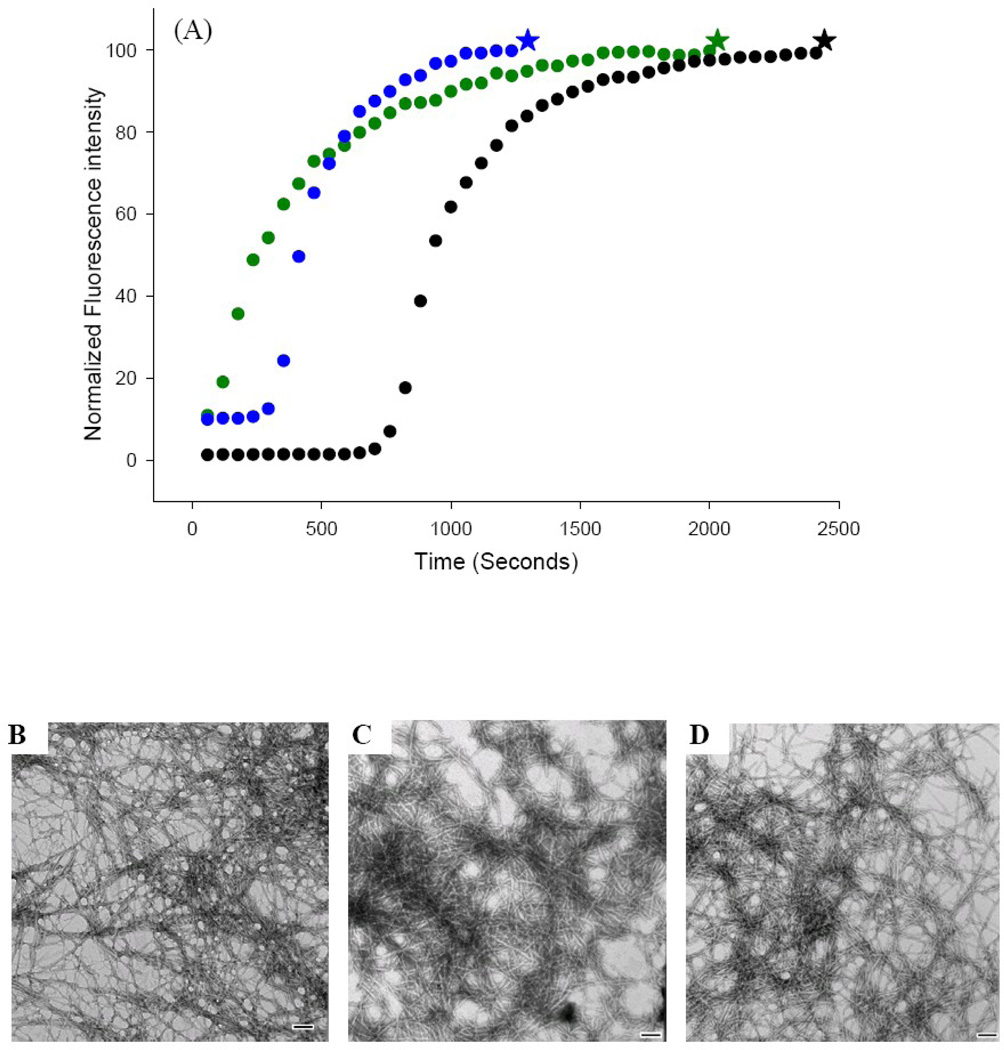
Amyloid formation can be seeded by preformed amyloid fibers. Addition of a small amount of “seeds” in the form of already formed fibers to an unaggregated solution bypasses the lag phase. Seeding is usually specific and, although cross seeding can sometimes be observed, seeds are much more effective at catalyzing amyloid formation by their own monomers.39–40 Seeding specificity can result from differences in fiber structure between different peptides and because fibers formed by different peptides may present different surfaces to which soluble peptide can bind. Seeding studies thus provide another, albeit indirect, probe of the similarity of different fibers. Cross seeding is more likely if the fibers formed by two different peptides are similar and if the structures at the fiber tips are similar. We seeded wild type IAPP with wild type IAPP fibers and, as expected, observed a bypass of the lag phase and a significant increase in the rate of amyloid formation (Fig. 3A, green curve). We then tested the ability of preformed S20G-IAPP amyloid fibers to seed wild type IAPP. S20G-IAPP fibers seed amyloid formation by wild type IAPP; reducing the lag phase by a factor of 2.6 and producing fibers that have a morphology similar to that of wild type fibers (Fig. 3A, blue curve, Fig. 3D). S20G-IAPP seeds appeared to be somewhat less effective at seeding wild type IAPP than were wild type seeds. The ability of the S20G mutant fibers to seed fiber formation by wild type IAPP is consistent with the mutant fibers adopting a structure which shares similarities to the wild type amyloid fibers. The relatively small apparent differences in seeding effectiveness may be due to differences in the concentration of seed-competent fiber ends in the two preparations. The same concentration of peptide, in monomer units, was used in both experiments, but it is not known if the distribution of fiber lengths is the same in each sample and this could lead to differences in the relative concentration of fiber ends. It is also possible that the different seeding behavior reflects small differences in the structure of the fiber ends. It is not possible to distinguish between these two possibilities, but the important observation is that S20G-IAPP fibers can effectively seed wild type fiber formation and this is consistent with the two peptides adopting similar fiber geometries.
Figure 3.
S20G-IAPP amyloid fibers can seed amyloid formation by wild type IAPP. (A) Thioflavin-T fluorescence experiments: unseeded wild type IAPP (black); wild type IAPP seeded by wild type IAPP amyloid fibers (green); wild type IAPP seeded by S20G-IAPP amyloid fibers (blue). (B) TEM images of IAPP amyloid fibers formed in the unseeded reaction (black star). (C) TEM images of IAPP amyloid fibers formed by seeding with wild type amyloid fibers (green star). (D) TEM images of IAPP amyloid fibers formed by seeding with S20G-IAPP amyloid fibers (blue star). Scale bars represent 100 nm. Experiments were conducted at 25°C, 20 mM Tris-HCl, pH 7.4, 2% HFIP (v/v), 16 µM IAPP, with constant stirring. Seeds, when present, were at 10% concentration in monomer units. The higher initial thioflavin-T fluorescence intensity for the seeded reactions at time=0 is due to the fact that the seeds bind thioflavin-T.
The sensitivity of amyloid formation to the identity of residue 20 can be exploited to develop slow aggregating variants of IAPP which inhibit amyloid formation by wild type IAPP
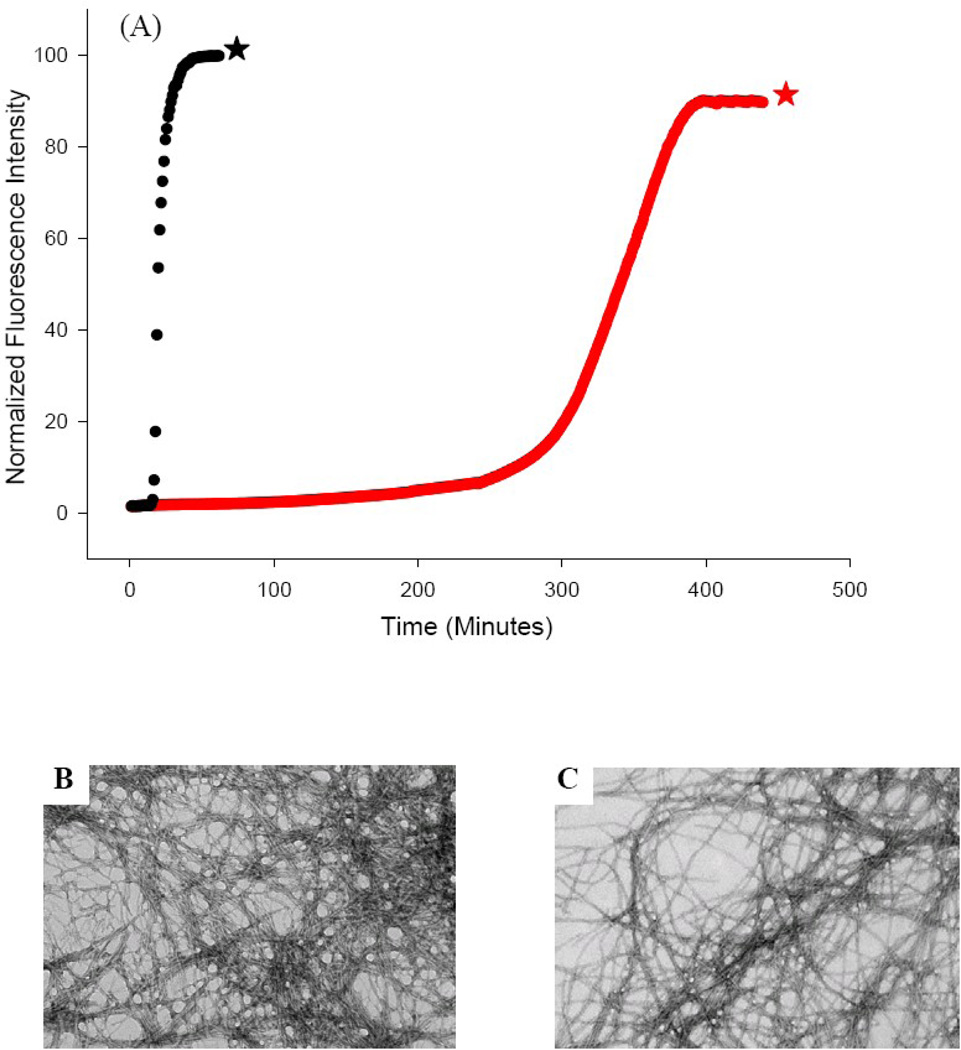
Amyloid formation by IAPP is clearly sensitive to the identity of position 20. We reasoned that if substitution of Ser by the smaller Gly accelerated amyloid formation, then substitution with a larger residue might reduce the rate of amyloid formation, particularly if the larger residue was charged. The parallel, in-registry arrangement of monomers within the amyloid fiber means that a charged residue will form a quasi-infinite array which is expected to destabilize the fibers via unfavorable electrostatic interactions. IAPP contains no acidic residues and has a net positive charge at neutral pH. Thus we choose to introduce another positively charged residue, reasoning that the increased total charge and the additional bulk at position 20 would slow amyloid formation. This is exactly what we observe upon substituting Ser-20 with a lysine. The resulting S20K mutant had a significant effect upon amyloid formation, lengthening the lag phase by a factor of 18 (Fig. 4A, red curve). This is a remarkable effect for a single point mutation, particularly for one located outside of the β-sheet core of the amyloid fibers. Almost all other reported single non-proline mutants of IAPP have much smaller effects.41–42 TEM images show that the S20K-IAPP amyloid fibers have a similar morphology as wild type amyloid fibers, at least at this level of resolution (Fig. 4B&C). This is not surprising since molecular modeling shows that a lysine can be accommodated into either the NIH or UCLA structure with standard sidechain dihedral angles. CD confirms that the S20K-IAPP fibers are rich in β-sheet (Supplementary Material).
Figure 4.
The S20K mutant of IAPP significantly reduces the rate of amyloid formation. (A) Thioflavin-T fluorescence experiments: wild type IAPP (black), S20K-IAPP (red). (B) TEM image of a sample of the amyloid fibers formed by wild type IAPP at the end of the reaction (black star). (C) TEM image of a sample of the amyloid fibers formed by S20K-IAPP at the end of the reaction (red star). Experiments were conducted at 25°C, 20 mM Tris-HCl, pH 7.4, 2% HFIP (v/v), 16 µM IAPP, with constant stirring. Scale bars in the TEM images represent 100 nm.
We also tested the ability of S20K-IAPP fibers to seed formation of wild type fibers and observed effects that were similar to those obtained with the S20G mutant; the S20K mutant does seed wild type IAPP amyloid formation (Supplementary Material). This is consistent with the TEM analysis, and shows that the S20K-IAPP amyloid fibers share similarity with wild type fibers.
Several models of IAPP amyloid formation postulate that initial events might involve association in the region involving residues 7 to 18 or 5 to 22.42–46 These models can rationalize the ability of rat IAPP and certain designed mutants to inhibit amyloid formation by human IAPP.47–49 These models suggest that the S20K mutant might inhibit wild type amyloid formation since the mutant should be able to interact with wild type IAPP in the same fashion. The mutant does indeed inhibit amyloid formation by wild type IAPP. We examined a 1:1 mixture of wild type IAPP and S20K-IAPP. Thioflavin-T experiments show that the time to reach 50% of the total fluorescence change, t50, is 3.5-fold longer in the presence of the S20K mutant (Fig. 5A, blue curve). This is a larger effect than was observed with a 1:1 mixture of the rat polypeptide, which is a known inhibitor of amyloid formation by human IAPP.47 TEM images collected at the end of the reaction show that the mixture eventually forms amyloid fibers which are similar to those generated by wild type (Fig. 5C&D).
Figure 5.
The S20K mutant of IAPP inhibits amyloid formation by wild type IAPP. (A) Thioflavin-T monitored kinetic experiments. Black: wild type IAPP; Blue: 1:1 mixture of wild type IAPP and S20K-IAPP. (B) p-Cyanophenylalanine fluorescence studies. The black curve is the kinetic trace for F15 p-cyanoPhe IAPP in the absence of inhibitor. The blue curve is the kinetic trace in the presence of a 1:1 ratio of S20K-IAPP. (C) TEM image of amyloid fibers formed by F15 p-cyanoPhe IAPP in the absence of S20K-IAPP (black star). (D) TEM image of amyloid fibers formed by the 1:1 mixture of F15 p-cyanoPhe IAPP and S20K-IAPP (blue star). Experiments were conducted at 25°C, 20 mM Tris-HCl, pH 7.4, 2% HFIP (v/v) with constant stirring. IAPP was at 16 µM, S20K-IAPP when present was also at 16 µM. Scale bars in the TEM images represent 100 nm. F15 p-cyanoPhe IAPP was used as the wild type for both kinetic experiments to allow direct comparison of the thioflavin-T and p-cyanoPhe fluorescence data from the sample. The p-cyanoPhe substitution has been shown not to perturb amyloid formation.
Thioflavin-T fluorescence reports on amyloid formation, but cannot discriminate between fibers formed directly by the mutant or by wild type or mixed fibers, thus there is an inherent ambiguity in this thioflavin-T experiment. We used p-cyanophenylalanine fluorescence measurements to directly probe amyloid formation by the human peptide in the presence of S20K-IAPP. p-Cyanophenylalanine (p-cyanoPhe) is an analog of Phe/Tyr which contains a cyano group at the para-position of the ring. Its fluorescence quantum yield is controlled by hydrogen bonding to the cyano group and by quenching from Tyr or neutral His side chains.50–54 We have previously demonstrated that substitution of any of the three aromatic residues in IAPP by p-cyanoPhe does not perturb the kinetics of amyloid formation and we have shown that the time course of amyloid formation monitored by p-cyanoPhe fluorescence is identical to the one probed by thioflavin-T fluorescence.50–51 Note that this helps to validate the use of thioflavin-T assays to study IAPP. Thus the p-cyanoPhe variants can be used to specifically monitor amyloid formation by IAPP in the presence of the S20K mutant. Fig. 5B displays the p-cyanoPhe fluorescence time course for the F15 p-cyanoPhe variant of human IAPP. The time course for amyloid formation by F15 p-cyanoPhe-IAPP in the absence of the S20K-IAPP mutant is very similar to that observed in previous studies (Fig. 5B, black curve).51 The kinetic curve collected in the presence of S20K-IAPP is very different (Fig. 5B, blue curve), with a 3.5-fold increase in t50. The values of t50 determined by thioflavin-T fluorescence and p-cyanoPhe fluorescence are in excellent agreement. This confirms conclusively that the S20K mutant inhibits amyloid formation by wild type IAPP.
The effect of the Ser-20 mutants is not a consequence of the solution conditions used
Amyloid formation by IAPP has been probed under a wide range of conditions including dilution from DMSO solutions, dilution from HFIP solutions, dilution from TFE solutions, in solutions containing high concentrations of acetic acid, at neutral and at low pH by adding buffer to dried films of peptide or to lyophilized samples of peptide.55–59 In some cases samples are stirred or shaken, while in others quiescent reactions are monitored. The observed kinetics depend on the conditions used and it can be difficult to quantitatively compare data collected using different protocols. Different protocols have been used for purifying the peptide and seemingly subtle changes, such as the choice of counter ion used in HPLC purification, can affect experimental results. There have been reports of different behaviors from samples of IAPP obtained from commercial sources when the only difference is the lot number of the peptide.59 Thus it is very important to verify that the results are not an artifact of the solution conditions used, although this is often not done with in vitro studies of IAPP.
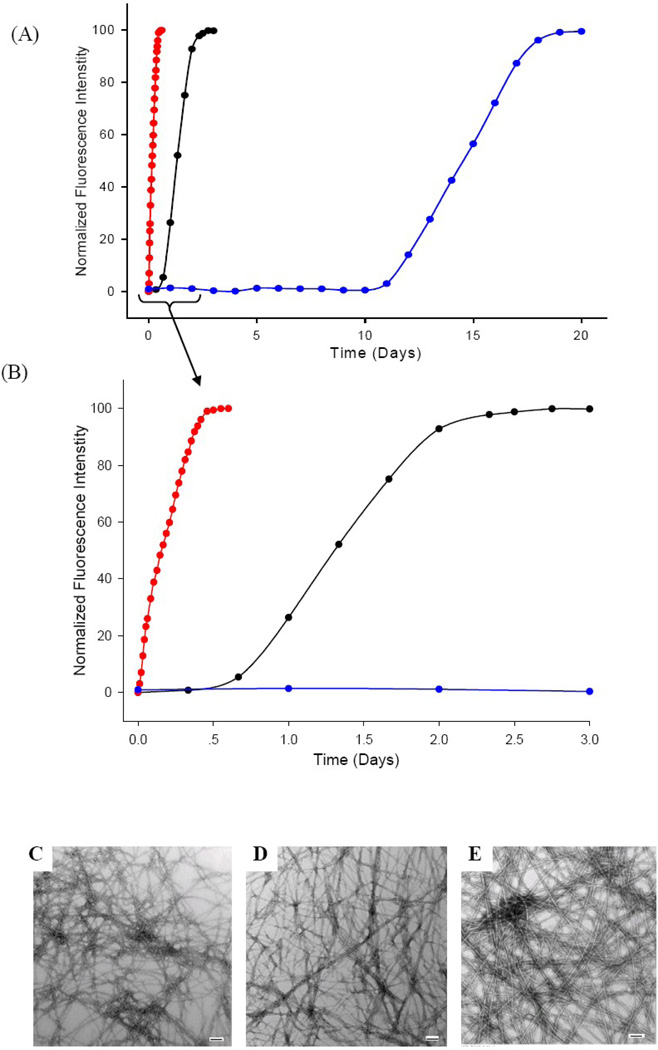
The experiments described in the previous sections were conducted by diluting a stock solution of IAPP which had been dissolved in HFIP into buffer. This leads to a final concentration of 2% HFIP by volume. These are standard conditions for IAPP biophysical experiments and we used them in order to facilitate comparison with other studies; however, trace amounts of residual HFIP are known to accelerate amyloid formation by IAPP.55–56 The experiments were also conducted with continuous stirring which is known to accelerate amyloid formation, probably by increasing secondary nucleation.60–63 We conducted additional experiments in the absence of HFIP and without stirring (see Methods) to ensure that the observed effects were not a consequence of the small amount of residual co-solvent, or induced by mixing and shear forces. The lag phase of wild type IAPP is longer in buffer, as expected (Fig. 6, black curve), but the same relative effects are observed with the two mutants. No lag phase is observed for the S20G-IAPP peptide (red curve), while the S20K-IAPP peptide has an 18-fold longer lag phase than wild type (blue curve). TEM images collected of the final products reveal that the all three form typical amyloid fibers under these conditions (Fig. 6C–E). CD spectra confirm that the samples contain β-structure (Supplementary Material).
Figure 6.
The effects of the Ser-20 substitutions are not a consequence of low levels of co-solvents. (A) Thioflavin-T monitored kinetic experiments of wild type IAPP (black), S20G-IAPP (red) and S20K-IAPP (blue) in the absence of HFIP. (B) An expansion of the first 3 days of panel A. (C) TEM image of the amyloid fibers formed by wild type IAPP. (D) TEM image of the amyloid fibers formed by S20G-IAPP. (E) TEM image of the amyloid fibers formed by S20K-IAPP. Experiments were conducted at 25°C, 20 mM Tris-HCl, pH 7.4, 20 µM IAPP and without stirring or agitation. Scale bars in the TEM images represent 100 nm.
S20G-IAPP is toxic to cultured cells
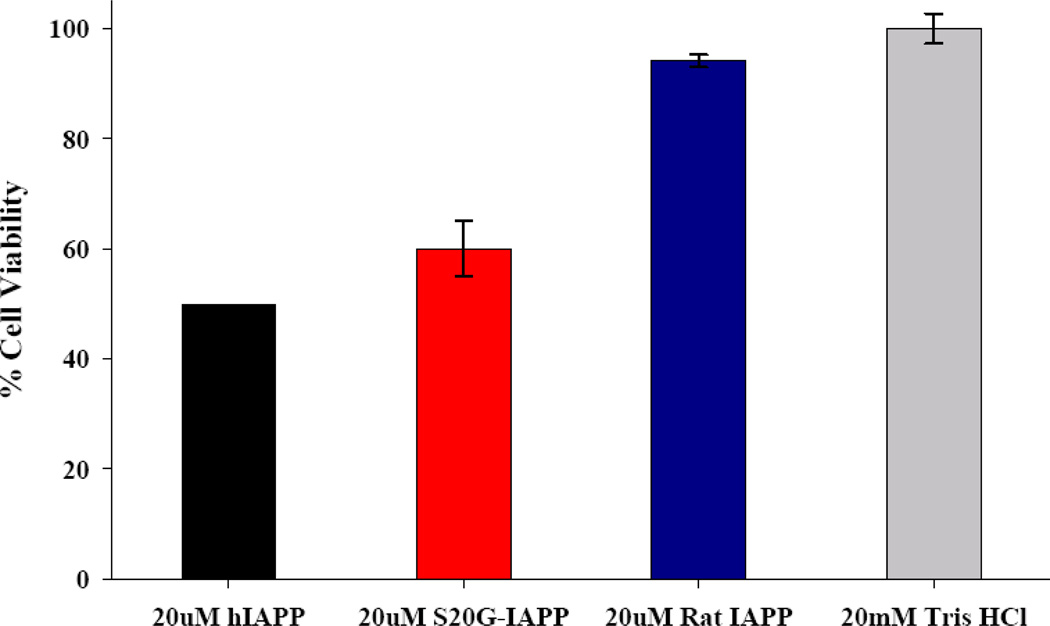
We tested the effects of the S20G mutant on cell viability using transformed rat insulinoma-1 (INS-1) β-cells. INS-1 β-cells are a standard pancreatic cell line for in vitro studies of cytotoxicity. Human IAPP was used as a positive control and Rat IAPP, which is known to be non-toxic, was used as a negative control. Cell viability was monitored using Alamar Blue reduction assays. Under these experimental conditions, the S20G mutant exhibits similar cytotoxicity as wild type human IAPP (Fig. 7). Of course in vitro experiments, such as those presented here, do not allow one to deduce if the S20G mutation leads to increased production of toxic species in vivo or affects their clearance, but the data clearly shows that the mutant is toxic.
Figure 7.
The S20G-IAPP peptide is toxic to cultured rat INS-1 β-cells. Cell viability was determined by Alamar Blue reduction assays. Rat IAPP was used as negative control. All peptide solutions were incubated for 4 hrs at room temperature in 20mM Tris-HCl buffer at pH 7.4 and then applied to rat INS-1 β-cells in 96-well plates. INS-1 β-cells were stimulated with IAPP solution for 5 hours prior to cell viability measurement.
Conclusions
Glycines have been proposed to play a role in preventing amyloid formation by globular proteins owing to their low β-sheet propensity and the increase in local flexibility associated with a glycine substitution.64 The S20G mutation provides an interesting counter example in which a glycine noticeably enhances amyloid formation. Another example is offered by the Aβ peptide. Position 25 in Aβ is a Gly in the wild type sequence and, like Ser-20 in IAPP, is located in the loop which connects the two β–strands. Mutation of this site to Ala destabilizes the Aβ1–40 fibers.65
The physiochemical basis for the enhanced in vitro aggregation rate of S20G-IAPP is not known, but simple steric effects in the fiber state are unlikely to be the cause as judged by analysis of existing models of IAPP amyloid fibers. X to Gly mutations which increase the stability or folding rate of globular proteins usually do so because the residue in question is involved in steric clashes in the native state or is required to adopt a conformation with a positive value of the backbone dihedral angle ϕ.66 This does not appear to be the case based upon current models of the IAPP fibers. Ser-20 adopts normal ϕ, ψ, dihedral angles in each of the two IAPP protofiber models and the Ser-20 side chain does not make any steric clashes, arguing that the enhanced kinetics are not due to the relief of unfavorable geometric clashes in the final fiber state. Of course, there is always the possibility that Ser-20 may adopt an ordered conformation in the actual fibers that differs somewhat from that formed in the models and which leads to steric clashes that could be relieved by a Gly substitution.
Computational studies using small fragments of IAPP have attempted to rationalize the effect of the S20G mutant based upon local conformational preorganization in the monomer induced by the Ser to Gly mutation,67 while experimental studies of a different smaller fragment have led to the suggestion that changes in secondary structure propensity could be important.68 Alternatively, the S20G mutant has also been proposed to promote assembly of the nucleus by increasing local flexibility that facilitates formation of early oligomers.42 Irrespective of the details, the analysis presented here confirms that the S20G mutation accelerates amyloid formation in vitro in physiologically relevant buffers and suggests that the final fiber state is similar to that of wild type human IAPP.
The pronounced effects of the S20K mutation highlight the sensitivity of amyloid formation to the identity of position-20. The dramatic effect of the S20K mutant likely results from both electrostatic and steric effects. If it were due only to increased electrostatic repulsion, then one would expect the relative effect of the mutation to be much less pronounced in high salt. Amyloid formation by wild type IAPP and S20K-IAPP is faster in 500 mM salt, but the t50 for S20K-IAPP is still 3.4 fold longer than that of wild type IAPP, indicating that electrostatic and steric are both likely to play a role (Supplementary Material). The ability of the S20K mutant to inhibit amyloid formation by the wild type peptide is consistent with the proposed mode of action of other inhibitors which have been designed to target oligomeric intermediates by interacting with the N-terminal region of IAPP.47
Materials and Methods
Peptide synthesis and purification
Human IAPP and the mutants were synthesized on a 0.25 mmol scale using a CEM microwave peptide synthesizer, by 9-fluornylmethoxycarbonyl (Fmoc) chemistry. Fmoc protected pseudoproline dipeptide derivatives were incorporated to facilitate the synthesis. 5-(4'-fmoc-aminomethyl-3', 5-dimethoxyphenol) valeric acid (PAL-PEG) resin was used to generate an amidated C terminus. IAPP contains an amidated C-terminus which is important for function. The N-termini of all peptides were free. Double couplings were performed for the pseudoprolines, the residues following each pseudoproline, Arg, and all of the β-branched residues. Peptides were cleaved from the resin through the use of standard trifluoroacetic acid (TFA) methods. Crude peptides were dissolved in 20 % acetic acid (V/V), frozen in liquid nitrogen and lyophilized to increase their solubility prior to additional work up. The disulfide bond was formed via oxidation by DMSO.69 The peptides were purified by reverse-phase HPLC using a Vydac C18 preparative column (10 mm × 250 mm). Two buffer solutions were used: buffer A consists of 100 % H2O and 0.045 % HCl (V/V) and buffer B includes 80 % acetonitrile, 20 % H2O and 0.045 % HCl. HCl was used as the ion-pairing agent since residual TFA can cause problems with cell toxicity assays and has been shown to influence the aggregation kinetics of some IAPP derived peptides.70 The identity of the pure products was confirmed by mass spectrometry using a Bruker MALDI-TOF MS: Wild type IAPP, expected 3903.3, observed 3903.1; S20G-IAPP, expected 3873.3, observed 3873.5; S20K-IAPP, expected 3944.5, observed 3945.1; hIAPP-F15 cyanoPhe, expected 3928.3, observed 3928.8. Analytical HPLC was used to check the purity of the peptides before each experiment. This is an important control because deamidation can be a complicating factor with IAPP.71
Sample preparation
A 1.6 mM peptide solution was prepared in 100 % hexafluoroisopropanol (HFIP) and stored at −20°C. For kinetic experiments with HFIP, the solution was prepared by diluting a 17 µL aliquot of filtered stock solution into 20 mM Tris-HCl buffer at pH 7.4. The final protein concentration was 16 µM peptide in 2% HFIP.
For experiments without HFIP, reaction solutions were prepared by adding Tris-HCl buffer (20 mM, pH 7.4) to dry lyophilized peptide. The dried samples were formed by filtering an HFIP stock solution of peptide (1.6 mM in HFIP) through a 0.22 µm filter, aliquoting the desired amount and then extensively freeze drying to remove the solvent. We have found that this leads to reproducible aggregation kinetics, whereas solvent removal by evaporation under N2 leads to samples which aggregate faster. The final concentration of IAPP was 20 µM peptide in 20 mM Tris-HCl.
Thioflavin-T and p-cyanoPhe fluorescence assays
Fluorescence experiments were performed on an Applied Phototechnology fluorescence spectrophotometer at an excitation wavelength of 450 nm and emission wavelength of 485 nm. p-cyanoPhe fluorescence was excited at 240 nm and emission was monitored at 296 nm. The excitation and emission slits were set at 6 nm, and a 1.0 cm cuvette was used. For kinetic experiments with HFIP, solutions were prepared by diluting filtered stock solution (GHP Acrodisc 0.22 µm Syringe filter) into Tris-HCl buffer and thioflavin-T solution immediately before the measurement. The final concentration of peptide was 16 µM peptide, with 32 µM Thioflavin-T and 2% HFIP (V/V) and 20 mM Tris HCl at pH 7.4. All solutions were stirred during these fluorescence experiments.
For kinetic experiments without HFIP, reaction solutions were prepared by adding Tris-HCl buffer (20 mM, pH 7.4) to dry lyophilized peptide for a final peptide concentration of 20 µM. Aliquots of the peptide solution were removed from the reaction solution and added into a thioflavin-T solution right before measurements. These samples were not stirred.
For seeding experiments, a fibril solution was prepared by dilution of a filtered HFIP stock solution into 20 mM Tris-HCl buffer to give a final peptide concentration of 16 µM. Aliquots of this solution were removed after amyloid formation was complete and used to seed other solutions. The solution was used within 8 hours to ensure reproducibility of the seeding experiments. The concentration of the seeds was 1.6 µM in monomer units.
Circular Dichroism (CD) experiments
CD experiments were performed using an Applied Photophysics Chirascan CD spectrometer. Aliquots were removed from the kinetic studies when the time course of amyloid formation was complete. Far-UV CD experiments were performed using a 0.1 cm quartz cuvette. Wavelength scans were recorded at 25°C, pH 7.4 over a range of 190 to 260 nm, at 1 nm intervals with an averaging time of 0.5 second and the result of 3 repeats. Background spectra were subtracted from the collected data.
Transmission electron microscopy (TEM)
TEM was performed at the Life Science Microscopy Center at Stony Brook University. TEM samples were prepared from the same solutions used for the fluorescence measurements. 15 µL of peptide solution was removed at the end of the kinetic reactions and blotted on a carbon-coated Formvar 300 mesh copper grid for 1 min and then negatively stained with saturated uranyl acetate for 1 min.
Analysis of the effect of the S20G mutation on IAPP-induced toxicity
Transformed rat insulinoma-1 (INS-1) β-cells were used to assess IAPP cytotoxicity. INS-1 β-cells were grown in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 11 mM glucose, 10 mM Hepes, 2 mM L-glutamine, 1 mM sodium pyruvate, 50 µM beta-mercaptoethanol, 100 U/ml penicillin, and 100 U/ml streptomycin. Cells were maintained at 37°C/5% CO2.
Alamar Blue cell viability assays were conducted with cells seeded at a density of 30,000 cells per well in 96-well plates and cultured for 24 hours prior to addition of peptide. Wild type IAPP and S20G-IAPP were dissolved and incubated in Tris HCl (pH 7.4, 25°C) prior to addition to cells in 96-well plates. Cytotoxicity was measured by Alamar Blue reduction after 5 hrs of IAPP stimulation. Alamar Blue was diluted ten-fold in culture media and cells were incubated for 5 additional hours at 37°C before read out. Fluorescence (excitation 530; emission 590 nm) was measured with a Beckman model D880 plate reader. Values calculated were relative to those of control cells treated with buffer only. Rat IAPP which is known to be non-toxic under these conditions was used as a negative control. All values represent means ± standard deviation (n=3).
Supplementary Material
Acknowledgements
We thank Professor David Eisenberg and Dr. Robert Tycko for providing the coordinates of their structures of the IAPP protofiber. We thank Dr. Annette Plesner for helpful discussions. This work was supported by NIH grants GM078114 to D. P. R.; and F32 DK089734-02 to A. A., and V. P. was supported by NIH grant GM086199 to David F. Green.
Abbreviations
- 2D-IR
two-dimensional infrared spectroscopy
- CD
Circular Dichroism
- Fmoc
9-fluorenylmethoxycarbonyl
- HFIP
hexafluoroisopropyl alcohol
- HPLC
high-performance liquid chromatography
- IAPP
human islet amyloid polypeptide
- INS-1 β-cells
transformed rat insulinoma-1 beta cells
- MALDI-TOF MS
matrix-assisted laser desorption ionization-time-of-flight mass spectrometry
- PAL-PEG
5-(4’-Fmoc-aminomethyl-3’,5-dimethoxyphenyl)-valeric acid
- p-cyanoPhe, p-Cyanophenylalanine
a derivative of phenyalanine with a cyano group at the 4-position of the phenyl ring
- S20G-IAPP
a point mutant of human IAPP in which Ser-20 has been replaced by glycine
- S20K-IAPP
a point mutant of human IAPP in which Ser-20 has been replaced by lysine
- TEM
Transmission Electron Microscopy
- TFA
trifluoroacetic acid
- TFE
Trilfluoroethanol
- t50
the time required to achieve 50% of the final thioflavin-T intensity in a kinetic experiment
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
CD spectra of the final reaction products for the kinetic experiments with S20G-IAPP, wild type human IAPP, S20K-IAPP and the 1:1 mixture of S20K-IAPP and wild type human IAPP. Seeding experiments comparing the ability of S20K-IAPP and wild type fibers to seed amyloid formation by wild type IAPP. Kinetic studies of amyloid formation by wild type human IAPP and S20K-IAPP in 500 mM salt.
References
- 1.Sipe JD. Amyloidosis. Crit. Rev. Cl. Lab. Sci. 1994;31:325–354. doi: 10.3109/10408369409084679. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe DJ. Cell biology of protein misfolding: The examples of Alzheimer's and Parkinson's diseases. Nat. Cell. Biol. 2004;6:1054–1061. doi: 10.1038/ncb1104-1054. [DOI] [PubMed] [Google Scholar]
- 3.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 4.Lansbury PT, Lashuel HA. A century-old debate on protein aggregation and neurodegeneration enters the clinic. Nature. 2006;443:774–779. doi: 10.1038/nature05290. [DOI] [PubMed] [Google Scholar]
- 5.Westermark P, Wernstedt C, Wilander E, Hayden DW, Obrien TD, Johnson KH. Amyloid fibrils in human insulinoma and islets of langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc. Natl. Acad. Sci. USA. 1987;84:3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, Reid KB. Purification and characterization of a peptide from amyloid-rich pancreases of type-2 diabetic-patients. Proc. Natl. Acad. Sci. USA. 1987;84:8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahn SE, Andrikopoulos S, Verchere CB. Islet amyloid: A long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. 1999;48:241–253. doi: 10.2337/diabetes.48.2.241. [DOI] [PubMed] [Google Scholar]
- 8.Clark A, Cooper GJ, Lewis CE, Morris JF, Willis AC, Reid KB, Turner RC. Islet amyloid formed from diabetes-associated peptide may be pathogenic in type-2 diabetes. Lancet. 1987;2:231–234. doi: 10.1016/s0140-6736(87)90825-7. [DOI] [PubMed] [Google Scholar]
- 9.Hull RL, Westermark GT, Westermark P, Kahn SE. Islet amyloid: A critical entity in the pathogenesis of type 2 diabetes. J. Clin. Endocr. Metab. 2004;89:3629–3643. doi: 10.1210/jc.2004-0405. [DOI] [PubMed] [Google Scholar]
- 10.Marzban L, Park K, Verchere CB. Islet amyloid polypeptide and type 2 diabetes. Exp. Gerontol. 2003;38:347–351. doi: 10.1016/s0531-5565(03)00004-4. [DOI] [PubMed] [Google Scholar]
- 11.Westermark P, Wernstedt C, Wilander E, Sletten K. A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochem. Biophys. Res. Com. 1986;140:827–831. doi: 10.1016/0006-291x(86)90708-4. [DOI] [PubMed] [Google Scholar]
- 12.Jaikaran ET, Higham CE, Serpell LC, Zurdo J, Gross M, Clark A, Fraser PE. Identification of a novel human islet amyloid polypeptide beta-sheet domain and factors influencing fibrillogenesis. J. Mol. Biol. 2001;308:515–525. doi: 10.1006/jmbi.2001.4593. [DOI] [PubMed] [Google Scholar]
- 13.Cooper GJ. Amylin compared with calcitonin-gene-related peptide - structure, biology, and relevance to metabolic disease. Endocr. Rev. 1994;15:163–201. doi: 10.1210/edrv-15-2-163. [DOI] [PubMed] [Google Scholar]
- 14.Hutton JC. The insulin secretory granule. Diabetologia. 1989;32:271–281. doi: 10.1007/BF00265542. [DOI] [PubMed] [Google Scholar]
- 15.Nishi M, Sanke T, Nagamatsu S, Bell GI, Steiner DF. Islet amyloid polypeptide - a new beta-cell secretory product related to islet amyloid deposits. J. Biol. Chem. 1990;265:4173–4176. [PubMed] [Google Scholar]
- 16.Lorenzo A, Razzaboni B, Weir GC, Yankner BA. Pancreatic-islet cell toxicity of amylin associated with type-2 diabetes-mellitus. Nature. 1994;368:756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- 17.Clark A, Wells CA, Buley ID, Cruickshank JK, Vanhegan RI, Matthews DR, Cooper GJ, Holman RR, Turner RC. Islet amyloid, increased alpha-cells, reduced beta-cells and exocrine fibrosis - quantitative changes in the pancreas in type-2 diabetes. Diabetes. Res. Clin. Ex. 1988;9:151–159. [PubMed] [Google Scholar]
- 18.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 19.Hayden MR, Karuparthi PR, Manrique CM, Lastra G, Habibi J, Sowers JR. Longitudinal ultrastructure study of islet amyloid in the HIP rat model of type 2 diabetes mellitus. Exp. Biol. Med. 2007;232:772–779. [PubMed] [Google Scholar]
- 20.Rocken C, Linke RP, Saeger W. Immunohistology of islet amyloid polypeptide in diabetes-mellitus - semiquantitative studies in a postmortem series. Virchows Arch. A. 1992;421:339–344. doi: 10.1007/BF01660981. [DOI] [PubMed] [Google Scholar]
- 21.Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol. Rev. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 22.Abedini A, Raleigh DP. Islet amyloid polypeptide, in protein misfolding diseases: Current and emerging principles and therapies. In: Ramirez-Alvarado M, Kelly JW, Dobson CM, editors. Islet Amyloid Polypeptide. New York: John Wiley & Sons, Inc.; 2010. pp. 517–541. [Google Scholar]
- 23.Westermark GT, Westermark P, Berne C, Korsgren O Nordic Network. Widespread amyloid deposition in transplanted human pancreatic islets. New Engl. J. Med. 2008;359:977–979. doi: 10.1056/NEJMc0802893. [DOI] [PubMed] [Google Scholar]
- 24.Westermark GT, Westermark P, Nordin A, Tornelius E, Andersson A. Formation of amyloid in human pancreatic islets transplanted to the liver and spleen of nude mice. Upsala J. Med. Sci. 2003;108:193–203. doi: 10.3109/2000-1967-113. [DOI] [PubMed] [Google Scholar]
- 25.Udayasankar J, Kodama K, Hull RL, Zraika S, Aston-Mourney K, Subramanian SL, Tong J, Faulenbach MV, Vidal J, Kahn SE. Amyloid formation results in recurrence of hyperglycaemia following transplantation of human IAPP transgenic mouse islets. Diabetologia. 2009;52:145–153. doi: 10.1007/s00125-008-1185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Potter KJ, Abedini A, Marek P, Klimek AM, Butterworth S, Driscoll M, Baker R, Nilsson MR, Warnock GL, Oberholzer J, Bertera S, Trucco M, Korbutt GS, Fraser PE, Raleigh DP, Verchere CB. Islet amyloid deposition limits the viability of human islet grafts but not porcine islet grafts. Proc. Natl. Acad. Sci. USA. 2010;107:4305–4310. doi: 10.1073/pnas.0909024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammarstrom P, Wiseman RL, Powers ET, Kelly JW. Prevention of transthyretin amyloid disease by changing protein misfolding energetics. Science. 2003;299:713–716. doi: 10.1126/science.1079589. [DOI] [PubMed] [Google Scholar]
- 28.Levy E, Carman MD, Fernandezmadrid IJ, Power MD, Lieberburg I, Vanduinen SG, Bots GTAM, Luyendijk W, Frangione B. Mutation of the Alzheimer's disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990;248:1124–1126. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- 29.Hardy J. Amyloid, the presenilins and Alzheimer's disease. Trends. Neurosci. 1997;20:154–159. doi: 10.1016/s0166-2236(96)01030-2. [DOI] [PubMed] [Google Scholar]
- 30.Sakagashira S, Sanke T, Hanabusa T, Shimomura H, Ohagi S, Kumagaye KY, Nakajima K, Nanjo K. Missense mutation of amylin gene (S20G) in Japanese NIDDM patients. Diabetes. 1996;45:1279–1281. doi: 10.2337/diab.45.9.1279. [DOI] [PubMed] [Google Scholar]
- 31.Morita S, Sakagashira S, Ueyama M, Shimajiri Y, Furuta M, Sanke T. Progressive deterioration of insulin secretion in Japanese type 2 diabetic patients in comparison with those who carry the S20G mutation of the islet amyloid polypeptide gene: A long-term follow-up study. J. Diabetes Invest. 2011;2:287–292. doi: 10.1111/j.2040-1124.2011.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SC, Hashim Y, Li JK, Ko GT, Critchley JA, Cockram CS, Chan JC. The islet amyloid polypeptide (amylin) gene S20G mutation in Chinese subjects: Evidence for associations with type 2 diabetes and cholesterol levels. Clin. Endocrinol. 2001;54:541–546. doi: 10.1046/j.1365-2265.2001.01244.x. [DOI] [PubMed] [Google Scholar]
- 33.Sakagashira S, Hiddinga HJ, Tateishi K, Sanke T, Hanabusa T, Nanjo K, Eberhardt NL. S20G mutant amylin exhibits increased in vitro amyloidogenicity and increased intracellular cytotoxicity compared to wild-type amylin. Am. J. Pathol. 2000;157:2101–2109. doi: 10.1016/S0002-9440(10)64848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma Z, Westermark GT, Sakagashira S, Sanke T, Gustavsson A, Sakamoto H, Engstrom U, Nanjo K, Westermark P. Enhanced in vitro production of amyloid-like fibrils from mutant (S20G) islet amyloid polypeptide. Amyloid. 2001;8:242–249. doi: 10.3109/13506120108993820. [DOI] [PubMed] [Google Scholar]
- 35.Luca S, Yau WM, Leapman R, Tycko R. Peptide conformation and supramolecular organization in amylin fibrils: Constraints from solid-state NMR. Biochemistry. 2007;46:13505–13522. doi: 10.1021/bi701427q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiltzius JJ, Sievers SA, Sawaya MR, Cascio D, Popov D, Riekel C, Eisenberg D. Atomic structure of the cross-beta spine of islet amyloid polypeptide (amylin) Protein Sci. 2008;17:1467–1474. doi: 10.1110/ps.036509.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Middleton CT, Singh S, Reddy AS, Woys AM, Strasfeld DB, Marek P, Raleigh DP, Pablo JJ, Zanni MT, Skinner JL. 2DIR spectroscopy of human amylin fibrils reflects stable β-sheet structure. J. Am. Chem. Soc. 2011;133:16062–16071. doi: 10.1021/ja204035k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levine H. Thioflavine-T interaction with amyloid beta-sheet structures. Amyloid. 1995;2:1–6. [Google Scholar]
- 39.O'Nuallain B, Williams AD, Westermark P, Wetzel R. Seeding specificity in amyloid growth induced by heterologous fibrils. J. Biol. Chem. 2004;279:17490–17499. doi: 10.1074/jbc.M311300200. [DOI] [PubMed] [Google Scholar]
- 40.Krebs MR, Morozova-Roche LA, Daniel K, Robinson CV, Dobson CM. Observation of sequence specificity in the seeding of protein amyloid fibrils. Protein Sci. 2004;13:1933–1938. doi: 10.1110/ps.04707004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koo BW, Hebda JA, Miranker AD. Amide inequivalence in the fibrillar assembly of islet amyloid polypeptide. Protein Eng. Des. Sel. 2008;21:147–154. doi: 10.1093/protein/gzm076. [DOI] [PubMed] [Google Scholar]
- 42.Wiltzius JJ, Sievers SA, Sawaya MR, Eisenberg D. Atomic structures of IAPP (amylin) fusions suggest a mechanism for fibrillation and the role of insulin in the process. Protein Sci. 2009;18:1521–1530. doi: 10.1002/pro.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abedini A, Raleigh DP. A role for helical intermediates in amyloid formation by natively unfolded polypeptides? Phys. Biol. 2009;6 doi: 10.1088/1478-3975/6/1/015005. 015005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abedini A, Raleigh DP. A critical assessment of the role of helical intermediates in amyloid formation by natively unfolded proteins and polypeptides. Protein Eng. Des. Sel. 2009;22:453–459. doi: 10.1093/protein/gzp036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williamson JA, Miranker AD. Direct detection of transient alpha-helical states in islet amyloid polypeptide. Protein Sci. 2007;16:110–117. doi: 10.1110/ps.062486907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williamson JA, Loria JP, Miranker AD. Helix stabilization precedes aqueous and bilayer-catalyzed fiber formation in islet amyloid polypeptide. J. Mol. Biol. 2009;393:383–396. doi: 10.1016/j.jmb.2009.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao P, Meng F, Abedini A, Raleigh DP. The ability of rodent islet amyloid polypeptide to Inhibit amyloid formation by human islet amyloid polypeptide has important implications for the mechanism of amyloid formation and the design of inhibitors. Biochemistry. 2010;49:872–881. doi: 10.1021/bi901751b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meng FL, Abedini A, Raleigh DP. Combination of kinetically selected inhibitors in trans leads to highly effective inhibition of amyloid formation. J. Am. Chem. Soc. 2010;132:14340–14342. doi: 10.1021/ja1046186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abedini A, Meng FL, Raleigh DP. A single-point mutation converts the highly amyloidogenic human islet amyloid polypeptide into a potent fibrillization inhibitor. J. Am. Chem. Soc. 2007;129:11300–11301. doi: 10.1021/ja072157y. [DOI] [PubMed] [Google Scholar]
- 50.Marek P, Gupta R, Raleigh DP. The fluorescent amino acid p-cyanophenylalanine provides an intrinsic probe of amyloid formation. Chembiochem. 2008;9:1372–1374. doi: 10.1002/cbic.200800052. [DOI] [PubMed] [Google Scholar]
- 51.Marek P, Mukherjee S, Zanni MT, Raleigh DP. Residue-specific, real-time characterization of lag-phase species and fibril growth during amyloid formation: A combined fluorescence and IR study of p-cyanophenylalanine analogs of islet amyloid polypeptide. J. Mol. Biol. 2010;400:878–888. doi: 10.1016/j.jmb.2010.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taskent-Sezgin H, Chung J, Patsalo V, Miyake-Stoner SJ, Miller AM, Brewer SH, Mehl RA, Green DF, Raleigh DP, Carrico I. Interpretation of p-cyanophenylalanine fluorescence in proteins in terms of solvent exposure and contribution of side-chain quenchers: A combined fluorescence, IR and molecular dynamics study. Biochemistry. 2009;48:9040–9046. doi: 10.1021/bi900938z. [DOI] [PubMed] [Google Scholar]
- 53.Taskent-Sezgin H, Marek P, Thomas R, Goldberg D, Chung J, Carrico I, Raleigh DP. Modulation of p-cyanophenylalanine fluorescence by amino acid side chains and rational design of fluorescence probes of alpha-helix formation. Biochemistry. 2010;49:6290–6295. doi: 10.1021/bi100932p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tucker MJ, Oyola R, Gai F. A novel fluorescent probe for protein binding and folding studies: p-cyano-phenylalanine. Biopolymers. 2006;83:571–576. doi: 10.1002/bip.20587. [DOI] [PubMed] [Google Scholar]
- 55.Padrick SB, Miranker AD. Islet amyloid: Phase partitioning and secondary nucleation are central to the mechanism of fibrillogenesis. Biochemistry. 2002;41:4694–4703. doi: 10.1021/bi0160462. [DOI] [PubMed] [Google Scholar]
- 56.Abedini A, Raleigh DP. The role of His-18 in amyloid formation by human islet amyloid polypeptide. Biochemistry. 2005;44:16284–16291. doi: 10.1021/bi051432v. [DOI] [PubMed] [Google Scholar]
- 57.Cao P, Raleigh DP. Ester to amide switch peptides provide a simple method for preparing monomeric islet amyloid polypeptide under physiologically relevant conditions and facilitate investigations of amyloid formation. J. Am. Chem. Soc. 2010;132:4052–4053. doi: 10.1021/ja910763m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Higham CE, Jaikaran ET, Fraser PE, Gross M, Clark A. Preparation of synthetic human islet amyloid polypeptide (IAPP) in a stable conformation to enable study of conversion to amyloid-like fibrils. Febs Lett. 2000;470:55–60. doi: 10.1016/s0014-5793(00)01287-4. [DOI] [PubMed] [Google Scholar]
- 59.Konarkowska B, Aitken JF, Kistler J, Zhang SP, Cooper GJ. The aggregation potential of human amylin determines its cytotoxicity towards islet beta-cells. Febs J. 2006;273:3614–3624. doi: 10.1111/j.1742-4658.2006.05367.x. [DOI] [PubMed] [Google Scholar]
- 60.Collins SR, Douglass A, Vale RD, Weissman JS. Mechanism of prion propagation: Amyloid growth occurs by monomer addition. Plos. Biol. 2004;2:1582–1590. doi: 10.1371/journal.pbio.0020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xue WF, Homans SW, Radford SE. Systematic analysis of nucleation-dependent polymerization reveals new insights into the mechanism of amyloid self-assembly. Proc. Natl. Acad. Sci. USA. 2008;105:8926–8931. doi: 10.1073/pnas.0711664105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knowles TP, Waudby CA, Devlin GL, Cohen SI, Aguzzi A, Vendruscolo M, Terentjev EM, Welland ME, Dobson CM. An analytical solution to the kinetics of breakable filament assembly. Science. 2009;326:1533–1537. doi: 10.1126/science.1178250. [DOI] [PubMed] [Google Scholar]
- 63.Bishop MF, Ferrone FA. Kinetics of nucleation-controlled polymerization - a perturbation treatment for use with a secondary pathway. Biophys. J. 1984;46:631–644. doi: 10.1016/S0006-3495(84)84062-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parrini C, Taddei N, Ramazzotti M, Degl'lnnocenti D, Ramponi G, Dobson CM, Chiti F. Glycine residues appear to be evolutionarily conserved for their ability to inhibit aggregation. Structure. 2005;13:1143–1151. doi: 10.1016/j.str.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 65.Williams AD, Shivaprasad S, Wetzel R. Alanine scanning mutagenesis of A beta(1–40) amyloid fibril stability. J. Mol. Biol. 2006;357:1283–1294. doi: 10.1016/j.jmb.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 66.Anil B, Song BB, Tang YF, Raleigh DP. Exploiting the right side of the ramachandran plot: Substitution of glycines by D-alanine can significantly increase protein stability. J. Am. Chem. Soc. 2004;126:13194–13195. doi: 10.1021/ja047119i. [DOI] [PubMed] [Google Scholar]
- 67.Xu WX, Jiang P, Mu YG. Conformation preorganization: effects of S20G mutation on the structure of human islet amyloid polypeptide segment. J. Phys. Chem. B. 2009;113:7308–7314. doi: 10.1021/jp8106827. [DOI] [PubMed] [Google Scholar]
- 68.Liu G, Prabhakar A, Aucoin D, Simon M, Sparks S, Robbins KJ, Sheen A, Petty SA, Lazo ND. Mechanistic studies of peptide self-assembly: transient alpha-helices to stable beta-sheets. J. Am. Chem. Soc. 2010;132:18223–18232. doi: 10.1021/ja1069882. [DOI] [PubMed] [Google Scholar]
- 69.Abedini A, Singh G, Raleigh DP. Recovery and purification of highly aggregation-prone disulfide-containing peptides: Application to islet amyloid polypeptide. Anal. Biochem. 2006;351:181–186. doi: 10.1016/j.ab.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 70.Nilsson MR, Raleigh DP. Analysis of amylin cleavage products provides new insights into the amyloidogenic region of human amylin. J. Mol. Biol. 1999;294:1375–1385. doi: 10.1006/jmbi.1999.3286. [DOI] [PubMed] [Google Scholar]
- 71.Nilsson MR, Driscoll M, Raleigh DP. Low levels of asparagine deamidation can have a dramatic effect on aggregation of amyloidogenic peptides: Implications for the study of amyloid formation. Protein Sci. 2002;11:342–349. doi: 10.1110/ps.48702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.