Abstract
During polychemotherapy, cytotoxic drugs are given in combinations to enhance their anti-tumor effectiveness. For most drug combinations, underlying signaling mechanisms responsible for positive drug–drug interactions remain elusive. Here, we prove a decisive role for the Bcl-2 family member NOXA to mediate cell death by certain drug combinations, even if drugs were combined which acted independently from NOXA, when given alone. In proof-of-principle studies, betulinic acid, doxorubicin and vincristine induced cell death in a p53- and NOXA-independent pathway involving mitochondrial pore formation, release of cytochrome c and caspase activation. In contrast, when betulinic acid was combined with either doxorubicine or vincristine, cell death signaling changed considerably; the drug combinations clearly depended on both p53 and NOXA. Similarly and of high clinical relevance, in patient-derived childhood acute leukemia samples the drug combinations, but not the single drugs depended on p53 and NOXA, as shown by RNA interference studies in patient-derived cells. Our data emphasize that NOXA represents an important target molecule for combinations of drugs that alone do not target NOXA. NOXA might have a special role in regulating apoptosis sensitivity in the complex interplay of polychemotherapy. Deciphering the differences in signaling of single drugs and drug combinations might enable designing highly effective novel polychemotherapy regimens.
Keywords: NOXA, p53, betulinic acid, doxorubicin, PUMA
Polychemotherapy involves the simultaneous application of various cytotoxic drugs. So far, effective drug combinations were selected empirically in clinical trials, while the mechanistic understanding of favorable drug–drug interactions remained largely elusive. Here, we characterized an important role for the Bcl-2 member NOXA in mediating effective drug combinations, even if the single drugs induced NOXA-independent cell death.
NOXA represents an important intracellular target for effective cell death induction in tumor cells. NOXA is a pro-apoptotic member of the BCL-2 family, which mediates induction of apoptosis via activation of mitochondria and the intrinsic apoptosis signaling pathway.1, 2 Cell death induction by cytotoxic drugs is mainly mediated by the intrinsic apoptosis signaling cascade and NOXA has an important role for apoptosis induction by cytotoxic drugs.3, 4, 5 As a so called ‘BH3-only' protein of the BCL-2 family, upregulation of NOXA counteracts the pro-survival function of anti-apoptotic BCL-2 family members.3, 4, 5 As described for PUMA, another pro-apoptotic BH3-only member, the expression of NOXA is regulated by the transcription factor p53, but also by, for example, p38 and ERK.4, 6, 7, 8, 9 Although its important role for apoptosis induction by single drugs was extensively shown,3 the role of NOXA for drug combinations remains elusive so far.
Betulinic acid (BA) represents a potent new drug for cell death induction in a broad spectrum of different tumor cells.10, 11 We recently identified BA as highly effective in ALL cells, especially upon drug resistance and relapse.10 For signaling apoptosis, BA directly targets mitochondria, but also regulates members of the BCL-2 family shifting the balance into a pro-apoptotic state.10, 12, 13, 14
Here, we found that NOXA had an important role for the effect of combinations of cytotoxic drugs, which induced NOXA-independent cell death, when given alone.
Results
The aim was to characterize the role of NOXA for mediating apoptosis induction by doxorubicin, vincristine and BA either given as single drugs or in drug combinations.
Super-additive apoptosis by the combination of BA and doxorubicin
BA induced super-additive, synergistic apoptosis in combination with doxorubicin (doxo) in all six tumor cell lines tested (Figure 1a). Throughout the work, doxorubicin was used in concentrations that were within the drug level ranges measured in patients upon anti-tumor treatment and BA was used in concentrations that were successfully used in animals as clinical data are not available yet.15, 16 For all cell line experiments, a significant increase in cell death induction by the combinatorial approach was testified by asterixes whenever the addition of apoptosis induction by each drug alone was inferior to the effect of the combined stimulation with a P-value of P<0.05 (for details see Supplementary Figure S1). In addition to its direct cytotoxic effects, the formation of new tumor colonies was markedly reduced (Figure 1b and data not shown). Most importantly, BA enabled induction of cell death in otherwise doxo-resistant leukemia cells and thus overcame resistance towards doxo (Supplementary Figure S2 and data not shown).
Figure 1.
Synergistic cell death and reduction of colony formation by the combination of BA and doxorubicin. (a) MCF-7 breast cancer, HCT116 colon cancer, A549 lung cancer, A498 renal cancer and CEM and JURKAT leukemia cells were stimulated with doxo and BA. Each drug was applied in a concentration that induced less than 25% specific apoptosis, when the drug was given alone. For all solid tumor cell lines, doxo was applied at 0.5 μM and BA at 20 μM, CEM and JURKAT cells were treated with 0.03 μM doxo and 2 μM BA. Apoptosis induction was measured after 72 h by forwardside scatter analysis for leukemia cells and after 96 h by Nicoletti staining for solid tumor cells. Statistical analysis was performed applying ANOVA. *P<0.05, comparing apoptosis induction after combined treatment and the addition of apoptosis induction after single-agent stimulation (for detailed description of statistical analysis compare Supplementary Figure S1). For all cell line experiments, data are presented as mean values of at least three independent experiments±S.E.M. if not stated differently. (b) JURKAT cells were seeded at 0.05 million cells/ml and stimulated as in Figure 1a. Colony formation was detected by Cellscreen light microscopy and pictures are representative image sections from the whole well of a 96-well plate. Percentages indicated within the picture represent simultaneously determined specific apoptosis induction. Numbers below light microscopy pictures indicate quantity of colonies over the whole well. Depicted is one representative experiment after 72 h of incubation time. (c) Cells from n=20 primary childhood acute leukemia samples were stimulated with doxorubicine (doxo; 0.5 μM) and BA (20 μM) directly after isolation from bone marrow aspirates for at least 48 h as described in Materials and Methods. Depicted are specific apoptosis data for the single and the combinatory stimulations. *P<0.05, ANOVA, comparing each single agent to the combinatorial stimulation
Beyond work with cell lines, favorable anti-leukemia effects of the combination of BA and doxo (BA/doxo) were detected in fresh primary tumor cells from n=20 children with newly diagnosed ALL or ALL relapse before onset of therapy (Figure 1c).
In addition to doxo, BA also induced synergistic apoptosis in combination with vincristine (data not shown).
Taken together, the combination of BA together with doxo or vincristine induced synergistic anti-tumor effects on several cell lines and primary ALL cells in vitro.
Common downstream apoptosis signaling for single drugs and drug combinations
To identify the intracellular target molecule responsible for the favorable cell death effect of the combination BA plus doxo, we studied the two T-cell leukemia cell lines JURKAT and CEM. Results for JURKAT cells and doxo together with BA are presented in printed figures; results for the combination of vincristine and BA (Supplementary Figure S7) and data for doxo plus BA on CEM cells (Supplementary Figure S8) were similar throughout all experiments, and decisive experiments are summarized in Supplementary Figures.
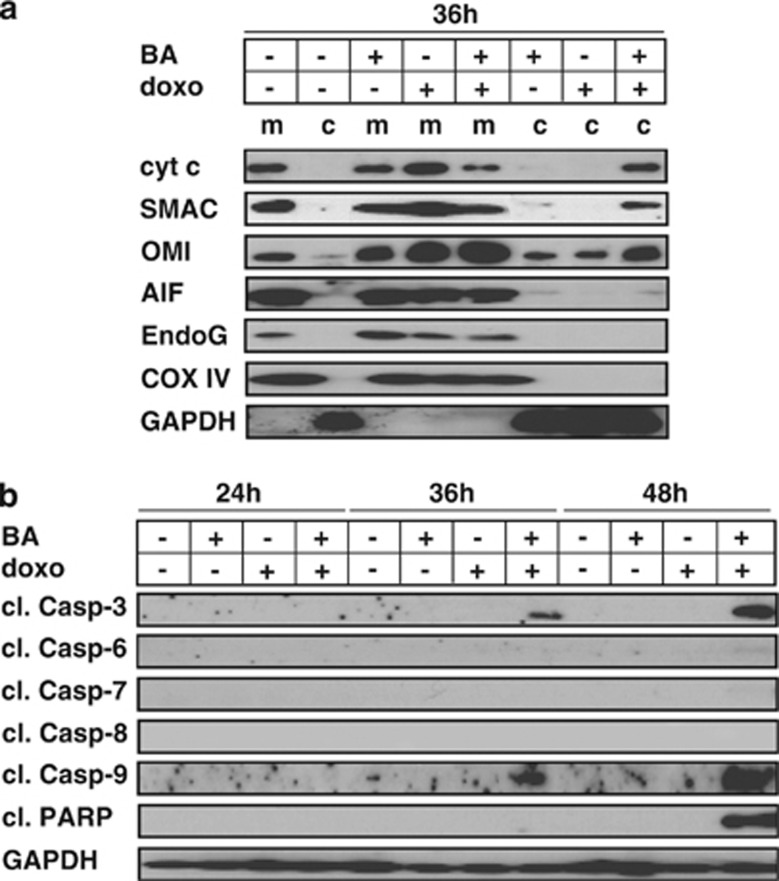
As BA and doxo are both known to activate the intrinsic apoptosis signaling cascade, we first studied the release of the pro-apoptotic factors cytochrome c, SMAC/DIABLO, OMI/HtrA2, AIF and EndoG from mitochondria by fractionated investigation of mitochondrial and cytosolic lysates. Compared with single-agent stimulations, the mitochondrial release of the apoptogenic factors cytochrome c, SMAC/DIABLO and OMI/HtrA2 was augmented upon combined stimulation with BA plus doxo. No release of AIF and Endonuclease G (EndoG) was detected (Figure 2a and data not shown). The release of apoptogenetic factors from mitochondria was followed by the activation of executioner caspases 9 and 3, while no activation of further effector caspases was observed (Figure 2b).
Figure 2.
Release of apoptogenetic factors and activation of caspases during synergistic apoptosis induction. (a) JURKAT cells were stimulated with doxo and BA for 36 h and fractionated investigation of cytosolic (c) and mitochondrial (m) extracts was performed by western blot. GAPDH and COX IV served as loading controls. For the clearness of presentation, the order of samples from the original blot was rearranged without any further modifications. (b) JURKAT cells were stimulated as in Figure 2a for time periods indicated and western blot analysis was performed of total cellular extracts. For the detection of all caspase-cleavage products, TRAIL-stimulated cells were used as positive control (data not shown). Experimental procedure and drug concentrations were applied as in Figure 1a
Pro-apoptotic factors are released from mitochondria via formation of membrane pores. Regulators of this pore formation are members of the BCL-2 family, which consists of both pro- and anti-apoptotic members. It is well known, that inhibition of mitochondrial pore formation reduces cell death induction by BA or doxo alone10, 17, 18 (own data not shown). In line, cell death induction of the combinatorial treatment with doxo plus BA was markedly reduced in JURKAT cells, which lacked simultaneously BAX and BAK expression and in cells with stable overexpression of BCL-2 and BCL-XL (Figure 3a).
Figure 3.
Mitochondrial pore formation and activation of caspases as prerequisite for synergistic apoptosis induction. (a) Parental JURKAT cells, JURKAT cells deficient for BAX and BAK (BAK−/−) and JURKAT cells overexpressing BCL-2 (BCL-2) or BCL-XL (BCL-XL) were stimulated with doxorubicin and BA. (b) Parental JURKAT cells, JURKAT cells pretreated with qVD (50 μM) for 6 h, JURKAT cells overexpressing XIAP (XIAP), with loss of caspase-9 (Casp-9−/−) and after retransfection of caspase-9 (Casp-9+/+) were stimulated with doxo and BA as in Figure 1a. Drug concentrations, experimental procedure, measurement of apoptosis induction, statistical analysis and presentation of data were identical as in Figure 1a and Supplementary Figure S1. NS, not significant
Downstream of mitochondrial pore formation, cell death induction mostly depends on the activation of caspases. It is well known, that biochemical or molecular inhibition of caspase activation reduces cell death induction by BA or doxo alone10, 17, 18, 19 (own data not shown). Next, we tested the impact of the irreversible broad-spectrum caspase inhibitor qVD, overexpression of XIAP, which is known to inhibit caspases activation, or the lack of caspase-9 on the combination of BA plus doxo. All described modifications markedly inhibited the cell death induction by BA plus doxo (Figure 3b). An important role of caspase-9 was proven by recombinant re-expression of caspase-9 in caspase-9-lacking cells, which rescued apoptosis induction by BA plus doxo (Figure 3b).
Taken together, these data show that the drug combination of BA plus doxo activated the identical downstream signaling steps that have been described before for BA or doxo alone.
Distinct role of NOXA for single drugs and drug combinations
BAX/ BAK form the mitochondrial membrane pore to release pro-apoptotic factors from mitochondria, unless they are inhibited by BCL-2 and/ or BCL-XL. One step upstream, the pro-apoptotic BH3-only members of the Bcl-2 family, such as BIM, BID, NOXA and PUMA, inhibit the anti-apoptotic BCL-2 members, such as BCL-2 and BCL-XL, and thus enable pro-apoptotic signaling by double inhibition.3, 20, 21 As we had found an important impact of BAX/BAK and BCL-2/BCL-XL on cell death induction by BA and doxo alone and for the combinatorial treatment, we next studied the putative role of BH3-only members of the BCL-2 family. For JURKAT cells, it has been described in the literature, that cell death induction by doxo does not depend on the most prominent members of the BH3-only family as long as only one member is targeted.19 In contrast, nothing is known for the signal initiation of BA upstream of BCL-2 and BCL-XL. So we first evaluated the impact of RNA interference against PUMA, NOXA, BID and BIM on cell death induction by BA and doxo if applied alone. We were not able to ascribe any of these BH3 members an impact on apoptosis induction by BA or doxo alone (Supplementary Figures S3A and B and data not shown).
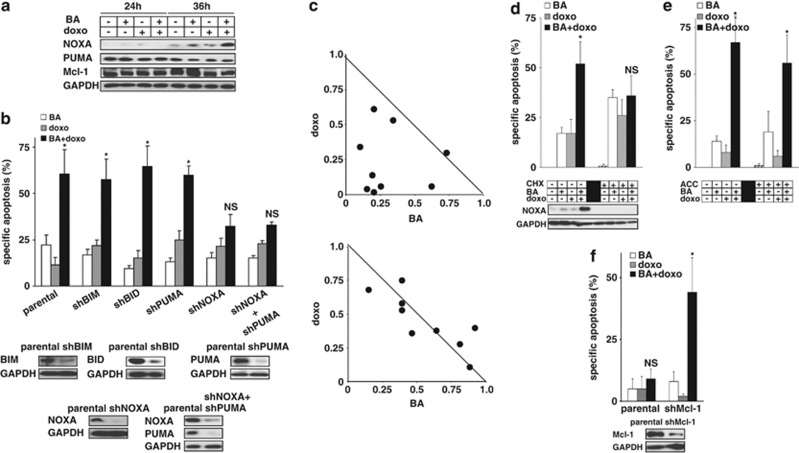
To evaluate the relevance for the combinatorial treatment approach of BA plus doxo, we first studied the expression of most members of the IAP and BCL-2 family after combined stimulation. Levels of all proteins remained unchanged, including PUMA and Mcl-1, with the exception of the pro-apoptotic BH3-only protein NOXA that showed a marked upregulation upon stimulation with BA plus doxo (Figure 4a, Supplementary Figures S3C and D). On a functional level, neither knockdown of BIM, BID nor PUMA by RNA interference altered apoptosis induction by BA plus doxo. In contrast, when NOXA was downregulated by shRNA, apoptosis induction by BA plus doxo was markedly reduced and the drug combination was unable to induce apoptosis efficiently. The effect of knockdown of NOXA could not be enhanced by simultaneous knockdown of PUMA as shown by double knockdown experiments arguing towards distinct functions of NOXA and PUMA upon BA plus doxo-induced cell death (Figure 4b). The decisive impact of NOXA was present for a broad range of different drug combinations (Figure 4c). The presence of either cycloheximide or actinomycin D inhibited both upregulation of NOXA and the synergistic activity of the drug combination suggesting an important role of protein neosynthesis (Figure 4d and data not shown). In contrast, addition of neither acetylcysteine nor glutathion esthers altered these signaling steps arguing against involvement of reactive oxygene species, which have an important role for regulating NOXA in other settings (Figure 4e and data not shown).7, 8 As candidate target of NOXA, knockdown of Mcl-1 promoted cell death induction for the combination of BA and doxo, but not for both single drugs (Figure 4f and Supplementary Figures S3E and F). These data highlight a unique role of NOXA for apoptosis induction by the combination of BA plus doxo, but not by either single agent alone.
Figure 4.
Differential involvement of NOXA after single-agent and combination therapy. (a) Total JURKAT cell lysates from Figure 2b were analyzed for the expression of NOXA, PUMA and Mcl-1. (b) Parental JURKAT cells, JURKAT cells stably transfected with shRNA against BIM (shBIM), BID (shBID), PUMA (shPUMA), NOXA (shNOXA) or NOXA and simultaneously PUMA (shNOXA+shPUMA) were stimulated with doxorubicin and BA. (c) Parental JURKAT cells (upper panel) and JURKAT cells stably transfected with shRNA against NOXA (lower panel) were stimulated with several drug combinations for doxo (0.01, 0.03, 0.06 μM) and BA (2, 6, 12 μM). Synergism was evaluated using isobolograms. (d and e) JURKAT cells were pretreated with cycloheximide (CHX, 0.3 ng/ml, d) or acetylcysteine (ACC, 500 μg/ml, e) for 6 h followed by stimulation with doxorubicin and BA. (f) Parental JURKAT cells and JURKAT cells stably transfected with shRNA against Mcl-1 (shMcl-1) were stimulated with doxorubicin and BA. Drug concentrations, experimental procedure, measurement of apoptosis induction, statistical analysis and presentation of data were identical as in Figure 1a and Supplementary Figure S1 if not stated differently. Statistical significance is indicated with an asterix, whenever the P-value was <0.05. NS, statistically not significant
Distinct role of p53 for single drugs and drug combinations
So far, we assigned NOXA a key role in enabling apoptosis induction by the combination of BA/doxo. NOXA is typically regulated by the transcription factor p53 and most cytotoxic drugs including doxo activate p53.3, 5, 22 We had recently demonstrated p53 functionality in the JURKAT cells used here, which showed p53-dependent upregulation of ,for example, caspase-8 upon stimulation with methotrexate.23, 24, 25, 26 BA is known to induce apoptosis independently from p53 in several cell systems.11, 12 Interestingly and in contrast to other cancer cells, cell death induction by doxo alone did not depend on the presence of p53 in JURKAT cells (Supplementary Figures S4A and B), although p53 was activated after stimulation with doxo (Figure 5a). When JURKAT cells were simultaneously stimulated with BA plus doxo, p53 activation and accumulation was much more pronounced (Figure 5a). Inhibition of the accumulation of p53 using RNA interference directed against p53 markedly reduced the upregulation of NOXA and apoptosis induction for the combination of BA plus doxo suggesting p53-dependent cell death signaling selectively for the combinatorial treatment (Figures 5b and c). The impact of p53 for efficient cell death was detected for a broad range of drug combinations tested (Figure 5d). The decisive role of p53 was confirmed in cells with p53 in wild-type status (Supplementary Figure S5).
Figure 5.
Distinct involvement of the transcription factor p53 after single-agent and combined stimulation. (a) JURKAT cells stimulated with doxorubicin and BA as indicated were fractionated into cytosolic and nuclear extracts for western blot. GAPDH and Histon H1 served as loading control. (b) Parental JURKAT cells and JURKAT cells stably transfected with a control sequence (mock) or shRNA against p53 (shp53) were stimulated with doxorubicin and BA. (c) JURKAT cells from Figure 5b were evaluated for the expression level of NOXA as in Figure 4a. (d) The experimental setting from Figure 5b of shRNA interference against p53 was applied for the drug combinations of doxo and BA as in Figure 4c. Data for parental JURKAT cells are presented in Figure 4c. Drug concentrations, experimental procedure, measurement of apoptosis induction, statistical analysis and presentation of data were identical as in Figure 1a and Supplementary Figure S1 if not stated differently
These data show that cell death induction by the combination of BA plus doxo, but not by each agent alone was dependent on the transcription factor p53 that regulated the BH3-only member NOXA.
Roles of NOXA and p53 in patient-derived ALL cells
Due to prolonged in vitro culture, leukemic cell lines inherit alterations and mutations not present in patient-derived cells.27, 28 For example, leukemic cell lines show frequent alterations in p53, whereas those are rare in patient-derived leukemia cells.23, 29, 30 We therefore aimed to survey the dependency of BA plus doxo-induced cell death on p53 and NOXA in patient-derived leukemia cells.
Towards this aim, we used our recently established technique for transient transfection of patient-derived acute leukemia cells with siRNA in cells from two different patients with pre-B-ALL (ALL-50, initial diagnosis, female, 7 years, Figure 6) and pre-B-ALL (ALL-169, initial diagnosis, female, 18 years, Supplementary Figures S6C–H)24 and siRNAs targeting p53, NOXA or PUMA. p53 functionality was proven using irradiation and Etoposide as classical p53 stimuli and regulation of PUMA as typical p53 target gene (Supplementary Figures S6A and B). As expected, inhibition of p53 or NOXA did not affect cell death induction by BA or doxo if applied alone (Supplementary Figures S6C–F). For the combinatorial treatment of BA plus doxo, cell death induction was significantly inhibited by transfection of siRNA directed against p53 or NOXA, but not PUMA (Figure 6a, Supplementary Figure S6G) and the cell death regulating function was present for a range of drug concentrations tested (Figure 6b and Supplementary Figure S6H). Transient knockdown efficiency of expression or upregulation of proteins was achieved as shown by Western Blot.
Figure 6.
Verification of the distinct involvement of NOXA and p53 during single-agent and combinatorial treatment in patient-derived tumor cells. (a) Leukemia cells from a patient with pre-B-ALL (ALL-50, initial diagnosis, female, 7 years) were studied after amplification in NOD/SCID mice. Cells were transfected with identical siRNAs as in Supplementary Figures S6C and D or siRNA against PUMA (siPUMA) were stimulated 6 h after transfection with doxo (0.05 μM) and BA (2 μM) for another 72 h. Impact of stimulation on the regulation of p53 (nuclear extracts, 36 h incubation time), NOXA and PUMA (cytosolic extracts, 48 h) was analyzed by western blot. Statistical analysis was performed out of four independent experiments, for each setting one representative western blot is presented. *P<0.05, ANOVA, significantly augmented apoptosis induction for the combined stimulation compared with the addition of apoptosis induction after single-agent stimulation. (b) The identical experimental setting from Figure 6a was performed for cells transfected with the control siRNA (upper panel) and siRNA against NOXA (middle) or p53 (lower panel) for a range of drug combinations of doxo (0.02, 0.05, 0.2 μM) and BA (0.2, 0.6, 2 μM). (c) Differential involvement of NOXA and p53 during single-agent and combined stimulation: Synergistic apoptosis induction by doxo or vincristine plus BA depends on the regulation of p53 and NOXA, whereas single-agent signaling bypasses p53 and NOXA. Downstream of NOXA, a common pathway is activated for single-agent and combinatorial treatment approaches. Measurement of apoptosis induction, statistical analysis and presentation of data were performed as in Figure 1a, Supplementary Figure S1, Figures 4c and 5d
These data show that the underlying signaling mechanism responsible for effective cell death induction by the combination BA plus doxo in patient-derived leukemia cells involves p53 and NOXA, but not PUMA.
The identical distinct involvement of NOXA and p53 only during combined drug stimulation was observed for the second combinatorial treatment approach investigated of BA together with VCR (Supplementary Figure S7) and in CEM cells, the second ALL cell line investigated (Supplementary Figure S8 and data not shown).
Taken together, the pro-apoptotic BH3-only member NOXA and p53 represent the critical target molecules, which mediate the super-additive cell death induction by the combination of BA together with doxo or vincristine. In contrast, cell death by each single drug does not rely on NOXA or p53. Downstream of NOXA, all single agents and drug combinations converge in a common pathway depending on mitochondrial pore formation and caspases activation (Figure 6c).
Discussion
Our data show that the pro-apoptotic BCL-2 family member NOXA can have a decisive role in mediating efficient apoptosis induction for certain drug combinations. Most importantly, molecular studies on tumor cells derived from children with ALL proved that only the drug combinations relied on signaling by NOXA and p53, whereas the single agents did not.
Upon stimulation with BA plus doxo, NOXA was upregulated, whereas further classical target genes of p53, such as BAX, PUMA or caspase-8, were not regulated. This can be explained by the complex network of p53 signal transduction described, where different p53-activating stimuli induce upregulation of different p53 target genes.22, 23, 31 The data are in line with our recent results that demonstrated drug-specific regulation of common p53 target genes.3, 24, 25, 31, 32 Our data are in line with published results, that assign NOXA and PUMA overlapping and distinct regulations by p53 depending on the experimental setting.3, 5, 20, 21
For exerting its pro-apoptotic function, NOXA inactivates anti-apoptotic Bcl-2 family members, mainly Mcl-1 and A1.4 As downregulation of Mcl-1-sensitized cells for apoptosis induction by BA and doxo, Mcl-1 might represent the putative target for NOXA upon stimulation with the combination of BA and doxo (Figure 4).
So far, NOXA was mainly studied with focus on its role for efficient apoptosis induction after stimulation with single cytotoxic drugs, where the activation of NOXA appeared critical for the intracellular cell death response.3, 4, 5 In contrast, the decisive role of NOXA for combination chemotherapy is a completely new finding. So far, solely two recent studies showed that drug combinations induced super-additive apoptosis via upregulation of NOXA in chronic lymphocytic leukemia and mantle-cell lymphoma.7, 8 In these papers and in contrast to our data, NOXA was shown to be regulated by ROS generation and independently from p53, suggesting cell type- and/or stimulus-dependent regulation of NOXA. Together, these and our data clearly point out the necessity to further evaluate the significance of NOXA for combination chemotherapy.
As single agent, BA can directly target mitochondria independent from p53 or NOXA and bypass apoptosis resistance mechanisms in proximal signaling steps.10, 11, 13 When BA is combined with other cytotoxic drugs, detailed mechanistic investigations of the synergistic effects are missing so far.11, 33, 34 Based on the mechanistic data obtained here and their proof in patient-derived ALL cells, our data suggest incorporating BA in close proximity to doxo or vincristine into future polychemotherapy trials of ALL to take advantage of the favorable regulation of p53 and NOXA.
Here we show that NOXA is critical for efficient apoptosis induction by the drug combinations of BA together with doxo or vincristine, whereas the single drugs induce NOXA-independent apoptosis. Of interest, both experimental settings converge on the identical downstream intrinsic apoptosis signaling cascade but differ in the initiating step. On a more general level, our data argue that identification of the intracellular target for a single agent does not implicate the understanding of how this drug acts in combination with other drugs. Understanding the differences in signaling between single drugs and drug combinations might enable designing new effective polychemotherapy regimens. Our data suggest that NOXA might represent an important intracellular target determining chemosensitivity in the complex context of polychemotherapy.
Materials and Methods
Materials
Acetylcysteine, glutathione esthers and the pan-caspase inhibitor qVD were obtained from Calbiochem (San Diego, CA, USA). All further reagents from Sigma (Deisenhofen, Germany). For western blot analysis, the following antibodies were used: anti AIF, anti BAK, anti BAX, anti BCL-2, anti cIAP-2, anti Histone H1, anti-Mcl-1 and anti p53 from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA); anti BCL-XL, anti BID, anti cleaved caspase-3, 6 and 7, anti cleaved PARP, anti cytochrome c, anti COX IV, anti Endonuclease G, anti OMI/HtrA2, anti PUMA and anti Survivin from Cell Signaling Technology Inc. (Danvers, MA, USA); anti α-Tubulin from Oncogene (San Diego, CA, USA); anti caspase-9 and anti XIAP from BD Biosciences (San Jose, SA, USA); anti GAPDH from Thermo Fisher (Waltham, MA, USA); anti NOXA from Calbiochem; anti BIM and anti caspase-8 from Alexis Corp. (Lausen, Switzerland); anti SMAC/DIABLO from Millipore (Billerica, MA, USA).
Cell lines, primary samples and stimulation experiments
All cell lines were maintained, seeded and stimulated as described.10, 25, 26 Drug-resistant derivative cell lines were established as specified.10 Cells were seeded and simultaneously stimulated with cytotoxic drugs and BA for 72 h. To enable visualization of synergistic apoptosis, drugs were used in concentrations that induced limited apoptosis as single drugs (10–25% specific apoptosis). Apoptosis was measured by DNA fragmentation and sub-G1 fraction in FACscan (Becton Dickinson, Heidelberg, Germany) for all solid tumor cell lines and by forwardside scatter analysis for leukemia cells. For primary leukemia cells and leukemic cell lines, Annexin V/propidium iodide staining was performed in parallel for selected experiments. Results of forwardside scatter analysis were highly correlated to the percentage of Annexin V/propidium iodide double-positive cells arguing towards apoptotic cell death. For biochemical inhibition, cells were pretreated for 6 h prior to stimulation. CFUs were performed using methylcellulose base media as described recently.25
Primary leukemia blasts were obtained from n=20 children treated for acute leukemia at the Ludwig Maximilian University Children's Hospital and the children's hospital of the TU Munich during 2006 and 2008. Samples were obtained, isolated, seeded, stimulated and incubated with cytotoxic drugs and BA as described.10, 24, 25, 26, 35, 36 All experiments were approved by the local ethics boards according to the declaration of Helsinki.
Western blot analysis
Western blot analysis of total cellular protein and separated evaluation of cytosolic and nuclear fractions was performed as described.10, 26, 36 Investigation of cytosolic and mitochondrial fractions was achieved by homogenizing cell pellets with preparation buffer (250 mM sucrose, 20 mM HEPES (pH 7.4), 1 mM EDTA, 1 mM EGTA, 10 mM KCl, 1,5 mM MgCl2 and 1 mM DTT). Lysates were passed three times through a 24G needle and centrifuged at 13 000 × g. Supernatants were collected as cytosolic extracts, pellets were resuspended in RIPA buffer (150 mM NaCl, 1% NP40, 0,5% Deoxycholate, 0,1% SDS, 50 mM Tris (pH 8.0)) to obtain mitochondrial extracts. For clearness of presentation, the order of presentation was rearranged for some of the blots presented without any further modifications.
RNAi transfection of cell lines
Parental JURKAT and CEM cell lines were stably transfected with previously specified vectors expressing shRNA against p53, NOXA, BID and BIM or a corresponding mock sequence using the Cell Line Nucleofector kit V (Lonza, Walkersville, MD, USA).24, 25, 26, 37, 38 For expression of shRNA against PUMA or Mcl-1oligonucleotides containing 19 bp sequences against PUMA mRNA (5′-TCTCATCATGGGACTCCTG-3′) or Mcl-1 mRNA (5′-CGGGACTGGCTAGTTAAAC-3′) and in parallel a scrambled sequence were cloned between BamHI and EcoRI sites of the vector pGreenPuro (System Biosciences (SBI), Mountain View, CA, USA). VSV-G pseudotyped high titers lentiviruses (4 × 109 transducing units per ml) were generated by transient co-transfection of HEK-293T cells in six-well tissue culture plates with a four-plasmid combination (2.3 μg pGreenPuro-shPUMA; 2.3 μg pRSV-Rev; 4.7 μg pMDLg/pRRE; 1.3 μg pMD2.G) using TransIT-293 (Mirus, Madison, WI, USA). Supernatants were collected 72 h after transfection, purified by 0.45-μm-filters, concentrated using an Amicon Ultra centrifugal filter unit (Millipore), aliquoted and frozen at −80 °C. For lentiviral transduction, 1 million JURKAT and CEM cells were seeded in 12-well tissue culture plates and infected with 2 μl/ml lentivirus in the presence of 8 μg/ml polybrene (Sigma). To generate stably transfected cell lines, cells were selected starting 24 h post infection up to 7 days in 20 μg/ml puromycin.
Amplification and transfection of patient-derived tumor cells
The animal work was approved by the Regierung von Oberbayern (55.2-1-54-2531-2-07) and the xenograft NOD/SCID mouse model was performed as described by others.39 Shortly, fresh primary childhood ALL cells were isolated by Ficoll gradient centrifugation from blood or bone marrow that had been obtained from leftovers of clinical routine sampling. A total of 10 million ALL cells were injected into 6- to8-week-old NOD/SCID mice and engrafted human ALL cells were isolated from spleens of diseased mice by pressing through a cell strainer (BD Biosciences) and Ficoll gradient centrifugation. Cells were separated and simultaneously injected into next generation of mice and subjected to in vitro experiments. Xenografted cells were transfected using the technique recently described.24, 40 Directly after transfection, cells were transferred to the previously specified medium and stimulated 6 h after transfection. The following siRNAs were used at a concentration of 20 μM: silencer-validated siRNA against p53 (5′-GGGUUAGUUUACAAUCAGC-3′, Ambion, Austin, TX, USA), siRNA against NOXA (5′-GUCGAGUGUGCUACUCAACU-3′); siRNA against PUMA (5′-UCUCAUCAUGGGACUCCUG-3′, both from MWG Biotech, Ebersberg, Germany) and All Star negative control siRNA (Qiagen, Hilden, Germany).
Statistical analysis
Specific apoptosis was calculated as [(apoptosis of stimulated cells at end − apoptosis of unstimulated cells at end)/ (100 − apoptosis of unstimulated cells at end) × 100]. Drug resistance was defined as specific apoptosis<10%. Isobolograms were performed using CompuSyn software version 1.0 (ComboSyn Incorporated, Paramus, NJ, USA). To test for statistically significant differences, one-way RM ANOVA was applied to compare the different experimental settings, including the expected (addition) and the measured cell death induction of the combinatorial treatment. Statistical significance was accepted with P-values <0.05.
Acknowledgments
The skilled technical work of L Mura and U Borgmeier is kindly appreciated. We thank the animal facility for caring for the mice, K Schneider and V Groiss for providing patient-derived xenograft leukemia cells and M Grunert for help with cloning strategies. JURKAT cells overexpressing XIAP were a kind gift from C Duckett, JURKAT cells overexpressing BCL-XL from C Thompson, JURKAT BAX/BAK−/− cells from H Rabinowich, JURKAT caspase-9−/− cells from K Schulze-Osthoff, plasmids containing shRNA against BID from X Jiang. This work was supported by Else Kroener Fresenius Stiftung (P45/05//A19/05//F0, to HE and IJ).
Glossary
- ALL
acute lymphoblastic leukemia
- BA
betulinic acid
- doxo
doxorubicin
- EndoG
endonuclease G
- ROS
reactive oxygen species
- VCR
vincristine
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by A Stephanou
Supplementary Material
References
- Suzuki S, Nakasato M, Shibue T, Koshima I, Tanigushi T. Therapeutic potential of proapoptotic molecule Noxa in the selective elimination of tumor cells. Cancer Sci. 2009;100:759–769. doi: 10.1111/j.1349-7006.2009.01096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Lopez H, George NM, Liu X, Pang X, Luo X. Selective involvement of BH3-only proteins and differential targets of Noxa in diverse apoptotic pathways. Cell Death Differ. 2010;18:864–873. doi: 10.1038/cdd.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labi V, Erlacher M, Kiessling S, Villunger A. BH3-only proteins in cell death initiation, malignant disease and anticancer therapy. Cell Death Differ. 2006;13:1325–1338. doi: 10.1038/sj.cdd.4401940. [DOI] [PubMed] [Google Scholar]
- Ploner C, Kofler R, Villunger A. NOXA: at the tip of the balance between life and death. Oncogene. 2009;27:S84–S92. doi: 10.1038/onc.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T, Taniguchi T. BH3-only proteins: Integrated control point of apoptosis. Int J Cancer. 2006;119:2036–2043. doi: 10.1002/ijc.21751. [DOI] [PubMed] [Google Scholar]
- Sheridan C, Brumatti G, Elgendy M, Brunet M, Martin SJ, An ERK. dependent pathway to Noxa expression regulates apoptosis by platinum-based chemotherapeutic drugs. Oncogene. 2010;29:6428–6441. doi: 10.1038/onc.2010.380. [DOI] [PubMed] [Google Scholar]
- Tonino SH, van Laar J, van Oers MH, Wang JY, Eldering E, Kater AP. ROS-mediated upregulation of NOXA overcomes chemoresistance in chronic lymphocytic leukemia. Oncogene. 2010;30:701–713. doi: 10.1038/onc.2010.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Galán P, Roué G, Villamor N, Montserrat E, Campo E, Colomer D. The proteasome inhibitor bortezomibe induces apoptosis in mantle-cell lymphoma through generation of ROS and NOXA activation independent of p53 status. Blood. 2006;107:257–264. doi: 10.1182/blood-2005-05-2091. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang L. PUMA, a potent killer with or without p53. Oncogene. 2009;27:S71–S83. doi: 10.1038/onc.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt H, Fulda S, Führer M, Debatin KM, Jeremias I. Betulinic acid-induced apoptosis in leukaemia cells. Leukemia. 2004;18:1406–1412. doi: 10.1038/sj.leu.2403406. [DOI] [PubMed] [Google Scholar]
- Fulda S. Betulinic acid: a natural product with anticancer activity. Mol Nutr Food Res. 2009;53:140–146. doi: 10.1002/mnfr.200700491. [DOI] [PubMed] [Google Scholar]
- Fulda S, Friesen C, Los M, Scaffidi C, Mier W, Benedict M, et al. Betulinic acid triggers CD95 (APO-1/Fas)- and p53-independent apoptosis via activation of caspases in neuroectodermal tumors. Cancer Res. 1997;57:4956–4964. [PubMed] [Google Scholar]
- Fulda S, Scaffidi C, Susin SA, Krammer PH, Kroemer G, Peter ME, et al. Activation of mitochondria and release of mitochondrial apoptogenetic factors by betulinic acid. J Biol Chem. 1998;273:33942–33948. doi: 10.1074/jbc.273.51.33942. [DOI] [PubMed] [Google Scholar]
- Wick W, Grimmel C, Wagenknecht B, Dichgans J, Weller M. Betulinic acid-induced apoptosis in glioma cells: a sequential requirement for new protein synthesis, formation of reactive oxygen species, and caspase processing. J Pharmacol Exp Ther. 1999;289:1306–1312. [PubMed] [Google Scholar]
- Estlin EJ, Ronghe M, Burke GA, Yule SM. The clinical and cellular pharmacology of vincristine, corticosteroids, L-asparaginase, anthracyclines and cyclophosphamide in relation to childhood acute lymphoblastic leukaemia. Br J Haematol. 2000;110:780–790. doi: 10.1046/j.1365-2141.2000.t01-1-02153.x. [DOI] [PubMed] [Google Scholar]
- Shin YG, Cho KH, Chung SM, Graham J, Das Gupta TK, Pezzuto JM. Determination of betulinic acid in mouse blood, tumor and tissue homogenates by liquid chromatography-electrospray mass spectrometry. J Chromatogr B Biomed Sci Appl. 1999;732:331–336. doi: 10.1016/s0378-4347(99)00291-1. [DOI] [PubMed] [Google Scholar]
- Gamen S, Anel A, Pérez-Galán P, Lasierra P, Johnson D, Piñeiro A, et al. Doxorubicin treatment activates a Z-VAD-sensitive caspase, which causes deltapsim loss, caspase-9 activity, and apoptosis in Jurkat cells. Exp Cell Res. 2000;258:223–235. doi: 10.1006/excr.2000.4924. [DOI] [PubMed] [Google Scholar]
- Ballestrero A, Nencioni A, Boy D, Rocco I, Garuti A, Mela GS, et al. Tumor necrosis factor-related apoptosis-inducing ligand cooperates with anticancer drugs to overcome chemoresistance in antiapoptotic Bcl-2 family members expressing jurkat cells. Clin Cancer Res. 2004;10:1463–1470. doi: 10.1158/1078-0432.ccr-1365-02. [DOI] [PubMed] [Google Scholar]
- López-Royuela N, Pérez-Galán P, Galán-Malo P, Yuste VJ, Anel A, Susín SA, et al. Different contribution of BH3-only proteins and caspases to doxorubicin-induced apoptosis in p53-deficient leukemia cells. Biochem Pharmacol. 2010;79:1746–1758. doi: 10.1016/j.bcp.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Letai AG. Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8:121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Resnick-Silverman L, Manfredi JJ. Gene-specific mechanisms of p53 transcriptional control and prospects for cancer therapy. J Cell Biochem. 2006;99:679–689. doi: 10.1002/jcb.20925. [DOI] [PubMed] [Google Scholar]
- Petitjean A, Mathe E, Kato S, Ishioka C, Tavtigian SV, Hainaut P, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28:622–629. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- Ehrhardt H, Wachter F, Maurer M, Stahnke K, Jeremias I. Important role of Caspase-8 for chemo-sensitivity of ALL cells. Clin Cancer Res. 2011;17:7605–7613. doi: 10.1158/1078-0432.CCR-11-0513. [DOI] [PubMed] [Google Scholar]
- Ehrhardt H, Schrembs D, Moritz C, Wachter F, Haldar S, Graubner U, et al. Optimized anti-tumor effects of anthracyclines plus vinca alkaloids using a novel, mechanism-based application schedule. Blood. 2011;118:6123–6113. doi: 10.1182/blood-2010-02-269811. [DOI] [PubMed] [Google Scholar]
- Ehrhardt H, Haecker S, Wittmann S, Maurer M, Borkhardt A, Toloczko A, et al. Cytotoxic drug-induced, p53-mediated upregulation of Caspase-8 in tumor cells. Oncogene. 2008;27:783–793. doi: 10.1038/sj.onc.1210666. [DOI] [PubMed] [Google Scholar]
- Greshock J, Nathanson K, Martin AM, Zhang L, Coukos G, Weber BL, et al. Cancer cell lines as genetic models of their parent histology: analyses based on array comparative genomic hybridization. Cancer Res. 2007;67:3594–3600. doi: 10.1158/0008-5472.CAN-06-3674. [DOI] [PubMed] [Google Scholar]
- Sandberg R, Ernberg I. Assessment of tumor characteristic gene expression in cell lines using a tissue similarity index (TSI) Proc Natl Acad Sci USA. 2005;102:2052–2057. doi: 10.1073/pnas.0408105102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peller S, Rotter V. TP53 in hematological cancer: low incidence of mutations with significant clinical relevance. Hum Mutat. 2003;21:277–284. doi: 10.1002/humu.10190. [DOI] [PubMed] [Google Scholar]
- Royds JA, Iacopetta B. p53 and disease: when the guardian angel fails. Cell Death Differ. 2006;13:1017–1026. doi: 10.1038/sj.cdd.4401913. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. p53: an ubiquitous target of anticancer drugs. Int J Cancer. 2002;98:161–166. doi: 10.1002/ijc.10158. [DOI] [PubMed] [Google Scholar]
- Joerger AC, Fersht AR. Structure-function-rescue: the diverse nature of common p53 cancer mutants. Oncogene. 2007;26:2226–2242. doi: 10.1038/sj.onc.1210291. [DOI] [PubMed] [Google Scholar]
- Sawada N, Kataoka K, Kondo K, Arimochi H, Fujino H, Takahashi Y, et al. Betulinic acid augments the inhibitory effects of vincristine on growth and lung metastasis of B16F10 melanoma cells in mice. Br J Cancer. 2004;90:1672–1678. doi: 10.1038/sj.bjc.6601746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer E, Pimentel E, Wacheck V, Schlegel W, Pehamberger H, Jansen B, et al. Effects of betulinic acid alone and in combination with irradiation in human melanoma cells. J Invest Dermatol. 2000;114:935–940. doi: 10.1046/j.1523-1747.2000.00972.x. [DOI] [PubMed] [Google Scholar]
- Baader E, Toloczko A, Fuchs U, Schmid I, Beltinger C, Ehrhardt H, et al. TRAIL-mediated proliferation of tumor cells with receptor-close apoptosis defects. Cancer Res. 2005;65:7888–7895. doi: 10.1158/0008-5472.CAN-04-4278. [DOI] [PubMed] [Google Scholar]
- Ehrhardt H, Fulda S, Schmid I, Hiscott J, Debatin KM, Jeremias I. TRAIL-induced survival and proliferation in cancer cells resistant towards TRAIL-induced apoptosis mediated by NFκB. Oncogene. 2003;22:3842–3852. doi: 10.1038/sj.onc.1206520. [DOI] [PubMed] [Google Scholar]
- Gao Z, Shao Y, Jiang X. Essential roles of the Bcl-2 family of proteins in caspase-2-induced apoptosis. J Biol Chem. 2005;280:38271–38275. doi: 10.1074/jbc.M506488200. [DOI] [PubMed] [Google Scholar]
- Obexer P, Geiger K, Ambros PF, Meister B, Ausserlechner MJ. FKHRL1-mediated expression of Noxa and Bim induces apoptosis via the mitochondria in neuroblastoma cells. Cell Death Differ. 2007;14:534–547. doi: 10.1038/sj.cdd.4402017. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- Höfig I, Ehrhardt H, Jeremias I. Efficient RNA interference in patientśacute lymphoblastic leukemia cells amplified as xenografts in mice. Cell Commun Signal. 2012;10:8. doi: 10.1186/1478-811X-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.