Abstract
Toll-like receptor 9 (TLR9) triggering is a promising novel strategy to combat cancer as it induces innate and adaptive immunity responses. B-cell lymphoma is unique in this context as tumor cells express TLR9 and may harbor latent Epstein-Barr virus (EBV), a gamma-herpesvirus with remarkable oncogenic potential when latent. Latent EBV may be promoted by TLR9 triggering via suppression of lytic EBV. Here, we elaborated an initial assessment of the impact of TLR9 triggering on EBV-positive and EBV-negative B-cell lymphoma using Burkitt's lymphoma (BL) cell lines as an in vitro model. We show that, independent of the presence of EBV, the TLR9 ligand oligodeoxynucleotide (ODN) CpG-2006 may or may not induce caspase-dependent cell death in BL cells. Moreover, ODN CpG-2006-induced cell death responses of BL cells were associated with TLR9 single-nucleotide polymorphisms (SNPs) rs5743836 or rs352140, which we detected in primary BL tumors and in peripheral blood from healthy individuals at similar frequencies. Thus, our findings suggest that the effect of TLR9 agonists on BL cells should be tested in vitro before installment of therapy and TLR9 SNPs in BL patients should be determined as potential biological markers for the therapeutic response to treatment targeting innate immunity.
Keywords: Burkitt's lymphoma, Epstein-Barr virus, Toll-like receptor TLR 9 agonists, polymorphism, CpG
Toll-like receptors (TLRs) are important players of the innate immune system,1 and expression of the 10 TLRs known in humans2, 3 depends on the cell subset and differentiation status.4, 5, 6 TLR9 is preferentially expressed by B cells and plasmacytoid dendritic cells (reviewed in reference Iwasaki and Medzhitov1). Synthetic TLR9 ligands, short oligodeoxynucleotides (ODN) with unmethylated CpG motifs,7 activate TLR9 that recruits the adapter protein myeloid differentiation factor 88 (MyD88) and induces a cascade leading to the nuclear translocation of the transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB).2
The immune response following TLR9 engagement is highly desirable for cancer treatment.8 Synthetic TLR9 agonists are regarded as potential anti-cancer agents. Clinical trials have shown promising results for the treatment of various cancer types with CpG ODNs.9 Their effects can be indirect by enhancing the anti-tumor immune response or direct by inducing apoptosis in the malignant cells. Direct effects would be expected for B-cell malignancies as they express TLR9. B-cell activation can eventually lead to activation-induced cell death of cancer cells and therefore support anti-cancer treatment.10 Nevertheless, the TLR9 agonist effects vary strongly between B-cell cancer types, and the responses of B-cell lymphomas to ODN CpG-2006 show high variability.11 Thus, the effects of CpG ODNs on B-cell malignancies are not predictable. Moreover, CpG ODN treatment has tumor-promoting effects on benign B cells and strongly enhances their proliferation and differentiation.12, 13, 14
Stimulation of TLR9 with ODN CpG-2006 suppresses lytic reactivation of Epstein-Barr virus (EBV), a B-cell tropic gamma-herpesvirus, and can thereby promote latent EBV.15, 16 The latter is associated with several types of B-cell lymphomas including Burkitt's lymphoma (BL).17 EBV is present in nearly all cases of the high-incidence form of BL (‘endemic BL'), in up to 85% of the intermediate-incidence cases, and in 15% of the low-incidence cases (‘sporadic' BL). Up to 40% of BL in human immunodeficiency virus carriers harbor EBV.17 As latent EBV exhibits unique growth transformation potential on B cells in vitro18 and TLR9 triggering enhances EBV-induced B-cell transformation19 and promotes latent EBV in vitro,15, 16 TLR9 stimulation in the treatment of EBV-positive BL could provoke detrimental rather than beneficial outcomes.
Single-nucleotide polymorphisms (SNPs) of TLR9 are associated with increased risk for certain B-cell lymphomas20 and have the potential to exhibit deregulation of signaling thereby promoting tumorigenesis. The responses of TLR9 SNPs in B-cell tumors to CpG ODN treatment are not reported.
Here, we used BL-cell lines to model direct effects of TLR9 stimulation on malignant cells, investigate the influence of EBV, and assess the impact of TLR9 SNPs, which we found in primary BL samples or in healthy primary cells.
Results
TLR9 triggering alters gene expression and activates Akata cells in a MyD88-dependent manner
CpG ODNs activate B cells by impacting on gene expression.21 We asked how the gene expression pattern of BL cells is affected by TLR9 triggering using CpG ODN. We performed a microarray analysis comparing ODN CpG-2006-treated versus untreated Akata cells. Most of the ≥2-fold changes in gene expression were upregulation and only few downregulation. Among upregulated genes, we were mainly interested in those involved in cytokine (human interleukin 10; hIL-10) and chemokine expression (CXCL10), B-cell activation (CD40), transcription (NF-κB) and apoptosis (FAS) as these genes are those that preferentially determine cell proliferation and survival (Supplementary Table 1). Thirteen genes, including two regulating cell cycle, one transcription factor, one involved in B-cell differentiation and apoptosis, four of diverse functions and four of unknown function, showed downregulation following ODN CpG-2006 treatment (Supplementary Table 2).
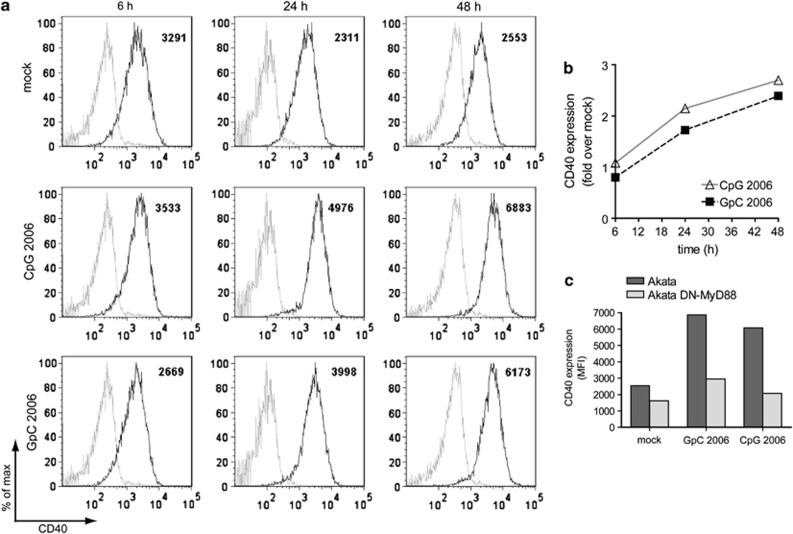
We earlier confirmed that TLR9 triggering of Akata cells with ODN CpG-2006 increases hIL-10 expression and leads to the translocation of NF-κB to the nucleus.16 To validate a marker for B-cell activation, in this context, we measured CD40 expression by flow cytometry. CD40 expression was increased upon ODN CpG-2006 treatment (Figure 1a). After 48 h, CD40 expression on ODN CpG-2006-treated cells was increased 2.7-fold compared with untreated cells (Figures 1a and b). Surprisingly, the control ODN GpC-2006, which is similar to ODN CpG-2006 but lacks the CpG motifs, also led to a 2.4-fold increase in CD40 expression (Figures 1a and b). This might reflect a TLR9-independent mechanism or the ability of the control ODN to stimulate TLR9 in BL cells.
Figure 1.
TLR9 agonists induce CD40 expression in Akata cells in a MyD88-dependent manner. Akata cells or DN-MyD88 Akata cells were left untreated or treated with 0.5 μM ODN CpG-2006 or ODN GpC-2006 for 48 h. After 6, 24 and 48 h, 105 cells were harvested, washed with PBS and stained with PE-Cy5-conjugated mouse anti-human CD40 antibody or a PE-Cy5 mouse IgG1κ isotype control and analyzed with a flow cytometer. (a) FACS plots of Akata cells. Isotype control: shaded gray line; anti-CD40 PE-Cy5: black line. Numbers indicate the mean fluorescence intensity (MFI) (b) Quantification of (a). (c) Comparison of CD40 expression in Akata cells and DN-MyD88 Akata cells
To clarify this point, we used Akata cells overexpressing a dominant-negative mutant of the adaptor protein MyD88 (DN-MyD88).16 Treatment with ODN CpG-2006 or ODN GpC-2006 induced increased CD40 expression, corroborating our first observation (Figure 1c). By contrast, neither ODN CpG-2006 nor ODN GpC-2006 significantly induced an increase in CD40 expression in DN-MyD88 Akata cells (Figure 1c). Finally, we validated the ODN CpG-2006 treatment-induced upregulation of STAT3 (Supplementary Table 1) by quantitative real-time polymerase chain reaction (qRT-PCR) and confirmed activation of STAT3 by western blot (data not shown).
Collectively, we validated our microarray data showing that triggering with ODN CpG-2006 activates Akata cells to induce CD40 and STAT3 expression. Moreover, our results indicated that both ODNs used here act in a MyD88-dependent manner and that the ODN lacking CpG motifs activates Akata cells to a similar extent as the ODN containing CpG motifs.
TLR9 triggering by CpG ODN does not impact on the cell cycle of Akata cells
TLR9 triggering leads to cell cycle entry and proliferation of B chronic lymphatic leukemia cells.11, 12, 22 We analyzed the cell cycle of mock-treated or ODN CpG-2006-treated Akata cells after initiation of treatment by propidium iodide staining and flow cytometry. Most cells were in the S phase during the time frame of 48 h (Supplementary Figure 1). Treatment with ODN CpG-2006 did not alter the cell cycle compared with mock treatment. Similar results were obtained with Akata31 cells (data not shown), an EBV-negative subclone of Akata cells.23 Thus, although TLR9 triggering by ODN CpG-2006 resulted in BL-cell activation based on increased expression of certain genes, it did not seem to influence the cell cycle.
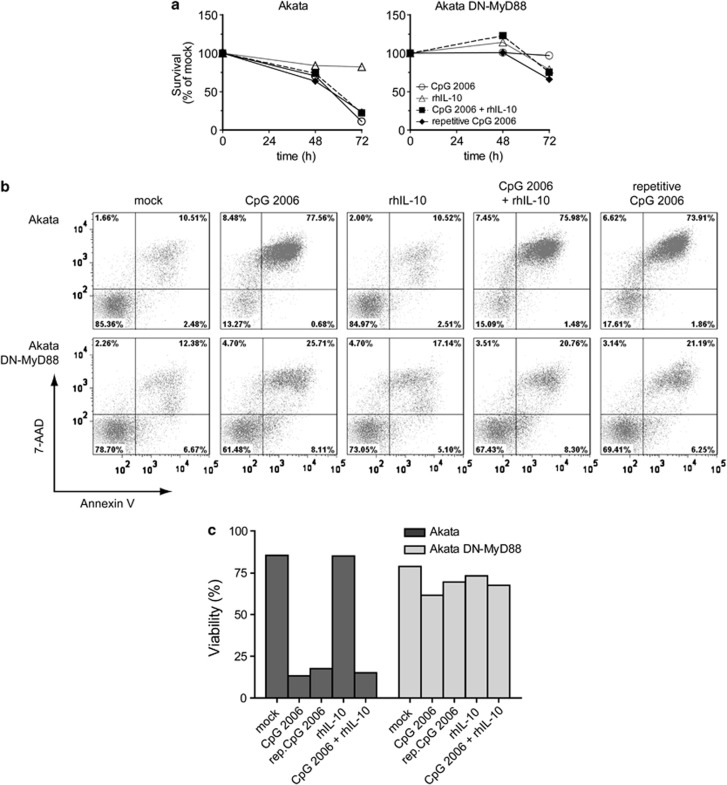
ODN-induced cell death of BL Akata cells is MyD88-dependent
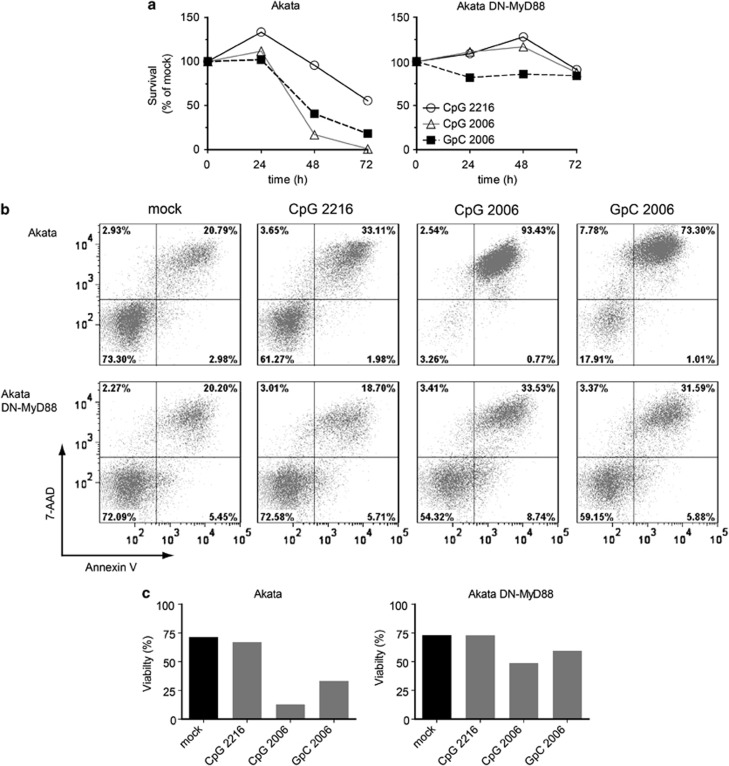
Treatment with ODN GpC-2006 unexpectedly induced CD40 upregulation in Akata cells in a MyD88-dependent manner similar to treatment with ODN CpG-2006. We determined whether treatment with distinct TLR9 ligands would similarly result in a MyD88-dependent effect on cell survival. Also, we explored whether a class A ODN, that displays low specificity for B cells, exerts similar effects on BL cells as class B ODNs that possess a high specificity for B cells. Therefore, we treated Akata cells or DN-MyD88 Akata cells with ODN CpG-2216 (type A), ODN CpG-2006 (type B) and ODN GpC-2006 (type B control), respectively, and analyzed the viability of the cells by Trypan Blue exclusion assay and PE-Annexin V/7-amino-actinomycin (7-AAD) staining. The Trypan Blue exclusion assay showed that treatment of Akata cells with ODN CpG-2006 or ODN GpC-2006 reduced the survival of cells within 48 h to below 50% of not treated cells and treatment with ODN CpG-2216 to almost 50% of not treated cells after 72 h (Figure 2a). By contrast, the survival of DN-MyD88 Akata cells was not, if at all, reduced by treatment with any of the three ODNs for 72 h (Figure 2a). These findings were corroborated by the Annexin V/7-AAD assay revealing that the viability of Akata cells was drastically affected by treatment with class B ODNs and to a lesser extent with class A ODN, contrasting DN-MyD88 Akata cells that were not affected by treatment with ODNs for 72 h (Figure 2b and quantification in Figure 2c). Thus, treatment of Akata cells with ODNs CpG-2006 and GpC-2006 triggers TLR9 signaling via MyD88 and results in cell death.
Figure 2.
The cell death induced by TLR9 agonists is MyD88 dependent. Akata cells and DN-MyD88 Akata cells were incubated with medium only, ODN CpG-2216, ODN CpG-2006 or ODN GpC-2006. (a) The cell survival was assessed by Trypan Blue exclusion assay 0, 24, 48 and 72 h after treatment. (b) Apoptosis assay after 72 h of treatment. 105 cells were stained with PE-Annexin V/7-AAD and analyzed by flow cytometry. The double-negative population represents viable cells whereas PE-Annexin V-positive or double-positive populations represent non-viable cells. (c) Quantification of the Annexin V/7-AAD assay in (b). Shown are the results of a representative experiment of two experiments (showing mean values)
ODN-induced cell death of BL Akata cells is caspase-dependent
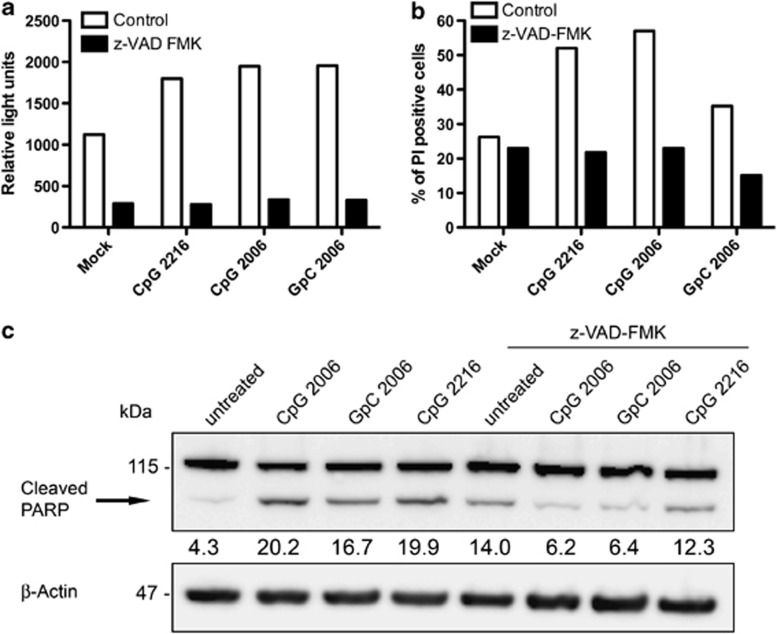
To explore the mechanism of ODN-induced cell death of Akata cells we set out to investigate whether caspases were involved. Thus, the activity of caspase-3 and -7 in ODN-treated Akata cells was detected using a luminogenic substrate, which gives rise to a luminescent signal proportional to caspase-3/7 activity. Triggering with TLR9 ligands for 48 h increased the luminescent signal about twofold as compared with no triggering. This increase was abolished by treatment with the caspase inhibitor z-VAD-FMK (Figure 3a), indicating that TLR9 triggering resulted in increased caspase activity in Akata cells and that this activity was not increased if cells were concomitantly treated with a caspase inhibitor. These findings were corroborated using PI staining, which indicate that Akata cell death induced by treatment with ODNs can be abrogated by the caspase inhibitor z-VAD-FMK (Figure 3b); and by western blotting showing that PARP cleavage is increased by ODN treatment and can be reduced by the inhibitor (Figure 3c). Thus, ODN-induced cell death of Akata cells was found to be caspase-3/7 dependent and therefore due to apoptosis.
Figure 3.
TLR9 ligands induce cell death of Akata cells via caspase activation. (a) Caspase activity in 0.5 × 106 Akata cells measured 48 h after treatment with the TLR9 ligands ODN CpG-2006, ODN GpC-2006 and ODN CpG-2216 without or with z-VAD-FMK. Cells were incubated with the Caspase-Glo luminogenic substrate to detect activated caspases and analyzed with a luminometer. Results represent the relative light units generated by active caspases 3 and 7. Shown in the bar graph are the results of a representative experiment out of three performed in duplicate. (b) Treatment of Akata cells with caspase inhibitor z-VAD-FMK reduces TLR9 ligand-induced cell death. 106 Akata cells were treated with the TLR9 ligands ODN CpG-2006, ODN GpC-2006 or ODN CpG-2216 with or without addition of z-VAD-FMK. After 48 h, cells were stained with PI and analyzed by flow cytometry. Shown are the results of a representative experiment out of two experiments performed in duplicate. (c) Treatment of Akata cells with TLR9 ligand induces PARP cleavage. Lysates from 106 Akata cells 48 h after incubation with the TLR9 ligands ODN CpG-2216, ODN CpG-2006 and ODN GpC-2006 with or without z-VAD-FMK were analyzed by immunoblotting for PARP cleavage. The relative density of the cleaved PARP was calculated using densitometry and normalized to β-actin
Survival of distinct BL cells following treatment with CpG ODNs differs considerably, independently of the presence of EBV
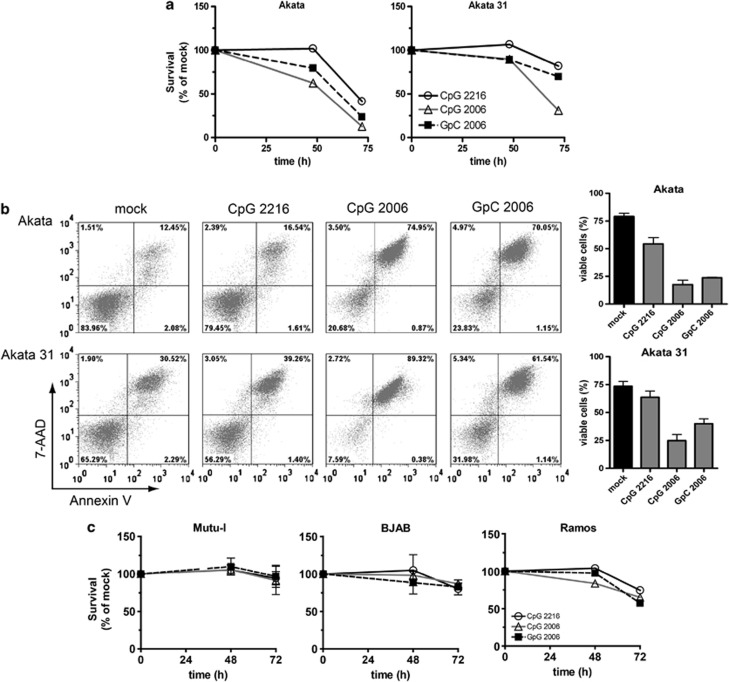
ODN CpG-2006 suppresses EBV lytic gene expression in Akata cells and primary B cells promoting latent EBV that may provide cell survival signals.15, 16 As we observed Akata cell death upon TLR9 triggering, we asked whether the absence of EBV would result in even more pronounced cell death following TLR9 triggering. Hence, we tested whether TLR9 agonists affect the survival of EBV-positive Akata cells and of EBV-negative Akata31 cells in a similar fashion. Treatment with ODN CpG-2006 or ODN GpC-2006 strongly decreased the percentages of surviving Akata cells or Akata 31 cells between 24 and 72 h compared with untreated cells. Nevertheless, the effect on Akata 31 cells was less marked (Figure 4a). The percentages of apoptotic Akata 31 cells assessed by Annexin V/7-AAD staining after 72 h of treatment, however, were comparable to those of Akata cells (Figure 4b). Treatment with ODN CpG-2216, ODN CpG-2006 or ODN GpC-2006 resulted in similar respective decreases in viability of Akata cells versus Akata31 cells due to apoptosis. Treatment with class A ODN CpG-2216 exhibited almost no negative impact on cell survival compared with treatment with the class B ODNs CpG-2006 and GpC-2006 (Figure 4b). These results showed that EBV-negative Akata31 cells exhibited a higher level of spontaneous cell death by apoptosis compared with EBV-positive Akata cells and corroborated the lower effect of TLR9 triggering by class A ODN compared with triggering by class B ODNs. Moreover, the results suggested that the presence of EBV does not prevent EBV-positive Akata cells from TLR9 triggering-induced cell death compared with EBV-negative Akata31 cells.
Figure 4.
TLR9 agonists induce cell death of Akata cells and Akata31 BL cells, but not of Mutu-I BL cells or BJAB BL cells, while Ramos BL cells are intermediately affected. 106 cells were treated with the TLR9 ligands ODN CpG-2216, ODN CpG-2006 and ODN GpC-2006. (a) Survival of untreated, ODN CpG-2006-treated or ODN GpC-2006-treated Akata cells and Akata 31 BL cells was assessed by Trypan Blue exclusion assay and counting of viable and dead cells and compared with the untreated control. (b) Apoptosis of Akata and Akata 31 BL cells after 72 h of treatment. 105 cells were stained with PE-Annexin V/7-AAD and analyzed by flow cytometry (left panel); percentages of PE-Annexin V/7-AAD negative cells (right panel). (c) The survival of untreated, ODN CpG-2006 or ODN GpC-2006-treated Mutu-I, BJAB and Ramos BL cells was evaluated by Trypan Blue exclusion. Shown are the results of a representative experiment out of at least two experiments performed in duplicate (showing mean values) or in triplicate (showing mean values±S.E.M., indicated by error bars)
Next, we investigated whether our observations in EBV-positive versus EBV-negative BL cells hold true in BL cells other than Akata. To this end, we used EBV-positive BL cells Mutu-I and EBV-negative BL cells BJAB and Ramos. Treatment with ODN CpG-2006 or with ODN GpC-2006 did not affect Mutu-I cell or BJAB cell survival (Figure 4c) but it decreased Ramos cell survival between 48 and 72 h (Figure 4c). Thus, the survival of BL cells following TLR9 triggering considerably differs between distinct BL cells and does not seem to depend on the presence or absence of EBV.
Exogenous hIL-10 does not prevent ODN CpG-2006-induced cell death
hIL-10 has an important role in B-cell lymphoma biology as it seems to act as autocrine growth factor.22, 24, 25, 26 We previously reported 16 and corroborate here, a strong hIL-10 induction in Akata cells following TLR9 triggering with ODN CpG-2006 (Supplementary Table 1). Nevertheless, the very same treatment resulted in drastic Akata cell death (Figure 2), suggesting that the latter could not be prevented by the induction of hIL-10 expression. Otherwise, a recent report suggested that treatment with ODN CpG-2006-induced hIL-10 in chronic lymphocytic leukemia B cells mediating their cell death.10 Therefore, we treated Akata cells and DN-MyD88 Akata cells with ODN CpG-2006, recombinant hIL-10 (rhIL-10) or a combination thereof. The concentration of rhIL-10 was based on the peak hIL-10 protein concentration induced by ODN CpG-2006 treatment in Akata cells.16 Additionally, ODN CpG-2006 treatment was reiterated at 48 h in a fraction of BL cells.
As expected, treatment with ODN CpG-2006 for 72 h reduced the viability of Akata cells to <20% but did not reduce viability of DN-MyD88 Akata cells compared with no treatment (Figures 5a–c). Reiterated treatment with ODN CpG-2006 after 48 h did not further reduce viability of Akata cells compared with one-time treatment and did not reduce viability of DN-MyD88 Akata cells (Figure 5a–c). Treatment with rhIL-10 for 72 h also did not reduce viability of Akata cells or DN-MyD88 Akata cells compared with no treatment. Treatment with rhIL-10 did not counteract ODN CpG-2006 treatment-induced reduction of BL cell viability or further decreased their viability. DN-MyD88 Akata cells were not affected by any of the treatments (Figures 5a–c). Thus, maximal negative impact of ODN CpG-2006 on cell survival in Akata cells occurred after one treatment. Moreover, rhIL-10 did not change the number of viable Akata cells, or rescue Akata cells from ODN CpG-2006 treatment-induced cell death. This suggested that hIL-10 cannot counteract TLR9 triggering-induced cell activation resulting in death of Akata cells and is not responsible for their cell death.
Figure 5.
hIL-10 does not influence ODN CpG-2006-induced cell death. Akata cells and DN-MyD88 Akata cells were incubated with medium only, 0.5 μM ODN CpG-2006, 900 pg/ml rhIL-10 or combination of ODN CpG-2006 and rhIL-10. To one ODN CpG-2006-stimulated sample, 0.5 mM ODN CpG-2006 was added again after 48 h (rep. CpG-2006). (a) The cell viability was monitored by the Trypan Blue exclusion test 0, 48 and 72 h after treatment. (b) After 72 h cells were harvested and 105 cells were stained with PE-Annexin V/7-AAD and analyzed by flow cytometry. (c) Quantification of (b)
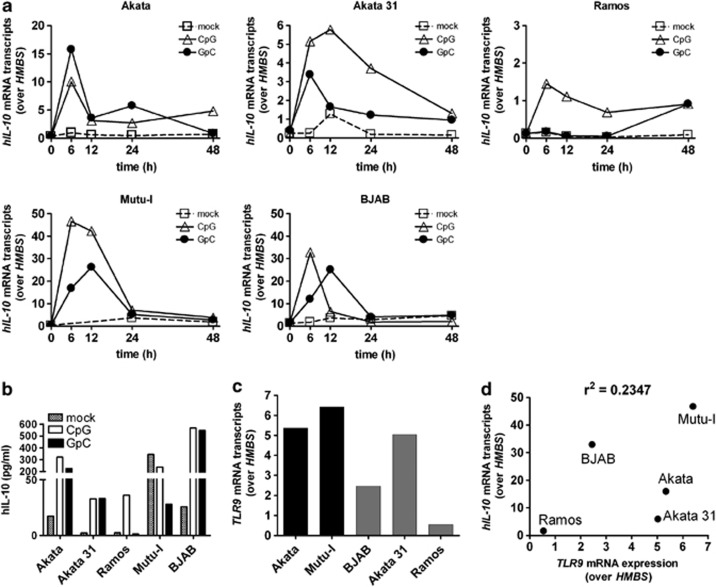
BL cells express distinct hIL-10 mRNA and hIL-10 levels following TLR9 triggering
The distinct survival of BL cells following TLR9 triggering with ODN CpG could be because of distinct magnitudes of ensuing cellular responses. The induction of hIL-10 following ODN CpG treatment can be regarded as a surrogate marker for the magnitude of cell activation mediated by TLR9 triggering. Thus, we assessed hIL-10 mRNA expression in BL cells treated with ODN CpG-2006 or ODN GpC-2006. All five BL cell lines tested expressed hIL-10 mRNA following treatment with ODN CpG-2006, the values peaking preferentially at 6 h post treatment (Figure 6a). Peak hIL-10 mRNA expression levels varied from 1.3 transcripts over HMBS in Ramos BL cells to 48 transcripts in Mutu-I BL cells. Treatment with ODN GpC-2006 also resulted in induction of hIL-10 mRNA expression but in lower levels, except in Akata cells. Similar variation of results was observed when analyzing hIL-10 protein levels (Figure 6b). Thus, TLR9 triggering of distinct BL cells resulted in a broad range of hIL-10 mRNA and hIL-10 protein expression levels, and the magnitude of the level seemed independent of EBV status.
Figure 6.
Treatment of BL cell lines with TLR9 agonists induces distinct hIL-10 mRNA and hIL-10 expression levels. (a) TLR9 triggering results in a wide range of hIL-10 mRNA expression levels. BL cells were left untreated or treated with 0.5 μM ODN CpG-2006 or ODN GpC-2006 for the indicated time points. (b) hIL-10 protein expression levels in the culture supernatants of BL cell lines 6 h after treatment or no treatment as in (a) measured by ELISA. (c) TLR9 mRNA expression levels differ between BL cell lines by up to sixfold. EBV-positive (black bars) and EBV-negative (gray bars) BL cells. (d) The hIL-10 mRNA peak expression responses to ODN CpG-2006 did not correlate with the corresponding relative TLR9 mRNA levels. Total RNA of 106 cells was isolated and transcribed into cDNA. HMBS, hIL-10 or TLR9 mRNA expression levels were determined by qRT-PCR. The Ct values for hIL-10 and TLR9 were normalized to the Ct values of the housekeeping gene HMBS. Maximal values of relative hIL-10 mRNA expression were correlated with the respective relative TLR9 mRNA expression using a two-tailed Pearson's correlation. Shown are the results of a representative experiment out of at least two experiments performed in duplicate (showing mean values)
Next, we determined TLR9 mRNA expression levels in BL cells by qRT-PCR. Although distinct BL cells showed up to sixfold different relative TLR9 mRNA expression levels (Figure 6c), ODN CpG-2006 treatment-induced hIL-10 mRNA peak expression levels did not correlate with the relative TLR9 mRNA expression levels (Figure 6d). Collectively, TLR9 triggering in distinct BL cells resulted in distinct hIL-10 mRNA expression, not linked to presence or absence of EBV, and not correlated to TLR9 mRNA expression levels.
TLR9 polymorphisms of BL cells might correlate with distinct responses to TLR9 triggering
TLR9 polymorphisms in patients are linked to different outcomes of inflammatory diseases and the development of cancer.27, 28 We hypothesized that TLR9 polymorphisms could be a possible explanation for our observations. Thus, we isolated the genomic DNA from BL cell lines and analyzed it for the presence of TLR9 polymorphisms. We found specific SNPs for each cell line, allowing segregation of the BL cell lines into three groups (Table 1): −1237 TT/1635 GA (Akata and Akata 31), −1237 TT/1635 GG (Ramos) and −1237 TC/1635 GG (Mutu-I and BJAB). This sub-grouping correlated with the degree of BL-cell survival in response to ODN treatment (Figure 4c), suggesting that the distinct BL-cell responses to TLR 9 triggering may depend on the SNPs present.
Table 1. TLR9 single-nucleotide polymorphisms in the five investigated BL-cell lines.
|
Single-nucleotide polymorphism |
|||
|---|---|---|---|
| BL cell | Epstein-Barr | rs5743836 | rs352140 |
| line | virus | −1237 T/C | 2848 G/A (P545P) |
| Akata | Positive | T/T | G/A |
| Akata 31 | Negative | T/T | G/A |
| Ramos | Negative | T/T | G/G |
| Mutu-I | Positive | T/C | G/G |
| BJAB | Negative | T/C | G/G |
Next, we analyzed TLR9 SNPs in genomic DNA from primary BLs or blood from healthy individuals. Indeed, we found the TLR9 SNPs detected in our BL cell lines in primary BL samples and blood of healthy individuals (Table 2). Importantly, the TLR9 SNP frequencies were statistically not significantly different between BL cell lines, primary BLs and blood from healthy individuals, respectively. This suggested that if the direct response to TLR9 agonists depends on the TLR9 SNP, this could be an important factor regarding the treatment of BL patients by TLR9 triggering.
Table 2. Frequencies of TLR9 single-nucleotide polymorphisms in five BL-cell lines, 15 primary BL tumors and blood samples from 402 healthy individuals.
| Single-nucleotide polymorphism | ||||
|---|---|---|---|---|
|
rs5743836 −1237 T/C |
rs352140 2848 G/A (P545P) |
BL cell lines N |
BL tumors N (%) |
Healthy individuals N (%) |
| T/T | G/G | 1 | 4 (27) | 178 (44) |
| T/T | G/A | 2 | 5 (33) | 165 (41) |
| T/T | A/A | — | 2 (13) | — |
| T/C | G/G | — | — | 1 (0) |
| T/C | G/A | 2 | 1 (7) | 55 (14) |
| T/C | A/A | — | 3 (20) | 5 (1) |
The frequencies of the polymorphisms were statistically not significantly different
Discussion
We analyzed the effects of TLR9 agonists on BL cell lines as an in vitro model for B-cell tumor. We found that treatment with TLR9 ligands induces distinct cytokine expression and cell death responses in distinct BL cells. Cell death was (i) dependent on TLR9-MyD88 signaling, (ii) occurred concomitantly with activation and could be suppressed by pan caspase inhibitors, (iii) was not dependent on the presence or absence of EBV in the tumor cells and (iv) was associated with SNPs in the TLR9 gene. Our results suggest that individualized in vitro pretesting of BL responses to CpG ODNs could help to predict the outcome of therapeutic TLR9 triggering and tailor adjuvant molecular treatment of BL.
Our observation that BL cells of different origin show distinct cell survival following TLR9 triggering is novel. We previously demonstrated that TLR9 triggering counteracts lytic EBV reactivation in BL cells and promotes latent EBV that is associated with B-cell lymphoproliferation.16 Here, we observed CpG type B ODN-induced cell death by apoptosis in EBV-positive Akata and EBV-negative Akata31 cells, but not or to a much lower extent in the other EBV-positive or EBV-negative BL cell lines tested. Thus, the EBV status of the tumor cells does not rule the responses of BL cells to TLR9 triggering.
TLR9-triggered BL cells consistently upregulated hIL-10 expression. hIL-10 influences the development and growth of B cells29 and acts as an autocrine growth factor for different B-cell lymphomas.25, 26, 30 Importantly, here, rhIL-10 did neither prevent nor enhance cell death induced by TLR9 triggering. hIL-10, as an anti-inflammatory cytokine, inhibits the Th1 immune response31 and this effect would be detrimental in cancer therapy that is based on intact or enhanced immune responses,9 as an unintended proliferation of the malignant cells could be provoked. Thus, the function of CpG type B ODN-induced hIL-10 expression in BL cells seems to considerably differ from that in chronic lymphocytic leukemia B cells that were reported to undergo hIL-10-mediated apoptosis.10
Surprisingly, ODN GpC-2006 induced cell death to the same extent as ODN CpG-2006. ODN GpC-2006 binds to TLR9, but in contrast to CpG-2006 it does not lead to TLR9 signaling in HEK293 cells or to changes in the secondary structure of the ectodomain.32 Therefore, we examined whether downstream signaling of TLR9 via MyD88 is involved in the induction of cell death. Indeed, experiments using Akata cells overexpressing a MyD88 dominant-negative mutant indicated that both ODNs induce cell death of BL cells in a MyD88-dependent manner. Others demonstrated that ODNs induce cell death independently of the CpG motif, of TLR933 or even of the whole-ODN sequence.34 Another pathway that is triggered by CpG ODNs, but is TLR9-independent, was described in monocytes and involves the activation of the Src family kinases Lyn and Hck.35 In our experiments, BL cell death induced by TLR9 triggering correlated with increased caspase activity and was caspase-dependent, thus most likely due to apoptosis.
The BL cell lines tested differed in their TLR9 mRNA expression level and TLR9 SNPs. mRNA expression did not correlate with cell death responses to TLR9 triggering, but the distinct TLR9 SNPs did. The role of TLR9 SNPs in BL is unknown. To start to investigate this we analyzed the frequencies of given SNPs in BL patients and healthy individuals and found them to be similar. A recent report suggests that the rs5743836 polymorphism, which was also detected here, confers an increased risk for non-Hodgkin lymphoma in people from Portugal and Italy but not from the United States.20 The association of the C allele rs5743836 with lack of cell death upon TLR9 triggering observed here in the Mutu-I and BJAB BL cell lines is striking. It does not allow establishing a causal link, but justifies the hypothesis that the distinct cell death responses upon CpG ODN treatment in BL cells may be linked to TLR9 SNPs. Notably, the C allele of rs5743836 exhibits greater NF-κB-binding affinity because of an additional NF-κB transcriptional binding site that may lead to increased production of proinflammatory cytokines.36 Hence, the presence of the C allele seems to result in enhanced NF-κB activation following TLR9 triggering. This may lead to protection of ODN CpG treatment-induced apoptosis that can be observed in BL cells without the C allele.
In conclusion, therapeutic TLR9 triggering appears to be a double-edge sword that may induce apoptosis, or enhance lymphoproliferation. Our findings suggest that the effect of TLR9 agonists on BL cells should be tested in vitro before installment of therapy and that TLR9 SNPs in BL patients should be evaluated as potential biological markers for the response to treatment targeting innate immunity.
Materials and Methods
Cell lines
The EBV-positive BL cell lines Akata and Mutu-I, and the EBV-negative BL cell lines Akata 31, BJAB and Ramos were grown in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, streptomycin (100 mg/ml), penicillin (100 U/ml) and L-glutamine (2 mM). EBV-positive BL Akata cells expressing a dominant-negative MyD88 (DN-MyD88 Akata)16 were grown in the same medium supplemented with 0.4 mg/ml G418 (Promega, Mannheim, Germany).
Primary BL tissue samples and peripheral blood from healthy individuals
BLs were retrieved from the database of the Institute of Surgical Pathology, University Hospital of Zurich (PathoPro Software, Institute of Medical Software, Saarbrücken, Germany). The BL diagnosis was performed according to the WHO classification of tumors of hematopoietic and lymphoid tissues.37 DNA was extracted from paraffin-embedded whole-tissue sections, according to standard procedures. This study was in accordance with Swiss laws and approved by the official authorities of the ethical committee of the Canton of Zurich (StV2-2007). Peripheral blood was collected from 402 healthy blood donors (aged 19–70 years) who were randomly selected according to the criteria of the Swiss Red Cross.38
Single-nucleotide polymorphism analysis
Whole-genomic DNA was extracted with the DNeasy Blood and Tissue Kit (Qiagen, Hombrechtikon, Switzerland) according to the manufacturer's instructions. Genotyping of the TLR9 polymorphisms –1237 T/C (rs5743836) and 2848 G/A (rs352140) was done by tetra-primer assays as previously described.39
Treatment with TLR9 agonists
Cells were split to a density of 0.5 × 106 cells/ml. The TLR9 ligands ODN CpG-2216 (type A), ODN CpG-2006 (type B) and ODN GpC-2006 (type B control) were applied at concentrations of 0.5 μM. ODN (LabForce-InvivoGen, Nunningen, Switzerland) sequences: ODN CpG-2216: 5′-ggGGGACGATCGTCgggggg-3′, bases are phosphodiester (capital letters) or phosphorothioate (lower case); ODN CpG-2006: 5′-tcgtcgttttgtcgttttgtcgtt-3′, full phosphorothioate backbone; ODN GpC-2006: 5′-tgctgcttttgtgcttttgtgctt-3′, full phosphorothioate backbone.
Microarray analysis
Akata cells were mock treated or treated with CpG-2006, and harvested 6 h later. Total RNA was isolated with RNeasy mini kit (Qiagen) according to the manufacturer's instructions. Microarray analysis was performed at the Functional Genomic Center Zurich (University of Zurich, Zurich, Switzerland). Human Exon 1.0 ST Array chips (Affymetrix, Santa Clara, CA, USA) and Genespring GX software (Agilent Technologies, Basel, Switzerland) were used for the analysis. An at least twofold change in gene expression (upregulation or downregulation) in the treated versus the mock-treated sample was regarded as significant.
qRT-PCR (Taqman)
Total RNA was extracted from 106 cells with the RNeasy Kit (Qiagen) and DNA was removed with DNA-free (Ambion Europe, Huntingdon, Cambridgeshire, UK). 1 μg of the purified RNA was used to generate cDNA with the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Rotkreuz, Switzerland). Quantitative real-time PCR was performed with specific Taqman primers and probes on ABI 7200 (Applied Biosystems). Data were analyzed with the software SDS2.2 (Applied Biosystems). Delta cycle threshold (Ct) values for the respective genes were normalized to HMBS.
Staining for surface antigens
105 cells were stained with FITC mouse anti-human IgM (Clone G20-127), PE-Cy5 mouse anti-human IgG (Clone G18-145), PE mouse anti-human IgD (Clone IA6-2) or the respective isotype controls, FITC IgG1κ isotype control (Clone MOPC-21), PE-Cy5 mouse IgG1κ isotype control (Clone MOPC-21), PE mouse IgG2aκ isotype control (Clone G155-178) or PE-Cy5 anti-human CD40 (Cat. 555590) (all from BD Biosciences, Allschwil, Switzerland) for 30 min at 4 °C in the dark. Cells were counted with a flow cytometer (FACSCanto II, BD Biosciences).
Annexin V/7-AAD staining
To assess apoptotic, necrotic or dead and viable cells, 105 cells were harvested, washed with phosphate-buffered saline (PBS) and resuspended in 100 μl Annexin V binding buffer (140 mM NaCl, 2.5 mM CaCl2, 10 mM HEPES, pH 7.4) and incubated with 5 μl Annexin V and 5 μl 7-AAD (BD Biosciences) for 15 min at room temperature in the dark. After addition of 200 μl Annexin V binding buffer, cells were analyzed with the FACSCanto II flow cytometer (BD Biosciences).
Propidium Iodide staining
To discern between dead and viable cells, treated cells were stained with 50 μg/ml PI (Sigma-Aldrich Chemie GmbH, Buchs, Switzerland) for 15 min at room temperature and then analyzed with the FACSCanto II flow cytometer (BD Biosciences).
Protein isolation, quantification and Western blot
Total protein lysates were obtained after lysing the cells in RIPA complete buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 2 mM EDTA, 1% NP40 complemented with 0.1% SDS, 1 × EDTA-free protease inhibitor cocktail (Roche, Rotkreuz, Switzerland)). Cell extracts were passed 10 times through a 25-G syringe. Protein content was determined using the Pierce BCA Protein Assay Kit (ThermoScientific, Erembodegem, Belgium), according to the manufacturer's instructions. To analyze protein expression by western blot, protein (20 μg/well) was loaded into a NuPAGE 4–12% Bis-Tris Gel (life technologies, Zug, Switzerland) and subjected to SDS-PAGE electrophoresis, transferred electrophoretically to a nitrocellulose membrane (GE Healthcare, Glattbrugg, Switzerland), incubated with rabbit anti-PARP antibody or rabbit anti-β-Actin antibody, and subsequently with anti-rabbit IgG, HRP-linked antibody (all diluted 1:1000 and from, Cell Signaling Technology (Allschwil, Switzerland). The signal was detected with the ECL Western Blotting Detection Reagents (GE Healthcare) and imaged using the LAS-3000 (Fujifilm, Dielsdorf, Switzerland) and image reader LA-3000 (Fujifilm).
hIL-10 ELISA
hIL-10 protein concentrations were determined in supernatants from TLR9-stimulated cultures by standard capture ELISA (Ready-SET-Go, eBioscience, Vienna, Austria) according to the manufacturer's instructions Plates were read at 450 nm once the substrate had developed, and cytokine concentration was determined by extrapolation from the standard curve.
Caspase-3/7 activity
Activity of caspase-3/7 was assessed with the Caspase-Glo 3/7 Assay according to the manufacturer's protocol (Promega, Wallisellen, Switzerland). Cells, dispensed at 0.5 × 106 cells/ml/well in a 24-well plate were treated with TLR9 ligands and incubated at 37°C for 48 h. At the end of the incubation period, 100 μl of Caspase-Glo 3/7 reagent was added to 100 μl cell suspension in a white-walled 96-well plate and incubated at room temperature for 1 h. Luminescence intensity was determined using a Synergy HT Multi-Detection Microplate Reader (BioTek, Luzern, Switzerland).
Treatment of BL cells with rhIL-10
One day before the experiment, the cells were split to a density of 0.5 × 106 cells/ml. The next day, 106 cells were incubated with 900 pg rhIL-10/ml (Immunotools, Friesoythe, Germany) for the indicated time points. This concentration corresponds to the hIL-10 protein concentration induced by ODN CpG-2006 in Akata cells.16
Acknowledgments
This work was funded by the Swiss National Foundation (310040-114118), Oncosuisse and the Cancer League of the Canton of Zurich.
Glossary
- BL
Burkitt's lymphoma
- Ct
Cycle threshold
- EBV
Epstein-Barr virus
- hIL-10
human interleukin 10
- MFI
mean fluorescence intensity
- MyD88
myeloid differentiation factor D 88
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- ODN
oligodeoxynucleotide
- PBS
phosphate-buffered saline
- qRT-PCR
quantitative real-time polymerase chain reaction
- rhIL-10
recombinant human interleukin 10
- TLR
Toll-like receptor
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by H-U Simon
Supplementary Material
References
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- Chiron D, Bekeredjian-Ding I, Pellat-Deceunynck C, Bataille R, Jego G. Toll-like receptors: lessons to learn from normal and malignant human B cells. Blood. 2008;112:2205–2213. doi: 10.1182/blood-2008-02-140673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin IR, Leadbetter EA, Busconi L, Viglianti G, Marshak-Rothstein A. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol Rev. 2005;204:27–42. doi: 10.1111/j.0105-2896.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- Bourke E, Bosisio D, Golay J, Polentarutti N, Mantovani A. The toll-like receptor repertoire of human B lymphocytes: inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood. 2003;102:956–963. doi: 10.1182/blood-2002-11-3355. [DOI] [PubMed] [Google Scholar]
- Dorner M, Brandt S, Tinguely M, Zucol F, Bourquin JP, Zauner L, et al. Plasma cell toll-like receptor (TLR) expression differs from that of B cells, and plasma cell TLR triggering enhances immunoglobulin production. Immunology. 2009;128:573–579. doi: 10.1111/j.1365-2567.2009.03143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, et al. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- Vollmer J, Krieg AM. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv Drug Deliv Rev. 2009;61:195–204. doi: 10.1016/j.addr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Weiner GJ. CpG oligodeoxynucleotide-based therapy of lymphoid malignancies. Adv Drug Deliv Rev. 2009;61:263–267. doi: 10.1016/j.addr.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Krieg AM. Toll-like receptor 9 (TLR9) agonists in the treatment of cancer. Oncogene. 2008;27:161–167. doi: 10.1038/sj.onc.1210911. [DOI] [PubMed] [Google Scholar]
- Liang X, Moseman EA, Farrar MA, Bachanova V, Weisdorf DJ, Blazar BR, et al. Toll-like receptor 9 signaling by CpG-B oligodeoxynucleotides induces an apoptotic pathway in human chronic lymphocytic leukemia B cells. Blood. 2010;115:5041–5052. doi: 10.1182/blood-2009-03-213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahrsdorfer B, Muhlenhoff L, Blackwell SE, Wagner M, Poeck H, Hartmann E, et al. B-cell lymphomas differ in their responsiveness to CpG oligodeoxynucleotides. Clin Cancer Res. 2005;11:1490–1499. doi: 10.1158/1078-0432.CCR-04-1890. [DOI] [PubMed] [Google Scholar]
- Decker T, Schneller F, Sparwasser T, Tretter T, Lipford GB, Wagner H, et al. Immunostimulatory CpG-oligonucleotides cause proliferation, cytokine production, and an immunogenic phenotype in chronic lymphocytic leukemia B cells. Blood. 2000;95:999–1006. [PubMed] [Google Scholar]
- Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, et al. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65:5009–5014. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- Wolska A, Lech-Maranda E, Robak T. Toll-like receptors and their role in carcinogenesis and anti-tumor treatment. Cell Mol Biol Lett. 2009;14:248–272. doi: 10.2478/s11658-008-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladell K, Dorner M, Zauner L, Berger C, Zucol F, Bernasconi M, et al. Immune activation suppresses initiation of lytic Epstein-Barr virus infection. Cell Microbiol. 2007;9:2055–2069. doi: 10.1111/j.1462-5822.2007.00937.x. [DOI] [PubMed] [Google Scholar]
- Zauner L, Melroe GT, Sigrist JA, Rechsteiner MP, Dorner M, Arnold M, et al. TLR9 triggering in Burkitt's lymphoma cell lines suppresses the EBV BZLF1 transcription via histone modification. Oncogene. 2010;29:4588–4598. doi: 10.1038/onc.2010.203. [DOI] [PubMed] [Google Scholar]
- Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- Rickinson A, Kieff E.Epstein-Barr virusIn: Knipe DM (ed)Fields Virology4th edn.Lippincott Williams & Wilkins: Philadelphia, PA; 20012575–2627. [Google Scholar]
- Traggiai E, Becker S, Subbarao K, Kolesnikova L, Uematsu Y, Gismondo MR, et al. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat Med. 2004;10:871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A, Cunha C, Almeida AJ, Osorio NS, Saraiva M, Teixeira-Coelho M, et al. The rs5743836 polymorphism in TLR9 confers a population-based increased risk of non-Hodgkin lymphoma. Genes Immun. 2012;13:197–210. doi: 10.1038/gene.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng SL. Signaling in B cells via Toll-like receptors. Curr Opin Immunol. 2005;17:230–236. doi: 10.1016/j.coi.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Jahrsdorfer B, Hartmann G, Racila E, Jackson W, Muhlenhoff L, Meinhardt G, et al. CpG DNA increases primary malignant B cell expression of costimulatory molecules and target antigens. J Leukoc Biol. 2001;69:81–88. [PubMed] [Google Scholar]
- Jenkins PJ, Binne UK, Farrell PJ. Histone acetylation and reactivation of Epstein-Barr virus from latency. J Virol. 2000;74:710–720. doi: 10.1128/jvi.74.2.710-720.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Beatty PR, Krams SM, Martinez OM. Involvement of IL-10 in the autonomous growth of EBV-transformed B cell lines. J Immunol. 1997;158:4045–4051. [PubMed] [Google Scholar]
- Jones KD, Aoki Y, Chang Y, Moore PS, Yarchoan R, Tosato G. Involvement of interleukin-10 (IL-10) and viral IL-6 in the spontaneous growth of Kaposi's sarcoma herpesvirus-associated infected primary effusion lymphoma cells. Blood. 1999;94:2871–2879. [PubMed] [Google Scholar]
- Ng MT, Van't Hof R, Crockett JC, Hope ME, Berry S, Thomson J, et al. Increase in NF-kappaB binding affinity of the variant C allele of the toll-like receptor 9 -1237T/C polymorphism is associated with Helicobacter pylori-induced gastric disease. Infect Immun. 2010;78:1345–1352. doi: 10.1128/IAI.01226-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano-Sarabia N, Vallejo A, Ramirez-Lorca R, Rodriguez Mdel M, Salinas A, Pulido I, et al. Influence of the Toll-like receptor 9 1635A/G polymorphism on the CD4 count, HIV viral load, and clinical progression. JAIDS. 2008;49:128–135. doi: 10.1097/QAI.0b013e318184fb41. [DOI] [PubMed] [Google Scholar]
- Rousset F, Garcia E, Defrance T, Peronne C, Vezzio N, Hsu DH, et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci USA. 1992;89:1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masood R, Zhang Y, Bond MW, Scadden DT, Moudgil T, Law RE, et al. Interleukin-10 is an autocrine growth factor for acquired immunodeficiency syndrome-related B-cell lymphoma. Blood. 1995;85:3423–3430. [PubMed] [Google Scholar]
- Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- Latz E, Verma A, Visintin A, Gong M, Sirois CM, Klein DC, et al. Ligand-induced conformational changes allosterically activate Toll-like receptor 9. Nat Immunol. 2007;8:772–779. doi: 10.1038/ni1479. [DOI] [PubMed] [Google Scholar]
- Grauer OM, Molling JW, Bennink E, Toonen LW, Sutmuller RP, Nierkens S, et al. TLR ligands in the local treatment of established intracerebral murine gliomas. J Immunol. 2008;181:6720–6729. doi: 10.4049/jimmunol.181.10.6720. [DOI] [PubMed] [Google Scholar]
- Castro JE, Prada CE, Aguillon RA, Kitada S, Fukuda T, Motta M, et al. Thymidine-phosphorothioate oligonucleotides induce activation and apoptosis of CLL cells independently of CpG motifs or BCL-2 gene interference. Leukemia. 2006;20:680–688. doi: 10.1038/sj.leu.2404144. [DOI] [PubMed] [Google Scholar]
- Sanjuan MA, Rao N, Lai KT, Gu Y, Sun S, Fuchs A, et al. CpG-induced tyrosine phosphorylation occurs via a TLR9-independent mechanism and is required for cytokine secretion. J Cell Biol. 2006;172:1057–1068. doi: 10.1083/jcb.200508058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng MT, Van't Hof R, Crockett JC, Hope ME, Berry S, Thomson J, et al. Increase in NF-kappaB binding affinity of the variant C allele of the toll-like receptor 9 -1237T/C polymorphism is associated with Helicobacter pylori-induced gastric disease. Infect Immun. 2010;78:1345–1352. doi: 10.1128/IAI.01226-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC: Lyon; 2008. [Google Scholar]
- Muntwyler J, Marti-Jaun J, Luscher TF, Hanseler E, Hersberger M. The Asp298 but not the C-786 genotype of the endothelial nitric oxide synthase is reduced with age in healthy Swiss men. Clin chem lab med: CCLM/FESCC. 2005;43:971–973. doi: 10.1515/CCLM.2005.167. [DOI] [PubMed] [Google Scholar]
- Krayenbuehl PA, Hersberger M, Truninger K, Mullhaupt B, Maly FE, Bargetzi M, et al. Toll-like receptor 4 gene polymorphism modulates phenotypic expression in patients with hereditary hemochromatosis. Eur J Gastroenterol Hepato. 2010;22:835–841. doi: 10.1097/MEG.0b013e3283322067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.