Abstract
Context:
Isolated 17,20 lyase deficiency is commonly defined by apparently normal 17α-hydroxylase activity but severely reduced 17,20 lyase activity of the bifunctional enzyme cytochrome P450 (CYP) enzyme 17A1 (CYP17A1), resulting in sex steroid deficiency but normal glucocorticoid and mineralocorticoid reserve. Cytochrome b5 (CYB5A) is thought to selectively enhance 17,20 lyase activity by facilitating the allosteric interaction of CYP17A1 with its electron donor P450 oxidoreductase (POR).
Objective:
We investigated a large consanguineous family including three siblings with 46,XY disorder of sex development (DSD) presenting with isolated 17,20 lyase deficiency.
Design:
We investigated the clinical and biochemical phenotype, conducted genetic analyses, and functionally characterized the identified CYB5A mutation in cell-based CYP17A1 coexpression assays.
Results:
All three siblings presented with 46,XY DSD, sex steroid deficiency, normal mineralocorticoids and glucocorticoids, and a urine steroid metabolome suggestive of isolated 17,20 lyase deficiency. CYP17A1 and POR sequences were normal, but we detected a homozygous CYB5A missense mutation (g.28,400A→T; p.H44L). Functional in vitro analysis revealed normal CYP17A1 17α-hydroxylase activity but severely impaired 17,20 lyase activity. In silico analysis suggested the disruption of CYB5A heme binding by p.H44L.
Conclusion:
We have identified the first human CYB5A missense mutation as the cause of isolated 17,20 lyase deficiency in three individuals with 46,XY DSD. Detailed review of previously reported cases with apparently isolated 17,20 lyase deficiency due to mutant CYP17A1 and POR reveals impaired 17α-hydroxylase activity as assessed by steroid metabolome analysis and short cosyntropin testing. This suggests that truly isolated 17,20 lyase deficiency is observed only in individuals with inactivating CYB5A mutations.
Androgen synthesis in humans crucially relies on the cytochrome P450 (CYP) enzyme 17A1 that is primarily expressed in the adrenals and the gonads. CYP17A1 exhibits two distinct catalytic activities: its 17α-hydroxylase activity converts pregnenolone and progesterone to 17α-hydroxypregnenolone (17-Preg) and 17α-hydroxyprogesterone (17OHP), respectively, thereby facilitating adrenal glucocorticoid synthesis. The 17,20 lyase activity of CYP17A1 further converts 17-Preg to the principal androgen precursor dehydroepiandrosterone (DHEA) and, with 50-fold lesser efficiency (1), 17OHP to androstenedione. Both the 17α-hydroxylase and the 17,20 lyase activities of CYP17A1 require electron transfer from reduced nicotinamide adenine dinucleotide phosphate via the electron donor enzyme P450 oxidoreductase (POR), which serves as the universal electron donor to all microsomal CYP enzymes. Inactivating mutations in the POR gene result in P450 oxidoreductase deficiency (PORD), a unique CAH variant that is associated with glucocorticoid and sex steroid deficiency (2, 3). CYP17A1 17,20 lyase activity requires additional interaction of CYP17A1 and POR with the heme-containing protein cytochrome b5 (CYB5A) (4). Previous studies suggest that CYB5A is not directly involved in electron transfer but rather facilitates allosteric interaction of the POR and CYP17A1 proteins, thereby enhancing electron flux (1). Importantly, 17α-hydroxylase activity of CYP17A1 is not modulated by CYB5A (1, 5).
Isolated 17,20 lyase deficiency results in sex steroid deficiency and clinically presents with male undermasculinization, i.e. 46,XY disorder of sex development (DSD) and with absent or disturbed pubertal development in both 46,XY and 46,XX individuals. In accordance with lack of impairment of 17α-hydroxylase activity, affected patients have been reported not to present with cortisol deficiency or mineralocorticoid excess, which are typically observed in classic, complete CYP17A1 deficiency (6–13). To date, four different missense mutations in CYP17A1 have been reported to cause apparently isolated 17,20 lyase deficiency (7, 10, 11). A recent case report described a patient presenting with isolated 17,20 lyase deficiency and normal CYP17A1 sequence, in whom an inactivating mutation in the POR gene was identified as the cause of disease (8). Recently, a nonsense mutation resulting in early truncation of the CYB5A protein has been described in a 46,XY DSD patient diagnosed with isolated 17,20 lyase deficiency (13).
Here we report clinical, genetic, and biochemical findings in a large consanguineous family with three children presenting with 46,XY DSD and biochemical evidence of isolated 17,20-lyase deficiency. No mutations in CYP17A1 or POR were detected, but we identified the first human CYB5A missense mutation, p.H44L, which was found in the homozygous state in all affected siblings and was associated with greatly reduced 17,20 lyase activity, whereas 17α-hydroxylase activity was completely preserved.
Patients and Methods
Patients
We have investigated three siblings with 46,XY DSD from a consanguineous family of Pakistani origin; the parents were first cousins (see Fig. 2B).
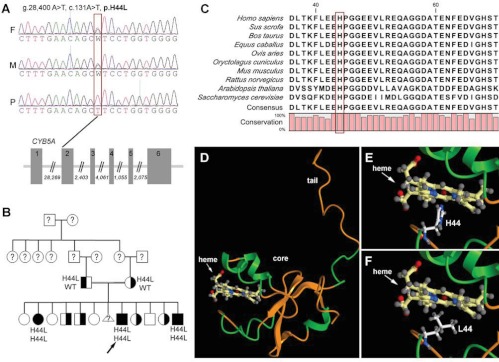
Fig. 2.
A, Electropherogram depicting the homozygous missense mutation in the CYB5A gene identified in the index patient (P), with heterozygosity in mother (M) and father (F). The mutation g.28,400 A→T is located at the beginning of exon 2 as indicated in the schematic representation of the CYB5A gene. Exons are shown as gray rectangles and introns as thin lines; numbers indicate the sizes of the intronic regions. B, Pedigree of the index family with segregation analysis of the identified CYB5A mutation. The arrow depicts the index patient. C, Multiple CYB5A alignments of amino acid sequences derived from different species. A red box highlights the H44 residue; red bars at the bottom indicate the degree of conservation. Species specification: Homo sapiens, human; Sus scrofa, wild boar; Bos taurus, domestic cattle; Equus caballus, domestic horse; Ovis aries, domestic sheep; Oryctulagus cuniculus, domestic rabbit; Mus musculus, house mouse; Rattus norvegicus, Norway rat; Arapidopsis thaliana, thale cress; Saccharomyces cerevisiae, baker's yeast. D, Three-dimensional model of CYB5A. The protein is composed of a core domain containing the heme molecule and a hydrophobic tail domain. E, Magnification of the core domain highlights residue H44 in close proximity to the central iron atom of the heme molecule. F, L44 disrupts the interaction with the heme molecule.
The index patient (case 1) was born at 41 wk of gestation after an uneventful pregnancy and delivery (birth weight 3460 g, height 36 cm). Routine ultrasound examinations during pregnancy had predicted a female baby. However, at birth, ambiguous, predominantly female genitalia were observed, with enlarged, mildly scrotalized labia majora that contained palpable gonads. The phallus appeared similar to a female clitoris (7 mm stretched length). Due to a skinfold between the labia, the urethral orifice and vaginal opening were not clearly detectable, but the baby was able to pass urine. Uro-genitogram and pelvic ultrasound revealed a proximal perineal opening of the urethra and absence of female internal genitalia; both adrenals appeared normal in shape and size. The karyotype was 46,XY. Hormonal measurements 3 d after birth revealed elevated 17OHP [120 nmol/liter; normal range (NR) < 5.0], a normal testosterone (7.2 nmol/liter; NR 5–15), normal androstenedione (1.8 nmol/liter; NR < 7.7), and low gonadotropins (LH < 0.5 U/liter; FSH < 0.5 U/liter). Serum electrolytes were normal when measured 3 wk after birth (Na 140 nmol/liter, NR 135–145; K 5.2 nmol/liter, NR 3.5–5.5). A human chorionic gonadotropin (hCG) stimulation test was performed at the age of 6 wk and again at 5 months, both times revealing a low baseline testosterone that did not increase sufficiently 3 d after hCG administration (Table 1). The decision was taken to raise the baby as a boy. He received testosterone treatment (25 mg im every 4 wk) from 5–8 months of age and underwent two-stage hypospadias repair and correction of the bifid scrotum between ages 2 and 4 yr. This resulted in penile growth from 7 to 27 mm and largely normal male genital appearance.
Table 1.
Summary of biochemical and genetic findings in three siblings with 46,XY DSD and isolated 17,20 lyase deficiency found to carry the homozygous CYB5A mutation p.H44L
| Sibling 1 (index case) | Sibling 2 | Sibling 3 | |
|---|---|---|---|
| Age at presentation | Neonatal | 12.75 yr | Neonatal |
| Karyotype | 46,XY | 46,XY | 46,XY |
| Age at time of investigation | 5 yr | 15 yr | 8 months |
| Cortisol (nmol/liter) | |||
| At baseline | 194 | 107 | 386 |
| 60 min after ACTH1–24 | 807 (>550) | 726 (>550) | 901 (>550) |
| 17OHP (nmol/liter) | 1.1 (2.0–9.0) | 9.5 (2.0–9.0) | 30.0 (0–12.0) |
| DHEA-sulfate (μmol/liter) | |||
| At baseline | <0.4 (0.4–3.7) | <0.4 (8.3–49) | <0.4 (0.5–20) |
| 60 min after ACTH1–24 | <0.4 | <0.4 | <0.4 |
| Androstenedione (nmol/liter) | |||
| At baseline | <1.1 (1.5–2.7) | <1.1 (1.8–4.8) | <1.1 (1.5–2.7) |
| 60 min after ACTH1–24 | <1.1 | <1.1 | <1.1 |
| Testosterone (nmol/liter) | |||
| At baseline | 0.2a (0.5–2.0) | 1.1 (female NR, 0–2.8.0) | 0.6 (0.5–2.0) |
| 3 d after 1500 U hCG | 0.2a | NM | |
| Estradiol (pmol/liter) | 60b | ||
| LH (U/liter) | 0.8 | 2.1b (0.3–2.5) | |
| FSH (U/liter) | 2.8 | 3.2b (1.3–6.6) | |
| MetHb (mmol/mmol Hb) | 0.063 (<0.015) | 0.061 (<0.015) | 0.085 (<0.015) |
Hb, Hemoglobin; MetHb, methemoglobin; NM, not measured.
hCG test performed at 5 months of age.
Measured at age 13 yr before the removal of bilateral intraabdominal gonads.
The diagnosis of 46,XY DSD in the index patient prompted investigations in one of his older sisters (case 2) who had not developed any signs of puberty at the age of 13 yr. On examination, her genitalia looked predominantly female but with a single perineal opening, separate labioscrotal folds with no palpable gonads and a mildly enlarged clitoris (15 mm). Clinically, she had an entirely prepubertal appearance including absence of public or axillary hair, indicating lack of adrenarche (Tanner stages B1, PH1). Her karyotype was 46,XY. Biochemical investigations revealed low androgens, a prepubertal estradiol, and early pubertal gonadotropins (Table 1). There was a normal cortisol response in the short cosyntropin test, but adrenal androgen precursors remained undetectable; 17OHP was mildly elevated (Table 1). The bilateral intraabdominal gonads were removed at age 13.5 yr. Estrogen replacement therapy was initiated, which prompted appropriate breast development and female body shape.
The youngest child of the family (case 3; 46,XY) was born with ambiguous genitalia, including bifid scrotum, a very small penis with hardly any corporal tissue present, and perineal hypospadias. His testes were not descended into the scrotum but palpable in the inguinal region. He had an elevated 17OHP and a normal cortisol response to ACTH (Table 1). Corrective surgery for hypospadias and bifid scrotum are scheduled for after the child's first birthday.
All three affected siblings had elevated methemoglobin levels (Table 1). However, methemoglobinemia was clinically inapparent, with no evidence of episodes of cyanosis, dyspnea, or respiratory distress in any of them.
Urinary steroid metabolome analysis
Analysis of urinary steroid metabolite excretion was performed as described previously by a quantitative gas chromatography/mass spectrometry (GC/MS) selected ion-monitoring method (3, 14). In brief, steroids were enzymatically released from conjugation and, after extraction, chemically derivatized before GC/MS selected ion-monitoring analysis. Steroids quantified included metabolites of corticosterone: tetrahydrocorticosterone (THB), 5α-THB, tetrahydro-11-dehydrocorticosterone (THA), 5α-THA, progesterone (pregnanediol), 17OHP (pregnanetriol), 17-hydroxypregnanolone, 17-Preg (pregnenetriol), 21-deoxycortisol (pregnanetriolone), cortisol [tetrahydrocortisol (THF), 5α-THF, and tetrahydrocortisone], and androgens/androgen precursors (androsterone, etiocholanolone, DHEA, and 16-OH-DHEA).
After quantification of steroid metabolites by GC/MS, we calculated substrate metabolite to product metabolite ratios to reflect the in vivo net activity of 17α-hydroxylase, i.e. corticosterone over cortisol metabolites (THA + 5α-THA + THB + 5α-THB)/(THF + 5αTHF + tetrahydrocortisone), and 17,20-lyase, i.e. 17OHP over androgen metabolites (17-hydroxypregnanolone + pregnanetriol)/(androsterone + etiocholanolone).
Genetic analysis
DNA analysis was performed after obtaining informed assent and consent from patients and parents, respectively, with approval from institutional research ethics committees. Genomic DNA was extracted from peripheral blood leukocytes for cases 1–3 and the parents, using a DNA blood and cell culture kit (QIAGEN, GmbH, Hilden, Germany). From all other siblings, DNA was extracted from saliva using a commercially available kit (Oragene DNA OG250 and OG300; DNA Genotek, Ontario, Canada).
The coding sequence of the CYB5A gene including exon-intron boundaries was amplified in five PCR fragments (for primer sequences, see Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). The CYP17A1 and POR coding sequences including exon-intron boundaries were amplified as previously described (3, 15). Direct sequencing was carried out using an automated ABI3730 Sequencer (Applied Biosystems Inc., Foster City, CA). Sequences were analyzed using the DNAStar Lasergene software package (DNASTAR Inc., Madison, WI) in accordance with Human Genome Variation Society recommendations (www.hgvs.org/rec.html) using the following reference sequences: CYB5A GenBank NG_023211.1 (genomic DNA), GenBank NM_148923.3 (cDNA; transcript variant 1), GenBank NP_683725.1 (protein). For DNA numbering, the nucleotide designated +1 was the A of the ATG start codon.
In silico analysis
The x-ray structure of human oxidized microsomal CYB5A was used as a template for in silico analysis (PDB ID 2I96; Nunez-Quintana, M., G. Truan, C. van Heijenhoort. Solution structure of human cytochrome b5, published online at http://www.pdb.org/pdb/explore/explore.do?structureId=2I96). The structural representations were generated using the program Molsoft ICM Browser Pro (Molsoft LLC, La Jolla, CA).
In vitro assessment of enzymatic activities
Human CYP17A1 cDNA was tagged with the V5 epitope as described previously (16) and subsequently cloned into the linearized bicistronic mammalian expression vector pIRES (Clontech, Mountain View) using the NheI restriction site at multiple cloning site A of the construct. Subsequently, wild-type (WT) human CYB5A cDNA was PCR amplified and cloned into pIRES-CYP17A1-V5 at the SalI restriction site of multiple cloning site B. Site-directed mutagenesis was performed using the QuikChange XL site-directed mutagenesis kit (Stratagene, Amsterdam, The Netherlands). Correct insertion of the mutation and integrity of the inserts were checked by direct sequencing.
For in vitro functional analysis, the human embryonic kidney cell line HEK293 was used for transient transfection with the generated pIRES constructs, and analysis of both catalytic activities of CYP17A1 was performed as follows. Cells were grown in Eagle's MEM (Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal calf serum and seeded at approximately 75 × 104 per well in six-well plates (cell bind surface; Corning Inc., Corning, NY) before transfection. After 24 h, cells were transfected with 2 μg plasmid DNA and 6 μl Fugene HD transfection reagent (Roche, Basel, Switzerland) according to the manufacturer's protocol. Kinetic constants were determined 48 h after transfection. For 17α-hydroxylase activity assays, cells were incubated in 500 μl MEM full-growth medium with 0.5, 1, 2, and 5 μmol/liter progesterone in the presence of 0.2 μCu 3H-labeled progesterone (101.3 Ci/mol; PerkinElmer, Waltham, MA). For 17,20 lyase activity assays, cells were incubated with 0.25, 0.5, 1.25, and 2.5 μmol/liter 17-Preg containing 0.2 μCu 3H-labeled 17-Preg (53.7 Ci/mol; PerkinElmer). To enhance the efficiency of the enzymatic reaction, reduced nicotinamide adenine dinucleotide phosphate was added to the medium in a final concentration of 200 nmol/liter. Steroids were extracted and then separated by thin-layer chromatography and quantified using a Bioscan 2000 image analyzer (Lablogic, Sheffield, UK). Kinetic parameters were calculated in GraphPad Prism software version 4.0 (GraphPad Inc., San Diego, CA), using the Michaelis-Menten equation.
Protein amounts were measured using the Bradford method (Bio-Rad, Hempel-Hempstead, UK) after lysing the cells in PBS containing protease inhibitors and 0.1% Triton X-100. To ensure similar expression levels, Western blotting was performed as described previously (16, 17) with some minor modifications. In brief, cell lysates (8–15 μg) were separated by SDS-PAGE, transferred on nitrocellulose membranes (Amersham Biosciences) at 30 V for 90 min, and blocked with 5% skim-milk powder (wt/vol) in PBS with 0.1% (vol/vol) Tween 20 for 1–2 h. Primary antibodies were incubated overnight employing the following dilutions: rabbit polyclonal to CYB5A (Abcam, Cambridge, MA) at 1:250, mouse monoclonal to V5 (Invitrogen) at 1:1000, and mouse monoclonal to β-actin (Abcam) at 1:25,000. Secondary horseradish peroxidase-conjugated antibodies and detection by enhanced chemiluminescence (Immobilon, Millipore, Watford, UK) was performed according to the manufacturer's protocol.
Results
Biochemical analysis by urinary steroid profiling
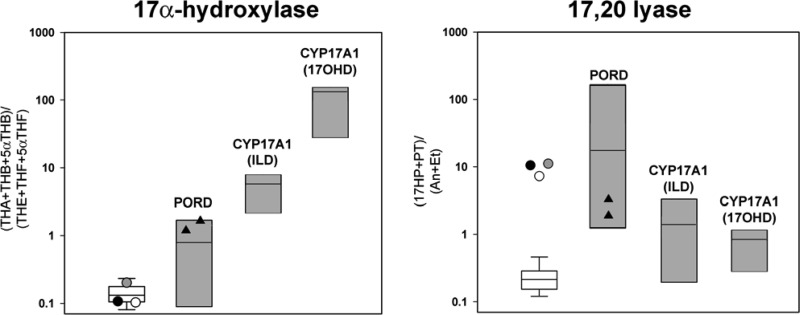
GC/MS analysis of urinary steroid metabolites revealed low androgen metabolite excretion with increased excretion of the 17-Preg metabolite pregnenetriol, suggestive of 17,20 lyase deficiency; concurrently, mineralocorticoid and glucocorticoid metabolite excretion appeared normal, suggesting preserved 17α-hydroxylase activity (Supplemental Fig. 1). In keeping with these findings, 17α-hydroxylation as assessed by the ratio of corticosterone over cortisol metabolites was not compromised in either sibling, whereas 17,20 lyase activity as determined by the ratio of 17OHP over androgen metabolites was significantly elevated, suggestive of isolated 17,20 lyase deficiency (Fig. 1). By contrast, 17α-hydroxylase activity reflected by the ratio of corticosterone over cortisol metabolites was significantly impaired in previously described patients with CYP17A1 and POR mutations as the cause of apparently isolated 17,20 lyase activity (12, 18) (Fig. 1).
Fig. 1.
In vivo assessment of CYP17A1 17α-hydroxylase and 17,20 lyase activities as indicated by urinary steroid metabolite analysis. Diagnostic steroid metabolite ratios in the three siblings with isolated 17,20 lyase deficiency due to homozygous p.H44L CYB5A are represented by circles (white, case 1; black, case 2; gray, case 3). White box plots represent the interquartile ranges of the reference cohort (healthy males and females, 4–20 yr; n = 98), whiskers represent the 5th and 95th percentiles, respectively. Gray box plots indicate the ranges and medians for the same steroid ratios measured in 20 patients with classic 17α-hydroxylase deficiency (CYP17A1 17OHD) (18), six patients with apparently isolated 17,20 lyase deficiency due to CYP17A1 p.E305G (CYP17A1 ILD) (12), and 21 patients with P450 oxidoreductase deficiency (our own data). The triangles represent two patients with the POR mutation p.G539R reported as associated with apparently isolated 17,20 lyase deficiency (8). For steroid abbreviations, please see Subjects and Methods.
Molecular genetic analysis
Sequencing of the coding regions of the CYP17A1 and POR genes did not reveal mutations in any of the three affected siblings. However, we identified a homozygous missense mutation at the beginning of exon 2 of the CYB5A gene (g.28,400 A→T), changing a histidine at position 44 to leucine (p.H44L) (Fig. 2A). Segregation analysis demonstrated heterozygosity for the p.H44L variant in both father and mother. Further sequencing analysis of the other clinically unaffected siblings revealed heterozygous p.H44L CYB5A and WT CYB5A in four and four of the remaining siblings, respectively (Fig. 2B).
In silico analysis
An alignment of the amino acid sequences of CYB5A from different species indicates that histidine at position 44 is highly conserved throughout the animal kingdom, yeast, and plants (Fig. 2C).
The histidine residue at position 44 is localized in the heme-binding pocket within the core domain of the CYB5A protein (Fig. 2D). The imidazole side chain of H44 appears to coordinate the heme iron and keep the redox active heme molecule attached to the CYB5A protein (Fig. 2E) (19). Substitution with apolar lysine in the same position appears to disrupt the interaction between the CYB5A core domain and the heme molecule (Fig. 2F).
Functional enzyme activity assays
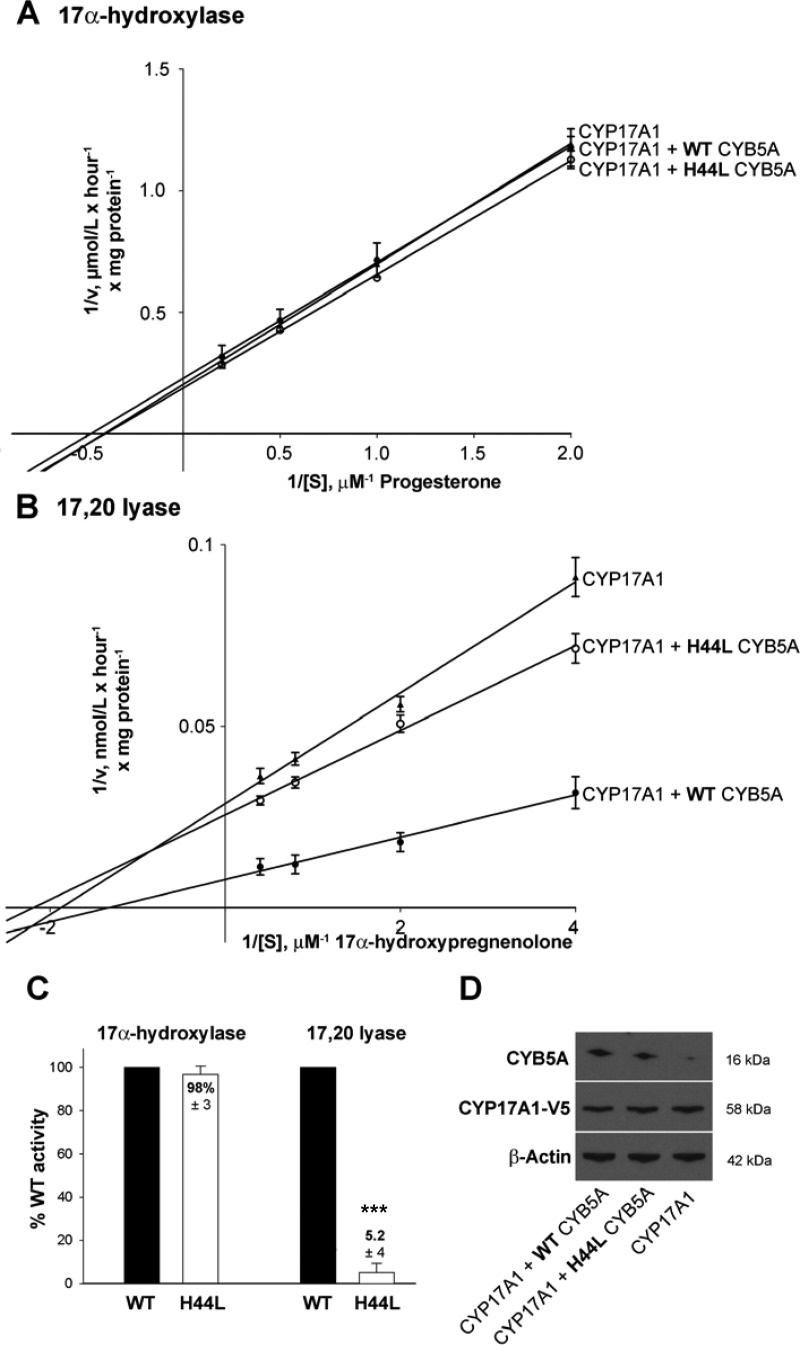
The observed catalytic constants for the 17α-hydroxylase reaction did not differ after coexpression of CYP17A1 with and without WT or mutant CYB5A (Fig. 3A and Table 2). In contrast, CYP17A1 17,20 lyase activity was greatly reduced in the presence of p.H44L CYB5A compared with incubations with WT CYB5A, almost down to the level observed after the expression of CYP17A1 without CYB5A (Fig. 3B). This decrease in catalytic efficiency was mainly a consequence of a marked reduction of the maximal reaction velocity Vmax, whereas the Michaelis-Menten constant Km (indicative of substrate affinity) appeared not to be affected by p.H44L (Table 2). The residual activity of the p.H44L mutant on the 17,20-lyase reaction was about 5% of WT (Fig. 3C). Western blotting confirmed equal expression of CYP17A1 and CYB5A, respectively, and the low endogenous CYB5A expression in the employed HEK293 cell line, rendering it a suitable model for functional analysis of CYB5A function (Fig. 3D).
Fig. 3.
A and B, Results of kinetic analysis of CYP17A1 activities. The panels show Lineweaver-Burk plots of 17α-hydroxylase (A) and 17,20 lyase (B) activities as assessed in HEK293 cells transiently transfected with CYP17A1 with or without WT or mutant p.H44L CYB5A. Cells were incubated with 0.5, 1, 2, and 5 μm progesterone (for CYP17A1 17α-hydroxylase activity) (A) or 0.25, 0.5, 1, and 2.5 μm 17-Preg (for CYP17A1 17,20 lyase activity (B). C, Residual enzyme activity expressed as percentage of WT activity, defined as 100%, based on measurements carried out at substrate concentrations around Km, i.e. 1 and 0.5 μm for 17α-hydroxylase and 17,20 lyase activity, respectively. For defining the impact of p.H44L on 17,20 lyase activity, the conversion rate observed after expression of CYP17A1 alone was subtracted from those observed after coexpression of CYP17A1 with WT and mutant CYB5A, respectively, before calculation of residual activities. ***, P < 0.001. In A–C, error bars represent the mean ± sem (percentage) of at least three independent triplicate experiments. D, A representative Western blot demonstrating equal expression levels of WT and mutant CYB5A and CYP17A1 with β-actin as a control for equal loading.
Table 2.
Kinetic constants (± sem) of human CYP17A1 catalytic activities after equal coexpression of human WT CYP17A1 with either human WT or mutant (p.H44L) CYB5A as compared withythe expression of CYP17A1 without CYB5A in HEK293 cells
| 17α-Hydroxylase | 17,20 Lyase | |
|---|---|---|
| Vmaxa | ||
| CYP17A1 + CYB5A WT | 4.71 ± 0.24 | 118 ± 5 |
| CYP17A1 + CYB5A H44L | 5.24 ± 0.16 | 41 ± 2 |
| CYP17A1 | 4.99 ± 0.19 | 35 ± 2 |
| Km (μm) | ||
| CYP17A1 + CYB5A WT | 2.3 ± 0.25 | 0.55 ± 0.07 |
| CYP17A1 + CYB5A H44L | 2.4 ± 0.15 | 0.48 ± 0.06 |
| CYP17A1 | 2.4 ± 0.20 | 0.48 ± 0.07 |
| Catalytic efficiency (Vmax/Km) | ||
| CYP17A1 + CYB5A WT | 2.05 | 214 |
| CYP17A1 + CYB5A H44L | 2.18 | 85 |
| CYP17A1 | 2.08 | 73 |
Vmax results are micomoles per milligram per hour for 17α-hydroxylase and nanomoles per milligram per hour for 17,20 lyase.
Discussion
Here we have described the first human CYB5A missense mutation identified in three children from a large consanguineous family presenting with 17,20 lyase deficiency, 46,XY DSD, and mild methemoglobinemia. Isolated 17,20 lyase deficiency is a rare condition, and only around 25 cases have been described in the literature so far (6–13). The commonly agreed hormonal phenotype of isolated 17,20 lyase deficiency comprises a selective decrease in DHEA and androstenedione biosynthesis and normal glucocorticoid and mineralocorticoid production (20). Importantly, the initial reports on the condition provided only the clinical and hormonal characterizations without identifying a distinct genetic abnormality in six families with 46,XY DSD patients (21–26) and two families with 46,XX patients that presented with lack of pubertal development (27, 28). These reports have been summarized and discussed in the review of Yanase et al. (6).
So far, 15 cases of isolated 17,20 lyase deficiency with a complete clinical, hormonal, genetic, and functional work-up have been reported (Table 3). Underlying causes were three distinct missense mutations in the CYP17A1 gene (p.R347H, p.R358Q, and p.E305G) (7, 10, 11) and one POR missense mutation (p.G539R) (8). It appears likely that another patient suggested to have 17,20 lyase deficiency, presenting with 46,XX DSD and low circulating androgens (28), may also have suffered from POR deficiency, but at the time, no genetic work-up was performed. Recently, a CYB5A nonsense mutation (p.W27X) has been reported resulting in early protein truncation and thus loss of CYB5A function (13) (Table 3). A unifying characteristic of all these individuals is severe sex steroid deficiency, with hormonal measurements confirming a lack of adrenal and gonadal androgen synthesis. However, it is noteworthy that all patients with underlying CYP17A1 or POR mutations concurrently showed significant impairment of glucocorticoid production, with insufficient cortisol responses to ACTH stimulation (Table 3). This is further reflected by in vitro results that showed a reduction of 17α-hydroxylase activities to 13–65 and 46% of WT activity for the CYP17A1 and POR mutations, respectively (Table 3). The only exception is the CYP17A1 mutant E305G for which normal or enhanced 17α-hydroxylase activity has been found in vitro (11). However, patients homozygous for this mutation failed to show an appropriate cortisol response to ACTH, and urinary steroid metabolite analysis demonstrated significantly impaired 17α-hydroxylase activity (12). Hence, although the impairment of 17,20 lyase activity dominates, none of the previously reported mutations in CYP17A1 or POR cause truly isolated 17,20-lyase deficiency. This is of clinical importance, because those patients will require not only sex steroid but also hydrocortisone replacement to avoid adrenal crisis during intercurrent illness or surgery. Future studies in patients with isolated 17,20 lyase deficiency including a complete genetic and hormonal characterization will show whether preservation of CYP17A1 17α-hydroxylase activity can only be observed in CYB5A deficiency.
Table 3.
Overview of patients with 17,20 lyase deficiency that have been reported with complete information on clinical, biochemical, and genetic work-up, including the functional work-up of the causative mutations
| Gene | Mutation | Ref. | Clinical presentation | Hormonal investigations |
In vitro functional analysis |
||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline 17OHP (nmol/liter) (<2) | Baseline cortisol (nmol/liter) | Maximal cortisol after ACTH (nmol/liter) (>550) | 17α -Hydroxylase (% WT activity) | 17,20 Lyase (% WT activity) | In vitro expression system | ||||
| CYP17A1 | p.R347H | Geller et al., 1997 (7) | 46,XY DSD (bifid scrotum, perineal hypospadias), both testes descended, female gender assignment | 1.2 | 223 | NA | 65% | <5% | Transient transfection in COS1 cells, scintillation counting |
| van den Akker et al., 2002 (10) | Two siblings with 46,XY DSD | 1.3 | 190–270 | 385–427 | |||||
| CYP17A1 | p.R358Q | Geller et al., 1997 (7) | 46, XY DSD, male gender assignment, gynecomastia at 14 yr (Tanner B5, PH4), 4.5 cm phallus, bifid scrotum, left testes descended, right in inguinal canal | 36.4 | 469 | 469 | 65% | <5% | Transient transfection in COS1 cells, scintillation counting |
| CYP17A1 | p.E305G | Sherbet et al., 2003 (11) Tiosano et al. 2008 (12) | A large kindred with four cases of 46,XY DSD and two 46,XX individuals (one is prepubertal; the other had delayed puberty and ovarian cysts) | 2.2–21.0 | 67–192 | 200–383 | Not impaired | decreased for Δ5 pathway, increased for Δ4 pathway | Yeast microsomes, kinetic analysis, thin-layer chromatography |
| POR | p.G539R | Hershkowitz et al., 2008 (8) | Four patients with 46,XY DSD from a large consanguineous Israeli family, various degrees of undermasculinization (micropenis to bifid scrotum/scrotal hypospadias) | 16.3–41.8 | 193–224 | 209–292 | 46% | 8% | Yeast microsomes, kinetic analysis, thin-layer chromatography |
| CYB5A | p.W28X | Kok et al., 2010 (13) | 46, XY DSD (bifid scrotum, scrotal hypospadias), testes descended | 20.3 | 714 | 888 | Not done | ||
| CYB5A | p.H44L | This article | Three siblings with 46,XY DSD, various degrees of undermasculinization from clitoral enlargement and intraabdominal testes (46,XY female) to ambiguous genitalia (hypospadias, bifid scrotum) with inguinal testes | 1.1–30.0 | 107–386 | 726–901 | 96% | 5.5% | Transient transfection in HEK293 cells, kinetic analysis, thin-layer chromatography |
All mutations were found in the homozygous state. NA, Not available.
In our family, clinical investigations, hormonal measurements, and in vitro functional assays clearly document true isolated 17,20 lyase deficiency in the presence of completely preserved 17α-hydroxylase activity. Similarly, the recently reported patient carrying a homozygous CYB5A nonsense mutation showed an entirely normal cortisol response in the short cosyntropin test (13) (Table 3). The low excretion of urinary androgen metabolites was consistent with the documented low or nondetectable circulating androgens, with the exception of an apparently normal early neonatal testosterone level in case 1, an observation most likely due to cross-reactivity of the assay with fetal steroids that are still circulating in high concentrations during the early days of life. The urinary steroid metabolome analysis carried out in our patients also had a pivotal role in establishing the correct diagnosis in our family, but also impressively illustrated that CYB5A deficiency is the only condition that shows normal 17α-hydroxylase activity in the presence of 17,20 lyase deficiency (Fig. 1). By contrast, 17α-hydroxylase activity as reflected by the corticosterone over cortisol metabolite ratio was clearly compromised in previously reported patients with apparently isolated 17,20 lyase deficiency due to mutant CYP17A1 or POR, although to a lesser degree than in patients considered to have classical, complete 17OHD (12, 18) (Fig. 1).
At the molecular level, it has been reported that CYB5A is not directly involved in electron transfer when facilitating the interaction between CYP17A1 and POR (1), even though it carries a heme molecule and is therefore potentially able to reduce interaction partners. This is based on in vitro observations that CYP17A1 17,20 lyase activity was not altered if coincubated with apo-b5, i.e. CYB5A devoid of the heme, or with the heme-containing holo-protein, which has also been demonstrated for the interaction of CYB5A with the drug-metabolizing enzyme CYP3A4 (1, 29). Distinct amino acid residues within CYB5A, specifically p.E48 and p.E49, have been shown to retain normal electron transfer properties when mutated, although resulting in severely reduced capacity to support 17,20 lyase activity, lending support to the assumption that CYB5A acts as an allosteric facilitator rather than as an electron donor in supporting 17,20 lyase activity (30). In silico analysis of the CYB5A mutation detected in our patient reveals close proximity of WT histidine 44 to the heme molecule, with polar bonds between the positively charged imidazole ring of histidine and the core heme iron atom (Fig. 2E). Replacement with leucine at this position, i.e. creating p.H44L, appears to disrupt this interaction (Fig. 2F), which is highly likely to result in loss of the heme moiety. This assumption is further supported by previous studies with rat CYB5A that have shown that the heme-holding ability of CYB5A depends mostly on the strong axial ligation provided by histidine residues 39 and 63 (31–33), which are equivalent to histidines 44 and 68 in the human CYB5A protein (Fig. 2C). Loss of the heme, i.e. the transition from the CYB5A apo-protein to the holo-protein, has been shown to result in a striking conformational change (33), which is likely to occur in p.H44L. From this we conclude that the heme group of CYB5A may be of more significance in supporting CYP17A1 17,20 lyase activity than previously assumed, which challenges the previous concept that the heme is not required (1).
Of note, in addition to supporting 17,20 lyase activity, CYB5A is also involved in a number of other processes requiring interaction with this distinct heme-containing protein, including drug metabolism (29), fatty acid desaturation (34), and importantly, hemoglobin reduction. Thus, CYB5A deficiency represents another multisystem disorder due to cofactor mutations, similar to POR deficiency, which results not only in disordered steroidogenesis but also in impaired drug metabolism (35) and 3′-phosphoadenosine 5′-phosphosulfate synthase 2 (PAPSS2) deficiency (36) that disrupts DHEA sulfation to cause androgen excess as well as other sulfation processes including hepatic drug metabolism.
In our three affected siblings we found mild, clinically inapparent methemoglobinemia, in keeping with the findings in the recently reported patient with a CYB5A nonsense mutation (13) resulting in a nonfunctional protein. Of note, one additional CYB5A mutation has been reported previously, resulting in a 16-bp deletion due to a splice site mutation (37). This patient presented with severe cyanosis 7 d after birth and significant methemoglobinemia of 12–19% (38), which is more similar to the range of methemoglobin increases commonly found in CYB5A reductase (CYB5R3) deficiency, i.e. recessive congenital methemoglobinemia (39). A detailed clinical work-up later revealed 46,XY DSD with an entirely female genital phenotype (37); however, the patient did not undergo endocrine assessment. Importantly, because methemoglobinemia is not expected in 17,20 lyase deficiency due to mutant CYP17A1 or POR, assessing methemoglobin levels in patients with isolated 17,20 lyase deficiency is very likely to represent a useful and presumably highly predictive screening tool for the detection of CYB5A deficiency.
In conclusion, we have described the first human CYB5A missense mutation, which results in true isolated 17,20 lyase deficiency as documented by in vivo and in vitro assessment. The mutation is highly likely to result in loss of CYB5A heme binding, suggesting that presence of the heme is crucial for support of 17,20 lyase deficiency. Lack of impairment of 17α-hydroxylase activity can be considered as highly suggestive of CYB5A mutations in patients presenting with biochemical evidence of 17,20 lyase deficiency, whereas impaired 17α-hydroxylation points toward mutant CYP17A1 or POR as the cause of disease. Future studies will need to clarify the impact of CYB5A mutations on other organs and systems, with methemoglobinemia commonly present.
Supplementary Material
Acknowledgments
We are grateful to Dr. Ghassan Maghzal, University of Sydney, Australia, for helpful suggestions regarding CYB5A Western blot analysis.
This work was supported by Medical Research Council UK (Research Fellowship G1001964, to J.I. and Program Grant 0900567 to W.A.), the European Society for Pediatric Endocrinology (Research Fellowship to J.I.), the European Community's Seventh Framework Program (Collaborative Research Project EuroDSD GA-2008-201444 to W.A.), and the Wellcome Trust (Clinician Scientist Fellowship GR079865MA, to N.K.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CYB5A
- Cytochrome b5
- CYP
- cytochrome P450
- DHEA
- dehydroepiandrosterone
- DSD
- disorder of sex development
- GC/MS
- gas chromatography/mass spectrometry
- hCG
- human chorionic gonadotropin
- NR
- normal range
- 17OHP
- 17α-hydroxyprogesterone
- POR
- P450 oxidoreductase
- 17-Preg
- 17α-hydroxypregnenolone
- THA
- tetrahydro-11-dehydrocorticosterone
- THB
- tetrahydrocorticosterone
- THF
- tetrahydrocortisol.
References
- 1. Auchus RJ, Lee TC, Miller WL. 1998. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem 273:3158–3165 [DOI] [PubMed] [Google Scholar]
- 2. Flück CE, Tajima T, Pandey AV, Arlt W, Okuhara K, Verge CF, Jabs EW, Mendonça BB, Fujieda K, Miller WL. 2004. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat Genet 36:228–230 [DOI] [PubMed] [Google Scholar]
- 3. Arlt W, Walker EA, Draper N, Ivison HE, Ride JP, Hammer F, Chalder SM, Borucka-Mankiewicz M, Hauffa BP, Malunowicz EM, Stewart PM, Shackleton CHL. 2004. Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: analytical study. Lancet 363:2128–2135 [DOI] [PubMed] [Google Scholar]
- 4. Onoda M, Hall PF. 1982. Cytochrome b5 stimulates purified testicular microsomal cytochrome P-450 (C21 side-chain cleavage). Biochem Biophys Res Commun 108:454–460 [DOI] [PubMed] [Google Scholar]
- 5. Geller DH, Auchus RJ, Miller WL. 1999. P450c17 mutations R347H and R358Q selectively disrupt 17,20-lyase activity by disrupting interactions with P450 oxidoreductase and cytochrome b5. Mol Endocrinol 13:167–175 [DOI] [PubMed] [Google Scholar]
- 6. Yanase T, Simpson ER, Waterman MR. 1991. 17α-Hydroxylase/17,20-lyase deficiency: from clinical investigation to molecular definition. Endocr Rev 12:91–108 [DOI] [PubMed] [Google Scholar]
- 7. Geller DH, Auchus RJ, Mendonça BB, Miller WL. 1997. The genetic and functional basis of isolated 17,20-lyase deficiency. Nat Genet 17:201–205 [DOI] [PubMed] [Google Scholar]
- 8. Hershkovitz E, Parvari R, Wudy SA, Hartmann MF, Gomes LG, Loewental N, Miller WL. 2008. Homozygous mutation G539R in the gene for P450 oxidoreductase in a family previously diagnosed as having 17,20-lyase deficiency. J Clin Endocrinol Metab 93:3584–3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simsek E, Ozdemir I, Lin L, Achermann JC. 2005. Isolated 17,20-lyase (desmolase) deficiency in a 46,XX female presenting with delayed puberty. Fertil Steril 83:1548–1551 [DOI] [PubMed] [Google Scholar]
- 10. Van Den Akker EL, Koper JW, Boehmer AL, Themmen AP, Verhoef-Post M, Timmerman MA, Otten BJ, Drop SL, De Jong FH. 2002. Differential inhibition of 17α-hydroxylase and 17,20-lyase activities by three novel missense CYP17 mutations identified in patients with P450c17 deficiency. J Clin Endocrinol Metab 87:5714–5721 [DOI] [PubMed] [Google Scholar]
- 11. Sherbet DP, Tiosano D, Kwist KM, Hochberg Z, Auchus RJ. 2003. CYP17 mutation E305G causes isolated 17,20-lyase deficiency by selectively altering substrate binding. J Biol Chem 278:48563–48569 [DOI] [PubMed] [Google Scholar]
- 12. Tiosano D, Knopf C, Koren I, Levanon N, Hartmann MF, Hochberg Z, Wudy SA. 2008. Metabolic evidence for impaired 17alpha-hydroxylase activity in a kindred bearing the E305G mutation for isolate 17,20-lyase activity. Eur J Endocrinol 158:385–392 [DOI] [PubMed] [Google Scholar]
- 13. Kok RC, Timmerman MA, Wolffenbuttel KP, Drop SL, de Jong FH. 2010. Isolated 17,20-Lyase deficiency due to the cytochrome b5 mutation W27X. J Clin Endocrinol Metab 95:994–999 [DOI] [PubMed] [Google Scholar]
- 14. Shackleton C, Marcos J, Malunowicz EM, Szarras-Czapnik M, Jira P, Taylor NF, Murphy N, Crushell E, Gottschalk M, Hauffa B, Cragun DL, Hopkin RJ, Adachi M, Arlt W. 2004. Biochemical diagnosis of Antley-Bixler syndrome by steroid analysis. Am J Med Genet A 128A:223–231 [DOI] [PubMed] [Google Scholar]
- 15. Dhir V, Reisch N, Bleicken CM, Lebl J, Kamrath C, Schwarz HP, Grötzinger J, Sippell WG, Riepe FG, Arlt W, Krone N. 2009. Steroid 17α-hydroxylase deficiency: functional characterization of four mutations (A174E, V178D, R440C, L465P) in the CYP17A1 gene. J Clin Endocrinol Metab 94:3058–3064 [DOI] [PubMed] [Google Scholar]
- 16. Parajes S, Kamrath C, Rose IT, Taylor AE, Mooij CF, Dhir V, Grötzinger J, Arlt W, Krone N. 2011. A novel entity of clinically isolated adrenal insufficiency caused by a partially inactivating mutation of the gene encoding for P450 side chain cleavage enzyme (CYP11A1). J Clin Endocrinol Metab 96:E1798–E1806 [DOI] [PubMed] [Google Scholar]
- 17. Maghzal GJ, Thomas SR, Hunt NH, Stocker R. 2008. Cytochrome b5, not superoxide anion radical, is a major reductant of indoleamine 2,3-dioxygenase in human cells. J Biol Chem 283:12014–12025 [DOI] [PubMed] [Google Scholar]
- 18. Neres MS, Auchus RJ, Shackleton CH, Kater CE. 2010. Distinctive profile of the 17-hydroxylase and 17,20-lyase activities revealed by urinary steroid metabolomes of patients with CYP17 deficiency. Arq Bras Endocrinol Metabol 54:826–832 [DOI] [PubMed] [Google Scholar]
- 19. Mathews FS. 1985. The structure, function and evolution of cytochromes. Prog Biophys Mol Biol 45:1–56 [DOI] [PubMed] [Google Scholar]
- 20. Gupta MK, Geller DH, Auchus RJ. 2001. Pitfalls in characterizing P450c17 mutations associated with isolated 17,20-lyase deficiency. J Clin Endocrinol Metab 86:4416–4423 [DOI] [PubMed] [Google Scholar]
- 21. Goebelsmann U, Zachmann M, Davajan V, Israel R, Mestman JH, Mishell DR. 1976. Male pseudohermaphroditism consistent with 17,20-desmolase deficiency. Gynecol Invest 7:138–156 [DOI] [PubMed] [Google Scholar]
- 22. Campo S, Stivel M, Nicolau G, Monteagudo C, Rivarola M. 1979. Testicular function in post pubertal male pseudohermaphroditism. Clin Endocrinol (Oxf) 11:481–490 [DOI] [PubMed] [Google Scholar]
- 23. Forest MG, Lecornu M, de Peretti E. 1980. Familial male pseudohermaphroditism due to 17–20-desmolase deficiency. I. In vivo endocrine studies. J Clin Endocrinol Metab 50:826–833 [DOI] [PubMed] [Google Scholar]
- 24. Campo S, Moteagudo C, Nicolau G, Pellizzari E, Belgorosky A, Stivel M, Rivarola M. 1981. Testicular function in prepubertal male pseudohermaphroditism. Clin Endocrinol (Oxf) 14:11–22 [DOI] [PubMed] [Google Scholar]
- 25. Zachmann M, Werder EA, Prader A. 1982. Two types of male pseudohermaphroditism due to 17,20-desmolase deficiency. J Clin Endocrinol Metab 55:487–490 [DOI] [PubMed] [Google Scholar]
- 26. Kaufman FR, Costin G, Goebelsmann U, Stanczyk FZ, Zachmann M. 1983. Male pseudohermaphroditism due to 17,20-desmolase deficiency. J Clin Endocrinol Metab 57:32–36 [DOI] [PubMed] [Google Scholar]
- 27. Larrea F, Lisker R, Bañuelos R, Bermúdez JA, Herrera J, Núñez Rasilla V, Pérez-Palacios G. 1983. Hypergonadotrophic hypogonadism in an XX female subject due to 17,20 steroid desmolase deficiency. Acta Endocrinol 103:400–405 [DOI] [PubMed] [Google Scholar]
- 28. de Peretti E, Pradon M, Forest MG. 1984. 17,20-desmolase deficiency in a female newborn, paradoxically virilized in utero. J Steroid Biochem 20:455–458 [DOI] [PubMed] [Google Scholar]
- 29. Yamazaki H, Johnson WW, Ueng YF, Shimada T, Guengerich FP. 1996. Lack of electron transfer from cytochrome b5 in stimulation of catalytic activities of cytochrome P450 3A4. Characterization of a reconstituted cytochrome P450 3A4/NADPH-cytochrome P450 reductase system and studies with apo-cytochrome b5. J Biol Chem 271:27438–27444 [DOI] [PubMed] [Google Scholar]
- 30. Naffin-Olivos JL, Auchus RJ. 2006. Human cytochrome b5 requires residues E48 and E49 to stimulate the 17,20-lyase activity of cytochrome P450c17. Biochemistry 45:755–762 [DOI] [PubMed] [Google Scholar]
- 31. Falzone CJ, Mayer MR, Whiteman EL, Moore CD, Lecomte JT. 1996. Design challenges for hemoproteins: the solution structure of apocytochrome b5. Biochemistry 35:6519–6526 [DOI] [PubMed] [Google Scholar]
- 32. Wang WH, Lu JX, Yao P, Xie Y, Huang ZX. 2003. The distinct heme coordination environments and heme-binding stabilities of His39Ser and His39Cys mutants of cytochrome b5. Protein Eng 16:1047–1054 [DOI] [PubMed] [Google Scholar]
- 33. Mukhopadhyay K, Lecomte JT. 2004. A relationship between heme binding and protein stability in cytochrome b5. Biochemistry 43:12227–12236 [DOI] [PubMed] [Google Scholar]
- 34. Guillou H, D'Andrea S, Rioux V, Barnouin R, Dalaine S, Pedrono F, Jan S, Legrand P. 2004. Distinct roles of endoplasmic reticulum cytochrome b5 and fused cytochrome b5-like domain for rat Δ6-desaturase activity. J Lipid Res 45:32–40 [DOI] [PubMed] [Google Scholar]
- 35. Tomalik-Scharte D, Maiter D, Kirchheiner J, Ivison HE, Fuhr U, Arlt W. 2010. Impaired hepatic drug and steroid metabolism in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. Eur J Endocrinol 163:919–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Noordam C, Dhir V, McNelis JC, Schlereth F, Hanley NA, Krone N, Smeitink JA, Smeets R, Sweep FC, Claahsen-van der Grinten HL, Arlt W. 2009. Inactivating PAPSS2 mutations in a patient with premature pubarche. N Engl J Med 360:2310–2318 [DOI] [PubMed] [Google Scholar]
- 37. Giordano SJ, Kaftory A, Steggles AW. 1994. A splicing mutation in the cytochrome b5 gene from a patient with congenital methemoglobinemia and pseudohermaphrodism. Hum Genet 93:568–570 [DOI] [PubMed] [Google Scholar]
- 38. Hegesh E, Hegesh J, Kaftory A. 1986. Congenital methemoglobinemia with a deficiency of cytochrome b5. N Engl J Med 314:757–761 [DOI] [PubMed] [Google Scholar]
- 39. Percy MJ, Lappin TR. 2008. Recessive congenital methaemoglobinaemia: cytochrome b(5) reductase deficiency. Br J Haematol 141:298–308 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.