Abstract
Hyaluronan is a polysaccharide, which is ubiquitous in vertebrates and has been reported to be strongly hydrated in a biological environment. We study the hydration of hyaluronan in solution using the rotational dynamics of water as a probe. We measure these dynamics with polarization-resolved femtosecond-infrared and terahertz time-domain spectroscopies. Both experiments reveal that a subensemble of water molecules is slowed down in aqueous solutions of hyaluronan amounting to ∼15 water molecules per disaccharide unit. This quantity is consistent with what would be expected for the first hydration shell. Comparison of these results to the water dynamics in aqueous dextran solution, a structurally similar polysaccharide, yields remarkably similar results. This suggests that the observed interaction with water is a common feature for hydrophilic polysaccharides and is not specific to hyaluronan.
In recent years, it has become evident that the biological function of macromolecules is not solely determined by their structure and chemical composition. In fact, the interaction with water molecules can provide the driving forces for the structure and stability of biomolecules through both enthalpic and entropic contributions (1). Hence, it is clear that water as a solvent cannot be viewed as a continuum, but the specific interaction with the polymer plays a key role for the macroscopic properties of biomolecules. Moreover, water mediates the interaction between biomolecules (polysaccharides, proteins, lipids, nucleic acids) and is thus crucial for molecular recognition and biological function (2).
Hyaluronan (HA) is an important macromolecule that is widely used for medical, pharmaceutical, and cosmetic applications (3). It is present in the vitreous body, the synovial fluid, and the extracellular matrix, and is a major structural component of biological tissues. In particular, the high viscosity and viscoelasticity is seemingly unique to aqueous solutions of HA (3,4). These peculiar hydrodynamic properties of aqueous HA solutions have been related to the ability of HA to strongly bind water molecules (5,6). In addition, the high affinity toward water is considered to be important for the biological function of HA (7). However, computer simulations predict the hydration shell of the carbohydrate to be rather short-lived and quite dynamic (8).
Herein, we report a study of the hydration of HA in solution. To investigate whether there is any specific or extraordinary interaction of HA with water, we compare our results for HA to the hydration of dextran, a structurally similar polysaccharide, whose hydrodynamic properties differ significantly from HA in solution. We use the rotational dynamics of water as a probe for the interaction strength with a solute. Due to the three-dimensional hydrogen-bonded structure of water, rotation of water molecules takes place via large-angle jumps (9). These jumps require breaking and formation of a new hydrogen bond and the change in reorientation dynamics have thus been shown to be very sensitive to the interaction with its environment. We study the rotational dynamics using two complementary experimental techniques: polarization-resolved femtosecond infrared spectroscopy (fs-IR) (10) and terahertz (THz) time-domain spectroscopy (11).
First, in the fs-IR experiment we monitor the rotation of the OH or equivalent OD bond of water (for experimental details, see the Supporting Material). This is achieved by labeling an OD stretching vibration in an isotopically diluted (typically 8% HOD in H2O) solution via vibrational excitation with an intense midinfrared (pump) laser-pulse. The excitation occurs most efficiently for OD oscillators aligned parallel to the pump pulse polarization and thus the excitation is anisotropic. The evolution of this excitation anisotropy is probed with a second weak infrared pulse by measuring the transient infrared spectra as a function of the delay time, t, for polarizations both parallel and perpendicular to the pump polarization. After correcting for the heating of the sample due to the dissipated energy, we obtain from the data the anisotropy parameter R. The value R(t) directly corresponds to the second-order rotational correlation function of the excited OD oscillators.
For HOD molecules in bulk water, R(t) decays monoexponentially with a characteristic rotation time of τb,IR = 2.5 ps (10). As solutes such as salts (11) or amphiphiles (12) are added, a subensemble of water molecules in the hydration shell of the solute is slowed down. This leads to a distinctive biexponential decay of R(t). The magnitude of the slow component provides a direct measure for the number of water molecules hydrating the solute.
In Fig. 1 we show the measured R(t) decays for solutions of HA and dextran. It is evident that for both polysaccharides the anisotropy decays slow down, as the concentration of the polymer increases. The observed slow-down of the rotational dynamics of water molecules is characteristic for the presence of a subensemble of water molecules with distinctly slower rotational dynamics and cannot be described with a slowdown of all water molecules (i.e., a single exponential decay of R(t); see the Supporting Material). Hence, we fit the measured R(t) decays to a biexponential decay,
| (1) |
where R0 is the anisotropy at t = 0. The fraction fb,IR has the same rotational dynamics as neat water (τb,IR = 2.5 ps). A second fraction of water molecules, fs,IR (= 1–fb,IR), exhibits significantly slower rotational dynamics. Fits of Eq. 1 to the experimental R(t) decays indicate that these molecules are static on the 5-ps timescale of our experiment (τ/τs,IR ≈ 0; see the Supporting Material). This assumption is supported by a Brillouin scattering study (6) revealing ∼20 times slower relaxation in the first hydration shell of HA.
Figure 1.
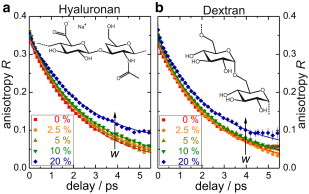
Anisotropy parameter, R(t), for the OD stretch vibration of 8% HOD in aqueous solutions of hyaluronan (a) and dextran (b) at different weight fractions, w, of the polysaccharide ranging from 0 to 20%. (Insets) Chemical structure of both polysaccharides.
As can be seen in Fig. 2, the thus-obtained fraction of slow water molecules, fs,IR, increases approximately linearly as the concentration of both polysaccharides increases. It should be noted that HA and dextran themselves contain OH groups. Due to fast isotopic exchange, these fragments will contribute to the fs-IR signals. However, these OD groups very likely have a relatively short vibrational lifetime due to efficient coupling to the saccharide (13) and do thus not contribute significantly to our measurements. Further, the determined values of fs,IR are significantly higher (ten and three times for HA and dextran, respectively) than what would be expected if fs,IR were completely due to the saccharide OH groups. Hence, our results show that the observed subensemble of slow OD groups originates predominantly from the interaction of water with the biopolymers. The linear increase of fs,IR with polymer weight fraction indicates that water molecules in the direct vicinity of the solute molecules are affected and that the polysaccharide does not have any long-range effects on the rotational dynamics of water molecules. The steeper slope of fs,IR versus weight fraction of polymer, w, for HA points toward a slightly enhanced hydration of HA compared to dextran.
Figure 2.

Fraction of rotationally slowed-down water molecules for aqueous solution of hyaluronan (blue circles) and dextran (red squares). (Solid symbols) fs-IR experiments, fs,IR. (Open symbols) Results obtained using THz spectroscopy, fs,THz. (Lines) Linear fits. Error bars show the typical reproducibility within two measurements.
Complementary to the fs-IR study, we perform THz spectroscopy experiments. With this technique, we probe the rotational dynamics of the permanent electric dipoles of water. We apply a THz pulse, which corresponds to a single oscillation (∼1 ps) of an electric field to the samples. This field leads to a partial alignment of the dipolar water molecules, which in turn couple to the THz pulse. This results in refraction and absorption of the pulse in the sample. We quantify the interaction of the pulses with the sample by extracting the complex dielectric function, as a function of field frequency, ν. Here, the real, ε′, and the imaginary part, ε″, of the permittivity correspond to the in-phase and out-of-phase polarization components of the sample, respectively (11).
For neat water, the dielectric spectrum is dominated by an intense relaxation at ∼20 GHz (12) with a peak in the dielectric loss, ε″, and a dispersion in the dielectric permittivity, ε′. This relaxation is characteristic for the rotational dynamics of the dipolar water molecules. Due to its broad nature, the rotational relaxation extends to THz frequencies (Fig. 3) and, in particular, the wing of the peak in ε″ dominates the spectra at these frequencies.
Figure 3.
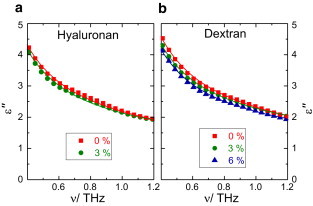
Dielectric loss spectra, ε″(ν), for aqueous solutions of HA (a; w = 0 and 3%) and dextran (b; w = 0, 3, and 6%). (Symbols) Experimental data. (Lines) Fits with two Debye-type relaxations. (See the Supporting Material.)
As can be seen in Fig. 3, the dielectric loss decreases as the concentration of dextran and HA increases. This decrease in ε″ originates from a decrease of the amplitude of the rotational relaxation peak and indicates that fewer water molecules exhibit bulklike rotational dynamics. Following previous practice (11), we extract the amplitude of the rotational relaxation peak (see the Supporting Material). This amplitude is proportional to the detected concentration of bulklike water molecules, cb. From the difference between the analytical (total) water concentration, , and cb, we obtain the fraction of water molecules that have significantly longer rotational correlation times than bulk-like water, The thus-obtained fraction of slow water molecules (Fig. 2) is consistent with the fs,IR values. Due to the high viscosity of these solutions, the applicable concentration range is limited to low polysaccharide concentrations (i.e., ≤3% for HA and ≤6% for dextran).
One salient feature of the experiments is that the two techniques probe rotations around different axes of the water molecule. That is, fs-IR experiments measure the rotation of the OD vector of HOD molecules, whereas THz experiments probe the rotation of the permanent electric dipole of water. Whereas for salt solutions both approaches are observed to give different results, due to local anisotropy induced by the ions (11), in this study both experiments yield the same fraction of water molecules that is slowed down. This finding indicates that the rotation of the water molecules hydrating the polysaccharide is slowed down isotropically (compared to bulk-water). Hence, we find no evidence for distinct types of hydrogen-bond-donating or -accepting water molecules around the saccharides.
We can readily relate the observed fs values to hydration numbers, i.e., the number of slow water molecules per disaccharide unit. For HA we find 15 ± 3 water molecules per disaccharide to be affected. This finding is in remarkably good agreement with results using differential scanning calorimetry that reveal ∼15 tightly bound water molecules per disaccharide unit (14,15). The hydration number is further consistent with 10–15 water molecules in the hydration shell, as inferred from molecular-dynamics simulations (16). For aqueous solutions of dextran, we determine 11 ± 3 water molecules in the hydration shell to be slowed down. Comparison of the two polysaccharides shows that their hydration and thus interaction with water is rather similar within the experimental uncertainty and seems to be a common feature of hydrophilic carbohydrates. In fact, the slightly larger hydration number for HA might be due to its ionic nature (dextran is neutral), i.e., the charged carboxylic group and/or the Na+ ions additionally slow down some water molecules. Thus, from the study of water mobility in polysaccharide solution, we find no evidence for exceptional binding of water by HA and the hydration appears to be similar to another polysaccharide, dextran. Therefore, the peculiar hydrodynamic behavior of HA appears not to be related to its hydration.
Acknowledgments
The authors acknowledge funding from the Stichting voor Fundamenteel Onderzoek der Materie with financial support from the Nederlandse Organisatie voor Wetenschappelijk Onderzoek. J.H. thanks the Deutsche Forschungsgemeinschaft for a research fellowship.
Supporting Material
References and Footnotes
- 1.Levy Y., Onuchic J.N. Water mediation in protein folding and molecular recognition. Annu. Rev. Biophys. Biomol. Struct. 2006;35:389–415. doi: 10.1146/annurev.biophys.35.040405.102134. [DOI] [PubMed] [Google Scholar]
- 2.Lemieux R.U. How water provides the impetus for molecular recognition in aqueous solution. Acc. Chem. Res. 1996;29:373–380. [Google Scholar]
- 3.Lapcík L., Jr., De Smedt S., Chabrecek P. Hyaluronan: preparation, structure, properties, and applications. Chem. Rev. 1998;98:2663–2684. doi: 10.1021/cr941199z. [DOI] [PubMed] [Google Scholar]
- 4.Day A.J., Sheehan J.K. Hyaluronan: polysaccharide chaos to protein organization. Curr. Opin. Struct. Biol. 2001;11:617–622. doi: 10.1016/s0959-440x(00)00256-6. [DOI] [PubMed] [Google Scholar]
- 5.Ogston A.G., Stanier J.E. Further observations on the preparation and composition of the hyaluronic acid complex of ox synovial fluid. Biochem. J. 1952;52:149–156. doi: 10.1042/bj0520149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S.A., Flowers M.R., Lindsay S.M. Brillouin-scattering study of hyaluronic acid: dynamic coupling with the water of hydration and phase transitions. Phys. Rev. E. 1993;47:677–683. doi: 10.1103/physreve.47.677. [DOI] [PubMed] [Google Scholar]
- 7.Kogan G., Soltés L., Gemeiner P. Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007;29:17–25. doi: 10.1007/s10529-006-9219-z. [DOI] [PubMed] [Google Scholar]
- 8.Almond A., Brass A., Sheehan J.K. Oligosaccharides as model systems for understanding water-biopolymer interaction: hydrated dynamics of a hyaluronan decamer. J. Phys. Chem. B. 2000;104:5634–5640. [Google Scholar]
- 9.Laage D., Hynes J.T. A molecular jump mechanism of water reorientation. Science. 2006;311:832–835. doi: 10.1126/science.1122154. [DOI] [PubMed] [Google Scholar]
- 10.Rezus Y.L., Bakker H.J. On the orientational relaxation of HDO in liquid water. J. Chem. Phys. 2005;123:114502. doi: 10.1063/1.2009729. [DOI] [PubMed] [Google Scholar]
- 11.Tielrooij K.J., van der Post S.T., Bakker H.J. Anisotropic water reorientation around ions. J. Phys. Chem. B. 2011;115:12638–12647. doi: 10.1021/jp206320f. [DOI] [PubMed] [Google Scholar]
- 12.Tielrooij K.J., Hunger J., Bakker H.J. Influence of concentration and temperature on the dynamics of water in the hydrophobic hydration shell of tetramethylurea. J. Am. Chem. Soc. 2010;132:15671–15678. doi: 10.1021/ja106273w. [DOI] [PubMed] [Google Scholar]
- 13.Gulmen T.S., 3rd, Sibert E.L. Vibrational energy relaxation of the OH(D) stretch fundamental of methanol in carbon tetrachloride. J. Chem. Phys. 2005;123:204508. doi: 10.1063/1.2131055. [DOI] [PubMed] [Google Scholar]
- 14.Jouon N., Rinaudo M., Desbrières J. Hydration of hyaluronic acid as a function of the counterion type and relative humidity. Carbohydr. Polym. 1995;26:69–73. [Google Scholar]
- 15.Průšová A., Šmejkalová D., Kučerík J. An alternative DSC approach to study hydration of hyaluronan. Carbohydr. Polym. 2010;82:498–503. doi: 10.1016/j.carbpol.2013.07.049. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann J., Möhle K., Arnold K. Molecular dynamics study of hyaluronic acid in water. J. Mol. Struct. 1998;422:109–121. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


