Abstract
Clostridium difficile is a significant problem in hospital settings as the most common cause of nosocomial diarrhea worldwide. C. difficile infections (CDIs) are characterized by an acute intestinal inflammatory response with neutrophil infiltration. These symptoms are primarily caused by the glucosylating toxins, TcdA and TcdB. In the past decade, the frequency and severity of CDIs have increased markedly due to the emergence of so-called hypervirulent strains that overproduce cytotoxic glucosylating toxins relative to historical strains. In addition, these strains produce a third toxin, binary toxin or C. difficile transferase (CDT), that may contribute to hypervirulence. Both the glucosylating toxins and CDT covalently modify target cell proteins to cause disassembly of the actin cytoskeleton and induce severe inflammation. This review summarizes our current knowledge of the mechanisms by which glucosylating toxins and CDT disrupt target cell function, alter host physiology and stimulate immune responses.
Key Words: Glucosylating toxin, Binary toxin, Clostridium difficile infection, Hypervirulence
Introduction
Clostridium difficile is a Gram-positive, spore-forming, obligate anaerobe that is the leading cause of nosocomial diarrhea worldwide [1, 2]. The spectrum of disease caused by C. difficile ranges from mild diarrhea to pseudomembranous colitis, i.e. severe inflammation of the colonic lining. C. difficile infection (CDI) typically occurs in patients whose normal gut flora has been disrupted by antibiotic treatment. Suppression of the intestinal flora permits C. difficile to colonize the colon and produce the glucosylating toxins that are primarily responsible for the symptoms associated with CDI [3].
Because C. difficile is naturally resistant to most antibiotics, CDIs are often difficult to treat [4]. High relapse rates are frequently observed because C. difficile spores readily persist in hospital settings and are resistant to common disinfectants [5]. Of the 1–3% of hospitalized patients receiving antibiotics who become infected with C. difficile, about 25% will experience recurrent infections [1]. Thus, CDI represents a significant problem in health care settings, costing US hospitals alone an estimated USD 1–3 billion annually [6, 7].
In the past decade, the severity, morbidity and frequency of CDI have increased markedly. This rise corresponds with the emergence of so-called hypervirulent C. difficile strains and the increased use of fluoroquinoline antibiotics [1]. Hypervirulent strains toxinotype III and ribotype 027 (B1/NAP1) cause CDI with higher rates of pseudomembranous colitis and mortality [8]. Whereas 027 strains accounted for less than 0.1% of strain types collected from 1984 to 1993, they accounted for approximately 50% of strains isolated from outbreaks from 2000 to 2003. The increased virulence of this strain has been attributed to its natural fluoroquinoline resistance [8], higher sporulation rates [9], and approximately 20-fold increase in glucosylating toxin production relative to historical C. difficile strains [10]. NAP1/027 strains also produce glucosylating toxin variants that are more cytotoxic [11, 12] and encode a third toxin, binary toxin or C. difficile transferase (CDT), which may further enhance their pathogenicity relative to historical strains [13].
The glucosylating toxins are primarily responsible for the colonic inflammation that characterizes C. difficile-associated disease. Although other surface proteins can contribute to the inflammatory response, this review focuses on recent advances in our understanding of how C. difficile toxins cause disease and their role in modulating innate immunity.
C. difficile Glucosylating Toxins
TcdA and TcdB are part of the large clostridial toxin family, which includes lethal toxin and hemorrhagic (C. sordellii), TpeL (C. perfringens), and α-toxin (C. novyi) [14]. Large clostridial toxins are multidomain toxins that transfer glycosyl residues to small Rho GTPases and cause cell rounding, inhibition of cell division and eventually cell death [14].
The Glucosylating Toxins Are Inflammatory Enterotoxins
The glucosylating toxins are primarily responsible for the symptoms associated with CDI. In animal models, TcdA and TcdB are sufficient to cause all the symptoms of CDI, including intestinal injury, mucosal inflammation and marked neutrophil recruitment (fig. 1) [15]. Notably, antibody-mediated neutralization of TcdA and/or TcdB is sufficient to alleviate disease symptoms [16], and high anti-TcdA antibody titers correlate with asymptomatic carriage and protection against recurrence [17].
Fig. 1.
Pathogenesis of Clostridium difficile. When C. difficile spores are ingested, they are stimulated to germinate by bile salts (e.g. taurocholate) in the small intestine. C. difficile can productively colonize the colon of individuals whose normal intestinal flora has been disrupted (e.g. by antibiotic treatment). Colonization likely depends upon adherence of the bacterium to the epithelium, although little is known about the factors that mediate adherence. CDT-producing strains may increase their adherence to intestinal epithelial cells by inducing microtubule protrusions that trap C. difficile. Glucosylating toxin-producing strains stimulate inflammation of the colonic lining by inducing cytoskeletal changes that compromise the epithelial barrier and inflammatory cytokine production. Disruption of tight junctions allows the toxins to cross the epithelium, where they can further induce inflammatory cytokine production in lymphocytes and mast cells. This leads to escalation of the inflammatory response due to neutrophil and lymphocyte influx, which can lead to pseudomembrane formation. Whether glucosylating toxins enter the bloodstream remains unclear, although in a zebrafish model of infection, the toxins can become systemic [78]. During colonization of the host, C. difficile produces spores that are shed by the patient and facilitate transmission of C. difficile to susceptible hosts.
TcdA and TcdB covalently modify and inactivate the Rho family GTPases Rho, Rac and Cdc42 [3], which play key roles in regulating signaling pathways. Glucosylation of Rho GTPases results in disaggregation of the actin cytoskeleton, cell rounding, cell death and loss of intestinal epithelium barrier function. The toxins also stimulate the release of proinflammatory cytokines (e.g. IL-1β, TNF-α, IL-8) from epithelial cells and resident mucosal immune cells [18, 19, 20] (fig. 1), which causes neutrophil influx and leads to further destruction of the intestinal lining. Notably, neutrophil influx into the peripheral blood (leukocytosis) correlates with poor prognosis [21], suggesting that an overzealous host inflammatory response is responsible for much of the pathology associated with CDI [15].
Despite the knowledge that TcdA and TcdB function as inflammatory enterotoxins, little is known about the specific mechanisms by which they activate innate immunity and stimulate proinflammatory cytokine release. As with other bacterial toxins [22], purified TcdA and TcdB activate an ASC-containing inflammasome, which leads to the production of proinflammatory cytokines like IL-1β [23]. Accordingly, inhibition of inflammasome signaling by an IL-1 receptor antagonist (anakinra) protected mice from C. difficile toxin-induced intestinal injury and mimicked the phenotype of administering toxin to ASC−/− mice [23]. These findings suggest that glucosylating toxin-induced activation of the inflammasome is largely responsible for the intestinal injury that characterizes severe CDI.
Glucosylating Toxins and Virulence
The relative importance of TcdA and TcdB during infection has been a matter of debate for decades. Although these toxins share a common domain structure, approximately 66% sequence similarity and similar enzymatic activities, TcdA has an extended C-terminus, different cell tropism and is generally less potent in cell cytotoxicity assays than TcdB (fig. 2) [3, 24]. Nevertheless, in early rodent models of infection, TcdA caused intestinal inflammation more efficiently than TcdB [3, 25]. Later studies using humanized mice, however, revealed that purified TcdB can recapitulate the intestinal pathology of CDI in humans [20], suggesting that the receptors for TcdB are poorly expressed in many animal models.
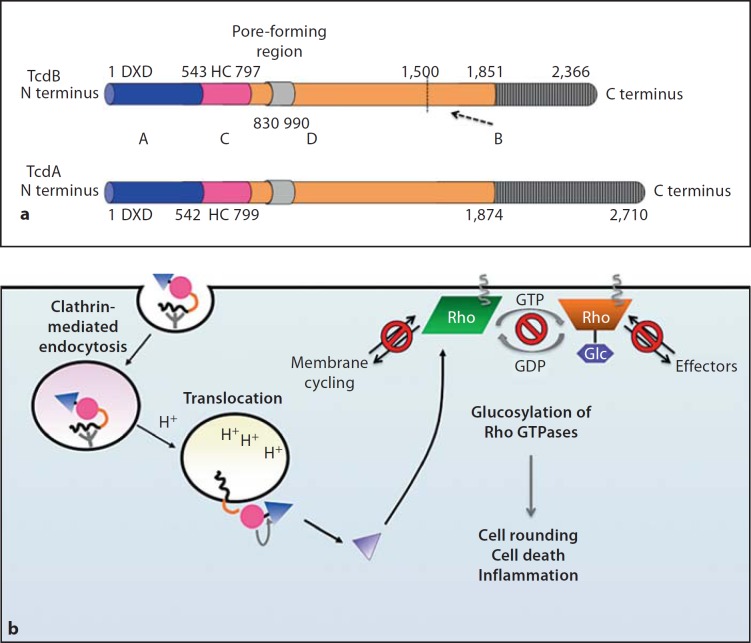
Fig. 2.
aABCD structure of glucosylating toxins TcdA and TcdB. Functional and structural domain boundaries are marked, with active site residues being indicated. A = ‘Activity’ domain of Glc (blue); C = ‘cutting’ domain/autoprocessing CPD (pink); D = ‘delivery’ domain/translocation domain (orange); B = ‘binding’ domain/receptor binding domain (grey). For the D domain, the minimal pore-forming region consists of residues 830–990, while residues 1–1,850 are sufficient to mediate intoxication of target cells. The B domain contains multiple repeat sequences (CROPs), which range in size from 21–50 residues and are repeated throughout the C-terminus of the protein. The CROPs are more divergent and less frequent in TcdB than in TcdA. The dashed arrow indicates a putative minor receptor binding domain (residues 1,500–1,850) identified by deletion analyses [38, 40]. b Intoxication of target cells by TcdA and TcdB. Binding of the B domain to unknown receptors on target cells results in clathrin-dependent receptor-mediated endocytosis. During acidification of the endosome, conformational changes occur within the toxin that result in pore formation by the D domain and translocation of the A domain into the cytosol. Exposure of the C domain to InsP6 (yellow) activates its protease function, resulting in toxin autoprocessing and release of the A domain into the cytosol. The A domain glucosylates Rho GTPases (Rho, Rac, Cdc42 family) on a conserved Thr residue, which prevents Rho GTPases from interacting with their cognate effectors and sequesters them at the membrane.
Recent genetic analyses of isogenic toxin mutants confirmed that TcdB is essential for virulence in a hamster model of infection [26, 27]. The requirement for TcdA during infection, however, remains controversial. While TcdA+, TcdB– mutants were less virulent in studies performed by Lyras et al. [27], the equivalent mutants in studies performed by Kuehne et al. [26] were as virulent as wild-type. Differences in the hamster models of infection or in the parent strains may account for the discrepancies observed between these two studies [28]. Nevertheless, it should be noted that in the context of human infection, TcdA+, TcdB– strains have never been isolated from patients with CDI, even though TcdA-, TcdB+ strains are routinely isolated from patients (frequency between 0.2 and 97% depending on location) [29]. These isolates cause the same spectrum of disease as TcdA+, TcdB+ strains and often cause more severe disease symptoms than TcdA+, TcdB+ strains for reasons that remain unclear [29].
Structure and Function of C. difficile-Glucosylating Toxins
Like other large clostridial toxin members, TcdA (308 kDa) and TcdB (270 kDa) have an ABCD domain structure (fig. 2a) [30]. The N-terminal glucosyltransferase (Glc) domain comprises the ‘A’ activity domain (aa 1–543, TcdB), the C-terminal receptor binding domain makes up the ‘B’ binding domain (aa 1,851–2,366, TcdB), the cysteine protease domain (CPD) comprises the ‘C’ cutting domain (aa 544–797, TcdB) and an internal hydrophobic region makes up the ‘D’ delivery domain (aa 800–1,850, TcdB).
During intoxication, the C-terminal receptor-binding domain binds to unknown carbohydrate receptors on the target cell surface, and the toxin is internalized via receptor-mediated endocytosis (fig. 2b). Acidification of the endosome causes conformational changes in the toxin, which allows the hydrophobic D domain to insert into the endosomal membrane and form a pore; this pore translocates the N-terminal Glc domain and internal CPD. The CPD is thought to bind inositol hexakisphosphate (InsP6) in the cytosol, which subsequently activates toxin autoprocessing and releases the Glc domain into the target cell cytosol. The liberated Glc domain then modifies and inactivates Rho GTPases, which leads to the inflammation that characterizes C. difficile-associated disease [3].
(I) Binding of Glucosylating Toxins to Cell Membranes
The C-terminal receptor binding domain of C. difficile glucosylating toxins consists of combined repetitive oligopeptides (CROPs) that can recognize carbohydrate structures such as Gal-A-(1,3)-Gal-β-(1,4)-GlcNAc on host epithelial cells [30]. Multiple carbohydrate binding sites are present in both TcdA and TcdB receptor binding domains [3, 31], and recent mass spectrometry analyses indicate that both toxins can interact with human milk oligosaccharides [32]. Although the receptors for glucosylating toxins in humans are unknown, the crystal structure of a portion of the receptor binding domain of TcdA has been solved in complex with a synthetic derivative of a carbohydrate ligand [33]. Extrapolation of the solved structure suggests that the C-terminal domain forms a β-solenoid left-handed helix; this structure increases the surface area of protein and is frequently observed in bacterial cell-surface binding proteins [33]. Consistent with this hypothesis, the cryoEM structure of TcdA by Pruitt et al. [34] indicates that the receptor binding region adopts an elongated, kinked-tail domain at neutral and low pH.
The greatest diversity in sequence between TcdA and TcdB is found in the C-terminal binding domain [3]. Accordingly, the toxins are thought to bind different receptors on target cells, with the receptor for TcdB likely being restricted to the basolateral membrane of some cell types [1, 35]. Notably, heterogeneity in the C-terminal region is also observed between TcdB toxins produced by historical and hypervirulent strains [11]. These differences may account for the observation that TcdB from hypervirulent strains can intoxicate a broader range of cell types [30, 36].
While the C-terminal domain is sufficient to induce clathrin-dependent receptor-mediated endocytosis [37], it is not absolutely required for receptor-mediated endocytosis, since a CROP-deficient toxin can still enter targets cells albeit with reduced uptake kinetics [38]. The CROP region may also function to hold the toxin in an inactive conformer at neutral pH, based on cryoEM analyses of full-length toxin by Pruitt et al. [34] and target cell binding studies by Olling et al. [38].
(II) Translocation of Glucosylating Toxins across the Endosomal Membrane
During glucosylating toxin entry into cells, endosomal acidification triggers dramatic rearrangements within the toxins that allow the central hydrophobic translocation domain to insert into the endosomal membrane [39]. This delivery domain (approx. 800–1,850 aa; fig. 2) is thought to form a pore that translocates the Glc domain into the target cell cytosol. Recent deletion and biochemical analyses indicate that residues 830–990 constitute the minimal pore-forming region for the toxins, with Glu970 and Glu976 acting as potential pH sensors for pore formation [40]. These studies also indicated that residues 1–1,500 are sufficient for toxin activity, i.e. translocation, autoprocessing and Glc function [40]. The authors further proposed that residues 1,500–1,850 of the delivery domain constitute a minor receptor binding domain, since a truncated toxin lacking the CROP sequences in the C-terminal receptor binding domain (TcdB residues 1–1,851) can still intoxicate cells with reduced kinetics [38, 40].
The translocation region is believed to function as a pH sensor for pore formation [24, 30]. Pruitt et al. [34] directly visualized pH-inducible changes in TcdA using cryoEM. At low pH, the ‘head-like’ delivery domain extends away from the ‘tail-like’ binding domain, and the Glc domain loses structural stability. At neutral pH, the delivery domain appears ‘locked’ in a non-pore-forming state, while low pH relieves this inhibition. Intriguingly, TcdB produced by hypervirulent strains (TcdBHV) undergoes pH-dependent hydrophobic transitions at a higher pH than TcdB produced by historical strains (TcdBhist) [11]. These pH-inducible structural changes correlate with more rapid kinetics of entry, providing a potential explanation for why TcdBHV is more cytotoxic than TcdBhist.
(III) Autoprocessing of Glucosylating Toxins in Target Cells
Upon translocation of the Glc domain into the target cell cytosol, the CPD autoproteolytically cleaves the toxin at a conserved Leu residue to release the Glc domain from the endosomal membrane [41]. The CPD is part of the C80 family of proteases (MEROPS peptidase classification [42]), which are site-specific cysteine proteases (catalytic Cys-His dyad) that autoprocess the multidomain glucosylating toxin and MARTX toxin family members in cis [43, 44]. The closest structural homologs of bacterial CPDs are the caspases, with which they share similar mechanisms of substrate recognition and catalysis [45]. Bacterial CPDs are unique in that they act as biosensors for the eukaryotic cell environment, since they are allosterically activated by the eukaryotic-specific small molecule InsP6 [43, 44]. Although the crystal structures of both TcdA CPD and TcdB CPD have been solved bound to InsP6 [46, 47, 48], the molecular details of this allosteric activation event remain unknown due to the absence of an apo-structure. Nevertheless, we recently showed that a conserved β-flap region in TcdB CPD acts as a mechanical couple between the InsP6 binding and active sites [48].
CPD-mediated autoprocessing is necessary for optimal glucosylating toxin function [41, 49], and chemical inhibition of TcdB CPD reduces toxin function in cells [47]. Interestingly, the CPD of TcdBHV autoprocesses more rapidly than the CPD of TcdBhist [Lanis et al., unpubl. results], a property that may accelerate the kinetics of intoxication for TcdBHV relative to TcdBhist [11].
(IV) Glucosylation of Rho GTPases
Autoprocessing of glucosylating toxins by the CPD releases the N-terminal 543-aa Glc domain into the cytosol of target cells [50]. This irreversible event presumably allows the Glc domain to more efficiently interact with its Rho GTPase substrates. Rho GTPases hydrolyze GTP and toggle between GTP- and GDP-bound forms. They function as molecular switches and regulate diverse cellular processes from actin dynamics, proliferation, apoptosis and gene expression [51].
The Glc domain transfers UDP-glucose to a conserved Thr37 in the switch region of Rho GTPases to directly interfere with GTP-GDP switching. This posttranslational modification prevents membrane cycling of Rho GTPases [52] and blocks their binding to downstream effector proteins [53]. As a result, glucosylated Rho GTPases effectively function like dominant-negative Rho GTPases [54]. The net result of Rho GTPase glucosylation is actin cytoskeletal disassembly, cell rounding and cell death.
Although the enzymatic activity of the Glc domain has been well characterized, the molecular links between Rho GTPase glucosylation and cell death still have to be established. Glc activity is necessary to induce apoptosis in a wide variety of cell lines [55, 56, 57, 58] but is dispensable for inducing inflammasome activation in mice [23]. The Glc domain of TcdA may also induce α-tubulin deacetylation by HDAC6 tubulin deacetylase [59] and thus reduce microtubule polymerization.
The Glc domain shares a similar fold with type A family Glcs and the catalytic DXD motif, which binds Mn2, UDP and glucose [60]. Mutational analyses of the Glc domain have identified a number of residues required for enzyme activity, cosubstrate binding and substrate recognition, respectively [35, 61]. TcdA and TcdB exclusively use the cosubstrate UDP-glucose; this substrate specificity can be modulated by mutating cosubstrate interacting residues [61]. The catalytic core of the Glc domain consists of 234 residues, with the remaining residues forming helices that surround the core [60]; the 4 N-terminal helices function as a membrane localization domain in green fluorescent protein fusion studies [62].
C. difficile Transferase
C. difficile strains can also produce a third toxin called CDT or binary toxin [1, 24]. CDT is an ADP-ribosyltransferase of the ADPRT family, which includes clostridial ADP-ribosylating toxins such as C. perfringens iota toxin and C. botulinum C2 toxin. ADPRT toxins are classical AB toxins, with an ADP-ribosyltransferase activity subunit and a binding/translocation subunit (CDTa and CDTb, respectively; fig. 3a) [63]. The binding subunit is activated by proteases at the target cell surface; proteolytic cleavage allows the binding subunit to oligomerize and form a complex with the activity subunit (fig. 3b) [63]. The AB toxin complex then binds to unknown receptors on the target cells and enters via endocytosis. During endosomal acidification, the binding/translocation subunit forms a pore that delivers the activity domain into the cytosol. The activity domain subsequently ADP-ribosylates actin, which leads to disassembly of the actin cytoskeleton, cell rounding, loss of fluid and ultimately cell death.
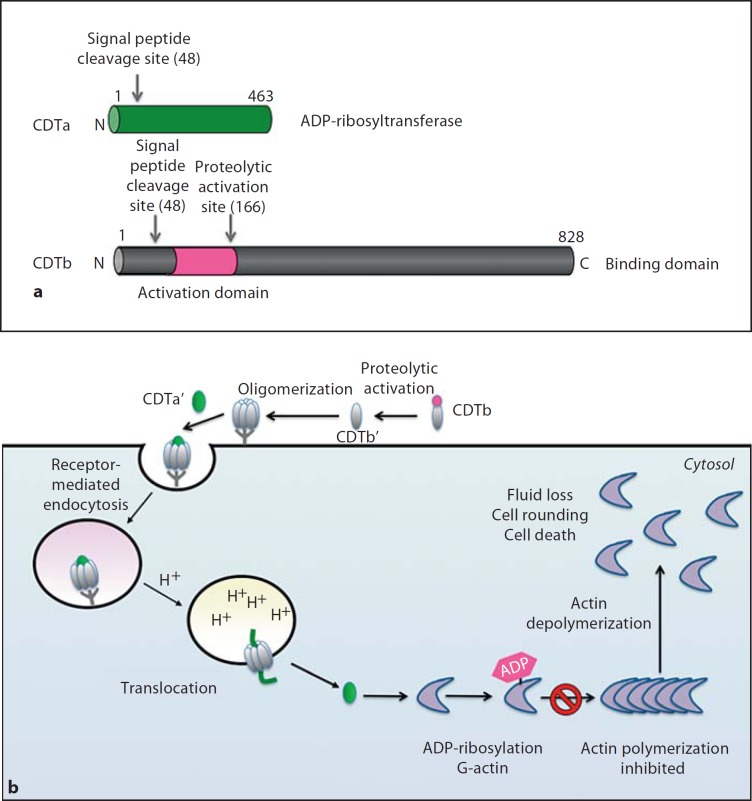
Fig. 3.
aCDT is comprised of 2 polypeptides, CDTa (ADP-ribosyltransferase, green) and CDTb (receptor binding domain, grey). Signal peptide sequences of both polypeptides are shown. CDTb contains a propeptide whose proteolytic removal allows the activated binding domain (CDTb’ in b) to interact with as yet unidentified receptors on target cells. b Intoxication of target cells by CDT. Following proteolytic activation on the target cell surface, the binding domain oligomerizes and binds to cell receptors. Binding results in receptor-mediated endocytosis. During acidification of the endosome, the binding domain undergoes conformational rearrangements that result in pore formation and translocation of the ADP-ribosyltransferase domain into the target cell cytosol in an Hsp90-dependent manner. The ADP-ribosyltransferase domain ADP-ribosylates monomeric G-actin on Arg177, which blocks actin polymerization and ultimately leads to the dissolution of the actin cytoskeleton. CDTa also induces formation of microtubule projections, which may enhance the adherence of C. difficile to the intestinal epithelium.
CDT and Virulence
The biological significance of CDT during infection remains an open question. The genes encoding CDT, cdtA and cdtB, are found in about 10% of clinical isolates [1], although they are conserved in NAP1/027 hypervirulent strains and other clinical strains with increasing frequency [64]. In animal models of infection, TcdA–, TcdB– and CDT+ strains do not cause disease despite high rates of colonization, although their culture supernatants induce massive edema in rabbit ileal loops [65]. These findings suggest that, although CDT is not sufficient to cause disease, it may enhance the pathogenesis of glucosylating toxin-positive strains. Consistent with this hypothesis, recent epidemiological analyses showed that patients infected with strains producing CDT had 60% higher fatality rates than patients infected with CDT-deficient strains [13]. Determining the specific contribution of CDT to pathogenesis will likely be facilitated by the recent development of genetic knock-out tools for C. difficile [66]. It is worth noting, however, that the animal models generally used to evaluate the role of C. difficile do not fully recapitulate human disease and thus may underestimate the contribution of CDT to C. difficile pathogenesis [28].
Structure and Function of CDT
CDT is composed of two separate polypeptides, CdtA (49 kDa) and CdtB (99 kDa), which function as the activity domain and receptor binding/translocation domains, respectively [24]. The structure of CDTa is similar to the enzymatic domain of C. perfringens Iota toxin, although they exhibit slight differences in their mode of ligand recognition [67]. Like other ADP-ribosylating toxins, the CDTa-CDTb complex induces cell rounding and cell death in Vero cells [68, 69], and the uptake of CDT into cells also requires endosomal acidification [70]. Kaiser et al. [70] recently showed that the translocation of CDTa into the cytosol is dependent on the target cell proteins Hsp90 and cyclophilin, which directly interact with CDTa in vitro. Since similar results were obtained with C. botulinum C2 toxin, the authors propose a common Hsp90/cyclophilin A-dependent translocation mechanism for ADPRT family members.
Following the CDTb-mediated translocation of CDTa into the cytosol, CDTa ADP-ribosylates G-actin on Arg177 [24]. This modification blocks actin polymerization and leads to dissolution of the actin cytoskeleton. CDT also induces the formation of dramatic ‘net-like’ microtubule protrusions (5–100 μΜ) on the surface of intestinal epithelial cells [71]. Formation of these microtubule protrusions, which are devoid of actin, preferentially occurs in cholesterol- and sphingolipid-rich lipid microdomains [72]. These dynamic protrusions increased C. difficile adherence to epithelial cells 5-fold in vitro and 4-fold in vivo in a murine model of infection, suggesting a mechanism by which CDT may promote colonization [71].
Modulation of Immune Responses as a Therapeutic Strategy
Although prior antibiotic treatment is a major predisposing factor to CDI, the antibiotics metronidazole and vancomycin are usually used to treat it [1, 4]. Because these therapies continue to suppress normal gut flora, CDIs recur in approximately 25% of cases. Since much of the pathology associated with CDI results from the acute host inflammatory response stimulated by the glucosylating toxins, it has been proposed that a combination of antibiotic and anti-inflammatory agents might be an effective treatment strategy [15]. Consistent with this proposal, a number of studies have shown that anti-inflammatory agents can reduce CDI severity in animal models of infection [18].
Nevertheless, certain types of innate immune signaling may actually prevent C. difficile-induced immunopathology. Jarchum et al. [73] recently demonstrated that administration of the TLR5-agonist Salmonella-derived flagellin was protective against C. difficile-induced colitis. The authors proposed that TLR5 signaling induced by intestinal microbiota stimulates productive innate immune responses that render mice resistant to CDI. This hypothesis is based on the observations that (1) antibiotic pretreatment sensitizes mice to CDI [74], (2) antibiotic pretreatment reduces TLR signaling in mice [73] and (3) mice defective in innate immune signaling (MyD88-deficient) are more susceptible to CDI [74].
Conclusions and Future Considerations
C. difficile has become a significant public health threat in the past decade largely due to the emergence/selection of hypervirulent strains that persist in healthcare-associated settings and cause more severe infections. These strains are now being associated with disease in healthy individuals who are not part of the population considered to be at risk [1]. A better understanding of how these new hypervirulent and historical strains colonize and cause disease will be critical to developing new therapies for ameliorating or preventing CDI. With the advent of new tools that permit the genetic manipulation of C. difficile [66], it will become possible to test the contribution of individual factors, such as the glucosylating toxins, to the pathogenesis of hypervirulent strains.
Understanding the mechanisms by which the glucosylating toxins produced by hypervirulent strains (TcdHV) are more cytotoxic than those produced by historical strains (TcdHist) will require a better understanding of the conformational rearrangements that lead to toxin activation during entry into target cells. As TcdHV appears to be more conformationally flexible than TcdHist [11], it will be important to identify and characterize the structural components that contribute to this enhanced flexibility, particularly in response to pH. Such studies may facilitate the development of new classes of inhibitors that allosterically regulate the conformational dynamics of glucosylating toxins and prevent their proper functioning [75].
A recent report (publ. during revision of this manuscript) has identified host S-nitrosylation as a previously unrecognized innate defense mechanism against C. difficile [76]. Using recently developed proteomic methods, Savidge et al. [76] demonstrated that the glucosylating toxins are S-nitrosylated during intoxication of mice and humans as part of the nitric oxide innate immune response. Their results indicate that this posttranslational modification inactivates toxin function by targeting the CPD domain. The authors further demonstrated that treatment of mice with exogenous InsP6 and S-nitrothi-ols can actually protect mice from CDI, suggesting that enhancing nitrothiol-based innate immunity may represent a viable therapeutic approach. Understanding the precise mechanism by which S-nitrosylation modulates toxin function is an area ripe for investigation.
Delineating how glucosylating toxins modulate other host innate immune responses, particularly how they stimulate the inflammasome, requires further investigation. While a link between between Rho GTPase signaling and inflammasome activation has been noted, the molecular mechanism(s) relating these two processes remain unknown [77]. Such analyses will be aided by the recent development of a more reliable murine model of CDI [74], which opens up the possibility for identifying host determinants that protect individuals or sensitize them to CDI. Lastly, future studies should also examine the role that host microbiota play in regulating innate immune responses to CDI.
Recent advances in genetic tools for C. difficile and improved animal models of infection will also permit the contribution of CDT to virulence to be determined. Further studies are required to determine whether CDT serves as a marker for severe infection and/or enhances pathogenesis. These analyses will be aided by studies examining the precise effects of CDT on the intestinal epithelium.
Disclosure Statement
The author declares no conflict of interests.
Acknowledgements
This work was supported by grants from the NIH National Institutes of General Medical Sciences (R00GM092934) and National Center of Research Resources (P20-RR021905).
References
- 1.Carroll KC, Bartlett JG. Biology of Clostridium difficile: implications for epidemiology and diagnosis. Annu Rev Microbiol. 2011;65:501–521. doi: 10.1146/annurev-micro-090110-102824. [DOI] [PubMed] [Google Scholar]
- 2.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nature Rev. 2009;7:526–536. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 3.Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev. 2005;18:247–263. doi: 10.1128/CMR.18.2.247-263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerding DN, Johnson S. Management of Clostridium difficile infection: thinking inside and outside the box. Clin Infect Dis. 2010;51:1306–1313. doi: 10.1086/657116. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett JG. Clinical practice: antibiotic-associated diarrhea. N Engl J Med. 2002;346:334–339. doi: 10.1056/NEJMcp011603. [DOI] [PubMed] [Google Scholar]
- 6.McGlone SM, Bailey RR, Zimmer SM, Popovich MJ, Tian Y, Ufberg P, Muder RR, Lee BY. The economic burden of Clostridium difficile. Clin Microbiol Infect. 2011 doi: 10.1111/j.1469-0691.2011.03571.x. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Brien JA, Lahue BJ, Caro JJ, Davidson DM. The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol. 2007;28:1219–1227. doi: 10.1086/522676. [DOI] [PubMed] [Google Scholar]
- 8.McDonald LC, Killgore GE, Thompson A, Owens RC, Jr, Kazakova SV, Sambol SP, Johnson S, Gerding DN. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433–2441. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 9.Akerlund T, Persson I, Unemo M, Noren T, Svenungsson B, Wullt M, Burman LG. Increased sporulation rate of epidemic Clostridium difficile type 027/nap1. J Clin Microbiol. 2008;46:1530–1533. doi: 10.1128/JCM.01964-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, Frost E, McDonald LC. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–1084. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 11.Lanis JM, Barua S, Ballard JD. Variations in TcdB activity and the hypervirulence of emerging strains of Clostridium difficile. PLoS Pathog. 2010;6:e1001061. doi: 10.1371/journal.ppat.1001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stabler RA, Dawson LF, Phua LT, Wren BW. Comparative analysis of bi/nap1/027 hypervirulent strains reveals novel toxin B-encoding gene (TcdB) sequences. J Med Microbiol. 2008;57:771–775. doi: 10.1099/jmm.0.47743-0. [DOI] [PubMed] [Google Scholar]
- 13.Bacci S, Molbak K, Kjeldsen MK, Olsen KE. Binary toxin and death after Clostridium difficile infection. Emerg Infect Dis. 2011;17:976–982. doi: 10.3201/eid1706.101483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schirmer J, Aktories K. Large clostridial cytotoxins: cellular biology of Rho/Ras-glucosylating toxins. Biochim Biophys Acta. 2004;1673:66–74. doi: 10.1016/j.bbagen.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Kelly CP, Kyne L. The host immune response to Clostridium difficile. J Med Microbiol. 2011;60:1070–1079. doi: 10.1099/jmm.0.030015-0. [DOI] [PubMed] [Google Scholar]
- 16.Babcock GJ, Broering TJ, Hernandez HJ, Mandell RB, Donahue K, Boatright N, Stack AM, Lowy I, Graziano R, Molrine D, Ambrosino DM, Thomas WD., Jr Human monoclonal antibodies directed against toxins A and B prevent Clostridium difficile-induced mortality in hamsters. Infect Immun. 2006;74:6339–6347. doi: 10.1128/IAI.00982-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson S. Antibody responses to clostridial infection in humans. Clin Infect Dis. 1997;25((suppl 2)):S173–S177. doi: 10.1086/516220. [DOI] [PubMed] [Google Scholar]
- 18.Pothoulakis C. Effects of Clostridium difficile toxins on epithelial cell barrier. Ann NY Acad Sci. 2000;915:347–356. doi: 10.1111/j.1749-6632.2000.tb05263.x. [DOI] [PubMed] [Google Scholar]
- 19.Warny M, Keates AC, Keates S, Castagliuolo I, Zacks JK, Aboudola S, Qamar A, Pothoulakis C, LaMont JT, Kelly CP. P38 map kinase activation by Clostridium difficile toxin A mediates monocyte necrosis, IL-8 production, and enteritis. J Clin Invest. 2000;105:1147–1156. doi: 10.1172/JCI7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savidge TC, Pan WH, Newman P, O'Brien M, Anton PM, Pothoulakis C. Clostridium difficile toxin B is an inflammatory enterotoxin in human intestine. Gastroenterology. 2003;125:413–420. doi: 10.1016/s0016-5085(03)00902-8. [DOI] [PubMed] [Google Scholar]
- 21.Pepin J, Vo TT, Boutros M, Marcotte E, Dial S, Dube S, Vasilevsky CA, McFadden N, Patino C, Labbe AC. Risk factors for mortality following emergency colectomy for fulminant Clostridium difficile infection. Dis Colon Rectum. 2009;52:400–405. doi: 10.1007/DCR.0b013e31819a69aa. [DOI] [PubMed] [Google Scholar]
- 22.Lamkanfi M, Dixit VM. Inflammasomes: guardians of cytosolic sanctity. Immunol Rev. 2009;227:95–105. doi: 10.1111/j.1600-065X.2008.00730.x. [DOI] [PubMed] [Google Scholar]
- 23.Ng J, Hirota SA, Gross O, Li Y, Ulke-Lemee A, Potentier MS, Schenck LP, Vilaysane A, Seamone ME, Feng H, Armstrong GD, Tschopp J, Macdonald JA, Muruve DA, Beck PL. Clostridium difficile toxin-induced inflammation and intestinal injury are mediated by the inflammasome. Gastroenterology. 2010;139:542–552. doi: 10.1053/j.gastro.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Davies AH, Roberts AK, Shone CC, Acharya KR. Super toxins from a super bug: structure and function of Clostridium difficile toxins. Biochem J. 2011;436:517–526. doi: 10.1042/BJ20110106. [DOI] [PubMed] [Google Scholar]
- 25.Lyerly DM, Saum KE, MacDonald DK, Wilkins TD. Effects of Clostridium difficile toxins given intragastrically to animals. Infect Immun. 1985;47:349–352. doi: 10.1128/iai.47.2.349-352.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. The role of toxin A and toxin B in Clostridium difficile infection. Nature. 2010;467:711–713. doi: 10.1038/nature09397. [DOI] [PubMed] [Google Scholar]
- 27.Lyras D, O'Connor JR, Howarth PM, Sambol SP, Carter GP, Phumoonna T, Poon R, Adams V, Vedantam G, Johnson S, Gerding DN, Rood JI. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009;458:1176–1179. doi: 10.1038/nature07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter GP, Rood JI, Lyras D. The role of toxin A and toxin B in Clostridium difficile-associated disease: past and present perspectives. Gut Microbes. 2010;1:58–64. doi: 10.4161/gmic.1.1.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drudy D, Fanning S, Kyne L. Toxin A-negative, toxin B-positive Clostridium difficile. Int J Infect Dis. 2007;11:5–10. doi: 10.1016/j.ijid.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Jank T, Aktories K. Structure and mode of action of clostridial glucosylating toxins: The ABCD model. Trends Microbiol. 2008;16:222–229. doi: 10.1016/j.tim.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Dingle T, Wee S, Mulvey GL, Greco A, Kitova EN, Sun J, Lin S, Klassen JS, Palcic MM, Ng KK, Armstrong GD. Functional properties of the carboxy-terminal host cell-binding domains of the two toxins, TcdA and TcdB, expressed by Clostridium difficile. Glycobiology. 2008;18:698–706. doi: 10.1093/glycob/cwn048. [DOI] [PubMed] [Google Scholar]
- 32.El-Hawiet A, Kitova EN, Kitov P, Eugenio L, Ng KK, Mulvey GL, Dingle TC, Szpacenko A, Armstrong GD, Klassen JS. Binding of Clostridium difficile toxins to human milk oligosaccharides. Glycobiology. 2011;21:1217–1227. doi: 10.1093/glycob/cwr055. [DOI] [PubMed] [Google Scholar]
- 33.Ho JG, Greco A, Rupnik M, Ng KK. Crystal structure of receptor-binding C-terminal repeats from Clostridium difficile toxin A. Proc Natl Acad Sci USA. 2005;102:18373–18378. doi: 10.1073/pnas.0506391102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pruitt RN, Chambers MG, Ng KK, Ohi MD, Lacy DB. Structural organization of the functional domains of Clostridium difficile toxins A and B. Proc Natl Acad Sci USA. 2010;107:13467–13472. doi: 10.1073/pnas.1002199107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jank T, Giesemann T, Aktories K. Clostridium difficile glucosyltransferase toxin B-essential amino acids for substrate binding. J Biol Chem. 2007;282:35222–35231. doi: 10.1074/jbc.M703138200. [DOI] [PubMed] [Google Scholar]
- 36.Stabler RA, He M, Dawson L, Martin M, Valiente E, Corton C, Lawley TD, Sebaihia M, Quail MA, Rose G, Gerding DN, Gibert M, Popoff MR, Parkhill J, Dougan G, Wren BW. Comparative genome and phenotypic analysis of Clostridium difficile 027 strains provides insight into the evolution of a hypervirulent bacterium. Genome Biol. 2009;10:R102. doi: 10.1186/gb-2009-10-9-r102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papatheodorou P, Zamboglou C, Genisyuerek S, Guttenberg G, Aktories K. Clostridial glucosylating toxins enter cells via clathrin-mediated endocytosis. PloS One. 2010;5:e10673. doi: 10.1371/journal.pone.0010673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olling A, Goy S, Hoffmann F, Tatge H, Just I, Gerhard R. The repetitive oligopeptide sequences modulate cytopathic potency but are not crucial for cellular uptake of Clostridium difficile toxin A. PloS One. 2011;6:e17623. doi: 10.1371/journal.pone.0017623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qa'Dan M, Spyres LM, Ballard JD. pH-Induced conformational changes in Clostridium difficile toxin B. Infect Immun. 2000;68:2470–2474. doi: 10.1128/iai.68.5.2470-2474.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Genisyuerek S, Papatheodorou P, Guttenberg G, Schubert R, Benz R, Aktories K. Structural determinants for membrane insertion, pore formation and translocation of Clostridium difficile toxin B. Mol Microbiol. 2011;79:1643–1654. doi: 10.1111/j.1365-2958.2011.07549.x. [DOI] [PubMed] [Google Scholar]
- 41.Egerer M, Giesemann T, Jank T, Satchell KJ, Aktories K. Auto-catalytic cleavage of Clostridium difficile toxins A and B depends on cysteine protease activity. J Biol Chem. 2007;282:25314–25321. doi: 10.1074/jbc.M703062200. [DOI] [PubMed] [Google Scholar]
- 42.Rawlings ND, Barrett AJ, Bateman A. MEROPS: the peptidase database. Nucleic Acids Res. 2010;38:D227–D233. doi: 10.1093/nar/gkp971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Egerer M, Satchell KJ. Inositol hexakisphosphate-induced autoprocessing of large bacterial protein toxins. PLoS Pathog. 2010;6:e1000942. doi: 10.1371/journal.ppat.1000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen A. Allosteric regulation of protease activity by small molecules. Mol Biosystems. 2010;6:1431–1443. doi: 10.1039/c003913f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen A, Lupardus PJ, Albrow VE, Guzzetta A, Powers JC, Garcia KC, Bogyo M. Mechanistic and structural insights into the proteolytic activation of Vibrio cholerae MARTX toxin. Nat Chem Biol. 2009;5:469–478. doi: 10.1038/nchembio.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pruitt RN, Chagot B, Cover M, Chazin WJ, Spiller B, Lacy DB. Structure-function analysis of inositol hexakisphosphate-induced autoprocessing in Clostridium difficile toxin A. J Biol Chem. 2009;284:21934–21940. doi: 10.1074/jbc.M109.018929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puri AW, Lupardus PJ, Deu E, Albrow VE, Garcia KC, Bogyo M, Shen A. Rational design of inhibitors and activity-based probes targeting Clostridium difficile virulence factor TcdB. Chem Biol. 2010;17:1201–1211. doi: 10.1016/j.chembiol.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen A, Lupardus PJ, Gersch MM, Puri AW, Albrow VE, Garcia KC, Bogyo M. Defining an allosteric circuit in the cysteine protease domain of Clostridium difficile toxins. Nat Struct Mol Biol. 2011;18:364–371. doi: 10.1038/nsmb.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kreimeyer I, Euler F, Marckscheffel A, Tatge H, Pich A, Olling A, Schwarz J, Just I, Gerhard R. Autoproteolytic cleavage mediates cytotoxicity of Clostridium difficile toxin A. Naunyn Schmiedebergs Arch Pharmacol. 2010;383:253–262. doi: 10.1007/s00210-010-0574-x. [DOI] [PubMed] [Google Scholar]
- 50.Rupnik M, Pabst S, Rupnik M, von Eichel-Streiber C, Urlaub H, Soling HD. Characterization of the cleavage site and function of resulting cleavage fragments after limited proteolysis of Clostridium difficile toxin B (TcdB) by host cells. Microbiology. 2005;151:199–208. doi: 10.1099/mic.0.27474-0. [DOI] [PubMed] [Google Scholar]
- 51.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 52.Genth H, Aktories K, Just I. Monoglucosylation of RhoA at threonine 37 blocks cytosol-membrane cycling. J Biol Chem. 1999;274:29050–29056. doi: 10.1074/jbc.274.41.29050. [DOI] [PubMed] [Google Scholar]
- 53.Herrmann C, Ahmadian MR, Hofmann F, Just I. Functional consequences of monoglucosylation of Ha-Ras at effector domain amino acid threonine 35. J Biol Chem. 1998;273:16134–16139. doi: 10.1074/jbc.273.26.16134. [DOI] [PubMed] [Google Scholar]
- 54.Just I, Selzer J, Wilm M, von Eichel-Streiber C, Mann M, Aktories K. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500–503. doi: 10.1038/375500a0. [DOI] [PubMed] [Google Scholar]
- 55.Gerhard R, Nottrott S, Schoentaube J, Tatge H, Olling A, Just I. Glucosylation of Rho GTPases by Clostridium difficile toxin a triggers apoptosis in intestinal epithelial cells. J Med Microbiol. 2008;57:765–770. doi: 10.1099/jmm.0.47769-0. [DOI] [PubMed] [Google Scholar]
- 56.Huelsenbeck J, Dreger S, Gerhard R, Barth H, Just I, Genth H. Difference in the cytotoxic effects of toxin B from Clostridium difficile strain VPI 10463 and toxin B from variant Clostridium difficile strain 1470. Infect Immun. 2007;75:801–809. doi: 10.1128/IAI.01705-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nottrott S, Schoentaube J, Genth H, Just I, Gerhard R. Clostridium difficile toxin A-induced apoptosis is p53-independent but depends on glucosylation of Rho GTPases. Apoptosis. 2007;12:1443–1453. doi: 10.1007/s10495-007-0074-8. [DOI] [PubMed] [Google Scholar]
- 58.Qa'Dan M, Ramsey M, Daniel J, Spyres LM, Safiejko-Mroczka B, Ortiz-Leduc W, Ballard JD. Clostridium difficile toxin B activates dual caspase-dependent and caspase-independent apoptosis in intoxicated cells. Cell Microbiol. 2002;4:425–434. doi: 10.1046/j.1462-5822.2002.00201.x. [DOI] [PubMed] [Google Scholar]
- 59.Nam HJ, Kang JK, Kim SK, Ahn KJ, Seok H, Park SJ, Chang JS, Pothoulakis C, Lamont JT, Kim H. Clostridium difficile toxin A decreases acetylation of tubulin, leading to microtubule depolymerization through activation of histone deacetylase 6, and this mediates acute inflammation. J Biol Chem. 2010;285:32888–32896. doi: 10.1074/jbc.M110.162743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reinert DJ, Jank T, Aktories K, Schulz GE. Structural basis for the function of Clostridium difficile toxin B. J Mol Biol. 2005;351:973–981. doi: 10.1016/j.jmb.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 61.Jank T, Reinert DJ, Giesemann T, Schulz GE, Aktories K. Change of the donor substrate specificity of Clostridium difficile toxin B by site-directed mutagenesis. J Biol Chem. 2005;280:37833–37838. doi: 10.1074/jbc.M506836200. [DOI] [PubMed] [Google Scholar]
- 62.Geissler B, Tungekar R, Satchell KJ. Identification of a conserved membrane localization domain within numerous large bacterial protein toxins. Proc Natl Acad Sci USA. 2010;107:5581–5586. doi: 10.1073/pnas.0908700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aktories K, Barth H. Clostridium botulinum C2 toxin – new insights into the cellular up-take of the actin-ADP-ribosylating toxin. Int J Med Microbiol. 2004;293:557–564. doi: 10.1078/1438-4221-00305. [DOI] [PubMed] [Google Scholar]
- 64.Barbut F, Decre D, Lalande V, Burghoffer B, Noussair L, Gigandon A, Espinasse F, Raskine L, Robert J, Mangeol A, Branger C, Petit JC. Clinical features of Clostridium difficile-associated diarrhoea due to binary toxin (actin-specific ADP-ribosyltransferase)-producing strains. J Med Microbiol. 2005;54:181–185. doi: 10.1099/jmm.0.45804-0. [DOI] [PubMed] [Google Scholar]
- 65.Geric B, Carman RJ, Rupnik M, Genheimer CW, Sambol SP, Lyerly DM, Gerding DN, Johnson S. Binary toxin-producing, large clostridial toxin-negative Clostridium difficile strains are enterotoxic but do not cause disease in hamsters. J Infect Dis. 2006;193:1143–1150. doi: 10.1086/501368. [DOI] [PubMed] [Google Scholar]
- 66.Heap JT, Kuehne SA, Ehsaan M, Cartman ST, Cooksley CM, Scott JC, Minton NP. The clostron: mutagenesis in Clostridium refined and streamlined. J Microbiol Methods. 2010;80:49–55. doi: 10.1016/j.mimet.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 67.Sundriyal A, Roberts AK, Shone CC, Acharya KR. Structural basis for substrate recognition in the enzymatic component of ADP-ribosyltransferase toxin CDTa from Clostridium difficile. J Biol Chem. 2009;284:28713–28719. doi: 10.1074/jbc.M109.043018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perelle S, Gibert M, Bourlioux P, Corthier G, Popoff MR. Production of a complete binary toxin (actin-specific ADP-ribosyltransferase) by Clostridium difficile CD196. Infect Immun. 1997;65:1402–1407. doi: 10.1128/iai.65.4.1402-1407.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sundriyal A, Roberts AK, Ling R, McGlashan J, Shone CC, Acharya KR. Expression, purification and cell cytotoxicity of actin-modifying binary toxin from Clostridium difficile. Protein Expr Purif. 2010;74:42–48. doi: 10.1016/j.pep.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 70.Kaiser E, Kroll C, Ernst K, Schwan C, Popoff M, Fischer G, Buchner J, Aktories K, Barth H. Membrane translocation of binary actin-ADP-ribosylating toxins from Clostridium difficile and Clostridium perfringens is facilitated by cyclophilin A and Hsp90. Infect Immun. 2011;79:3913–3921. doi: 10.1128/IAI.05372-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schwan C, Stecher B, Tzivelekidis T, van Ham M, Rohde M, Hardt WD, Wehland J, Aktories K. Clostridium difficile toxin CDT induces formation of microtubule-based protrusions and increases adherence of bacteria. PLoS Pathog. 2009;5:e1000626. doi: 10.1371/journal.ppat.1000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwan C, Noelke T, Kruppke AS, Schubert DM, Lang AE, Aktories K. Cholesterol- and sphingolipid-rich microdomains are essential for microtubule-based membrane protrusions induced by Clostridium difficile transferase CDT. J Biol Chem. 2011;286:29356–29365. doi: 10.1074/jbc.M111.261925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jarchum I, Liu M, Lipuma L, Pamer EG. Toll-like receptor 5 stimulation protects mice from acute Clostridium difficile colitis. Infect Immun. 2011;79:1498–1503. doi: 10.1128/IAI.01196-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lawley TD, Clare S, Walker AW, Goulding D, Stabler RA, Croucher N, Mastroeni P, Scott P, Raisen C, Mottram L, Fairweather NF, Wren BW, Parkhill J, Dougan G. Antibiotic treatment of Clostridium difficile carrier mice triggers a supershedder state, spore-mediated transmission, and severe disease in immunocompromised hosts. Infect Immun. 2009;77:3661–3669. doi: 10.1128/IAI.00558-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee GM, Craik CS. Trapping moving targets with small molecules. Science. 2009;324:213–215. doi: 10.1126/science.1169378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Savidge TC, Urvil P, Oezguen N, Ali K, Choudhury A, Acharya V, Pinchuk I, Torres AG, English RD, Wiktorowicz JE, Loeffelholz M, Kumar R, Shi L, Nie W, Braun W, Herman B, Hausladen A, Feng H, Stamler JS, Pothoulakis C. Host S-nitrosylation inhibits clostridial small molecule-activated glucosylating toxins. Nat Med. 2011;17:1136–1141. doi: 10.1038/nm.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muller AJ, Hoffmann C, Hardt WD. Caspase-1 activation via Rho GTPases: A common theme in mucosal infections? PLoS Pathog. 2010;6:e1000795. doi: 10.1371/journal.ppat.1000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hamm EE, Voth DE, Ballard JD. Identification of Clostridium difficile toxin B cardiotoxicity using a zebrafish embryo model of intoxication. Proc Natl Acad Sci USA. 2006;103:14176–14181. doi: 10.1073/pnas.0604725103. [DOI] [PMC free article] [PubMed] [Google Scholar]